Abstract
Whereas epidemiological data strongly link vitamin D (VD) deficiency to childhood asthma, the underlying molecular mechanisms remain unknown. Although VD is known to stimulate alveolar epithelial-mesenchymal interactions, promoting perinatal lung maturation, whether VD supplementation during this period protects against childhood asthma has not been demonstrated experimentally. Using an in vivo rat model, we determined the effects of perinatal VD deficiency on overall pulmonary function and the tracheal contraction as a functional marker of airway contractility. One month before pregnancy, rat dams were put on either a no cholecalciferol-added or a 250, 500, or 1,000 IU/kg cholecalciferol-added diet, which was continued throughout pregnancy and lactation. At postnatal day 21, offspring plasma 25(OH)D levels and pulmonary function (whole body plethysmography and tracheal contraction response to acetylcholine) were determined. 25(OH)D levels were lowest in the no cholecalciferol-supplemented group, increasing incrementally in response to cholecalciferol supplementation. Compared with the 250 and 500 IU/kg VD-supplemented groups, the no cholecalciferol-supplemented group demonstrated a significant increase in airway resistance following methacholine challenge. However, the cholecalciferol deficiency-mediated increase in tracheal contractility in the cholecalciferol-depleted group was only blocked by supplementation with 500 IU/kg cholecalciferol. Therefore, in addition to altering alveolar epithelial-mesenchymal signaling, perinatal VD deficiency also alters airway contractility, providing novel insights to asthma pathogenesis in perinatally VD-deficient offspring. Perinatal VD supplementation at 500 IU/kg appears to effectively block these effects of perinatal VD deficiency in the rat model used, providing a strong clinical rationale for effective perinatal VD supplementation for preventing childhood asthma.
Keywords: childhood asthma, lipofibroblast, epithelial-mesenchymal interactions, peroxisome proliferator-activated receptor-γ, Wnt signaling
asthma is a common disorder, affecting an estimated 300 million individuals worldwide, and it contributes a significant social and financial burden to the individuals and families affected (1, 37). Factors such as genetic predisposition, early allergen exposure, infections, diet, tobacco smoke exposure, pollution, and vitamin D (VD) status are all proposed to influence the development and/or severity of childhood asthma (8, 16, 31, 47, 49). Currently, there is a great deal of interest in VD's role in childhood asthma since recent epidemiological and experimental animal and human data suggest that dihydroxycholecalciferol [1α,25(OH)2D3] (1,25D), a physiological hormone, is a paracrine factor that modulates fetal lung maturation, airway smooth muscle cell proliferation, and differentiation (4, 23–25, 34, 39, 40, 45, 52) and that perinatal VD deficiency is associated with childhood asthma (28).
VD comes from two sources: skin exposure to ultraviolet B (UVB) rays and dietary intake. Dietary sources include fish oil, fish, liver, egg yolk, and dietary supplements (23, 52). Because very few foods contain VD, sunlight exposure is the primary determinant of VD status in humans. VD synthesis is initiated in the skin by solar UVB radiation (wavelength 290–315 nm), activating the precursor 7-dehydrocholesterol, which then circulates in the bloodstream to the liver, where it is converted to its main metabolite, 25(OH)D, which has blood levels about 1,000 times higher than the active metabolite, 1,25D. Until recently, it was thought that the conversion to 1,25D occurred only in the kidneys, but increasing evidence indicates that the cells of most organs express the VD receptor (VDR) and the capacity to synthesize 1,25D locally (23). This synthesis of 1,25D is dependent on serum 25(OH)D levels, the primary circulating form of VD (23). Although multiple studies have suggested that perinatal VD deficiency is associated with childhood asthma and some studies also suggested a role for VD in preventing and treating childhood asthma (28), the molecular mechanisms involved and how perinatal VD deficiency might predispose to childhood asthma and increased airway smooth muscle proliferation and differentiation have not been systematically investigated. Using a rat model, we have recently demonstrated VD's effects on alveolar epithelial-mesenchymal interactions that are known to be involved in perinatal lung maturation. In particular, VD was demonstrated to modulate alveolar type II (ATII) cell and lipofibroblast proliferation and differentiation, two critical steps in alveolar maturation (48). While VD promotes alveolar epithelial-mesenchymal interactions spatiotemporally, accelerating alveolar maturation and improving lung function, whether perinatal VD supplementation blocks asthma development in offspring has not been shown experimentally.
Here, using a rat model of VD deficiency during pregnancy and lactation (33), we tested the hypothesis that perinatal VD deficiency alters homeostatic signaling in the developing lung, resulting in altered lung structural and functional changes, characterized by an enhanced myogenic phenotype of both the proximal and distal airways, i.e., an asthma phenotype in the offspring. We further hypothesized that optimal VD supplementation during pregnancy and lactation blocks the development of the asthma phenotype in offspring.
MATERIALS AND METHODS
Animal protocol and lung tissue harvesting.
Sprague-Dawley rat dams (30 days old) were obtained from Charles River (Holister, CA), kept in a dark room, and were divided into four different dietary groups: no cholecalciferol added to rat chow (VD-deficient group; catalog no. 1811505) and 250 IU/kg cholecalciferol (catalog no. 1814547)-, 500 IU/kg cholecalciferol (catalog no. 1814548)-, and 1,000 IU/kg cholecalciferol (catalog no. 1814549)-supplemented groups. Rat diets were obtained from TestDiet (Richmond, IN). All diets contained 4.5 g/kg calcium. Animals were allowed food and water intake ad libitum. Diets were started 4 wk before mating. When the rat dams were 60 days old, they were mated with young adult males on a normal rat chow diet. The assigned dietary regimens were continued throughout pregnancy and lactation until death on postnatal day (PND) 21. At death, blood, lungs, and trachea were collected for further analysis. All animal studies were performed in accordance with the National Institutes of Health guidelines and approved by the Los Angeles Biomedical Research Institute's Institutional Animal Care and Use Committee (protocol no. 14033).
Serum 25(OH)D, alkaline phosphatase, and calcium levels.
Serum samples were kept frozen at −70°C until analysis, when samples were initially moved to −25°C for 6 h and then thawed at 4°C until complete dissolution was achieved. 25(OH)D levels were measured by the electrochemiluminescence technique using a Roche Modular Analytics E170 immunoassay analyzer (Roche Diagnostics, Indianapolis, IN). Serum alkaline phosphatase and calcium levels were measured using a Roche Cobas 8000 Autoanalyzer Spektrofortometrik (Roche Diagnostics).
Lung morphology.
An investigator unaware of the treatment groups performed lung morphometry, assessed objectively by determining radial alveolar counts and mean linear intercepts. For radial alveolar count determination, 50 randomly selected nonoverlapping fields from sections obtained from similar regions of each lung from each treatment group were examined (12 blocks/condition, and 2 blocks/animal). Each field was viewed at 200-fold magnification, scanned with a digital camera, and projected on a video monitor. For each field, the number of alveoli was counted visually and expressed per square millimeter. The mean linear intercept was determined as described previously using the intersection counting method (28).
Choline incorporation in saturated phosphatidylcholine assay.
[3H]choline (NEN Dupont, Boston, MA) incorporation into saturated phosphatidylcholine, which is the major surface-active lipid component of surfactant responsible for maintaining the stability of the alveoli, was determined in cultured explants as previously described (46).
Triglyceride uptake assay.
Triolein uptake, a key marker of alveolar lipofibroblast function, was used to quantitate triglyceride uptake by fetal rat lung explants using a previously described method (46). Briefly, culture medium was replaced with DMEM containing 20% adult rat serum mixed with [3H]triolein (5 μCi/ml). The explants were incubated at 37°C in 5% CO2-air balance for 4 h. At the termination of the incubation, the medium was decanted, the explants were rinsed two times with 1 ml of ice-cold PBS, and the tissue was thoroughly homogenized. An aliquot of the tissue homogenate was taken for protein assay, and the remaining tissue homogenate was extracted to determine its neutral lipid content.
Immunoblot analysis.
The isolated lungs were flash-frozen in liquid nitrogen and then homogenized and sonicated in four volumes of ice-cold lysis buffer containing 50 mM β-glycerophosphate (pH 7.4), 150 mM NaCl, 1.5 mM EGTA, 1 mM EDTA, 1% Triton X-100, 100 mM NaF, 2 mM Na3VO4, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 2 μg/ml pepstatin A. After centrifugation at 13,200 g for 15 min at 4°C, the supernatant was used for Western blot analysis for fibronectin, calponin, peroxisome proliferator-activated receptor (PPAR) γ, CCAAT/enhancer binding proein (C/EBP) α, and surfactant protein C (SP-C). The total protein concentration of the supernatant was measured by the Bradford method, using bovine serum albumin as the protein standard. Aliquots of the supernatant, each containing 30 μg of protein, were separated by SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific binding sites were blocked with Tris-buffered saline (TBS) containing 5% nonfat dry powdered milk (wt/vol) for 1 h at room temperature. After a brief rinse with TBS containing 0.1% Tween 20 (TBST), the protein blots were incubated in 1:250 diluted anti-fibronectin polyclonal antibody (sc-9068; Santa Cruz), 1:350 diluted anti-PPARγ polyclonal antibody (sc-7196; Santa Cruz), 1:3,000 diluted anti-calponin monoclonal antibody (Sigma-Aldrich, St. Louis, MO), 1:200 diluted anti-C/EBPα polyclonal antibody (sc-61; Santa Cruz), 1:350 diluted anti-SP-C polyclonal antibody (sc-13979; Santa Cruz), and 1:10,000 diluted anti-GAPDH monoclonal antibody (MAB-374; Millipore) overnight at 4°C. After three more washes in TBST, the blots were exposed to X-ray film using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL) and developed. The relative densities of the protein bands were determined with UN-SCAN-IT software (Silk Scientific, Orem, Utah) and normalized to that of GAPDH.
Immunofluorescence staining.
For tissue immunofluorescence staining for the relevant proteins, rat lungs were inflated in situ with 4% paraformaldehyde in phosphate buffer at a standard inflation pressure of 20 cmH2O for 4 h at 4°C. The lungs were subsequently transferred to PBS containing 30% sucrose (wt/vol) until equilibrated in the cold (4°C). After fixation, 5-μm paraffin sections were treated three times with Histo-Clear (National Diagnostics, Atlanta, GA) for 5 min and then rehydrated by a sequential ethanol wash. Sections were then washed two times for 10 min with PBS and blocked for 1 h in PBS-5% normal goat serum-0.2% Triton X-100. Sections were incubated with primary antibodies for 1 h at room temperature and then with the appropriate secondary antibody for 1 h, also at room temperature. Antibodies included calponin [primary antibody, 1:250 dilution, catalog no. C2687 (Sigma-Aldrich); secondary antibody, 1:100 dilution (Alexa Fluor), anti-rabbit 488 green]; vimentin [primary antibody, 1:100 dilution, catalog no. V6630 (Sigma); secondary antibody, 1:100 dilution (Alexa Fluor), anti-mouse 488 green]; α-smooth muscle actin (SMA) [primary antibody, 1:500 (Sigma), catalog no. A2547; secondary antibody, 1:250 (Alexa Fluor), goat anti-mouse 568 red]; PPAR-γ [primary antibody, 1:100 (Santa Cruz), catalog no. SC-7196; secondary antibody, 1:100 (Alexa Fluor), anti-rabbit 568 red]; and SP-C [primary antibody, 1:250 (Santa Cruz), catalog no. sc-13979; secondary antibody, 1:150, anti-rabbit 488 green] p180 lamellar [primary antibody, 1:500 dilution, catalog no. MMS-645R (Covance, Berkeley, CA); secondary antibody, 1:250, Alexa Fluor anti-mouse 568 red]. Sections were washed with PBS and then mounted on glass slides with ProLong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA) for visualization under a fluorescence microscope.
Pulmonary function test.
Pulmonary function tests (PFTs) were determined using whole body plethysmography by our previously described method (32, 42, 43). Briefly, plethysmography for restrained animals with the rat in the supine position was used. Dynamic compliance and resistance of the respiratory tract were concurrently calculated on sedated, tracheotomized, and ventilated rats at baseline and immediately following serial 3-min nebulizations of saline vehicle or methacholine (MCh, 0.5 and 2.0 mg/ml), with 3-min recovery periods allowed after each exposure to MCh, as previously described. The pups were deeply anesthetized and sedated with medetomidine (0.5 mg/kg, Domitor; Orion Pharma) and tiletamine-zolazepam (50 mg/kg, Telazol; Fort Dodge Laboratories, Overland Park, KS) and then ventilated at 7–8 ml/kg with a small animal ventilator (MiniVent; Harvard Apparatus, Cambridge, MA) for the duration of the procedure.
Tracheal tension studies.
The trachea was excised en bloc following death and freed of connective tissue in ice-cold modified Krebs-Ringer bicarbonate buffer (in mM: 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, and 11.1 glucose). Next, a roughly 6-mm ring was resected from the middle of the trachea and used for this procedure. The tracheal ring was suspended in an organ bath in 10 ml of modified Krebs-Ringer bicarbonate solution kept at 37 ± 0.5°C and aerated with 95% O2-5% CO2 (pH 7.4). The rings were suspended via two stirrups that were passed through the lumen, where one stirrup was anchored to the bottom of the organ bath while the other stirrup was connected to a strain gauge (model FT03C; Grass Instrument, Quincy, MA) to measure isometric force, as described previously (30, 40, 41).
Real-time-PCR.
RNA extraction from PND21 lung tissue and q-RT-PCR were performed according to previously described methods (44). RT-PCR primers used included: fibronectin, forward 5′-AGCACACCCGTTTTCATCCA-3′ and reverse 5′-TTTCACGTCGGTCACTTCCA-3′ (102 bp); vimentin, forward 5′-CGGACAGGTGATCAATGAGACTT-3′ and reverse 5′-ATCTTGCGCTCCTGAAAACTG-3′ (162 bp); β-catenin, forward 5′-CCGTTCGCCTTCATTATGGA-3′ and reverse 5′-GGGCAAGGTTTCGGATCAAT-3′ (105 bp); tropoelastin, forward 5′-AGAAGCCTCGACATTAGATTTGGT-3′ and reverse 5′-GGAGCTATTCCCAGTGTGAGAAGT-3′ (139 bp); elastin, forward 5′-ACCTGGGTTTGGACTTTCTCCTA-3′ and reverse 5′-GGGTCCCCAGAAGATCACTTTC-3′ (171 bp); glycogen synthase kinase (GSK)-3β, forward 5′-GAGCCACCGATTACACGTCTAGT-3′ and reverse 5′-CAGGAAATATTGGTTGTCCTAGCA-3′ (87 bp); and 18S, forward 5′-GGACAGGATTGACAGATTGATAGC-3′ and reverse 5′-TGGTTATCGGAATTAACCAGACAA-3′. 18S ribosomal RNA expression was used as the normalizing control. Data were analyzed using a fluorescence threshold level that was in the linear range of the RT-PCR reaction. The CT value for the 18S ribosomal RNA was subtracted from the CT value for the gene of interest to obtain a delta CT value (ΔCT). The relative fold change for each gene was calculated using the ΔΔCT method. Results were expressed as means ± SE and considered statistically significant at P < 0.05.
Statistics.
Experimental data were analyzed using two-way ANOVA and, as indicated, followed by Tukey's post hoc test. A P value of <0.05 was considered to indicate statistically significant differences between the experimental groups. Results are expressed as means ± SE. A minimum of 4 animals/group taken from 4 separate litters were used for data analysis. Because of a relatively small sample size (4–8 animals in each group for most parameters), the data were analyzed as combined gender data without comparing males with females.
RESULTS
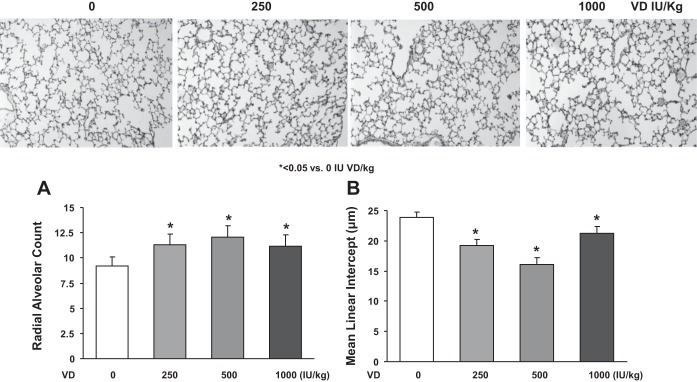
Initially, we determined the effect of VD supplementation on the circulating levels of 25(OH)D in various experimental groups (Fig. 1). 25(OH)D levels were lowest in the no cholecalciferol-supplemented, i.e., VD-deficient group, followed by a dose-dependent increase in the circulating levels of 25(OH)D, with the levels corresponding to the dietary intake of supplemental cholecalciferol (Fig. 1A). The amounts of serum calcium (Fig. 1B) were unaffected by either VD deficiency or by the supplemented cholecalciferol levels. Similarly, the levels of alkaline phosphatase (Fig. 1C) were unaffected by either VD deficiency or supplementation, with the exception of the 1,000 IU/kg cholecalciferol-supplemented group in which there was a significant decrease (P < 0.05 vs. VD-deficient and 250 and 500 IU/kg cholecalciferol-supplemented groups). Morphometrically, compared with the cholecalciferol-supplemented groups, in the VD-deficient group, the radial alveolar count was significantly decreased, and the mean linear intercept was significantly increased (P < 0.05, VD-deficient vs. VD-supplemented groups, Fig. 2).
Fig. 1.
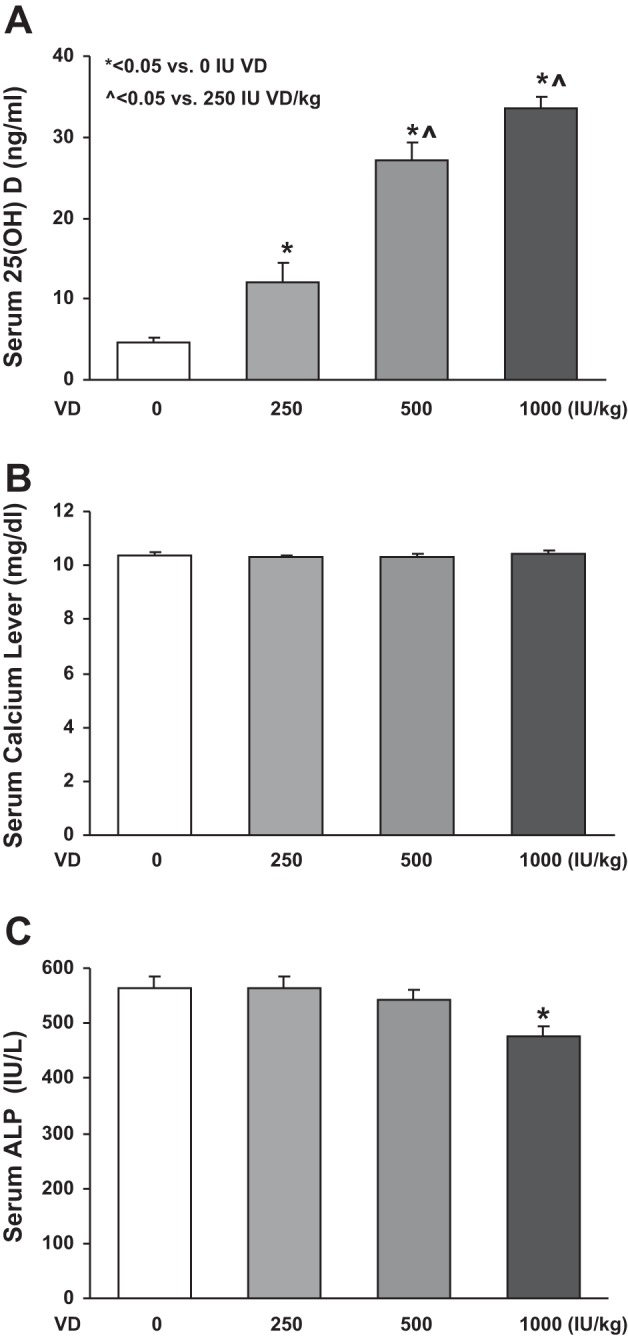
Serum 25(OH)D, calcium, and alkaline phosphatase (ALP) levels. 25(OH)D levels were lowest in the no cholecalciferol-supplemented group, followed by a dose-dependent increase in its levels corresponding to the dietary intake of supplemental cholecalciferol (A). The serum calcium levels were not significantly different among all of the different experimental groups (B). The levels of alkaline phosphatase were unaffected by either VD deficiency or supplementation, with the exception of the 1,000 IU/kg cholecalciferol-supplemented group in which there was a significant decrease. Values are means ± SE; n = 15–20 for each group. P < 0.05 vs. vitamin D (VD)-deficient (*) and 250 IU/kg-cholecalciferol supplemented (^) groups (C).
Fig. 2.
Effect of perinatal VD deficiency on lung morphometry. Perinatal VD deficiency alters lung structure in offspring, resulting in a significant decrease in alveolar number (P < 0.05 vs. VD-supplemented groups, n = 6) and a significant increase in mean linear intercept (P < 0.05 vs. VD-supplemented groups, n = 6) by morphometric analysis of lung samples from pups killed at postnatal day (PND) 21. Values are means ± SE; n = 6 for each group. *P < 0.05 vs. VD-deficient groups. Representative tissue sections from different experimental groups are shown (magnification ×10).
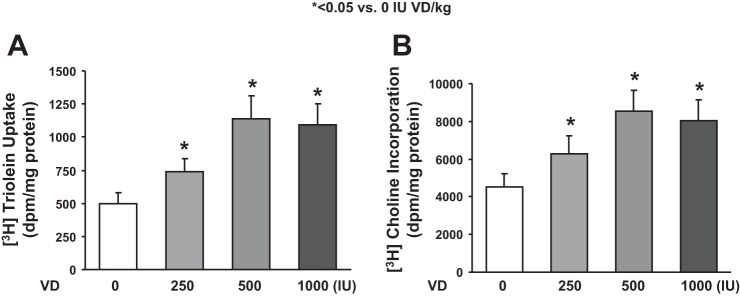
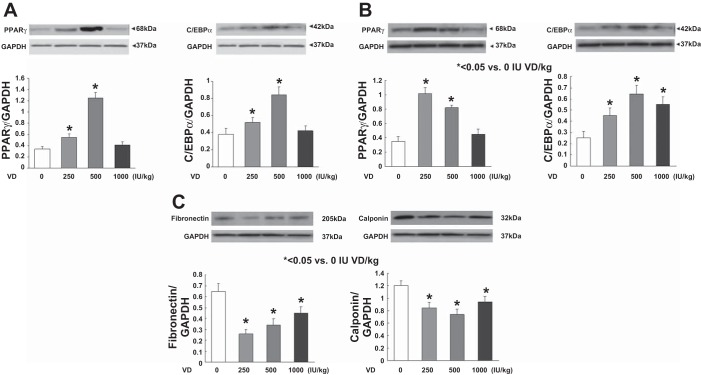
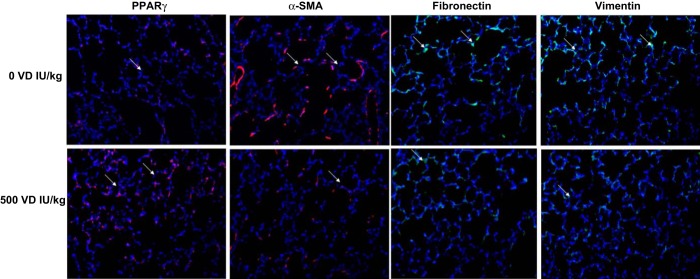
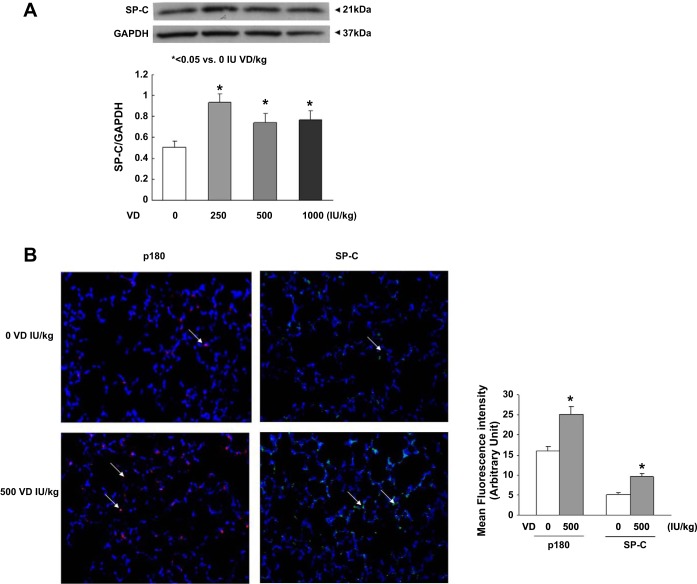
Assessing triglyceride uptake and de novo surfactant phospholipid synthesis, based on choline incorporation into saturated phosphatidylcholine, indicated that VD deficiency significantly inhibited both of these parameters (Fig. 3, P < 0.05, VD-deficient vs. VD-supplemented groups for both). Dietary cholecalciferol supplementation dose-dependently increased both triolein uptake and choline incorporation into saturated phosphatidylcholine. Examining the effects of VD supplementation on the mesenchymal lipogenic markers for alveolar differentiation (Fig. 4), PPARγ and C/EBPα protein levels on both PND1 (A) and PND21 (B) were lowest in the VD-deficient animals, increasing in the 500 and 250 IU/kg cholecalciferol-supplemented groups, respectively, with no significant effect at 1,000 IU/kg cholecalciferol supplementation. In contrast, on PND21, the myogenic mesenchymal markers fibronectin and calponin protein levels were highest in the lungs of the VD-deficient animals and were significantly inhibited by cholecalciferol supplementation in the 250, 500, and 1,000 IU/kg cholecalciferol-supplemented groups (Fig. 4C). Decrease in lipofibroblastic differentiation (PPARγ) and increase in myogenic differentiation (α-SMA, fibronectin, and vimentin) in the VD-deficient group, compared with the 500 IU/kg cholecalciferol-supplemented group, was also confirmed by immunostaining of lung sections from these conditions (Fig. 5). With respect to alveolar epithelial markers of differentiation (Fig. 6), SP-C was lowest in the VD-deficient group, showing elevated levels across all of the VD-supplemented animals (Fig. 6A). Lower SP-C, along with the lower lamellar body (p180) staining in the VD-deficient group, was also confirmed by immunostaining (Fig. 6B, P < 0.05 vs. 500 IU/kg cholecalciferol-supplemented group).
Fig. 3.
Effect of perinatal VD deficiency on surfactant phospholipid synthesis. Triglyceride uptake and de novo surfactant phospholipid synthesis by lung explants, based on triolein uptake and choline incorporation in saturated phosphatidylcholine, respectively, indicated that VD deficiency significantly inhibited both of these parameters. Values are means ± SE; n = 6 for each group. *P < 0.05, VD-deficient vs. VD-supplemented groups for both. Dietary cholecalciferol supplementation increased both triolein uptake and choline incorporation into saturated phosphatidylcholine.
Fig. 4.
Effect of perinatal VD deficiency on mesenchymal markers of alveolar differentiation. The lipogenic marker peroxisome proliferator-activated receptor (PPAR) γ and CCAAT/enhancer binding proein (C/EBP) α protein levels normalized to GAPDH were lowest in the VD-deficient animals on both PND1 (A) and -21 (B), increasing significantly in the 500 and 250 IU/kg cholecalciferol-supplemented groups, with no significant effect at 1,000 IU/kg cholecalciferol supplementation. In contrast, the myogenic mesenchymal marker fibronectin and calponin protein levels normalized to GAPDH were highest in the lungs of the VD-deficient animals and were significantly inhibited by cholecalciferol supplementation in the 250, 500, and 1,000 IU/kg cholecalciferol-supplemented groups (C). Values are means ± SE; n = 4 for each group. *P < 0.05 vs. the VD-deficient group.
Fig. 5.
Effect of perinatal VD deficiency on mesenchymal markers of alveolar differentiation. Decrease in lipofibroblastic differentiation (PPARγ, red fluorescence) and increase in myogenic differentiation [α-smooth muscle actin (SMA), red fluorescence; calponin, green fluorescence; and vimentin, green fluorescence] in the VD-deficient group, compared with the 500 IU/kg cholecalciferol-supplemented group, were also confirmed by immunostaining on lung sections from these conditions (n = 4; representative immunostaining blots are shown).
Fig. 6.
Effect of perinatal VD deficiency on epithelial marker [surfactant protein-C and lamellar body (p180)] differentiation. Surfactant protein-C protein levels were higher in the 1α,25(OH)2D3D-supplemented groups and lower in the VD-deficient group by both Western analysis (A) and immunofluorescence staining (green fluorescence) (B). Similarly, by immunostaining, p180 staining (red fluorescence) in lung sections from the VD-deficient group was less intense (B, representative immunofluorescence-stained sections are shown). Values for the density histogram for Western blots (A) and fluorescence intensities for immunostained sections (B) are means ± SE; n = 4 for each group. *P < 0.05 vs. VD deficient.
We then examined the effects of VD deficiency and supplementation on pulmonary function (Fig. 7). On methacholine challenge, compared with the 250 and 500 IU/kg cholecalciferol-supplemented groups, total pulmonary resistance was significantly (P < 0.05 for both) higher in the 0 and 1,000 IU/kg cholecalciferol-supplemented groups. However, total pulmonary compliance was not significantly different among all groups, either at baseline or on methacholine challenge. We then measured the tracheal contractility response to acetylcholine challenge (Fig. 8), observing the highest contractility in VD-deficient and 1,000 IU/kg cholecalciferol-supplemented animals (vs. the 500 IU/kg cholecalciferol-supplemented group).
Fig. 7.
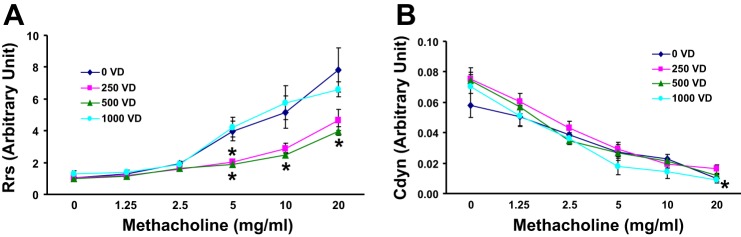
Effect of perinatal VD deficiency on total respiratory system resistance (Rrs, A) and compliance (Cdyn, B) at baseline and following methacholine (MCh) challenge. Compared with the 250 and 500 IU/kg cholecalciferol-supplemented groups, with methacholine challenge, total pulmonary system resistance was significantly increased in the 0 and 1,000 IU/kg cholecalciferol-supplemented groups; however, there was no change in total pulmonary compliance, either at baseline or following MCh challenge. Values are means ± SE; n = 6 for each group. *P < 0.05.
Fig. 8.
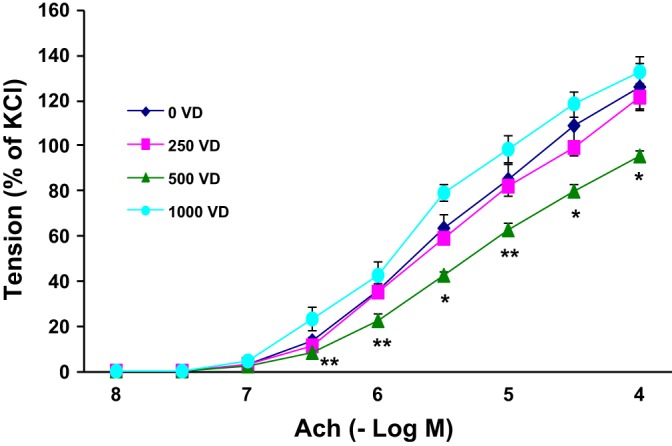
Effect of perinatal VD deficiency on tracheal contractility response to acetylcholine challenge. The tracheal constriction response to acetylcholine was significantly increased in the VD-deficient and 1,000 IU/kg cholecalciferol-supplemented animals. Values are means ± SE; n = 6 for each group. *P < 0.05 and **P < 0.01 vs. 500 IU/kg cholecalciferol-supplemented group.
Finally, we focused on the effects of VD deficiency and supplementation on the tracheal expression of functional myogenic markers, such as fibronectin and vimentin, and key Wnt signaling intermediates, GSK3β and β-catenin (Fig. 9). Compared with the 250 and 500 IU/kg cholecalciferol-supplemented groups, the expression of myogenic markers fibronectin and vimentin increased in the VD-deficient group, as was the case for the myogenic determinant Wnt signaling intermediates GSK3β and β-catenin. Although there were no significant differences in the expression of these markers between the 250 and 500 IU/kg cholecalciferol-supplemented groups, in general the 500 IU/kg cholecalciferol-supplemented group demonstrated the lowest expression of these markers, with the 250 IU/kg cholecalciferol-supplemented group demonstrating levels in between the 0 and 500 IU/kg-supplemented groups.
Fig. 9.
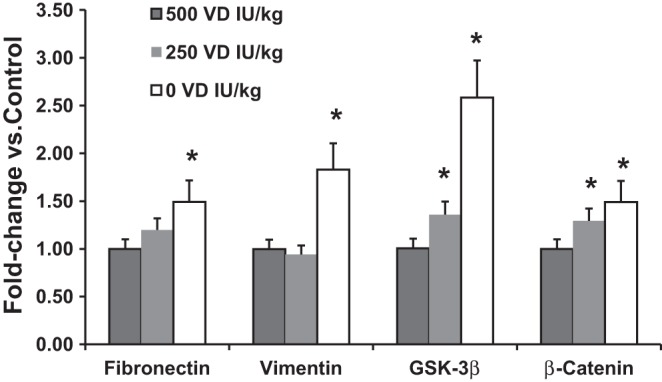
Effect of perinatal VD deficiency on the expression of key tracheal myogenic and Wnt signaling intermediates. When the expression of the myogenic markers fibronectin and vimentin and that of the Wnt signaling intermediates glycogen synthase kinase (GSK) 3β and β-catenin, by qRT-PCR, was compared with the 250 and 500 IU/kg-supplemented groups, the expression of both myogenic markers (fibronectin and vimentin) examined increased in the VD-deficient group as did the expression of the key Wnt signaling intermediates GSK3β and β-catenin. Values are means ± SE; n = 4–6. *P < 0.05 vs. VD-deficient group.
DISCUSSION
Our study demonstrated that perinatal VD deficiency causes offspring lung molecular and structural alterations, likely explaining the associated lung asthma phenotype. The altered lung structure and function, reflected by alterations in alveolar and tracheal mesenchymal differentiation markers, functional markers of surfactant phospholipid synthesis, PFT, and tracheal contractility, were largely blocked by 250 and/or 500 IU/kg VD dietary supplementation. Of note, this is the first study demonstrating, experimentally, both proximal and distal airway molecular and functional alterations with perinatal VD deficiency concurrently and prevention of these alterations with VD supplementation dose dependently.
Significantly lower VD levels have been noted in asthmatic children compared with healthy controls (18). Large cross-sectional studies have also shown that low serum VD levels are associated with reduced lung function in adolescents (11) and adults (5). A high frequency of VD insufficiency (35%) in 1,024 North American children with mild to moderate asthma has been reported (10). Moreover, a higher VD intake in pregnant women has been linked to reduced rates of wheezing and asthma in their offspring (13, 15, 17, 36). In asthmatic subjects, increased VD predicts reduced rates of asthma exacerbations in those both on and off inhaled corticosteroids (9, 10, 50). Coupled with these data, there is evidence for the rise in the incidence of VD insufficiency in women of child-bearing age (3, 38), underscoring the significance of the effects of perinatal VD deficiency on the developing lung (51).
Lung development begins in utero and continues through the first few years of life. Mammalian lung development occurs in five distinct anatomic phases: embryonic (4–7 wk), pseudoglandular (7–17 wk), canalicular (17–26 wk), saccular (27–36 wk), and alveolar (36 wk-about 2 yr) (12). At the end of fetal lung development, the alveolar epithelium undergoes abrupt differentiation as part of the preparation for gas exchange after birth. Fetal pulmonary maturation includes the differentiation of type II pneumocytes, with progressive disappearance of glycogen and the start of surfactant synthesis. Animal studies strongly suggest that VD plays a role in lung maturation during the saccular and alveolar stages of lung development, late in pregnancy, and support earlier observations such as the association of respiratory distress in VD-deficient preterm infants (“rachitic respiratory distress”) (21, 41). Decreased alveolar epithelial and lipofibroblastic differentiation, with the resultant decreased surfactant synthesis in VD-deficient animals on PND21, as observed by us, likely explains the respiratory distress seen in preterm VD-deficient infants. In fact, lower PPARγ and C/EBPα protein levels, two key lipofibroblastic differentiation markers vitally important for alveolar epithelial maturation, on PND1, suggest that the changes seen on PND21 are likely to be a sequela of the altered lung development started in utero.
There are ample rodent and human data suggesting VD's role in lung development. For example, rodent and human studies suggest the existence of a local alveolar VD autocrine/paracrine system, as evidenced by the production of physiologically active 1,25D by alveolar fibroblasts, the presence of the VDR on the adjoining ATII cells, and the fact that VD has been shown to modulate ATII cell and fibroblast proliferation and differentiation (32, 37, 38, 46). Determination of quasistatic pressure-volume curves of the respiratory system and of the lungs in 50-day-old rats, born to mothers deprived of dietary VD, demonstrated significantly decreased lung compliance but normal chest wall compliance, likely because of altered alveolar formation and lung mesenchyme development (20). In line with our data, the offspring mice of the VD-deficient mothers have also been recently demonstrated to exhibit reduced lung volumes and borderline reduced numbers of alveoli (55). Compared with the rat (43), in humans, the effect of VD on surfactant production appears to be rather complex since 1,25D decreased SP-A mRNA and protein levels in both fetal lung tissue and isolated ATII cells, whereas it increased SP-B mRNA and protein levels in ATII cells but had no effect on SP-C mRNA levels (41). It is worth noting that over 3,000 genes, including many that are involved in lung development, have VD response elements (7). In fact, VD- regulated genes are overrepresented in normal human and mouse developing lung transcriptomes (27). Therefore, it is not surprising that a strong link between VD deficiency and asthma has been observed, and rachitic preterm infants exhibit respiratory distress, likely because of altered lung structure and function resulting from VD deficiency (21).
Despite extensive evidence for VD's role on lung development, the mechanism(s) underlying increased predisposition to asthma in VD deficiency remains unknown. Our study provides novel mechanistic insights in this regard. In addition to corroborating VD's previously described role in alveolar maturation, our study demonstrates its effect on proximal airway structural and functional maturation, e.g., VD deficiency led to increased protein levels of myogenic proteins in the proximal airway, possibly because of Wnt signaling activation, likely explaining the increased airway responsiveness in asthma. In line with these data, persistent expression of early markers of myogenic differentiation in VDR knockout mice (14) and inhibition of airway smooth muscle proliferation on activation of VDR have previously been reported (22). However, it is important to point out that, in the present study, we used isolated tracheal ring contractility as a marker of airway reactivity rather than bronchi, which are more likely the site of airway contractility in asthma.
The optimal daily requirement for the purpose of lung maturation in neonates is not known. In fact, the normal VD levels for term and preterm infants are also not well-established; however, in older children and adolescents, currently 25(OH)D levels of ≥20 ng/ml (50 nmol/l) are considered sufficient, with levels between 15 and 20 ng/ml (37.5 and 50 nmol/l) being considered insufficient and levels of ≤15 ng/ml (37.5 nmol/l) being deficient (35). To ensure VD sufficiency in term and preterm infants, the American Academy of Pediatrics recommends 400 IU/day for VD intake (53). In the rat model used, although the optimal dose of VD dietary supplementation is not known, with 250 and 500 IU/kg cholecalciferol dietary supplementation to pregnant and lactating dams, serum 25(OH)D levels were 13 ± 3 and 24 ± 4 ng/ml, respectively. Although at these levels the lung structural, molecular, and functional effects of VD deficiency on the developing lung were largely prevented, it is difficult to translate these data from rats to humans. However, it is clear that the effects of VD supplementation were dose dependent, with 1,000 IU/kg VD supplementation largely exhibiting detrimental effects on the developing lung. Therefore, it will be interesting to study the impact of this change on human lung development, given the widespread evidence of VD deficiency in pregnant women (2) and the recent large body of evidence that perinatal VD deficiency may explain a significant portion of the recent asthma epidemic (29). However, it should also be noted that some studies have suggested a detrimental effect of VD supplementation on the development of asthma and allergy (19, 26, 54), suggesting a rather complex role of VD in the developing lung. The contradictory nature of these studies compared with our data may relate to the differences between the rodent model used by us and the human disease, or the critical differences between the dose, type, and timing of VD administration used in these studies. Although our data provide molecular insights to the possible benefits of VD supplementation during early development, results from the current ongoing intervention trials evaluating the effects of early life VD supplementation at a range of doses, in a range of populations and using a range of treatment schedules, are likely to provide more definitive answers in establishing the clinical significance of VD supplementation during development in preventing childhood asthma (13a, 30).
GRANTS
This work was supported by National Institutes of Health Grants HL-075405, HL-55268, HD-51857, HD-058948, HL-107118, and HD-071731.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
M.Y., J.L., R.S., M.G., S.M.H., M.A.S., M.H., P.V., and F.A. performed experiments; M.Y. and V.K.R. drafted manuscript; M.Y., J.L., R.S., M.G., S.M.H., M.A.S., M.H., P.V., J.S.T., and V.K.R. approved final version of manuscript; J.L. and V.K.R. analyzed data; J.L., R.S., and M.G. prepared figures; J.S.T. and V.K.R. edited and revised manuscript; V.K.R. conception and design of research; V.K.R. interpreted results of experiments.
ACKNOWLEDGMENTS
We are grateful to Dr. Harun Polat and Dr. Musa Dudukcu, Ataturk University, Medical Faculty, Biochemistry Department, Erzurum, Turkey, for generous help in performing the serum 25(OH)D, calcium, and alkaline phosphatase assays.
REFERENCES
- 1.Ait-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy 64: 123–148, 2009. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Prevention of Rickets, and Vitamin D Deficiency in Infants, Children, and Adolescents. www.pediatrics.org/cgi/doi/10.1542/peds.2008–1862. [DOI] [PubMed]
- 3.Andiran N, Yordam N, Ozon A. Risk factors for vitamin D deficiency in breast-fed newborns and their mothers. Nutrition 18: 47–50, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Awonusonu F, Srinivasan S, Strange J, Al-Jumaily W, Bruce MC. Developmental shift in the relative percentages of lung fibroblast subsets: role of apoptosis postseptation. Am J Physiol Lung Cell Mol Physiol 277: L848–L859, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest 128: 3792–3798, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bosse Y, Lemire M, Poon AH, Daley D, He JQ, Sandford A, White JH, James AL, Musk AW, Palmer LJ, Raby BA, Weiss ST, Kozyrskyj AL, Becker A, Hudson TJ, Laprise C. Asthma and genes encoding components of the vitamin D pathway (Abstract). Respir Res 10: 98, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy 67: 10–17, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, Litonjua AA. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 179: 765–771, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, Weiss ST, Litonjua AA. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 126: 52–58, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns JS, Dockery DW, Neas LM, Schwartz J, Coull BA, Raizenne M, Speizer FE. Low dietary nutrient intakes and respiratory health in adolescents. Chest 132: 238–245, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol 46: 617–628, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Camargo CA, Jr., Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 85: 788–795, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.ClinicalTirals.gov. Vitamin D supplementation during pregnancy for prevention of asthma in childhood (ABCvitaminD). ClinicalTrials.gov: NCT00856947.
- 14.Demay M. Muscle: a nontraditional 1,25-dihydroxyvitamin D target tissue exhibiting classic hormone-dependent vitamin D receptor actions. Endocrinology 144: 5135–5137, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr 85: 853–859, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Eder W, Ege MJ, von ME. The asthma epidemic. N Engl J Med 355: 2226–2235, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, Simell O, Knip M, Virtanen SM. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy 39: 875–882, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, Teach SJ. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr 156: 948–952, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 62: 68–77, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaultier C, Harf A, Balmain N, Cuisinier-Gleizes P, Mathieu H. Lung mechanics in rachitic rats. Am Rev Respir Dis 130: 1108–1110, 1984. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow JF, Thomas PS. Rachitic respiratory distress in small preterm infants. Arch Dis Child 52: 268–273, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosens R, Roscioni SS, Dekkers BG, Pera T, Schmidt M, Schaafsma D, Zaagsma J, Meurs H. Pharmacology of airway smooth muscle proliferation. Eur J Pharmacol 585: 385–397, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D deficiency. N Engl J Med 357: 266–281, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 39: 529–533, 1993. [PubMed] [Google Scholar]
- 25.Hollis BW, Napoli JL. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem 31: 1815–1819, 1985. [PubMed] [Google Scholar]
- 26.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann NY Acad Sci 1037: 84–95, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Kho AT, Bhattacharya S, Tantisira KG, Carey VJ, Gaedigk R, Leeder JS, Kohane IS, Weiss ST, Mariani TJ. Transcriptomic analysis of human lung development. Am J Respir Crit Care Med 181: 54–63, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knudsen L, Weibel ER, Gundersen HJ, Weinstein FV, Ochs M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol 108: 412–421, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Litonjua AA. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. 12: 179–185, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litonjua AA. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. 38: 37–50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 120: 1031–1035, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Naeem E, Tian J, Lombardi V, Kwong K, Akbari O, Torday JS, Rehan VK. Sex-specific perinatal nicotine-induced asthma in rat offspring. Am J Respir Cell Mol Biol 48: 53–62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maka N, Makrakis J, Parkington HC, Tare M, Morley R, Black MJ. Vitamin D deficiency during pregnancy and lactation stimulates nephrogenesis in rat offspring. Pediatr Nephrol 23: 55–61, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Marin L, Dufour ME, Nguyen TM, Tordet C, Garabedian M. Maturational changes induced by 1 alpha,25-dihydroxyvitamin D3 in type II cells from fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol 265: L45–L52, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 122: 398–417, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Maternal B vitamin intake during pregnancy and wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol 22: 69–74, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National surveillance for asthma–United States, 1980–2004. MMWR Surveill Summ 56: 1–54, 2007. [PubMed] [Google Scholar]
- 38.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 76: 187–192, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besancon F, Cayre YE, Garabedian M. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol 89–90: 93– 97, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol Lung Cell Mol Physiol 271: L392–L399, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1α,25-dihydroxyvitamin D3. Am J Physiol Lung Cell Mol Physiol 289: L617–L626, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Akbari O, Torday JS. Perinatal nicotine exposure induces asthma in second generation offspring (Abstract). BMC Med 10: 129, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol 305: L501–L507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rehan VK, Sakurai R, Li Y, Karadag A, Corral J, Bellusci S, Xue YY, Belperio J, Torday JS. Effects of maternal food restriction on offspring lung extracellular matrix deposition and long term pulmonary function in an experimental rat model. Pediatr Pulmonol 47: 162–171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehan VK, Torday JS, Peleg S, Gennaro L, Vouros P, Padbury J, Rao DS, Reddy GS. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab 76: 46–56, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Rehan VK, Wang Y, Sugano S, Santos J, Patel S, Sakurai R, Boros LG, Lee WP, Torday JS. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol 292: L323–L333, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Saglani S, Bush A. The early-life origins of asthma. Curr Opin Allergy Clin Immunol 7: 83–90, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, Torday JS, Rehan VK. 1α,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol 297: L496–L505, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutherland ER, Busse WW. Designing clinical trials to address the needs of childhood and adult asthma: The National Heart, Lung, and Blood Institute's AsthmaNet. J Allergy Clin Immunol 133: 34–38, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med 181: 699–704, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tare M, Emmett SJ, Coleman HA, Skordilis C, Eyles DW, Morley R, Parkington HC. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol 589: 4777–4786, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van EE, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 97: 93–101, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122: 1142–1152, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Wjst M, Dold S. Genes, factor X, and allergens: what causes allergic diseases? Allergy 54: 757–759, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med 183: 1336–1343, 2011. [DOI] [PubMed] [Google Scholar]