Abstract
Persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome that is characterized by high pulmonary vascular resistance due to changes in lung vascular growth, structure, and tone. PPHN has been primarily considered as a disease of the small pulmonary arteries (PA), but proximal vascular stiffness has been shown to be an important predictor of morbidity and mortality in other diseases associated with pulmonary hypertension (PH). The objective of this study is to characterize main PA (MPA) stiffness in experimental PPHN and to determine the relationship of altered biomechanics of the MPA with changes in extracellular matrix (ECM) content and orientation of collagen and elastin fibers. MPAs were isolated from control and PPHN fetal sheep model and were tested by planar biaxial testing to measure stiffness in circumferential and axial vessel orientations. Test specimens were fixed for histological assessments of the vascular wall ECM constituents collagen and elastin. MPAs from PPHN sheep had increased mechanical stiffness (P < 0.05) and altered ECM remodeling compared with control MPA. A constitutive mathematical model and histology demonstrated that PPHN vessels have a smaller contribution of elastin and a greater role for collagen fiber engagement compared with the control arteries. We conclude that exposure to chronic hemodynamic stress in late-gestation fetal sheep increases proximal PA stiffness and alters ECM remodeling. We speculate that proximal PA stiffness further contributes to increased right ventricular impedance in experimental PPHN, which contributes to abnormal transition of the pulmonary circulation at birth.
Keywords: persistent pulmonary hypertension of the newborn, biomechanics, collagen, elastin, extracellular matrix, lung development
postnatal gas exchange at birth requires the pulmonary circulation to undergo a rapid change in hemodynamics, which is characterized by a marked fall in pulmonary vascular resistance (PVR) and a dramatic increase in blood flow. Persistent pulmonary hypertension of the newborn (PPHN) represents the failure to achieve the normal neonatal transition of the lung due to sustained elevation of PVR at birth. (3). High PVR in PPHN causes extrapulmonary right-to-left shunting of deoxygenated blood across the foramen ovale and ductus arteriosus, which leads to marked hypoxemia. Although PPHN is associated with altered vascular tone and vasoreactivity, smooth muscle cell hyperplasia, and reduced arterial density, past studies of PPHN have largely focused on the contribution of small pulmonary arteries (PA) to high PVR (9, 29). More recently, proximal PA stiffness has been shown to be an important prognostic in the progression of disease in models of postnatal pulmonary hypertension (PH) (25). However, the proximal elastic PA has received little study in the developing lung or in experimental models of PPHN.
Past studies have shown that altered hemodynamic forces due to increased pulsatility, shear stress, and pressure cause endothelial cell (EC) dysfunction and pathological changes in vascular structure and function (3, 16a). During fetal and early neonatal lung development, these biomechanical factors are also important determinants of vascular cell growth and signaling that contribute to the PPHN phenotype (1). Postnatal survival depends on carefully choreographed changes in lung vascular biomechanics, including tone, structure, compliance, and growth in both central and distal PAs. However, the role of abnormal mechanical stiffness in proximal PA at birth is poorly understood.
Arterial impedance describes the importance of proximal elastic vessel compliance, total vascular resistance, and distal vascular remodeling (30). Proximal PA stiffness plays an important role in disease progression in adults with PH and is an independent predictor of morbidity and mortality in other diseases and postnatal models of PH (20, 23, 25). However, previous studies of the distal vasculature suggest that arterial collagen content is not altered in experimental PPHN (4, 5). Whether proximal stiffening occurs in PPHN and what mechanisms are responsible for vascular remodeling and cellular dysfunction are unclear.
To understand the role of proximal elastic PA remodeling in PPHN, we hypothesized that hemodynamic stress increases main pulmonary artery (MPA) stiffness in utero. Furthermore, we hypothesize increased stiffness changes the extracellular matrix (ECM) remodeling with increased collagen and altered elastin fiber deposition. Alternatively, hemodynamic stress increases proximal PA ECM production, which increases MPA stiffness and right ventricle (RV) afterload and alters hemodynamics within the distal lung. To test our hypotheses, we used a fetal sheep model to examine biaxial testing of the proximal compliance and remodeling in the pathophysiology of PPHN.
METHODS
Human PPHN.
Human lung histology samples from consented autopsies were acquired from Children's Hospital Colorado (Aurora, CO) through an exempt protocol approved by the Colorado Multiple Institutional Review Board. Lung sections were examined for proximal elastic arteries to compare structural remodeling in an infant who died from nonrespiratory causes and two patients who died of PPHN. The nonrespiratory death sample was obtained from a term infant who died at 3 days. PPHN samples were obtained from lungs of two term infants who lived 3 or 9 days. Both patients died from PPHN with associated meconium aspiration syndrome. Images were qualitatively assessed for vascular remodeling and morphological changes.
Fetal sheep model of PPHN.
Our studies used a previously described sheep model of PPHN that has been well established in our laboratory (3, 16a). All procedures and protocols were reviewed and approved by the Animal Care and Use Committee at the University of Colorado Denver Anschutz Medical Center (Aurora, CO). Surgery was performed on twin pregnant mixed-breed Columbia-Rambouillet ewes at day ∼127 of gestational age (n = 6; term = day 147). A left fetal thoracotomy exposed the heart and great vessels. A partial ligation of the ductus arteriosus in utero was performed in one twin while the other was used as the control. The MPA was harvested proximal to the ductus arteriosus from the PPHN and control fetal sheep 7–10 days after surgery.
Biaxial testing of compliance.
Isolated MPAs were tested in the axial (z) and circumferential (θ) directions by using a custom-made soft-tissue biaxial mechanical testing device, as described in Sacks (18) and Vande Geest et al. (27, 28). The biaxial device allows forces to be applied axis independently and equally to the water bath-mounted sample along the axial and circumferential directions of the sample. The stretch deformation is tracked optically in the axial-circumferential plane (10, 12, 19). The applied loads in the axial (PZ) and circumferential (Pθ) directions were measured with calibrated-load cells. Applied loading was controlled on the basis of physiological Cauchy stresses calculated as
| (1) |
| (2) |
where H is the undeformed thickness, Li is the undeformed lengths over which the applied loads act, and λZ and λθ are the in-plane stretches.
MPA samples were mounted with an initial preload of 0.5 kPa to ensure repeatability by consistently referencing the same tare configurations between samples. The specimen was preconditioned with 10 equibiaxial stress cycles at 20 kPa before the prescribed tests were performed. The 20-kPa stress was chosen to allow collagen engagement in test samples while preserving the elasticity in the tissue. MPAs were tested with a series of biaxial perturbations with a ratio of the maximal stress of 20 kPa in the axial and circumferential directions of 0:1 (axial uniaxial), 0.25:1, 0.5:1, 0.75:1, 1:1, 1:0.75, 1:0.5, 1:0.25, and 1:0 (circumferential uniaxial). The series of tests were designed to test the anisotropy, or directional, stiffness of the MPA samples.
Histological evaluation.
MPA samples were fixed in 10% neutral-buffered formalin after completion of the physiological studies. Gross dissections of the fixed samples were oriented in the axial or circumferential direction. Samples either were paraffin embedded or underwent optical clearing. Paraffin-embedded samples were serially sectioned at 5-μm intervals and stained with hematoxylin and eosin (H&E), Verhoff-Van Gieson, and Movat's Pentachrome to assess microstructure in the axial and circumferential directions. To achieve optical clearing, samples were dehydrated with ethanol treatment with progressive strengths of 50%, 70%, twice at 95%, and twice at 100%. These samples were then submerged in a solution of 1:1 ethanol to benzyl benzoate for 4 h before being placed in a solution of 1:2 benzyl alcohol to benzyl benzoate for at least 12 h. Images were viewed by second harmonic generation microscopy. All steps in the optical clearing were performed at room temperature.
Theoretical constitutive model of biaxial compliance.
MPA macroscopic mechanical behavior of the vessel walls was analyzed by using the four-fiber constitutive model as previously described (6, 12). MPA samples were modeled as single-layer fiber-reinforced isochoric, or incompressible, hyperelastic material. The fiber composite hyperelastic artery is related constitutively to experimental data by using a strain energy function to determine the stress required to deform the tissue. The strain energy function is composed of an isotropic function () that describes the ground substance and elastin dominant portion of the vessel low-load response and an anisotropic function () that accounts for collagen fiber orientation and engagement at higher loads with an exponential increase in material stiffness at high strains as collagen recruitment takes over the load response.
The isotropic function () employs a neo-Hookean model that describes the behavior of isotropic rubberlike materials. In the circumferential and axial planes this term becomes
| (3) |
| (4) |
where Î1 is the first invariant, c0 is a fitting constant, and λZ and λθ are the stretches in the circumferential and axial directions, respectively. The constant c0 is related to the shear modulus of the tissue. The anisotropic function () describes the exponential increase in load as collagen engagement begins. The collagen fiber contribution to the mechanics includes four families of collagen fibers: one oriented axially, one circumferentially, and two oriented symmetrically along the diagonals offset by an angle γ from the circumferential direction. The strain energy function is appropriately in exponential form:
| (5) |
| (6) |
in which we assume the diagonal fiber a mechanical equivalent, c13 = c14 = c13,4 and c23 = c24 = c23,4. This results in eight constitutive fit parameters (c0, c11, c21, c12, c22, c13,4, cs3,4, γ) that must be nonnegative to be physically realistic and 0 ≤ λ ≤ π/2. The invariant, Î4, characterizes the constitutive response of the fibers to mechanical loading.
To model the experimental response, we assumed a biaxially stretched membrane with no applied radial load, σ̄rr = 0. This results in boundary conditions described in the axial and circumferential directions (6, 12):
| (7) |
| (8) |
where σzz and σθθ denote the axial and circumferential stresses. Equations 7 and 8 define a system of nonlinear equations that can be solved numerically for a experimental stresses σ̄zz and σ̄θθ with experimental stretches λz and λθ.
The hyperelastic constitutive model was fit to experimental data by optimizing the correspondence between model-predicted behavior and experimental behavior provided from the experimental stress-displacement tests, by minimizing the stress-based nonlinear error function
| (9) |
where n is the number of experimental data points and the weighting factors are w1 and w2 with equal weighting given to each direction. Error function minimization was performed by using MATLAB (The MathWorks, Natick, MA) across a continuous spectrum of circumferential stretches and axial stretches, corresponding to the available experimental data. Constitutive parameters were found for the MPAs across individual data samples, minimizing the error function.
Statistical analysis.
Statistical analysis of the biomechanical characteristics was performed by repeated-measures ANOVA that compared control and PPHN MPAs and accounted for repeated stress and stretch measurements made in the same fetal vessel. Other measurements were analyzed by a two-sided Student's t-test, assuming unequal variance for parametric data, and a Mann-Whitney test for nonparametric data, as noted below.
RESULTS
Representative proximal arteries in human lung histology samples were examined for qualitative structural changes. The human elastic arteries show a slight increase in medial thickening and a striking increase in the thickness of the adventitia in the PPHN patients compared with the control patient (Fig. 1). The thicker adventitia in human PPHN was hypothesized to increase stiffness in the proximal arteries, which motivated our studies to test the stiffness in the remodeled proximal PA in an experimental model of PPHN in fetal sheep.
Fig. 1.
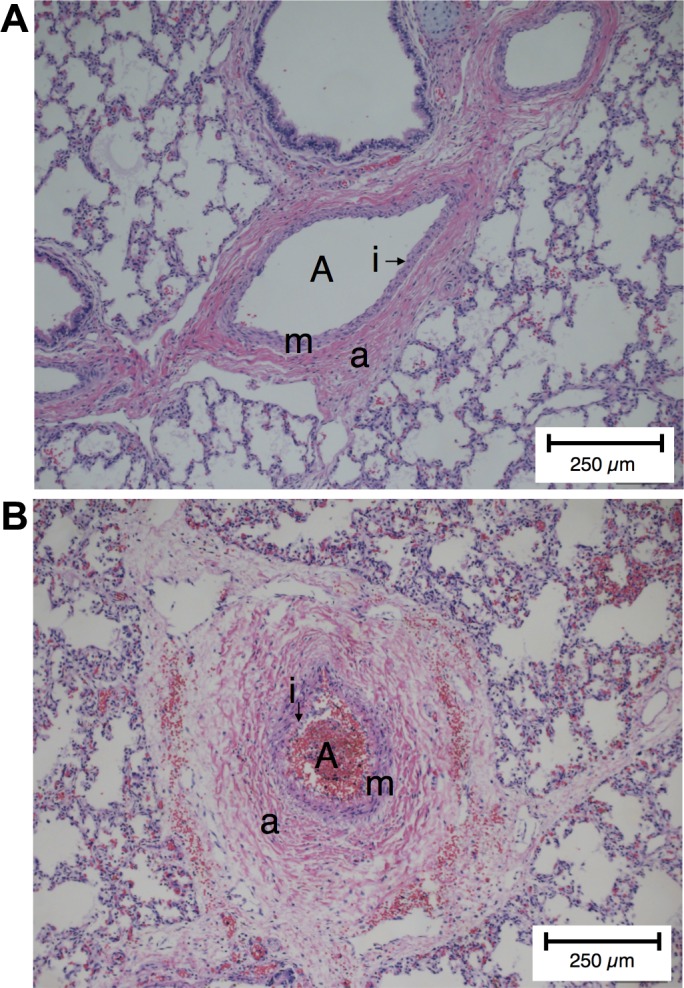
Proximal pulmonary artery remodeling in persistent pulmonary hypertension of the newborn (PPHN) human samples. A: hematoxylin and eosin (H&E)-stained clinical sample of the proximal pulmonary artery (A) of a human for the nonrespiratory death infant labeled with the intima (i), the media (m), and the adventitia (a). B: compared with nonrespiratory death infant, the PPHN artery (A) showed narrowed intima (i), a remodeled media (m), and a remodeled adventitia (a) that is thickened with evidence of fibrosis.
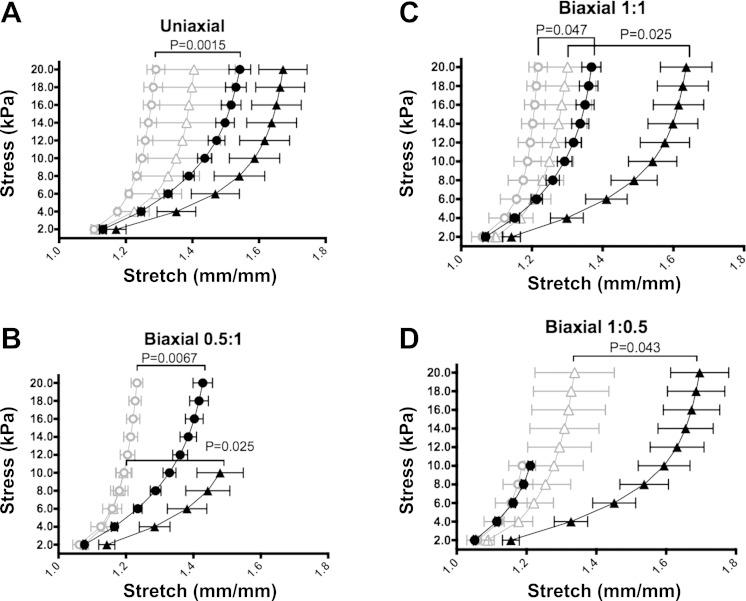
MPA stiffness was examined in control and PPHN fetal sheep by testing biaxial biomechanics across the prescribed stresses. (Fig. 2). Uniaxial tests show increased stiffness in the circumferential direction in PPHN compared with control samples (P < 0.05). The biaxial experimental data shows increased stiffness and demonstrated decreased anisotropy, or directionally dependent stiffness, in PPHN.
Fig. 2.
Experimental biomechanics show increased stiffness in the PPHN sheep model. Mean stretch and standard error of the mean for Cauchy stress-regulated biaxial testing in control axial (▲) and control circumferential (●) directions compared with PPHN axial (△) and PPHN circumferential (○) directions in uniaxial axial and circumferential tests (A), a ratio of 0.5 axial stress to 1 circumferential stress (B), an equal ratio of axial stress to circumferential stress (C), and a ratio of 1 axial stress to 0.5 circumferential stress (D).
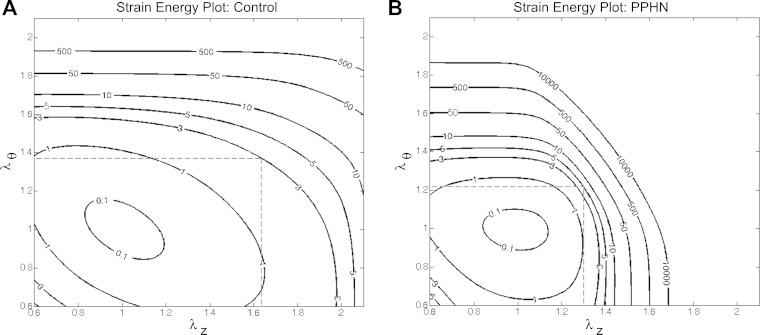
From the biomechanical data, the constitutive model relates the macroscopic biaxial function to the compositional changes with a goodness of fit greater than 0.95 for all samples. Table 1 shows less isotropic, or elastin and ground substance (c0), contribution to the stiffness from 3.17E−06 ± 3.17E−06 kPa (means ± SE) in PPHN to 1.75 ± 0.37 kPa in control MPAs. The contribution of collagen fibers in both axial and circumferential directions were increased in PPHN by 726% (c11) and a trend of 184% (c12), respectively, compared with control MPAs (P = 0.025 and P = 0.08). The diagonally aligned fibers showed a 29% increase in circumferential orientation (γ) in PPHN (P < 0.05). The constitutive model showed lower contributions of elastin and ground substance and a greater role for collagen engagement to PA stiffness. From the constitutive model, PPHN and control samples are plotted with solid strain energy contour lines to visualize the energy necessary to elastically stretch the MPA in the axial-circumferential plane with the average maximal stress plotted as the dashed line (Fig. 3). The strain energy contour confirms the decrease in anisotropy in the PPHN MPA compared with the control MPA, with the contour lines being almost isotropic in PPHN. The average stretch line demonstrates the shift in strain energy changes to preserve the average wall stress within the artery wall.
Table 1.
Fit coefficients for the 4-fiber model in control and PPHN sheep model samples
| Specimen | c0, kPa | c11, kPa | c21 | c12, kPa | c22 | c13,4, kPa | c23,4 | γ, ° | R2 |
|---|---|---|---|---|---|---|---|---|---|
| CTL | 1.75 ± 0.37 | 0.53 ± 0.45 | 0.99 ± 0.21 | 3.85 ± 2.32 | 1.03 ± 0.68 | 1.41 ± 0.30 | 1.66 ± 0.45 | 52.7 ± 3.6 | 0.98 ± 0.01 |
| PPHN | 3.17E-06 ± 3.17E-06 | 4.38 ± 1.44 | 2.51 ± 1.65 | 10.93 ± 2.46 | 0.44 ± 0.34 | 2.28 ± 1.79 | 5.82 ± 1.93 | 37.4 ± 1.3 | 0.97 ± 0.01 |
| P value | 0.004 | 0.025 | 0.33 | 0.08 | 0.5 | 0.6 | 0.05 | 0.009 |
Isotropic, or elastin and ground substance, contribution to the stiffness is described by c0. The contribution of the collagen fibers in both axial and circumferential directions are described by c11, c21 and c12, c22, respectively, while the diagonal collagen fibers are described by c13,4, c23,4 with the angle between the diagonal fibers γ. CTL, control; PPHN, persistent pulmonary hypertension of the newborn.
Fig. 3.
Strain energy contour shifts in PPHN sheep model. Representative strain energy plots for a control main pulmonary artery (MPA; A) and a PPHN MPA (B) with mean stretches in the axial-circumferential directions noted by the dashed line. λz and λθ, In-plane stretches.
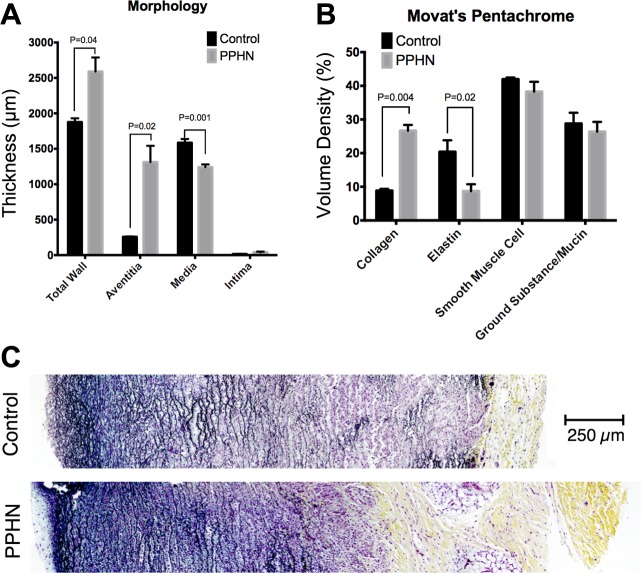
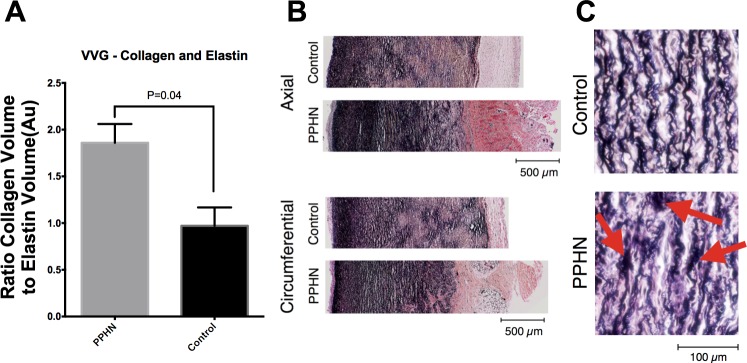
The MPA from the PPHN sheep has a markedly altered morphology and composition compared with the control (Fig. 4), including increased total wall thickness that is characterized by increased adventitia but decreased media layers compared with control MPA. The MPA from PPHN sheep has increased collagen, decreased elastin, and increased ratios of collagen to elastin compared with control MPA (Fig. 5). Qualitatively, elastic fibers in the PPHN MPA appear more globular and less organized than their control sample counterparts, which exhibited concentric elastic bands. H&E image analysis shows no change in cellular density in the PPHN and control vessels (Fig. 6). Second harmonic generation images of cleared MPA further demonstrate the structural changes in the PPHN vessel wall (Fig. 7 plus Supplemental Material for this article available on the Journal website). The constitutive collagen orientation correlates with the second harmonic generation images, which demonstrate greater organization of collagen fibers in the circumferential direction. The second harmonic generation z-stack images of the PPHN adventitial wall show increased density of microvessels compared with the control MPA (Fig. 7).
Fig. 4.
PPHN sheep model altered morphology and composition. A: morphology showed increased thickness with a increased adventitia and reduced media in the PPHN MPA compared with control. B: stereological quantification based on Movat's pentachrome staining showed increased collagen and decreased elastin in PPHN compared with control. C: representative Movat's pentachrome-stained MPAs for control and PPHN.
Fig. 5.
Increased collagen and reduced elastin in PPHN sheep model. A: stereological quantification based on Verhoff-van Gieson (VVG) staining showed increased collagen-to-elastin ratio in PPHN compared with control. B: representative VVG-stained MPAs for control and PPHN. C: close examination of the elastin fibers showed abnormal formation with “clumps” of elastin in PPHN (arrows) compared with the control MPA.
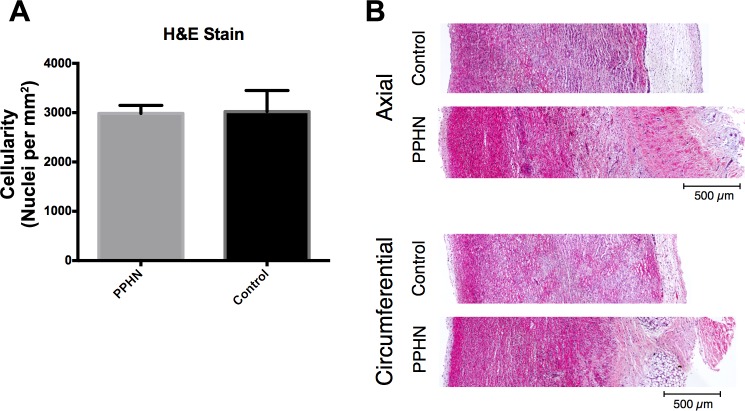
Fig. 6.
Constant cellular content in PPHN sheep model. A: H&E slides showed no increase in cells per area in PPHN compared with control MPAs. B: representative H&E-stained MPAs for control and PPHN.
Fig. 7.
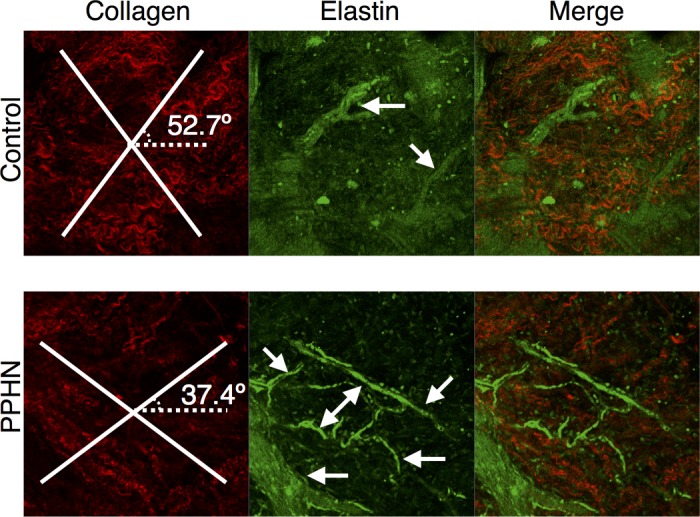
Reoriented collagen and increased microvessels in PPHN sheep model. Second harmonic generation of representative adventitia for control and PPHN MPAs for collagen (red) with modeled collagen fiber angles plotted, elastin (green) with microvessels, and the merged image.
DISCUSSION
We found that the proximal MPA from fetal sheep with PPHN is characterized by increased stiffness and altered ECM remodeling compared with normal fetal controls. Biaxial mechanics demonstrate that the stiffening occurs in both the axial and circumferential directions and reveal decreased anisotropy measured in MPA from PPHN sheep. The constitutive equations, which relate gross mechanical data to the microstructural components of the vessel wall, suggest a reduced role of elastin and more prominent role for collagen engagement in vascular mechanics in PPHN, which is primarily due to increased circumferentially oriented collagen fibers in the MPA wall. A series of morphometric techniques confirmed that MPA thickening occurs in PPHN with decreased elastin in the media and increased collagen deposition occurring in the adventitia. Interestingly, the adventitia in the MPA from PPHN sheep is further characterized by an increase in microvessel growth. Together, these findings demonstrate that the ECM in the MPA is more fibrotic and less compliant in experimental PPHN.
These findings are important because PPHN has long been considered as primarily a disease of the distal vasculature with little contribution of larger proximal arteries to abnormal pulmonary hemodynamics. PPHN is associated with smooth muscle cell hyperplasia and reduced arterial density in the distal lung (8, 9, 29). In contrast with small PAs, our findings show that significant medial hypertrophy does not occur in the MPA of PPHN sheep compared with control MPA. Past studies suggest that distal, second generation intralobar PA show no changes in ECM composition in PPHN compared with controls (4, 5). However, our study demonstrates that the proximal vessels in PPHN have decreased elastin, increased collagen content, and increased circumferential collagen orientation. Proximal vascular stiffness likely elevates RV afterload due to increased vascular impedance, as reflected by high RV systolic and pulse pressures in PPHN animals. Proximal PA stiffening may be an important consideration when addressing distal vascular vasoactive changes due to the influence of the Bayliss effect, in which stretch of proximal PAs augments downstream vasoconstriction (13).
In this model of PPHN, occlusion of the ductus arteriosus initially elevates pulmonary artery pressure (PAP) and pulmonary blood flow; however, blood flow progressively returns to baseline values despite sustained elevations of PAP (4). These findings suggest that the effects of hemodynamic stress in this model are primarily due to high pressure and pulsatility, and not to high flow (2). Past studies in this model have further demonstrated persistent EC dysfunction in vivo and in vitro, as characterized by impaired endothelium-dependent vasodilation; decreased EC growth and tube formation; and downregulation of VEGF, VEGF-R2, and eNOS protein contents (7).
The endothelium provides a critical role as an intermediary between blood flow and the vessel wall, serving as an effective biological mechanotransducer that senses and converts hemodynamic forces to biochemical signals that regulate angiogenesis, inflammation, vascular tone, and wall structure (24). Others have previously implicated increased stiffness and hemodynamic stress as a major cause of EC dysfunction in other experimental settings (11, 17). Proximal PA stiffening can further augment the effects of hemodynamic stress and EC dysfunction in the distal pulmonary circulation, as reflected by enhanced expression of proinflammatory molecules and increased endothelial to mesenchymal transitions (14–16, 22, 25).
Although impaired vascular reactivity and distal arterial growth contribute to PPHN physiology, collagen remodeling and stiffening of central PAs likely further contribute to abnormal hemodynamics, including increased pulsatility, shear stress, and pressure (20, 23). RV-PA coupling, as characterized by the ventricular-vascular coupling ratio (21), would also be adversely affected, since stiffening increases RV systolic pressure, which further alters arterial elastance. On the basis of our findings of decreased distensibility, we speculate that both proximal stiffness and distal vasoreactivity likely contribute to RV dysfunction and the pathogenesis of PPHN.
Limitations of this study include a lack of biochemical data to more precisely quantify changes in ECM proteins. Instead, we examined changes in MPA morphology to visualize the distribution and spatial organization of ECM and cellular structures.
In conclusion, we found that MPAs from fetal sheep with PPHN have increased stiffness and extensive ECM remodeling, which is characterized by a thin media but thicker adventitia with increased collagen content and orientation compared with controls. These finding suggest that in addition to changes in the distal pulmonary arteries, proximal remodeling and stiffness of the proximal PA may further contribute to impaired hemodynamics of PPHN by further increasing impedance and altering RV-PA coupling. We speculate that therapies aimed at preventing proximal vascular remodeling and enhancing distal vascular function will improve outcome through reduced RV afterload in severe PPHN.
GRANTS
Funding support provided by the Actelion Entelligence Young Investigator Award (R. B. Dodson), the Children's Hospital Colorado Research Scholar Award (R. B. Dodson), and a grant from the National Heart, Lung, and Blood Institute (HL68702; S. H. Abman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.B.D. and S.H.A. conception and design of research; R.B.D., M.M., and C.G. performed experiments; R.B.D., C.G., K.S.H., and S.H.A. analyzed data; R.B.D., C.G., K.S.H., and S.H.A. interpreted results of experiments; R.B.D. prepared figures; R.B.D. drafted manuscript; R.B.D., M.M., C.G., K.S.H., and S.H.A. edited and revised manuscript; R.B.D., M.M., C.G., K.S.H., and S.H.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Jason Gien for providing tissue, expertise, and assistance in using the PPHN model. Additionally, we thank the staff at the Perinatal Research Center for assistance and resources for this project. Second harmonic generation images were taken with the expertise at the Advanced Light Microscopy Core, School of Medicine, University of Colorado at Denver.
REFERENCES
- 1.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol 661: 323–335, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Accurso FJ. Acute effects of partial compression of ductus arteriosus on fetal pulmonary circulation. Am J Physiol Heart Circ Physiol 257: H626–H634, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary-hypertension in fetal lambs. J Clin Invest 83: 1849–1858, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belik J. Myogenic response in large pulmonary arteries and its ontogenesis. Pediatr Res 36: 34–40, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Belik J, Keeley FW, Baldwin F, Rabinovitch M. Pulmonary hypertension and vascular remodeling in fetal sheep. Am J Physiol Heart Circ Physiol 266: H2303–H2309, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Ferruzzi J, Vorp DA, Humphrey JD. On constitutive descriptors of the biaxial mechanical behaviour of human abdominal aorta and aneurysms. J R Soc Interface 8: 435–450, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gien J, Seedorf GJ, Balasubramaniam V, Markham N, Abman SH. Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor-nitric oxide signaling. Am J Respir Crit Care Med 176: 1146–1153, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grover TR, Parker TA, Balasubramaniam V, Markham NE, Abman SH. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 288: L648–L654, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Grover TR, Parker TA, Zenge JP, Markham NE, Kinsella JP, Abman SH. Intrauterine hypertension decreases lung VEGF expression and VEGF inhibition causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 284: L508–L517, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey JD. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. New York: Springer, 2002. [Google Scholar]
- 11.Johnson BD, Mather KJ, Wallace JP. Mechanotransduction of shear in the endothelium: basic studies and clinical implications. Vasc Med 16: 365–377, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Kamenskiy AV, Pipinos II, Dzenis YA, Lomneth CS, Kazmi SAJ, Phillips NY, MacTaggart JN. Passive biaxial mechanical properties and in vivo axial pre-stretch of the diseased human femoropopliteal and tibial arteries. Acta Biomater 10: 1301–1313, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levick JR. An Introduction to Cardiovascular Physiology. London, New York: Arnold; copublished in the USA by Oxford University Press, 2000, p. ix. [Google Scholar]
- 14.Li M, Scott DE, Shandas R, Stenmark KR, Tan W. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann Biomed Eng 37: 1082–1092, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Stenmark KR, Shandas R, Tan W. Effects of pathological flow on pulmonary artery endothelial production of vasoactive mediators and growth factors. J Vasc Res 46: 561–571, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Tan Y, Stenmark KR, Tan W. High pulsatility flow induces acute endothelial inflammation through overpolarizing cells to activate NF-κB. Cardiovasc Eng Techn 4: 26–38, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Morin FC. Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 25: 245–250, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Roman BL, Pekkan K. Mechanotransduction in embryonic vascular development. Biomech Model Mechanobiol 11: 1149–1168, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacks MS. Biaxial mechanical evaluation of planar biological materials. J Elast 61: 199–246, 2000. [Google Scholar]
- 19.Sacks MS, Sun W. Multiaxial mechanical behavior of biological materials. Annu Rev Biomed Eng 5: 251–284, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Safar M. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107: 2864–2869, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Sanz J, Garcia-Alvarez A, Fernandez-Friera L, Nair A, Mirelis JG, Sawit ST, Pinney S, Fuster V. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 98: 238–243, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Scott-Drechsel D, Su Z, Hunter K, Li M, Shandas R, Tan W. A new flow co-culture system for studying mechanobiology effects of pulse flow waves. Cytotechnology 64: 649–666, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shadwick R. Mechanical design in arteries. J Exp Biol 202: 3305–3313, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Stenmark KR. GROVER: Vascular stiffening in pulmonary hypertension: cause or consequence? Pulm Circ. http://www.jstor.org/stable/10.1086/677370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Z, Tan W, Shandas R, Hunter KS. Influence of distal resistance and proximal stiffness on hemodynamics and RV afterload in progression and treatments of pulmonary hypertension: a computational study with validation using animal models. Comput Math Methods Med 2013: 618326, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vande Geest JP, Sacks MS, Vorp DA. Age dependency of the biaxial biomechanical behavior of human abdominal aorta. J Biomech Eng 126: 815–822, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Vande Geest JP, Sacks MS, Vorp DA. A planar biaxial constitutive relation for the luminal layer of intra-luminal thrombus in abdominal aortic aneurysms. J Biomech 39: 2347–2354, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Villamor E, LeCras TD, Horan MP, Halbower AC, Tuder RM, Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 272: L1013–L1020, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Westerhof N, Stergiopulos N, Noble MIM. Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education. New York: Springer, 2010, p. xi, 271 p. [Google Scholar]