Abstract
Extracellular nucleotides via activation of P2 purinergic receptors influence hepatocyte proliferation and liver regeneration in response to 70% partial hepatectomy (PH). Adult hepatocytes express multiple P2Y (G protein-coupled) and P2X (ligand-gated ion channels) purinergic receptor subtypes. However, the identity of key receptor subtype(s) important for efficient hepatocyte proliferation in regenerating livers remains unknown. To evaluate the impact of P2Y2 purinergic receptor-mediated signaling on hepatocyte proliferation in regenerating livers, wild-type (WT) and P2Y2 purinergic receptor knockout (P2Y2−/−) mice were subjected to 70% PH. Liver tissues were analyzed for activation of early events critical for hepatocyte priming and subsequent cell cycle progression. Our findings suggest that early activation of p42/44 ERK MAPK (5 min), early growth response-1 (Egr-1) and activator protein-1 (AP-1) DNA-binding activity (30 min), and subsequent hepatocyte proliferation (24–72 h) in response to 70% PH were impaired in P2Y2−/− mice. Interestingly, early induction of cytokines (TNF-α, IL-6) and cytokine-mediated signaling (NF-κB, STAT-3) were intact in P2Y2−/− remnant livers, uncovering the importance of cytokine-independent and nucleotide-dependent early priming events critical for subsequent hepatocyte proliferation in regenerating livers. Hepatocytes isolated from the WT and P2Y2−/− mice were treated with ATP or ATPγS for 5–120 min and 12–24 h. Extracellular ATP alone, via activation of P2Y2 purinergic receptors, was sufficient to induce ERK phosphorylation, Egr-1 protein expression, and key cyclins and cell cycle progression of hepatocytes in vitro. Collectively, these findings highlight the functional significance of P2Y2 purinergic receptor activation for efficient hepatocyte priming and proliferation in response to PH.
Keywords: extracellular ATP, P2Y2 purinergic receptors, partial hepatectomy, hepatocyte proliferation, liver regeneration
liver regeneration in response to partial hepatectomy (PH) is a highly coordinated process involving a complex interplay of multiple humoral factors and integration of cell signaling in parenchymal and nonparenchymal cells (45). In response to PH, adult hepatocytes are “reprogrammed” to undergo cell cycle progression and proliferation until tissue restitution is achieved. The factor(s) that trigger cell cycle progression of hepatocytes in response to PH as well as cellular events that play a role in the reestablishment of quiescence upon the completion of liver growth are not well understood.
In response to 70% PH, the remnant livers are subjected to elevated shear stress and trigger the release of factors believed to play key roles in the initiation of proliferative response in hepatocytes (1, 15, 60). Cellular stress including shear stress is a potent trigger for ATP release. Extracellular ATP, via the activation of cell surface P2 purinergic receptors, influences cell signaling, activation of transcription factors, and gene expression (5, 18). In recent years, extracellular ATP is beginning to be recognized as a potential humoral factor influencing multiple liver functions in an autocrine and paracrine manner (58, 70). We have previously shown that extracellular ATP-mediated P2 purinergic receptor activation induces mitogenic signaling and hepatocyte proliferation of primary rat hepatocytes in vitro (67). Our studies in rats infused with P2 purinergic receptor antagonist PPADS prior to 70% PH suggested a role for extracellular ATP-mediated P2 purinergic receptor activation in regenerating livers (67). Recent studies have provided evidence for the rapid release of adenine nucleotides in response to PH and for the requirement of CD39/ENTPD1-mediated catalysis of extracellular nucleotides for efficient liver regeneration (6, 14, 25, 27). These studies highlight the importance of extracellular ATP-mediated purinergic signaling in liver regeneration.
Extracellular ATP effects could potentially be mediated via the activation of multiple isoforms of P2Y (G protein-coupled) and P2X receptors (ligand-gated ion channels) expressed in the liver, but the P2 purinergic receptor subtype(s) important for efficient hepatocyte proliferation remains unknown (5, 22, 26). P2Y2 purinergic receptors are beginning to be recognized as key mediators of proliferation in a number of cell types, but their role in hepatocyte cell cycle progression is not well characterized. In addition to activation of mitogenic signaling pathways that activate cell cycle progression, P2Y2 purinergic receptor activation has the potential to transactivate growth factor-mediated cell signaling and potentiate the effects of EGF on cell cycle progression. The present studies were designed to test the functional significance of P2Y2 purinergic receptor signaling in regenerating livers and to identify the P2Y2 receptor-mediated early events critical for efficient hepatocyte priming and proliferation in response to PH. Normal embryonic liver growth and development in P2Y2−/− mice renders this mouse model a valuable tool in the analysis of perinatal hepatocyte proliferation and liver regeneration in response to 70% PH (32).
Within minutes of PH, activation of mitogen-activated protein kinases (MAPK) and immediate early genes such as Egr-1 (early growth response-1) and activator protein-1 (AP-1) and degradation of extracellular matrix via the activation of the plasminogen-plasmin system and matrix metalloproteases contribute toward a well-coordinated gene expression program that drives cell cycle progression and proliferation of hepatocytes in remnant livers. Egr-1, a key zinc-finger transcription factor, is induced in the remnant livers within 30 min of PH, and Egr-1-deficient mice had impaired hepatocellular mitotic progression and liver regeneration in response to hepatectomy (40, 54, 64). Egr-1 drives cell cycle progression, presumably at G0/G1 transition via transcriptional activation of thymidine kinase (48). Egr-1 was required for timely hepatocyte cell cycle progression at both G0/G1 and G1/S transition after acute carbon tetrachloride exposure (56). However, the identity of humoral factor(s) responsible for the induction of Egr-1 during liver regeneration has remained elusive. Recent studies suggest that extracellular ATP is a potent stimulus for the induction of Egr-1 in multiple cellular systems in vitro (43, 55). Therefore, we reasoned that, in response to PH, elevated shear stress and ATP release into the extracellular milieu, via activation of P2Y2 purinergic receptors, could play a key role in the induction of Egr-1 and the early signaling events necessary for efficient hepatocyte proliferation.
Our findings suggest that hepatocyte priming and cell cycle progression in response to PH is impaired in P2Y2−/− mice. Early activation of p44/42 ERK MAPK signaling (5 min), induction of immediate early genes Egr-1 and AP-1 DNA-binding activity (30 min), as well as hepatocyte proliferation, as assessed by bromodeoxyuridine (BrdU) incorporation (24–72 h), were all impaired in P2Y2−/−. This demonstrates that extracellular ATP-mediated P2Y2 purinergic receptor activation is critical for efficient hepatocyte cell-cycle progression (G0/G1 and G1/S phase) in response to 70% PH. Moreover, extracellular ATP treatment alone was sufficient to induce p44/42 ERK MAPK signaling, Egr-1 protein, cyclins (D1, D3, and A), and BrdU incorporation in primary hepatocytes in vitro, which were dependent on intact P2Y2 purinergic receptor expression in hepatocytes.
MATERIALS AND METHODS
Partial hepatectomy.
Wild-type mice (WT; C57BL6/J) were purchased from Jackson Laboratory (Bar Harbor, ME) and P2Y2−/− mice in C57BL6/J background were a kind gift from Dr. Beverly Koller of University of North Carolina Chapel Hill (32). Mice were housed in a temperature-controlled animal facility with 12-h light-dark cycles and were maintained on standard diet and water. All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Adult male mice (10 to 14 wk; WT and P2Y2−/−) were subjected to 70% PH under isoflurane anesthesia based on the method described by Higgins and Anderson (31). Briefly, the left lateral and median lobes were individually ligated and excised, and the right lateral and caudate lobes (remnant livers) were harvested at various time points (5 min to 8 days). To assess hepatocyte proliferation in response to PH, mice subjected to hepatectomy were injected with BrdU (50 mg/kg) 2 h prior to the harvest of liver tissue and the liver sections were immunostained for BrdU incorporation (44).
Immunohistochemistry.
Formalin-fixed and paraffin-embedded liver sections or primary hepatocytes grown on collagen-coated coverslips were stained for BrdU-positive nuclei with the BrdU labeling and detection kit (Roche, Indianapolis, IN) according to manufacturer's instructions. We analyzed 20 randomly selected high-power fields (×20) either from liver sections from four to five mice per group or from hepatocytes on coverslips. The number of BrdU-positive hepatocytes was counted and expressed as a percentage of total number of hepatocytes, as visualized by counterstaining with hematoxylin. Liver sections were analyzed for β-catenin expression by incubation with anti-β-catenin antibody (BD Biosciences, San Jose, CA). Morphometry of hepatocytes was performed by using the digital photomicrographs of hematoxylin and eosin-stained liver sections of WT and P2Y2−/− mice that were acquired with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). The mean diameter of hepatocytes (in μm) was calculated with computer-assisted image analysis software (Image-Pro Plus 5.1, Media Cybernetics, Silver Spring, MD). A minimum of 10 randomly selected high-power fields (×40) were examined to determine the mean hepatocyte diameter.
Hepatocyte isolation and culture.
Hepatocytes were isolated from 8- to 12-wk-old WT and P2Y2−/− male mice by the two-step collagenase perfusion protocol as described previously, with modifications optimized for mice (7, 44, 67). Hepatocyte preparations with viability over 95%, as screened by Trypan blue exclusion assay, were seeded at a low density of 200,000 cells/35 mm on Primaria tissue culture wells (Becton Dickinson Labware, Franklin Lakes, NJ) or collagen-coated glass coverslips in Williams E complete media with additives (10% fetal bovine serum, 2 mM glutamine, 400 ng/ml dexamethasone, 2.5 μg/ml insulin, 4 ng/ml glucagon, 2.5 μg/ml transferrin, 2.5 ng/ml sodium selenite, 10,000 U/ml penicillin, 10,000 μg/ml streptomycin, 50 μg/ml gentamycin) for 3 h to ensure hepatocyte adherence to plates. Subsequently, hepatocytes were maintained in Williams E minimal media free from serum and growth factors for 24 h prior to treatment with ATP or ATPγS (7, 44, 67).
Western blotting.
Total protein extracts were obtained by homogenizing liver tissues in total lysis buffer (50 mM Tris·HCl, pH 7.5, 0.5 M NaCl, 2 mM EDTA, 2 mM EGTA, 1.0% Triton X-100, 0.25% deoxycholate, 1.0 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin, 1.0 μg/ml leupeptin, 1.0 μg/ml aprotinin, 2.0 mM NaF, 2.0 mM activated Na3VO4) and centrifuging at 14,000 rpm for 10 min (44). Nuclear protein extracts were prepared as described previously (33, 35). Briefly, liver tissues were gently homogenized in lysis buffer (25 mM Tris buffer, pH 7.5 containing 0.5 mM EDTA, 1.5 mM MgCl2, 420 mM NaCl, and protease inhibitors). Nuclear fractions were isolated by centrifugation at 4,000 rpm, and proteins were extracted with 25 mM Tris buffer, pH 7.5, containing 1 mM EDTA, 2 mM MgCl2, 50 mM KCl, 1 mM DTT, and protease inhibitors, by incubating at 4°C for 30 min and centrifuging at 14,000 rpm for 5 min (44). Equal amounts of total or nuclear proteins as determined by BCA protein assay (Pierce, Rockford, IL) were analyzed by Western blotting as described previously. Blots were probed with antibody specific for histone H3 (nuclear) or α-tubulin or β-actin (total protein) to ensure equal loading of proteins in each lane (44).
EMSA.
Nuclear protein extracts from the resected lobes (0 h) and remnant livers harvested at various time points post-PH were analyzed for Egr-1 (0.5 h), AP-1 (0.5 h), and NF-κB (1 h) DNA-binding activity by electrophoretic mobility shift assay (EMSA) as described previously (33). Briefly, 10 μg of nuclear protein extracts were incubated with poly-dI-dC (1 μg/ml) in binding buffer for 10 min at 4°C prior to incubation with 2 × 104 cpm of 32P end-labeled oligonucleotide as described previously (33). Specificity of DNA-binding activity was determined by the addition of 100-fold molar excess of the specific consensus or mutant unlabeled oligonucleotides in the binding reaction mixtures, along with the radiolabeled nucleotides. Gels were dried and autoradiographed by using Hyperfilm (Amersham) at −70°C for 1 to 3 days (44, 67).
Real-time qRT-PCR.
Total RNA was isolated from the resected lobes (0 min) and remnant livers harvested at 0.5 to 72 h post-PH, by using Qiagen RNeasy mini kit according to manufacturer's instructions (Qiagen Sciences, Germantown, MD). Complementary DNA (cDNA) synthesis was performed by reverse transcription of total RNA (2 μg) with high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). The cDNA product was amplified by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) in a ABI Prism 7,700 sequence-detection system using TaqMan Universal PCR master mix (Applied Biosystems) with SYBR Green. Primer sequences: p21 (Forward 5′-gctgtcttgcactctggtgt-3′; Reverse 5′-ctgcgcttggagtgatagaa-3′), p27 (Forward 5′-tctcaggcaaaactctgagga-3′, Reverse 5′-cttcctcatccctggacact-3′), Egr-1 (Forward 5′-agcgaacaaccctatgagcac-3′, Reverse 5′-tcgtttggctgggataactcg-3′), TNF-α (Forward 5′-catcttctcaaaattcgagtgacaa-3′, Reverse 5′-tgggagtagacaaggtacaaccc-3′), and IL-6 (Forward 5′-ccggagaggagacttcacaga-3′, Reverse 5′-agaattgccattgcacaactctt-3′). Quantitative expression values were extrapolated from standard curves and normalized to cyclophilin, as described previously (37).
Statistical analysis.
Data are presented as means ± SE. The statistical significance of difference between groups was analyzed by unpaired Student's t-test. Values of P < 0.05 were considered statistically significant.
RESULTS
Hepatocyte proliferation and cell-cycle progression in response to PH is impaired in P2Y2−/−.
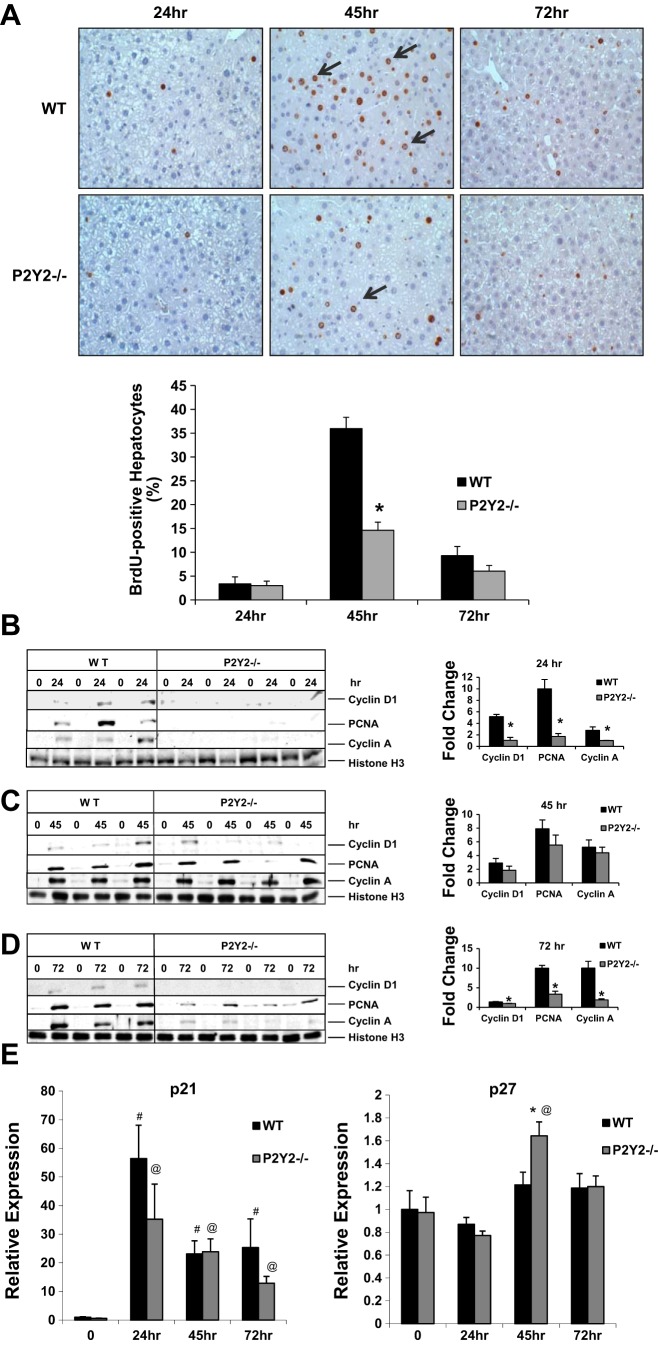
We first analyzed the influence of P2Y2 receptors on hepatocyte proliferation, as determined by the incorporation of BrdU in hepatocyte nuclei at 24, 45, and 72 h after hepatectomy. Partial hepatectomy induced robust DNA synthesis in WT, with 36% of hepatocytes BrdU positive at 45 h post-PH. Hepatocyte proliferation was significantly impaired in the P2Y2−/−, however, with BrdU labeling detected in only 15% of hepatocytes (Fig. 1A).
Fig. 1.
Hepatocyte proliferation in response to partial hepatectomy (PH) is impaired in P2Y2−/−. A: light microscopic images (×20) of bromodeoxyuridine (BrdU) immunostaining of liver sections. BrdU-positive hepatocytes expressed as a percentage of total hepatocytes; n = wild-type (WT): 5, knockout (KO): 4 (24 h); WT: 4, KO: 5 (45 h): WT: 5, KO: 4 (72 h). B: consecutive lanes (0, 24) represent Western blotting of nuclear extracts isolated from the resected lobes (0 h) and remnant livers harvested at 24 h post-PH (24 h) of the same animal; n = WT: 3, KO: 4. Histone H3, nuclear protein loading control. Fold change is calculated based on densitometric analysis of band intensities with reference to the respective resected lobe (0 h) of each animal. C: consecutive lanes (0, 45) represent Western blotting of nuclear extracts isolated from the resected lobes (0 h) and remnant livers harvested at 45 h post-PH (45 h) of the same animal; n = WT: 3, KO: 4. Histone H3, nuclear protein loading control. Fold change is calculated based on densitometric analysis of band intensities with reference to the respective resected lobe (0 h) of each animal. D: consecutive lanes (0, 72) represent Western blotting of nuclear extracts isolated from the resected lobes (0 h) and remnant livers harvested at 72 h post-PH (72 h) of the same animal; n = WT: 3, KO: 4. Histone H3, nuclear protein loading control. Fold change is calculated based on densitometric analysis of band intensities with reference to the respective resected lobe (0 h) of each animal. E: total RNA isolated from the resected lobes (0 h) and remnant livers harvested at 24–72 h post-PH of the WT and P2Y2−/− livers were analyzed by quantitative RT-PCR (qRT-PCR) for p21 and p27 mRNA expression. Fold change is calculated with reference to the expression in the respective resected lobes for each time point tested. Data represent means ± SE; n = WT: 20, KO: 20 (0 h); WT: 4, KO: 4 (24 h); WT: 4, KO: 4 (45 h); WT: 4, KO: 4 (72 h); #P < 0.05 vs. Control (WT, 0 h), @P < 0.05 vs. Control (P2Y2−/−, 0 h); *P < 0.05 vs. WT.
To characterize the role of P2Y2 purinergic receptor signaling in hepatocyte cell cycle progression, nuclear protein extracts isolated from the resected lobes (0 h) and remnant livers harvested at 24, 45, and 72 h post-PH were analyzed by Western blotting for key regulators of cell-cycle progression. Early induction of cyclin D1, PCNA, and cyclin A were attenuated in P2Y2−/− remnant livers (24 h post-PH), comparable at 45 h post-PH, and significantly attenuated at 72 h post-PH, compared with WT (Fig. 1, B–D).
In additions to cyclins, cyclin-dependent kinase inhibitors (CDKI) such as p21Waf1 and p27Kip1 are known to modulate the rate and extent of cell cycle progression in regenerating livers. p21 mRNA induction at 24, 45, and 72 h post-PH was comparable between the WT and P2Y2−/− (Fig. 1C). However, p27 mRNA expression was elevated in P2Y2−/− livers at 45 h post-PH, compared with WT (Fig. 1E).
Extracellular ATP-mediated activation of hepatocyte cell cycle progression in vitro is dependent on P2Y2 expression.
Isolated primary hepatocytes in culture is a well-established in vitro model system for the study of hepatocyte proliferation, especially the characterization of direct effects of growth factors on hepatocytes and their influence on stepwise induction of key cyclins driving cell cycle progression. These studies have established that hepatocytes undergo a “growth restriction point” at late G1 phase, based on observations that growth factor stimulation and induction of cyclin D1 is necessary for hepatocyte cell cycle progression beyond this point (42). We have previously shown that extracellular ATP treatment alone is sufficient to induce cell cycle progression of rat hepatocytes in vitro (67).
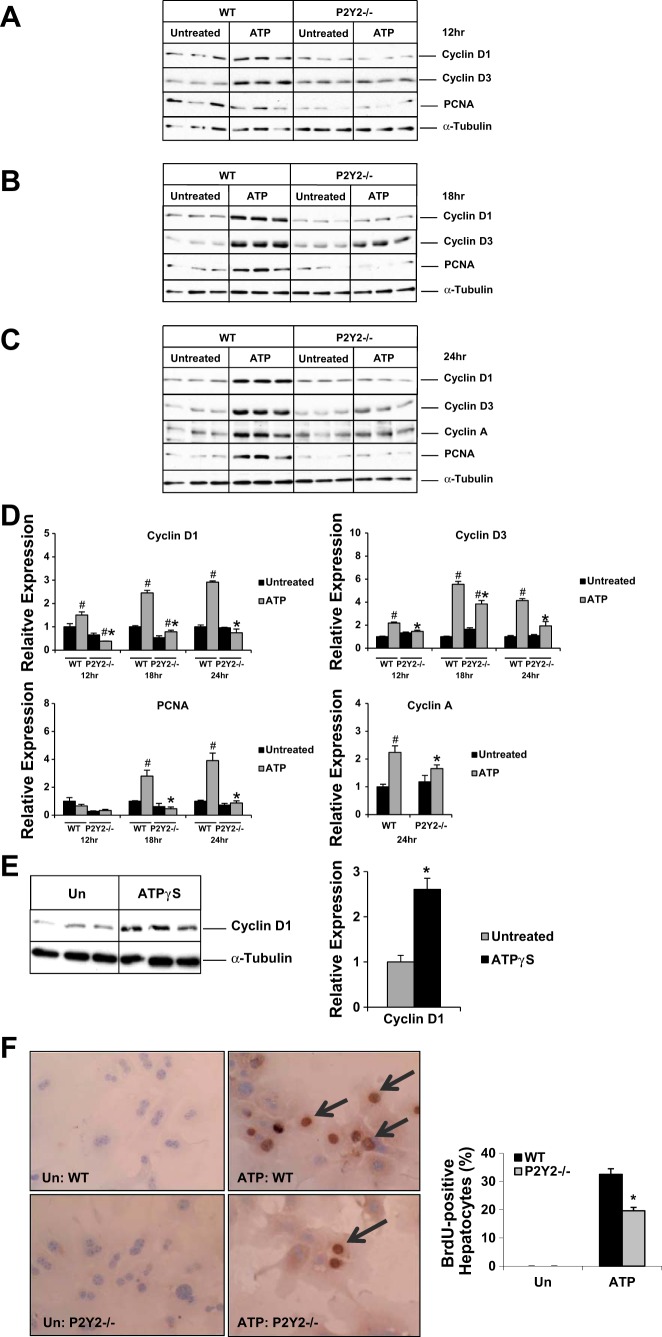
To determine whether P2Y2 purinergic receptor activation is necessary for ATP-mediated effects on hepatocyte cell cycle progression, primary hepatocytes isolated from the WT and P2Y2−/− mice were treated with ATP for 12–24 h. Our results suggest that ATP treatment alone was sufficient to induce cyclin D1 protein induction as early as 12 h in the WT hepatocytes. Cyclin D1 induction was significantly attenuated in P2Y2−/− hepatocytes at 12, 18, and 24 h, compared with WT controls (Fig. 2, A–D). Cyclin D3 induction was detectable at 12 h after ATP treatment with peak induction seen at 18 h. Similar to our findings with cyclin D1, cyclin D3 induction in response to ATP treatment at 12, 18, and 24 h was dependent on intact P2Y2 expression in hepatocytes (Fig. 2, A–D).
Fig. 2.
Hepatocyte proliferation in response to ATP treatment in vitro. Primary hepatocytes isolated from the WT and P2Y2−/− mice maintained in serum- and mitogen-free conditions for 24 h prior to treatment with ATP for 12 h; n = 3 each experimental group (WT-Untreated, WT-ATP Treated, KO-Untreated, KO-ATP Treated) (A), 18 h (B), or 24 h (C) and total protein extracts were analyzed by Western blotting. D: densitometric analysis of band intensities. Relative expression is calculated based on densitometric analysis of band intensities with reference to WT (Untreated). E: ATPγS treatment for 24 h; n = 3 each experimental group (WT-Untreated, WT-ATPγS Treated). Un, untreated. F: light microscopic images (×20) of BrdU immunostaining of primary hepatocytes, after 24 h of ATP treatment; n = 5 each experimental group (WT-Untreated, WT-ATP Treated, KO-Untreated, KO-ATP Treated). Data are represented as means ± SE, representative of 3 independent experiments; #P < 0.05 vs. WT (Untreated), *P < 0.05 vs. WT (Treated).
We next evaluated the induction of PCNA, a cofactor for DNA replicase and a well-established marker for DNA synthesis, induced at late G1 and G1/S transition. PCNA induction was detectable as early as 18 h after ATP treatment of WT hepatocytes, which was absent in P2Y2−/− hepatocytes. At 24 h after treatment, PCNA protein induction was significantly attenuated in P2Y2−/− hepatocytes (0.2-fold), compared with WT (3.9-fold) (Fig. 2, A–D). Cyclin A is a key cyclin necessary for cell cycle progression at G1/S and G2/M phases of the cell cycle. ATP treatment alone was sufficient for cyclin A induction in the WT and P2Y2−/− hepatocytes with 36% attenuation in the P2Y2−/− (fold induction: WT, 2.2; P2Y2−/−, 1.4) (Fig. 2, C and D).
ATP can be degraded to ADP and adenosine by ectonucleotidases expressed in hepatocytes (5, 39). To rule out the possibility that ATP treatment effects on hepatocyte proliferation are confounded by the generation of ATP breakdown products in culture, primary hepatocytes isolated from the WT mice were treated with ATPγS (nonhydrolyzable analog of ATP) for 24 h and total protein extracts were analyzed by Western blotting. ATPγS treatment alone was sufficient to induce cyclin D1 expression in primary hepatocytes (Fig. 2E).
To validate our observations that extracellular ATP-mediated activation of P2Y2 purinergic receptor alone was sufficient to induce cyclins (D1, D3, and A) and PCNA protein expression in hepatocytes in vitro and to assess the functional significance of P2Y2 purinergic receptor expression for hepatocyte proliferation, primary hepatocytes isolated from the WT and P2Y2−/− were treated with ATP for 24 h. Suggesting a role for P2Y2 purinergic receptors in extracellular ATP-mediated hepatocyte proliferation, ATP treatment alone was sufficient to induce hepatocyte proliferation in vitro as evidenced by 33% of BrdU labeling in WT, whereas hepatocyte proliferation was attenuated in P2Y2−/− (20%), compared with WT (Fig. 2F). These findings suggest that intact P2Y2 purinergic receptor expression is necessary for efficient hepatocyte proliferation in vitro, in response to ATP treatment.
Early activation of p44/42 ERK in response to PH is impaired in P2Y2−/−.
To assess the role of P2Y2 purinergic receptor activation in the induction of early signaling events, remnant livers (5 min post-PH) were analyzed by Western blotting for the activation of p44/42 ERK. ERK is a key component of mitogenic signaling cascades downstream of a wide array of hepatic mitogens. Partial hepatectomy induced p42/44 ERK phosphorylation and activation in WT mice, and early activation of p42/44 ERK in response to PH was attenuated in P2Y2−/− livers (Fig. 3A).
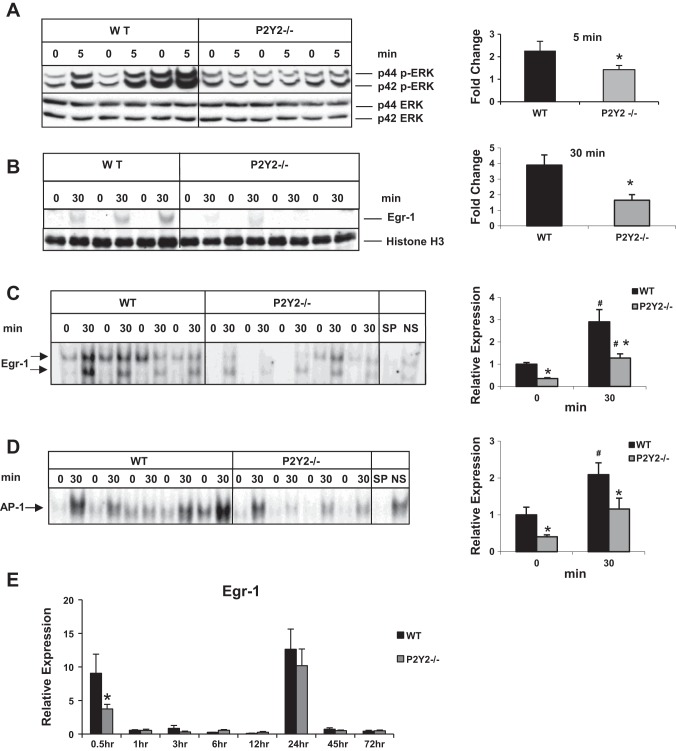
Fig. 3.
Early induction of p42/44 ERK MAPK and Egr-1 in response to PH is attenuated in P2Y2−/−. Western blotting for phospho-p42/44 ERK (Thr202/Tyr204) and total ERK of total proteins from (A) resected lobes (0 min) and remnant livers (5 min) of each animal; n = WT: 3, KO: 3; *P < 0.05 vs. WT. B: Western blotting for Egr-1 from nuclear protein extracts from the resected lobes (0 min) and remnant livers (30 min) of each animal; *P < 0.05 vs. WT; n = WT: 3, KO: 4. C: electrophoretic mobility shift assay (EMSA) for Egr-1 DNA-binding activity of nuclear protein extracts of the resected lobes (0 h) and remnant livers (30 min) of each animal; n = WT: 4, KO: 5. Arrows represent Egr-1-specific DNA binding activity. Specificity of binding was determined based on cold competition with 100-fold excess of specific and nonspecific oligonucleotides; *P < 0.05 vs. WT, #P < 0.05 (0 vs. 30 min). Protein loading control: histone H3 (nuclear). Data represent means ± SE. D: nuclear proteins isolated from the resected lobes (0 h) and remnant livers (30 min) of each animal were analyzed by EMSA for DNA-binding activity with radiolabeled double-stranded oligonucleotides containing AP-1 consensus binding sites. Specificity of AP-1 DNA-binding activity was determined by cold competition assay with 100-fold excess of cold specific and nonspecific oligonucleotides in the incubation mixture; n = WT: 5, KO: 4. E: total RNA isolated from the resected lobes and remnant livers harvested at 0.5–72 h of the WT and P2Y2−/− livers were analyzed by qRT-PCR for Egr-1 mRNA expression. Fold change is calculated with reference to the expression in the respective resected lobes for each time point tested. Data represent means ± SE; n = WT: 9, KO: 10 (0.5 h); WT: 4, KO: 4 (1 h); WT: 4, KO: 4 (3 h); WT: 4, KO: 4 (6 h); WT: 4, KO: 4 (12 h), WT: 12, KO: 11 (24 h); WT: 4, KO: 4 (45 h); WT: 4, KO: 4 (72 h). *P < 0.05 vs. WT, #P < 0.05 vs. WT Control (0 h).
Immediate early genes Egr-1 and AP-1 DNA-binding activity is attenuated in P2Y2−/−.
In response to 70% PH, hepatocytes in remnant livers are the first to enter cell cycle and transition from G0 to G1, which is characterized by the induction of immediate early genes, most notably Egr-1 (51, 54, 64). Partial hepatectomy activated a robust and early induction of Egr-1 protein expression (4.0-fold) in remnant livers, which was significantly attenuated in P2Y2−/− (1.6-fold) (Fig. 3B). Partial hepatectomy induced Egr-1 DNA-binding activity (2.9-fold) in the remnant livers of WT mice. Corresponding to the impairment of Egr-1 protein expression, Egr-1 DNA-binding activity of nuclear proteins from the remnant livers (30 min) of P2Y2−/− mice was impaired by 56% (Fig. 3C). Further validating the influence of P2Y2 purinergic receptor signaling on early events associated with regenerating livers, induction of AP-1 DNA-binding activity was attenuated in P2Y2−/− livers at 0.5 h post-PH (Fig. 3D).
In addition to its role as immediate early gene influencing hepatocyte priming (G0 to G1) in regenerating livers, studies with Egr-1−/− have highlighted important roles for Egr-1 in hepatocellular G1/S-phase transition and metaphase-to-anaphase mitotic progression following peak DNA synthesis. We evaluated induction of Egr-1 mRNA expression at early time points (0.5–6 h) as well as hepatocyte proliferative phase (12–72 h) in the WT and P2Y2−/− remnant livers post-PH. Egr-1 mRNA induction was significantly elevated at 0.5 h (G0/G1 transition) and 24 h (G1/S transition) post-PH. Interestingly, early induction of Egr-1 (0.5 h) was attenuated in P2Y2−/−; however, Egr-1 induction at 24 h was not dependent on P2Y2 (Fig. 3E).
ATPγS treatment alone is sufficient to induce ERK-dependent Egr-1 protein expression in primary mouse hepatocytes in vitro.
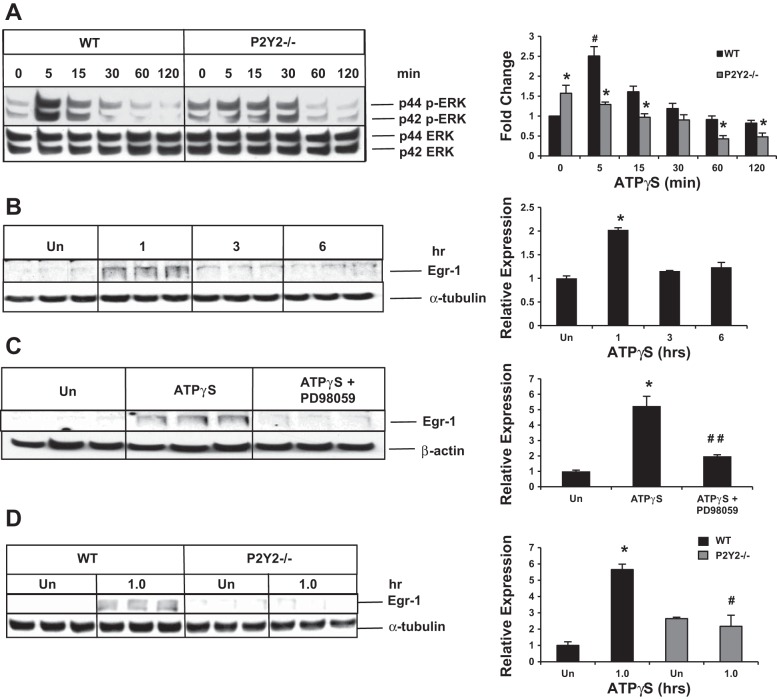
To determine whether ATP treatment alone, via the activation of P2Y2 purinergic receptors, was sufficient to activate hepatocyte p42/44 ERK activation and to rule out the possibility that ATP treatment effects are influenced by ATP breakdown products such as ADP and adenosine, primary hepatocytes isolated from WT and P2Y2−/− were treated with ATPγS, and total cell lysates were analyzed by Western blotting for the activation of p42/44 ERK (phosphorylation at Thr202 and Tyr404). ATPγS treatment alone was sufficient to induce p42/44 ERK activation in isolated mouse primary hepatocytes from WT mice in vitro, with significant impairment of the induction of p42/44 ERK activation in P2Y2−/− hepatocytes (Fig. 4A).
Fig. 4.
P2Y2 purinergic receptor-dependent p42/44 ERK/MAPK and Egr-1 induction in hepatocytes in vitro. A: representative Western blot of total protein extracts of WT and P2Y2−/− primary hepatocytes after ATPγS treatment (0–120 min); n = 3 each experimental group (WT: 0, 5, 15, 30, 60, 120 min; KO: 0, 5, 15, 30, 60, 120 min). Fold change is calculated based on densitometric analysis of band intensities with reference to WT (0 min). *P < 0.05 vs. WT, #P < 0.05 WT (0 vs. 5 min). B: ATPγS treatment for 1–6 h; n = 3 each experimental group (WT: Un, 1 h, 3 h, 6 h); *P < 0.05 vs. Un (Untreated). C: pretreatment with PD98059 (10 μM) for 30 min prior to treatment with ATPγS for 1 h; n = 3 each experimental group (WT: Un, ATPγS, ATPγS + PD98059); *P < 0.01 vs. Un, ##P < 0.05 vs. ATPγS treatment. D: Western blotting of total protein extracts of primary hepatocytes (WT and P2Y2−/−) after ATPγS for 1 h; n = 3 each experimental group (WT-Un, WT-ATPγS, KO-Un, KO-ATPγS); *P < 0.01 vs. Un; #P < 0.05 vs. WT-ATPγS.
To determine whether ATPγS treatment alone was sufficient to induce Egr-1 protein expression, primary mouse hepatocytes isolated from WT mice and maintained under serum- and mitogen-free conditions for 24 h were treated with 100 μM ATPγS, for 1–6 h. Total protein extracts were analyzed by Western blotting. ATPγS treatment alone was sufficient to induce a transient and robust induction of Egr-1 in primary mouse hepatocytes (Fig. 4B). Pretreatment of hepatocytes with p42/44 ERK inhibitor PD98059 (10 μM) blocked ATPγS-mediated induction of Egr-1, suggesting a role for p42/44 ERK in Egr-1 expression (Fig. 4C).
Egr-1 protein induction in response to ATPγS treatment is attenuated in P2Y2−/− hepatocytes.
To determine whether ATPγS-mediated effect on hepatocyte Egr-1 protein induction is mediated via the activation of P2Y2 purinergic receptors, primary hepatocytes isolated from the WT and P2Y2−/− were treated with ATPγS for 1 h. ATPγS treatment led to a 5.7-fold induction of Egr-1 protein expression in the WT. Despite a modest elevation of basal Egr-1 protein expression, no further induction of Egr-1 protein expression was detectable in P2Y2−/− hepatocytes (Fig. 4D).
Early induction of cytokines and cytokine-mediated signaling was comparable between the WT and P2Y2−/− livers.
Although extracellular ATP and P2 purinergic receptor activation can induce cytokine synthesis and release in cells of monocyte/macrophage lineage, there is no evidence for a role for P2Y2 purinergic receptor-mediated signaling in the elevation of cytokines such as TNF-α and IL-6 in response to PH. Our data suggest that induction of TNF-α and IL-6 (0.5–12 h post-PH) in response to PH were comparable between the WT and P2Y2−/− regenerating livers (Fig. 5A). Furthermore, early activation of NF-κB DNA binding activity (1 h) and phosphorylation of STAT-3 (3 h; Tyr785), targets of cytokine signaling were comparable between the WT and P2Y2−/− livers (Fig. 5, B and C).
Fig. 5.
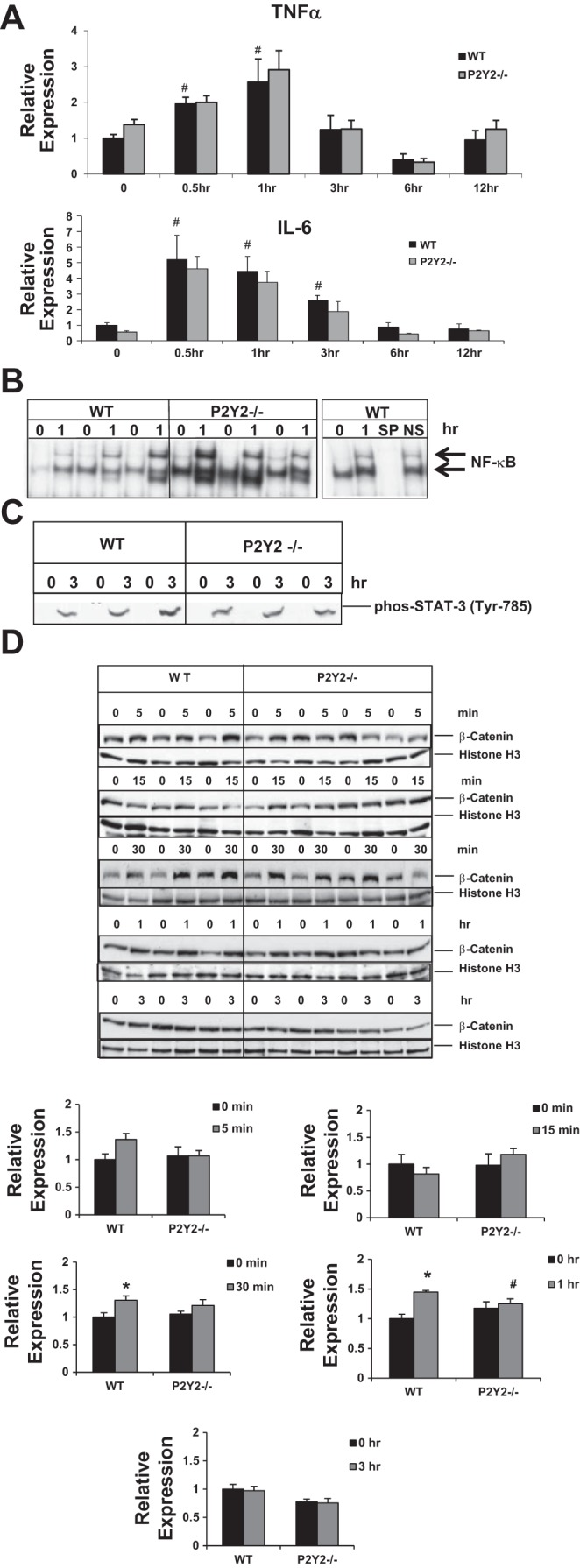
Comparable early induction of cytokines and cytokine-mediated signaling in WT and P2Y2−/− mice in response to 70% PH. WT and P2Y2−/− mice were subjected to 70% PH and the resected lobes (0 h), and remnant livers harvested at 0.5 to 12 h post-PH were analyzed by qRT-PCR (A) for the induction of cytokines (IL-6, TNF-α). Bar diagrams represent fold induction with reference to respective resected lobes (0 h); n = WT: 20, KO: 20 (0 h); WT: 4, KO: 4 (0.5 h); WT: 4, KO: 4 (1 h); WT: 4, KO: 4 (3 h); WT: 4, KO: 4 (6 h); WT: 4, KO: 4 (12 h). B: nuclear protein extracts isolated from the resected lobes (0 h) and remnant livers (1 h) of each animal were analyzed by EMSA for DNA binding activity with radiolabeled double-stranded oligonucleotides containing NF-κB consensus binding sites. Specificity of NF-κB DNA binding activity was determined by cold competition assay with 100-fold excess of cold specific and nonspecific oligonucleotides in the incubation mixture; n = WT: 3, KO: 3. C: total protein extracts were isolated from the resected lobes (0 h) and remnant livers (3 h) of each animal were analyzed by Western blotting for STAT-3 phosphorylation (Tyr-785), marker for the activation of IL-6-mediated activation of JAK-STAT signaling pathway. Data represent means ± SE; n = WT: 3, KO: 3. #P < 0.05 vs. Control (0 h); not significant between the WT and P2Y2−/−. D: nuclear proteins extracted from the respective resected lobes (0 min) and the remnant livers of each animal harvested at 5 min, 15 min, 30 min, 1 h, or 3 h were analyzed by Western blotting for β-catenin. Histone H3, protein loading control. Relative expression was calculated based on densitometric analysis of band intensities, with reference to WT (0 min); n = WT: 3, KO: 4 for each time point tested. Data represent means ± SE; *P < 0.05 vs. WT Control (0 h), #P < 0.05 vs. WT (1 h).
Early activation of β-catenin is attenuated in P2Y2−/−.
Activation and nuclear translocation of β-catenin is an early event in response to PH and toxic liver injury (2, 50). Activated β-catenin is a known mediator of cyclin D1 expression and hepatocyte proliferation in regenerating livers. To determine whether P2Y2 purinergic receptors influence early activation of β-catenin in regenerating livers, nuclear protein extracts of resected lobes (0 h) and remnant livers harvested at 5, 15, 30, and 60 min and 3 h post-PH from the WT and P2Y2−/− mice were analyzed by Western blotting for β-catenin. Nuclear accumulation of β-catenin was apparent at 30 min (31%, P < 0.05) and 60 min (45%, P < 0.05) post-PH in the WT, which was attenuated in P2Y2−/− livers (30 min, 15%; 60 min, 6%; P > 0.05) (Fig. 5D).
Phosphorylation and activation of c-Met is attenuated in P2Y2−/−.
Previous studies have established the importance of hepatocyte growth factor (HGFα)-mediated activation of c-Met signaling for hepatocyte proliferation in response to PH (10, 20). Our data suggest that induction of HGFα mRNA expression in response to PH was comparable between the WT and P2Y2−/− livers (Fig. 6A). However, it has been previously shown that P2Y2 purinergic receptor activation has the potential to modulate growth factor responses via ligand-independent transactivation of receptor tyrosine kinases (41, 62). Phosphorylation of c-Met at Tyr1234/1235 is critical for kinase activation and downstream mitogenic signaling. Suggesting a role for P2Y2 purinergic receptors' influence on HGFα c-Met signaling in regenerating livers, c-Met phosphorylation at Tyr1234/1235 was significantly attenuated in P2Y2−/− at 45 and 72 h post-PH, compared with WT (Fig. 6B).
Fig. 6.
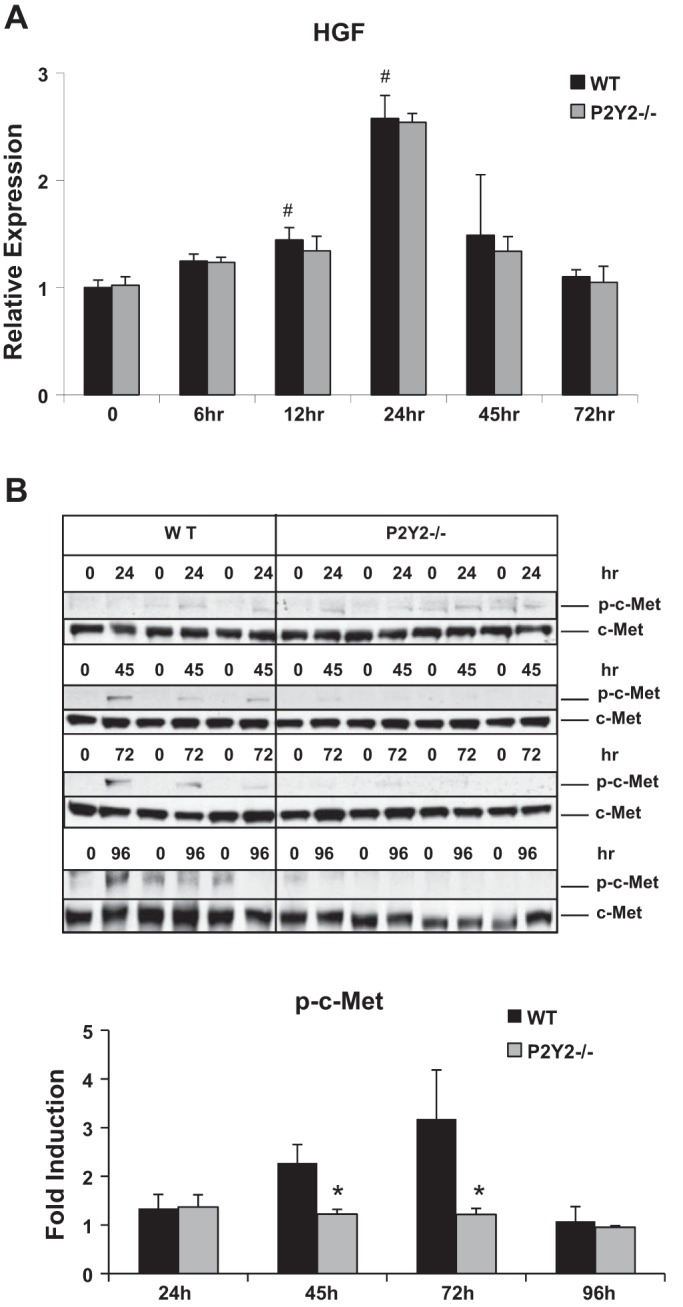
Activation of c-Met signaling in response to PH is attenuated in P2Y2−/−. A: WT and P2Y2−/− mice were subjected to 70% PH and the resected lobes (0 h) and remnant livers harvested at 6 to 72 h post-PH were analyzed by quantitative RT-PCR for the hepatocyte growth factor (HGF). Bar diagram represents fold induction with reference to respective resected lobes (0 h); n = WT: 20, KO: 20 (0 h); WT: 4, KO: 4 (6 h); WT: 4, KO: 4 (12 h); WT: 4, KO: 4 (24 h); WT: 4, KO: 4 (45 h); WT: 4, KO: 4 (72 h). B: total protein extracted from resected lobes (0 min) and remnant livers of each animal at respective 24, 45, 72, 96 h post-PH were analyzed by Western blotting for phospho-c-Met (Tyr1234/1235) and total c-Met. Fold induction of c-Met phosphorylation compared with respective resected lobes (0 min); n = WT: 3, KO: 4 for each time point tested (24, 45, 72, 96 h). Data represent means ± SE; #P < 0.05 vs. WT Control (0 h), *P < 0.05 vs. WT.
P2Y2−/− remnant livers undergo hepatocyte hypertrophy.
Despite impaired proliferation of hepatocytes in P2Y2−/− livers, liver regrowth as assessed by measuring liver weight-to-body weight ratios, were comparable between WT and P2Y2−/− mice, at 1–8 days after hepatectomy (Fig. 7A). A modest but statistically significant difference was observed at 72 h post-PH, which was not maintained at later time points. Interestingly, significant hepatocyte hypertrophy was evident in the P2Y2−/− livers at 8 days post-PH, with a 30% increase in cell size compared with the WT livers (Fig. 7B).
Fig. 7.
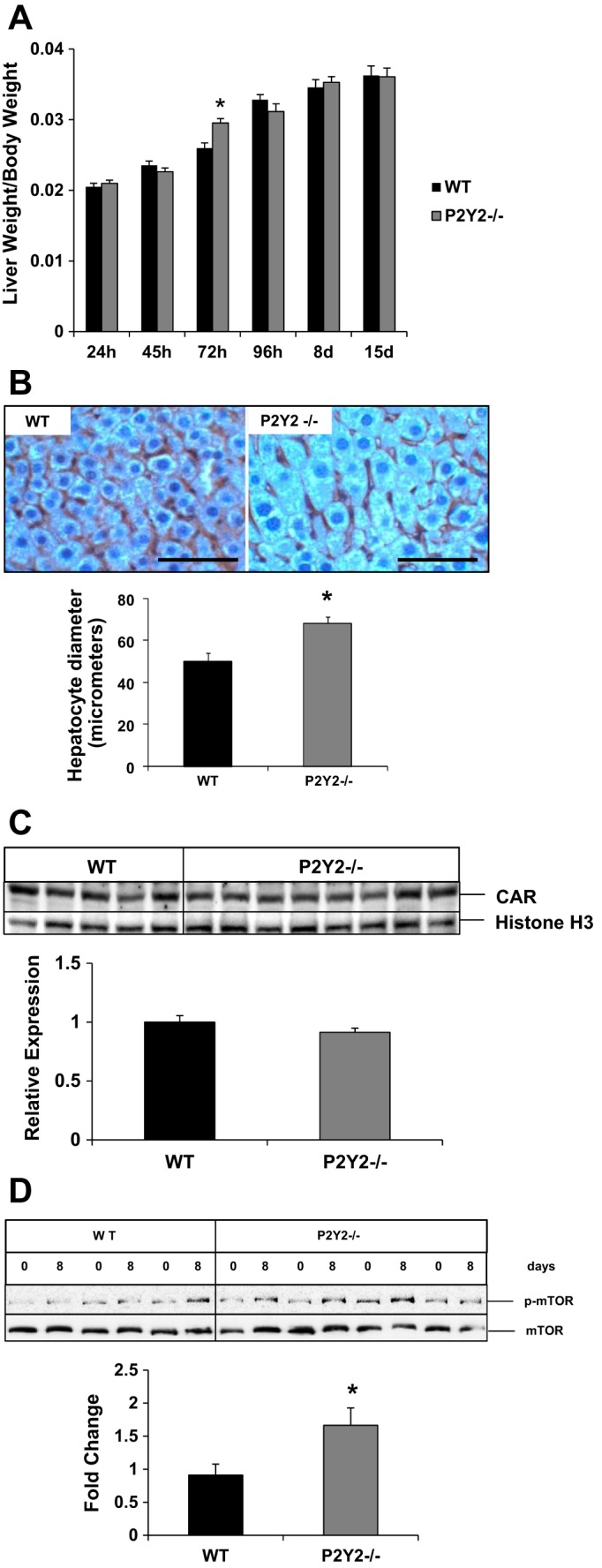
Compensatory hepatocyte hypertrophy occurs in response to PH in P2Y2−/− livers. A: liver growth in response to PH was evaluated at 1–15 days post-PH by analyzing the ratio of liver weight with reference to body weight for each animal; n = WT: 24, KO: 27 (24 h); WT: 16, KO: 24 (45 h); WT: 10, KO: 10 (72 h); WT: 10, KO: 9 (96 h); WT: 9, KO: 16 (8 day); WT: 19, KO: 22 (15 day). Data represent means ± SE; *P < 0.05 vs. WT. B: remnant livers harvested at 8 days of post-PH of WT and P2Y2−/− were subjected to immunohistochemical analysis for β-catenin, and hepatocyte mean diameter (μm) was calculated based on the analysis of light microscopic images (×40) of 10 fields of view with Image-Pro Plus 5.1 software. Bar diagrams represent mean diameter ± SE; n = WT: 3, KO: 3. *P < 0.05 vs. WT. Scale bar represents 100 μm. C: nuclear protein extracts of remnant livers at 8 days post-PH of the WT and P2Y2−/− livers analyzed by Western blotting. Analysis of relative expression was based on densitometric analysis of band intensities, with reference to WT. Histone H3, nuclear protein loading control; n = WT: 5, KO: 8. D: resected lobes (0 h) and remnant livers harvested at 8 days post-PH (8 days) were analyzed by Western blotting for total mammalian target of rapamycin (mTOR) and phosphorylated mTOR (Ser2248). Bar diagram represents densitometric analysis band intensities reflecting fold change with reference to the respective resected lobes (0 h). *P < 0.05 vs; n = WT: 3, KO: 4.
To begin to address the cellular mechanisms responsible for the induction of compensatory hepatocyte hypertrophy observed in P2Y2−/−, we first assessed the activation status of nuclear receptor CAR (constitutive androstane receptor) in response to PH of the WT and P2Y2−/−. CAR is a key mediator of xenobiotic stress, via its nuclear translocation, and activation of target genes induces both hepatocyte hypertrophy and hepatocyte proliferation (16, 59). Nuclear protein extracts isolated from the remnant livers were analyzed by Western blotting for CAR protein expression, which was comparable between the WT and P2Y2−/− at 8 days post-PH (Fig. 7C). Therefore, it is unlikely that CAR activation plays a predominant role in the induction of hepatocyte hypertrophy in P2Y2−/− remnant livers.
Previous studies have suggested a role for activation of mammalian target of rapamycin (mTOR) in the regulation of cell growth, especially when cell proliferation is impaired (28, 29). mTOR, a Ser/Thr protein kinase and a key ATP and nutrient sensor, upon phosphorylation and activation signals protein synthesis and cell growth (73). We reasoned that in the absence of optimal liver mass recovery due to attenuated hepatocyte proliferation in P2Y2−/−, hepatocyte hypertrophy mediated via activation of mTOR signaling may be necessary, to meet the metabolic demands of regenerating liver. Our analysis of total protein extracts by Western blotting revealed that mTOR phosphorylation (Ser2448) and activation were increased (66%) in the remnant livers of P2Y2−/− at 8 days post-PH (Fig. 7D).
DISCUSSION
Despite recent progress in our understanding of the mechanisms of liver regeneration, the molecular identity of humoral factor(s) that serve as the initial trigger for the activation of hepatocytes to undergo cell-cycle progression in response to PH is not well understood. It has long been speculated that shear stress experienced by the hepatic parenchyma in response to PH and the associated hemodynamic changes in the remnant livers play a major role in the initiation of liver regeneration (1). Discrete release of nucleotides in response to a variety of cellular stresses including shear stress has been observed in many cell types (12). Studies by Crumm et al. (14), focusing on very early time points (minutes) after PH, suggest that rapid release of ATP generates early stress signals that contribute to the onset of liver regeneration. Recent studies focusing on extracellular ATP phosphohydrolysis further highlight the importance of extracellular nucleotides as key regulators of liver regeneration (6, 8, 27).
Although the effects of extracellular ATP can be mediated via the activation of multiple P2 purinergic receptor isoforms expressed in the liver, and recent studies suggest a role for P2Y2 receptor activation in hepatocyte proliferation, the molecular mechanisms and mediators of P2Y2 receptor signaling in regenerating livers remain unexplored (5, 67). Moreover, studies performed in rodents and humans subjected to PH suggest that peak ATP release in response to hepatectomy occurs within 5 min of hepatectomy (25, 27). These findings prompted us to first analyze early events influenced by extracellular ATP and P2Y2 receptor activation in regenerating livers.
Our results suggest that early activation of p42/44 ERK MAPK signaling within 5 min of hepatectomy is dependent on intact P2Y2 receptor signaling in remnant livers. ERK activation influences transcriptional upregulation of several immediate early genes such as c-fos/c-jun (AP-1), serum response factor, and Egr-1, which are crucial for efficient hepatocyte priming in regenerating livers (17). In the present study, we identify that the efficient activation of Egr-1 and AP-1 DNA-binding activity as early as 30 min posthepatectomy depends on intact P2Y2 purinergic receptor signaling in regenerating livers. Peng et al. (54) first identified the role of Egr-1 activation as an early event in liver regeneration. Studies by Liao et al. (40) identified a role for Egr-1 in liver regeneration with Egr-1 null mice, which exhibited impaired hepatocellular metaphase-to-anaphase mitotic progression and liver regeneration. Pritchard et al. (56) reported that Egr-1 is required for timely cell cycle entry (G0 to G1) and G1/S phase transition of hepatocytes in response to toxic liver injury. Egr-1 expression is under the influence of a wide array of cytokines and growth factors in the liver. The present study provides experimental evidence that, in vitro, extracellular ATP alone was sufficient to induce ERK activation and Egr-1 protein expression in hepatocytes, and that, in vivo, P2Y2−/− mice had impaired ERK activation at 5 min and Egr-1 protein expression and function at 30 min post-PH. These findings are highly suggestive of the idea that extracellular ATP-mediated activation of P2Y2 purinergic receptor activation is a crucial early event important for efficient hepatocyte proliferation in regenerating livers (Figs. 3 and 4).
ATP treatment induces proliferative responses in multiple cell types, including vascular smooth muscle cells, endothelial cells, cardiac fibroblasts, and embryonic stem cells via activation of proproliferative ERK/MAPK, phosphatidylinositol-3-kinase (PI3K)/Akt, and PKC signaling cascades (11, 19, 30). Higher concentrations of ATP induce cell death in many cancer cell lines via activation of P2X receptor-mediated excessive calcium influx (13, 36, 71). A recent study suggests a negative regulatory role for ATP in regenerating livers via its effects on modulating natural killer cell (NK cell) function (27). Clearly, functional consequences of ATP-mediated signaling is dependent on multiple factors, i.e., acute and chronic changes in concentration of ATP in the extracellular milieu, cell type- and cell maturation-dependent changes in the expression of multiple P2Y and P2X receptor isoforms, and direct vs. indirect effects via its influence on nontarget cells.
Our in vivo studies highlight the importance of P2Y2 purinergic receptor function in the elicitation of effective proliferative response in response to 70% PH (Fig. 1). Adult hepatocytes are highly differentiated quiescent cells, which remain in G0 phase and rarely undergo cell division. Cell cycle progression in response to PH begins with hepatocyte “priming” marked by elevated cytokines such as TNF-α and IL-6 and cytokine-mediated induction of cell signaling (ERK, JNK, MAPK), transcription factor activation (NF-κB, STAT-3), and immediate early gene expression (Egr-1, c-fos, c-jun). During priming phase, hepatocytes transition from G0 to G1 and gain replicative competence. Significance of early priming events associated PH and their role in enhancing hepatocyte responsiveness to growth factors and efficient hepatocyte cell cycle progression is underscored by observations that growth factor infusion alone (without PH) was not sufficient to induce sustained hepatocyte proliferation and liver regeneration (68). Interestingly, TNF-α and IL-6 cytokine induction and transcriptional activation of NF-κB and STAT-3 were comparable between the WT and P2Y2−/−. P2Y2−/− livers exhibit distinct deficits in early activation of ERK signaling and transcriptional activation of immediate early genes (Egr-1, AP-1) in regenerating livers. Thus the present studies have uncovered cytokine-independent and P2Y2 purinergic receptor-dependent early priming events necessary for efficient hepatocyte proliferation in response to PH.
P2Y2 purinergic receptors are G protein (Gαq or Gα0)-coupled cell surface receptors. Extracellular nucleotide-mediated activation of P2Y2 purinergic receptors induce calcium signaling via activation of phospholipase C- and inositol triphosphate-3-dependent calcium release from intracellular stores. Recent studies have defined the functional significance of calcium signaling for efficient hepatocyte cell cycle progression from G0 to G1 and S phases in regenerating livers (15, 38, 52). Among the many intracellular mitogenic targets of calcium signaling, p44/42 ERK MAPKs are well-known mediators of hepatocyte cell cycle progression at the G1 phase and S phase entry (9, 24, 57, 65). In this study, we provide evidence that both ERK MAPK activation in response to 70% PH in vivo and ATP-treatment of isolated hepatocytes in vitro were dependent on intact P2Y2 purinergic receptor expression in hepatocytes.
Previous studies have identified that β-catenin is activated early on in regeneration in response to PH and acetaminophen-induced toxic liver injury (2, 50). Hepatocyte-specific deletion of β-catenin results in delayed hepatocyte proliferation in response to PH (61, 66). Activated β-catenin translocates to nuclei and formation of β-catenin-T-cell factor/lymphoid enhancement factor complex influence target gene expression, i.e., cyclin D1, a key mediator of cell cycle progression at the G1 phase of cell cycle (49). Key molecular mediators of upstream of β-catenin activation in regenerating livers are not well understood; however, both Wnt-dependent and Wnt-independent activation have been implicated (49). Recent studies have identified that growth factor-mediated activation of receptor tyrosine kinases (HGF, EGF) and activation of protein kinase A (thyroid hormone) regulate β-catenin activation via phosphorylation at specific residues (21, 49, 75).
Our results suggest that P2Y2 purinergic receptors influence early activation of ERK/MAPK signaling in regenerating livers. Interaction between ERK/MAPK and Wnt/β-catenin pathways has been described in many cell types (74). For example, GSK-3β is a negative regulator of Wnt/β-catenin signaling and a key component of a cytosolic protein complex, which targets cytosolic β-catenin to proteosomal degradation. ERK activation is known to inactivate GSK-3β activity by direct phosphorylation of S9. Alternatively, ERK-dependent phosphorylation and activation of a ribosomal S6 kinase (p90RSK or MAPK-activated protein kinase) phosphorylates and inactivates GSK-3β. Therefore, ERK activation has the potential to synergize with Wnt/β-catenin signaling by increasing the stability and subsequent nuclear translocation of β-catenin. Alternatively, there is evidence for cross talk between classical G protein-coupled receptors and β-arrestins and the scaffolding proteins critical for Wnt signaling such as Axins and members of the Disheveled (Dvl) family (23). Future studies are required to identify mechanisms responsible for the potential cross-talk between P2Y2 purinergic receptor signaling, and mediators of early activation and nuclear translocation of β-catenin in regenerating livers.
P2Y2 purinergic receptors have 2 Src-homology-3 (SH3) binding domains in the intracellular COOH-terminus facilitating transactivation of growth factor receptors such as epidermal growth factor receptor (EGFR), vascular endothelial growth factor-2 (VEGFR-2), and nerve growth factor/TrkA via Src-dependent phosphorylation of receptor tyrosine kinases (3, 4, 41, 62, 69). Interestingly, c-Met is a receptor tyrosine kinase and a key mediator of HGF signaling, which plays a major role in hepatocyte proliferation in regenerating livers (10, 34, 53). Recent studies have identified ligand-independent and c-Src-mediated transactivation of c-Met receptor signaling influence proliferation in squamous cell carcinoma cell lines (63). Our findings suggest that HGFα mRNA induction in response to PH was comparable between the WT and P2Y2−/− mice, yet c-Met phosphorylation and activation during hepatocyte proliferative phase (24–72 h) was attenuated (Fig. 6). It is likely that, in the absence of P2Y2 purinergic receptor-mediated transactivation, c-Met receptor phosphorylation is not optimal in regenerating livers.
Mirroring our observations on hepatocyte priming and proliferation in response to PH, our in vitro studies with isolated hepatocytes suggest that the P2Y2 purinergic receptors expressed in hepatocytes play a major role in extracellular ATP-mediated mitogenic responses (Fig. 2). Extracellular ATP-mediated stepwise induction of cyclins D1, D3 (late G1), and cyclin A (G1/S and G2/M) and hepatocyte proliferation (S-phase) as assessed by BrdU incorporation and PCNA protein induction were all attenuated in P2Y2−/− hepatocytes. ATP treatment alone was sufficient to induce BrdU incorporation in 36% of WT hepatocytes, whereas BrdU labeling was detected in only 15% of P2Y2−/− hepatocytes under the same conditions. These studies further validate the notion that extracellular ATP is a hepatic mitogen and its direct effects on hepatocyte cell cycle progression are largely dependent on intact P2Y2 purinergic receptor expressed in hepatocytes.
It is of interest to note that, despite defective hepatocyte proliferation in response to PH in P2Y2−/−, liver weight-to-body weight ratios were comparable potentially via the induction of compensatory hepatocyte hypertrophy in the P2Y2−/− remnant livers (Fig. 7B). Similar recovery of liver mass without proliferation was observed in mice lacking Skp2 (Skp2−/−), a component of ubiquitin ligase complex that targets cell cycle inhibitor p27kip1 for degradation (47). Hepatocyte proliferation in response to PH was attenuated in Skp2−/− as a result of accumulation of p27kip1. However, loss of ability to proliferate was compensated for by increased hepatocyte hypertrophy, and liver mass recovery of Skp2−/− was comparable to WT mice at 7 days post-PH (47). Similar observations were made with liver-specific STAT3-deficient mice (LS3-KO) subjected to 70% PH (28).
Furthermore, analysis of responses to PH of mice with liver-specific deletion and mutation of phosphoinositide-dependent protein kinase 1 (PDK1) highlighted the significance of PI3K/PDK1/Akt/mTOR-dependent survival pathways in modulation of liver regeneration through hepatocyte size rather than proliferation (29). mTOR is a key nutrient and amino acid sensor that regulates cell growth by promoting protein synthesis and inhibiting autophagy. Our results suggest that activation of mTOR signaling in the remnant livers of P2Y2−/− may play a role in the induction of compensatory hepatocyte hypertrophy and restoration of liver mass at 8 days post-PH (Fig. 7D).
Liver growth in response to xenobiotic stress has been well described in humans and rodents. Nuclear receptors such as CAR and PXR are key mediators of both hepatocyte proliferation and hypertrophy (16). Recent studies with “humanized” and knockout mice of CAR and PXR support their role in hepatocyte hypertrophy in response to xenobiotic stress in mice (59). CAR activation differs from the prototypic nuclear receptor activation. Both ligand-binding and ligand-independent mechanisms can induce activation and nuclear translocation of CAR (72). Our analysis of the nuclear extracts isolated from the remnant livers of the WT and P2Y2−/− at 8 days post-PH suggests that the induction of hepatocyte hypertrophy in P2Y2−/− remnant livers cannot be attributed to CAR activation (Fig. 7C).
Despite the fact that hepatocyte proliferation is attenuated in response to PH, mechanisms responsible for the maintenance of optimal liver weight-to-body weight ratios (“hepatostat”) are intact in P2Y2−/− (46). Further studies are necessary to pinpoint the cellular mechanisms responsible for the induction of compensatory hepatocyte hypertrophy in P2Y2−/− in response PH and to determine the impact of hepatocyte hypertrophy on recovery of liver functions and response to acute and chronic stress.
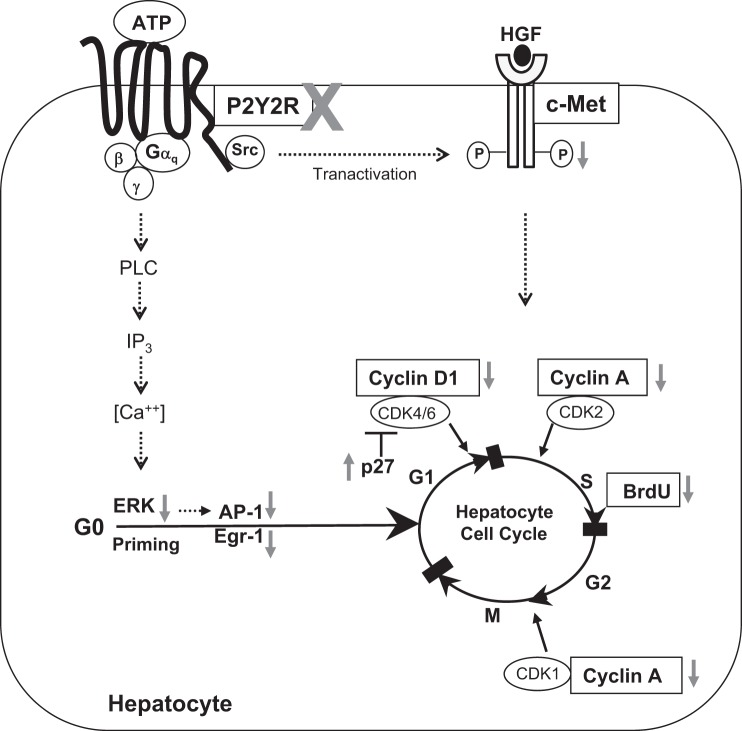
In conclusion, extracellular ATP-mediated activation of P2Y2 purinergic receptor signaling plays a crucial role in the activation of early priming events and hepatocyte cell cycle progression and proliferation in response to PH (Fig. 8). These findings underscore the importance of extracellular ATP as a potential humoral mediator of liver regeneration and highlight the functional significance of P2Y2 purinergic receptor signaling for efficient hepatocyte proliferation in regenerating livers.
Fig. 8.
Schematic representation of impact of P2Y2 purinergic receptor signaling on hepatocyte proliferation in response to 70% PH. Gray arrows indicate changes in P2Y2−/− mice livers in response to 70% PH, compared with WT. IP3, inositol triphosphate.
GRANTS
This study was supported in part by NIH RO1 DK069558 (ST), NIH/NIGMS T32 GM88129 (B. C. Tackett), NIH T32 007939, and NIH T32 DK07644 (Y. Mei), CPRIT Pre-Doctoral Training Grant RP101499 (J. P. Maynard), DK56338, which funds the Texas Medical Center Digestive Diseases Center, and generous grants from the Men of Distinction, Cade R. Alpard Foundation, Bauer Family Fund, and Spain Fund for Pediatric Liver Research at Texas Children's Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.C.T., H.S., Y.M., J.P.M., S.C., A.M., and A.H.-G. performed experiments; B.C.T., H.S., Y.M., J.P.M., S.C., A.M., A.H.-G., N.V., S.J.K., and S.T. analyzed data; B.C.T., H.S., Y.M., J.P.M., S.C., A.M., A.H.-G., N.V., S.J.K., and S.T. interpreted results of experiments; B.C.T. and S.T. prepared figures; B.C.T. and S.T. drafted manuscript; B.C.T., H.S., Y.M., J.P.M., S.C., A.M., A.H.-G., N.V., S.J.K., and S.T. approved final version of manuscript; S.T. conception and design of research; S.T. edited and revised manuscript.
REFERENCES
- 1.Abshagen K, Eipel C, Vollmar B. A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch Surg 397: 579–590, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol 175: 1056–1065, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur DB, Akassoglou K, Insel PA. P2Y2 and TrkA receptors interact with Src family kinase for neuronal differentiation. Biochem Biophys Res Commun 347: 678–682, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA 102: 19138–19143, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci 13: 2588–2603, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, Candinas D, Erb L, Robson SC. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology 135: 1751–1760, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol 43: 506–520, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besnard A, Julien B, Gonzales E, Tordjmann T. Innate immunity, purinergic system and liver regeneration: a trip in complexity. Hepatology 57: 1688–1690, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Bessard A, Coutant A, Rescan C, Ezan F, Fremin C, Courselaud B, Ilyin G, Baffet G. An MLCK-dependent window in late G1 controls S phase entry of proliferating rodent hepatocytes via ERK-p70S6K pathway. Hepatology 44: 152–163, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Borowiak M, Garratt AN, Wustefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA 101: 10608–10613, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JB, Liu WJ, Che H, Liu J, Sun HY, Li GR. Adenosine-5′-triphosphate up-regulates proliferation of human cardiac fibroblasts. Br J Pharmacol 166: 1140–1150, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3: re1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol 288: G1024–G1035, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Crumm S, Cofan M, Juskeviciute E, Hoek JB. Adenine nucleotide changes in the remnant liver: an early signal for regeneration after partial hepatectomy. Hepatology 48: 898–908, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doignon I, Julien B, Serriere-Lanneau V, Garcin I, Alonso G, Nicou A, Monnet F, Gigou M, Humbert L, Rainteau D, Azoulay D, Castaing D, Gillon MC, Samuel D, Duclos-Vallee JC, Tordjmann T. Immediate neuroendocrine signaling after partial hepatectomy through acute portal hyperpressure and cholestasis. J Hepatol 54: 481–488, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Donthamsetty S, Bhave VS, Kliment CS, Bowen WC, Mars WM, Bell AW, Stewart RE, Orr A, Wu C, Michalopoulos GK. Excessive hepatomegaly of mice with hepatocyte-targeted elimination of integrin linked kinase following treatment with 1,4-bis [2-(3,5-dichaloropyridyloxy)] benzene. Hepatology 53: 587–595, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur J Neurosci 17: 1617–1627, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflügers Arch 452: 552–562, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Erlinge D, Heilig M, Edvinsson L. Tyrphostin inhibition of ATP-stimulated DNA synthesis, cell proliferation and fos-protein expression in vascular smooth muscle cells. Br J Pharmacol 118: 1028–1034, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Factor VM, Seo D, Ishikawa T, Kaposi-Novak P, Marquardt JU, Andersen JB, Conner EA, Thorgeirsson SS. Loss of c-Met disrupts gene expression program required for G2/M progression during liver regeneration in mice. PLoS One 5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanti M, Singh S, Ledda-Columbano GM, Columbano A, Monga SP. Tri-iodothyronine induces hepatocyte proliferation by protein kinase A-dependent beta-catenin activation in rodents. Hepatology 59: 2309–2320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fausther M, Gonzales E, Dranoff JA. Role of purinergic P2X receptors in the control of liver homeostasis. Wiley Interdiscip Rev Membr Transp Signal 1: 341–348, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Force T, Woulfe K, Koch WJ, Kerkela R. Molecular scaffolds regulate bidirectional crosstalk between Wnt and classical seven-transmembrane-domain receptor signaling pathways. Sci STKE 2007: pe41, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Fremin C, Bessard A, Ezan F, Gailhouste L, Regeard M, Le Seyec J, Gilot D, Pages G, Pouyssegur J, Langouet S, Baffet G. Multiple division cycles and long-term survival of hepatocytes are distinctly regulated by extracellular signal-regulated kinases ERK1 and ERK2. Hepatology 49: 930–939, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Gonzales E, Julien B, Serriere-Lanneau V, Nicou A, Doignon I, Lagoudakis L, Garcin I, Azoulay D, Duclos-Vallee JC, Castaing D, Samuel D, Hernandez-Garcia A, Awad SS, Combettes L, Thevananther S, Tordjmann T. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol 52: 54–62, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzales E, Prigent S, Abou-Lovergne A, Boucherie S, Tordjmann T, Jacquemin E, Combettes L. Rat hepatocytes express functional P2X receptors. FEBS Lett 581: 3260–3266, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Graubardt N, Fahrner R, Trochsler M, Keogh A, Breu K, Furer C, Stroka D, Robson SC, Slack E, Candinas D, Beldi G. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis. Hepatology 57: 1969–1979, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Haga S, Ogawa W, Inoue H, Terui K, Ogino T, Igarashi R, Takeda K, Akira S, Enosawa S, Furukawa H, Todo S, Ozaki M. Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specific STAT3-deficient mice. J Hepatol 43: 799–807, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Haga S, Ozaki M, Inoue H, Okamoto Y, Ogawa W, Takeda K, Akira S, Todo S. The survival pathways phosphatidylinositol-3 kinase (PI3-K)/phosphoinositide-dependent protein kinase 1 (PDK1)/Akt modulate liver regeneration through hepatocyte size rather than proliferation. Hepatology 49: 204–214, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Heo JS, Han HJ. ATP stimulates mouse embryonic stem cell proliferation via protein kinase C, phosphatidylinositol 3-kinase/Akt, and mitogen-activated protein kinase signaling pathways. Stem Cells 24: 2637–2648, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12: 186–202, 1931. [Google Scholar]
- 32.Homolya L, Watt WC, Lazarowski ER, Koller BH, Boucher RC. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y2 receptor (−/−) mice. J Biol Chem 274: 26454–26460, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Hoppe-Seyler F, Butz K, Rittmuller C, von Knebel Doeberitz M. A rapid microscale procedure for the simultaneous preparation of cytoplasmic RNA, nuclear DNA binding proteins and enzymatically active luciferase extracts. Nucleic Acids Res 19: 5080, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA 101: 4477–4482, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang ST, Henning SJ. Hormonal regulation of expression of ileal bile acid binding protein in suckling rats. Am J Physiol Regul Integr Comp Physiol 278: R1555–R1563, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Janssens R, Boeynaems JM. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol 132: 536–546, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosters A, White DD, Sun H, Thevananther S, Karpen SJ. Redundant roles for cJun-N-terminal kinase 1 and 2 in interleukin-1beta-mediated reduction and modification of murine hepatic nuclear retinoid X receptor alpha. J Hepatol 51: 898–908, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagoudakis L, Garcin I, Julien B, Nahum K, Gomes DA, Combettes L, Nathanson MH, Tordjmann T. Cytosolic calcium regulates liver regeneration in the rat. Hepatology 52: 602–611, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8: 359–373, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao Y, Shikapwashya ON, Shteyer E, Dieckgraefe BK, Hruz PW, Rudnick DA. Delayed hepatocellular mitotic progression and impaired liver regeneration in early growth response-1-deficient mice. J Biol Chem 279: 43107–43116, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279: 8212–8218, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Loyer P, Cariou S, Glaise D, Bilodeau M, Baffet G, Guguen-Guillouzo C. Growth factor dependence of progression through G1 and S phases of adult rat hepatocytes in vitro. Evidence of a mitogen restriction point in mid-late G1. J Biol Chem 271: 11484–11492, 1996. [DOI] [PubMed] [Google Scholar]
- 43.McKee SC, Thompson CS, Sabourin LA, Hakim AM. Regulation of expression of early growth response transcription factors in rat primary cortical neurons by extracellular ATP. Brain Res 1088: 1–11, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Mei Y, Thevananther S. Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice. Hepatology 54: 1777–1789, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalopoulos GK. Liver regeneration. J Cell Physiol 213: 286–300, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 176: 2–13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minamishima YA, Nakayama K. Recovery of liver mass without proliferation of hepatocytes after partial hepatectomy in Skp2-deficient mice. Cancer Res 62: 995–999, 2002. [PubMed] [Google Scholar]
- 48.Molnar G, Crozat A, Pardee AB. The immediate-early gene Egr-1 regulates the activity of the thymidine kinase promoter at the G0-to-G1 transition of the cell cycle. Mol Cell Biol 14: 5242–5248, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monga SP. Role and regulation of beta-catenin signaling during physiological liver growth. Gene Expr 16: 51–62, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology 33: 1098–1109, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller L, Broering DC, Meyer J, Vashist Y, Goettsche J, Wilms C, Rogiers X. The induction of the immediate-early-genes Egr-1, PAI-1 and PRL-1 during liver regeneration in surgical models is related to increased portal flow. J Hepatol 37: 606–612, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Nicou A, Serriere V, Hilly M, Prigent S, Combettes L, Guillon G, Tordjmann T. Remodelling of calcium signalling during liver regeneration in the rat. J Hepatol 46: 247–256, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen K, Luo JH, Michalopoulos GK. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology 45: 1471–1477, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng Y, Du K, Ramirez S, Diamond RH, Taub R. Mitogenic up-regulation of the PRL-1 protein-tyrosine phosphatase gene by Egr-1. Egr-1 activation is an early event in liver regeneration. J Biol Chem 274: 4513–4520, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Pines A, Romanello M, Cesaratto L, Damante G, Moro L, D'Andrea P, Tell G. Extracellular ATP stimulates the early growth response protein 1 (Egr-1) via a protein kinase C-dependent pathway in the human osteoblastic HOBIT cell line. Biochem J 373: 815–824, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pritchard MT, Malinak RN, Nagy LE. Early growth response (EGR)-1 is required for timely cell-cycle entry and progression in hepatocytes after acute carbon tetrachloride exposure in mice. Am J Physiol Gastrointest Liver Physiol 300: G1124–G1131, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rescan C, Coutant A, Talarmin H, Theret N, Glaise D, Guguen-Guillouzo C, Baffet G. Mechanism in the sequential control of cell morphology and S phase entry by epidermal growth factor involves distinct MEK/ERK activations. Mol Biol Cell 12: 725–738, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roman RM, Fitz JG. Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology 116: 964–979, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Ross J, Plummer SM, Rode A, Scheer N, Bower CC, Vogel O, Henderson CJ, Wolf CR, Elcombe CR. Human constitutive androstane receptor (CAR) and pregnane X receptor (PXR) support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogens phenobarbital and chlordane in vivo. Toxicol Sci 116: 452–466, 2010. [DOI] [PubMed] [Google Scholar]
- 60.Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide 5: 453–464, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology 45: 361–368, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem 279: 35679–35686, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Stabile LP, He G, Lui VW, Thomas S, Henry C, Gubish CT, Joyce S, Quesnelle KM, Siegfried JM, Grandis JR. c-Src activation mediates erlotinib resistance in head and neck cancer by stimulating c-Met. Clin Cancer Res 19: 380–392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su AI, Guidotti LG, Pezacki JP, Chisari FV, Schultz PG. Gene expression during the priming phase of liver regeneration after partial hepatectomy in mice. Proc Natl Acad Sci USA 99: 11181–11186, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G1 phase progression in proliferating hepatocytes. Mol Cell Biol 19: 6003–6011, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology 131: 1561–1572, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Thevananther S, Sun H, Li D, Arjunan V, Awad SS, Wyllie S, Zimmerman TL, Goss JA, Karpen SJ. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology 39: 393–402, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology 28: 1226–1234, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, Gonzalez FA, Seye CI, Erb L. Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol 31: 169–184, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Woo K, Sathe M, Kresge C, Esser V, Ueno Y, Venter J, Glaser SS, Alpini G, Feranchak AP. Adenosine triphosphate release and purinergic (P2) receptor-mediated secretion in small and large mouse cholangiocytes. Hepatology 52: 1819–1828, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yaguchi T, Saito M, Yasuda Y, Kanno T, Nakano T, Nishizaki T. Higher concentrations of extracellular ATP suppress proliferation of Caco-2 human colonic cancer cells via an unknown receptor involving PKC inhibition. Cell Physiol Biochem 26: 125–134, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Yang H, Wang H. Signaling control of the constitutive androstane receptor (CAR). Protein Cell 5: 113–123, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan HX, Xiong Y, Guan KL. Nutrient sensing, metabolism, and cell growth control. Mol Cell 49: 379–387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeller E, Hammer K, Kirschnick M, Braeuning A. Mechanisms of RAS/beta-catenin interactions. Arch Toxicol 87: 611–632, 2013. [DOI] [PubMed] [Google Scholar]
- 75.Zeng G, Apte U, Micsenyi A, Bell A, Monga SP. Tyrosine residues 654 and 670 in beta-catenin are crucial in regulation of Met-beta-catenin interactions. Exp Cell Res 312: 3620–3630, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]