Abstract
Excess superoxide has been implicated in pulmonary hypertension (PH). We previously found lung overexpression of the antioxidant extracellular superoxide dismutase (EC-SOD) attenuates PH and pulmonary artery (PA) remodeling. Although comprising a small fraction of total SOD activity in most tissues, EC-SOD is abundant in arteries. We hypothesize that the selective loss of vascular EC-SOD promotes hypoxia-induced PH through redox-sensitive signaling pathways. EC-SODloxp/loxp × Tgcre/SMMHC mice (SMC EC-SOD KO) received tamoxifen to conditionally deplete smooth muscle cell (SMC)-derived EC-SOD. Mice were exposed to hypobaric hypoxia for 35 days, and PH was assessed by right ventricular systolic pressure measurements and right ventricle hypertrophy. Vascular remodeling was evaluated by morphometric analysis and two-photon microscopy for collagen. We examined cGMP content and soluble guanylate cyclase expression and activity in lung, lung phosphodiesterase 5 (PDE5) expression and activity, and expression of endothelial nitric oxide synthase and GTP cyclohydrolase-1 (GTPCH-1), the rate-limiting enzyme in tetrahydrobiopterin synthesis. Knockout of SMC EC-SOD selectively decreased PA EC-SOD without altering total lung EC-SOD. PH and vascular remodeling induced by chronic hypoxia was augmented in SMC EC-SOD KO. Depletion of SMC EC-SOD did not impact content or activity of lung soluble guanylate cyclase or PDE5, yet it blunted the hypoxia-induced increase in cGMP. Although total eNOS was not altered, active eNOS and GTPCH-1 decreased with hypoxia only in SMC EC-SOD KO. We conclude that the localized loss of PA EC-SOD augments chronic hypoxic PH. In addition to oxidative inactivation of NO, deletion of EC-SOD seems to reduce eNOS activity, further compromising pulmonary vascular function.
Keywords: extracellular superoxide dismutase; pulmonary vascular remodeling; endothelial nitric oxide synthase; guanosine 3′,5′-cyclic monophosphate; hypoxia; guanylate cyclase; phosphodiesterase; nitric oxide synthase
pulmonary hypertension (PH) is a progressive lethal disease affecting children and adults. The currently available therapies for PH have not adequately improved patient outcomes; therefore, a better understanding of the mechanisms underlying the development of PH is necessary to develop effective therapeutic approaches (18, 26, 29). Oxidative stress and reactive oxygen species (ROS) signaling are now recognized to have a critical role in the pathogenesis of human PH and animal models of PH (2, 7, 8, 15). This area of investigation has important translational implications since antioxidant treatments interrupt multiple pathological signaling pathways, but it is complicated by the observations that low levels of ROS are essential to normal cell signaling, and the reactions of ROS are highly compartmentalized. This makes it necessary to consider the dysregulation of specific ROS and antioxidants involved in the pathological processes as well as the tissue and cellular compartmentalization when interrogating mechanisms or designing new therapeutic strategies.
The sole extracellular enzymatic defense against superoxide (O2·−) is the antioxidant extracellular superoxide dismutase (EC-SOD or SOD3). EC-SOD is one of three known mammalian isoforms of SOD, which also include cytosolic Cu-Zn SOD (SOD1) and mitochondrial Mn-SOD (SOD2). Although EC-SOD constitutes a small fraction of total SOD activity in most tissues, it comprises up to 70% of the total SOD in arteries (17, 24, 28, 30). The high prevalence of EC-SOD in arteries under normal conditions results from its high expression by vascular cells as well as its positively charged COOH-terminus (heparin binding domain), which enables it to bind avidly to the matrix-rich vessel wall (1, 6, 17). In one human study testing bronchial tissue from individuals with end-stage idiopathic pulmonary arterial hypertension (PAH) as well as a number of animal models of PH, EC-SOD expression and activity are decreased with established PH (25, 33). Total body deletion of EC-SOD in animal models of PH worsens outcome, whereas overexpression of lung EC-SOD activity protects against both elevated pulmonary artery pressures and vascular remodeling (3, 17, 27, 36). Based on this information, investigating the consequence of insufficient vascular EC-SOD is necessary to more fully understand the role of ROS in PH. We therefore hypothesized that the specific loss of EC-SOD within the pulmonary artery would worsen PH caused by chronic hypoxia, a well-established and reproducible mouse model of PH.
To test our hypothesis, we used a genetically engineered mouse in which loxP sites had been cloned flanking the EC-SOD coding region. These mice were crossed with transgenic mice in which the myosin heavy chain gene promoter drives expression of tamoxifen-inducible Cre-recombinase (SMC EC-SOD KO). These mice exhibit pronounced EC-SOD knockdown in aorta (23). We now report that EC-SOD was also markedly reduced in pulmonary artery while total lung EC-SOD content was unchanged. Despite stable total lung EC-SOD, the knockdown of EC-SOD in the pulmonary vasculature augmented development of chronic hypoxic PH and pulmonary vascular remodeling. EC-SOD increases NO· bioavailability by limiting the reaction of NO· with O2·−; the original description of these mice demonstrated both increased vascular O2·− and decreased vascular NO· (23). Because decreased EC-SOD activity is associated with impaired cGMP signaling in a different model of PH (14, 34), we evaluated the soluble guanylate cyclase (sGC)-cGMP-phosphodiesterase 5 (PDE5) axis. Although we did not find evidence that vascular loss of EC-SOD impaired cGMP-dependent signaling, our data suggested that, in addition to its known effects to cause O2·−-mediated inactivation of NO·, loss of vascular EC-SOD may augment chronic hypoxic PH via cGMP-independent pathways through loss of eNOS activation. These data provide strong evidence that the compartmentalization of the production of ROS and antioxidant scavenging, and in particular the loss of extracellular vascular-derived EC-SOD, is critically important in the development of pulmonary vascular disease.
MATERIALS AND METHODS
Mouse model.
Animal studies were approved by the University of Colorado Institutional Animal Care and Use Committee. We used EC-SODloxp/loxp × Tgcre/SMMHC mice (SMC EC-SOD KO) with a conditional knockdown of EC-SOD specifically in smooth muscle cells (SMC) and EC-SODloxp/loxp (EC-SOD control) mice (23). Cre-recombinase (Cre) was activated via an estrogen receptor by intraperitoneal injection of tamoxifen suspended in sterile sesame oil (Sigma-Aldrich) at a dosage of 0.4 mg/20 g of body wt for five consecutive days. Cre was driven by smooth muscle cell myosin heavy chain (SMMHC) promoter to target SMC and was located on the Y chromosome; therefore, only male mice were used. All mice received tamoxifen injections beginning at 6 wk of age and were recovered for 14 days to allow for elimination of tamoxifen. Mice were then maintained in normobaric normoxia or hypobaric hypoxia for up to 35 days. For hypoxia, mice were placed in hypobaric chambers at a simulated altitude of 18,000 ft above sea level (395 Torr), conditions equivalent to 10% atmospheric oxygen. Normoxic mice remained in Denver (5,280 ft) ambient air. Wild-type C57/Bl6 and total body EC-SOD KO mice were used for isolation of pulmonary artery smooth muscle cells (PASMC) (Jackson Laboratories).
Hemodynamic measurements and tissue harvesting.
Mice were anesthetized with inhaled isofluorane (1.5–4%) under normoxic conditions. As previously described, PH was evaluated by right ventricular systolic pressure (RVSP), measured by direct right ventricle (RV) puncture in a closed chest (27). A 25-gauge needle attached to a pressure transducer was introduced in the RV, and live pressure tracings were measured using the Cardiomax III Cardiac Output program (Columbus Instruments). The chest was opened, and lungs were flushed with 5 ml cold PBS via the RV. The right lung was flash-frozen while the left lung was inflation fixed at 20 cmH2O pressure in 4% paraformaldehyde for 30 min and then dissected from the chest cavity and placed in 4% paraformaldehyde at 4°C for 2 days followed by 70% ethanol and paraffin embedding. The hearts were removed, and the RV was separated from the left ventricle under a dissecting microscope and then weighed to determine the ratio of the RV/left ventricle + septum weights.
Protein isolation.
Pulverized lung and thoracic aorta tissue was homogenized in T-PER (Thermo Scientific) containing protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich) and centrifuged to remove tissue debris. Protein concentration was determined using the Pierce 660-nm protein assay reagent (Thermo Scientific). For studies of sGC and PDE5 expression and activity, frozen lung was pulverized and homogenized in 1× Mg2+ lysis buffer (EMD Millipore) supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and a phosphatase inhibitor cocktail (EMD Biosciences). Lung protein extracts were sonicated, and protein concentration was determined using the Bradford assay (13, 20). Pulmonary artery protein was isolated from pulmonary arteries as previously described (13). Briefly, lungs were perfused with warmed agarose containing iron particles. The lung was cooled to solidify the agarose and then minced. The minced lung was then treated with collagenase and sheared with an 18-gauge needle to enable separation of the vessels from lung tissue. The iron-containing vessel segments were separated from the surrounding lung using a DynaMag-50 (Invitrogen). For protein homogenates, the vessel-iron pellet was resuspended in lysis buffer and sonicated on ice. The iron particles were separated from the vessel homogenate, and protein concentration was measured.
Western blot analysis.
For Western blot analysis, 20–40 mg of lung, aortic, or pulmonary artery protein were loaded on a 4–12% Bis-Tris Criterion XT Precast Gel (Bio-Rad), and protein was separated by gel electrophoresis. Proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad) or Hybond-ECL nitrocellulose (GE Healthcare Biosciences) using a Novex Semi-Dry Blotter (Invitrogen). Blots were blocked with 5% milk in Tris-buffered saline with 0.05% Tween 20 and probed with the following primary antibodies: polyclonal rabbit polyclonal EC-SOD antibody at 1:1,000 (Santa Cruz), mouse monoclonal endothelial nitric oxide synthase (eNOS) at 1:1,000 (BD Transduction), rabbit polyclonal phospho-eNOS at 1:1,000 (Abcam), rabbit polyclonal GTP cyclohydrolase-1 (GTPCH-1) at 1:800 (Abcam), rabbit polyclonal PDE5 at 1:1,000 (Santa Cruz Biotechnology), rabbit polyclonal sGCα at 1:1,000 (Cayman Chemical), sGCβ at 1:1,000 (Cayman Chemical), or mouse monoclonal β-actin at 1:5,000 (Sigma-Aldrich) overnight at 4°C followed by an anti-rabbit or mouse horseradish peroxidase-conjugated secondary antibody at the appropriate dilution (EMD Millipore, Cell Signaling). Blots were developed with enhanced chemiluminescence (ECL) (Thermo Scientific). For normalization, bands were quantified by densitometry using ImageJ software from the National Institutes of Health (NIH) or Image Lab Software (Bio-Rad). Data are shown as fold ± SE relative to EC-SOD control mice (13, 20).
SOD activity assay.
Lung tissue was homogenized in SOD activity assay buffer, and EC-SOD was separated from intracellular SOD (SOD1 and SOD2) using the Glycoprotein Isolation Kit, ConA (Pierce Biotechnology), as recently described (16). Activity levels were determined using the SOD assay kit-WST (Dojindo Molecular Technologies), according to the manufacturer's instructions. Data were expressed as units of EC-SOD activity per gram of lung tissue.
Measurement of muscularization and medial wall thickness.
Lung sections were immunostained with mouse monoclonal α-smooth muscle actin (α-SMA) antibody (1:1,000, Clone 1A4) (Sigma-Aldrich), using the Mouse on Mouse Basic Kit and the Elite ABC reagent (Vector Laboratories). The slides were developed with ImmPACT DAB peroxidase substrate and counterstained with hematoxylin QS (Vector Laboratories). Tissue sections were examined by light microscopy and photographed on a Zeiss Axiovert S100. The number of small vessels (<50 μm) with positive α-SMA staining was counted in 10 fields of view (×10 magnification) to evaluate muscularized small pulmonary vessels. For medial wall thickness (MWT), four measurements of the perpendicular lumen radius and medial wall were taken in small muscularized arteries (<200 μm). MWT was expressed as the average MWT divided by the average vessel radius. The analysis of α-SMA staining was evaluated by an investigator blinded to treatment groups.
Collagen analysis.
Collagen deposition around pulmonary arteries <200 μm associated with terminal bronchioles was analyzed by nonlinear second harmonic generation (SHG) imaging, as recently described (16). Collagen fibrils are able to intrinsically generate an SHG signal, which can be quantified to reflect collagen content (22). Briefly, deparaffinized lung sections (100 μm) were imaged on a Zeiss LSM510-Meta microscope with a ×63 1.4 NA Plan-Apochromat oil immersion objective, using a 800-nm mode-locked 100-fs pulsed Ti:sapphire laser (Chameleon, Coherent) for nonlinear excitation. SHG was detected with a narrow band 390- to 410-nm bandpass filter (Chroma), and autofluorescence was detected with a 450- to 700-nm broad bandpass filter. The area of SHG signal around a single pulmonary artery, measured as pixels, was calculated by an investigator blinded to treatment groups and expressed relative to the vessel perimeter, using Matlab (Mathworks) and ImageJ (NIH).
cGMP measurements by enzyme immunoassay.
cGMP levels were measured as previously described (13, 20). Briefly, lung tissue was homogenized in 10 volumes of 5% TCA (Sigma-Aldrich). Precipitate was removed by centrifugation at 1,500 g for 10 min. TCA was extracted using water-saturated ether (Sigma-Aldrich), and samples were acetylated according to the manufacturer's protocol. cGMP content was measured by enzyme immunoassay (EIA) in duplicate in the absence of any phosphodiesterase inhibitor, using a commercially available kit (Cayman Chemical). Results were measured using a Bio-Rad iMark automated plate reader at 405 nm. Results are shown as picomoles cGMP per milliliter per milligram total protein.
PDE5 activity assay.
Total lung protein was freshly prepared for PDE5 activity assays, as previously described (10). The protein was purified over a CentriSpin 10 column to remove any phosphate contamination (Princeton Separations). Protein concentration was determined as described above. Total protein (5 μg) was assayed for cGMP hydrolytic activity using a commercially available colorimetric cyclic nucleotide PDE assay kit (Enzo Life Sciences). Each sample was read in duplicate, both without sildenafil and with 100 nM sildenafil (Sigma-Aldrich), to determine PDE5-specific cGMP hydrolytic activity. The samples were incubated at 30°C for 30 min and then incubated with the BIOMOL Green reagent (Enzo Life Sciences) at room temperature for 20 min. Results were measured using a Bio-Rad iMark automated plate reader (Hercules, CA) at 620 nm. The difference between the picomoles cGMP hydrolyzed per milligram total protein per minute without sildenafil and the picomoles cGMP hydrolyzed per milligram total protein per minute with sildenafil represents the PDE5-specific cGMP hydrolytic activity. Results are shown as the PDE5-specific picomole cGMP hydrolyzed per milligram total protein per minute.
sGC activity assay.
Total lung protein was freshly prepared for sGC activity assays, as previously described (10, 20). Each sample was dried, resuspended in cGMP EIA buffer, and acetylated according to the manufacturer's protocol. cGMP was measured by EIA in duplicate using a commercially available kit (Cayman Chemical). Results were measured using a Bio-Rad iMark automated plate reader at 405 nm. sGC activity results are shown as picomoles cGMP per milligram total protein per minute.
Statistical analysis.
Data were analyzed by unpaired t-test or two-way ANOVA to determine differences between treatment groups and strain, followed by Bonferroni post hoc analysis using Prism software (GraphPad Software). Data are expressed as means ± SE. Significance was defined as P < 0.05.
RESULTS
Loss of SMC-derived EC-SOD did not affect total lung EC-SOD but significantly decreased vascular EC-SOD content.
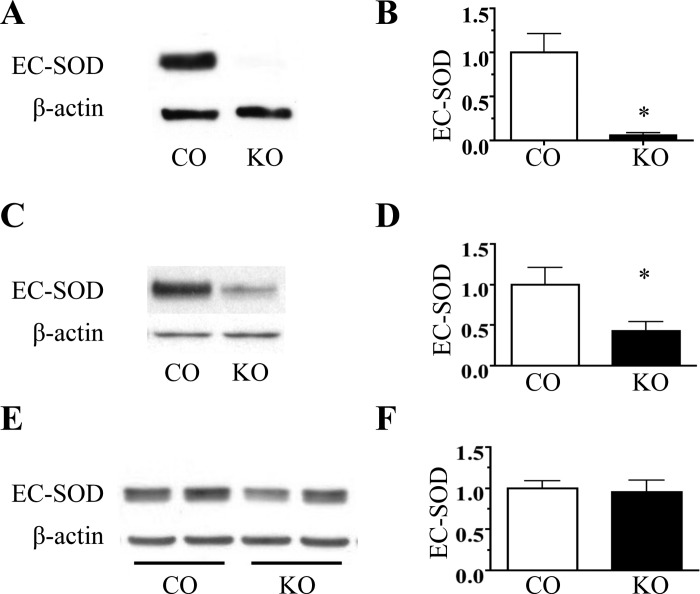
To evaluate the contribution of SMC-derived EC-SOD to lung and vascular EC-SOD content, we measured EC-SOD expression in the lung, aorta, and pulmonary artery of SMC EC-SOD KO and EC-SOD control mice. Conditional knockout of SMC-derived EC-SOD markedly decreased EC-SOD content in both the aorta and pulmonary artery (Fig. 1, A–D); however, it did not change total lung EC-SOD protein expression (Fig. 1, E and F) or activity (EC-SOD activity in U/g tissue: 264 ± 13 EC-SOD CO vs. 243 ± 18 SMC EC-SOD KO, P = 0.46 by unpaired t-test, n = 3–6).
Fig. 1.
Conditional depletion of smooth muscle cell (SMC) extracellular superoxide dismutase (EC-SOD) depletes vascular EC-SOD without decreasing lung EC-SOD expression. Representative Western blot analysis for EC-SOD in aorta (A), pulmonary artery (PA) (C), and lung (E) from male SMC EC-SOD KO (KO) and EC-SOD control (CO) mice. Corresponding relative densitometry for EC-SOD/β-actin in aorta (B), PA (D), and lung (F). Both mouse strains expressed EC-SODloxP/loxP, but only the KO mice expressed Cre-recombinase, driven by the smooth muscle myosin heavy chain promoter and induced by tamoxifen. Both strains were treated with 5 sequential days of tamoxifen (0.4 mg/20 g body wt ip in sesame oil) followed by 2 wk of recovery. *P < 0.05 by unpaired t-test, n = 3–6.
Conditional depletion of SMC-derived EC-SOD augmented chronic hypoxic PH.
The severity of hypoxia-induced PH was evaluated after 35 days of chronic hypoxia in SMC EC-SOD KO mice and EC-SOD control mice compared with normoxic mice. Chronic hypoxia PH, as shown by elevated RVSP, was worse in SMC EC-SOD KO mice compared with the EC-SOD control mice (Fig. 2A). Whereas there were no differences in RVSP between the strains under normoxic conditions, the RVSP was higher at baseline in tamoxifen-treated EC-SOD control mice compared with mice not receiving tamoxifen (22 ± 1.3 mmHg in untreated vs. 31.3 ± 1.4 mmHg tamoxifen-treated EC-SOD control mice). The SMC EC-SOD KO mice exhibited RV hypertrophy at baseline. Chronic hypoxia-induced RV hypertrophy was further augmented in SMC EC-SOD KO mice compared with EC-SOD control mice (Fig. 2B).
Fig. 2.

Conditional depletion of SMC EC-SOD augments chronic hypoxic pulmonary hypertension. A: right ventricular systolic pressures (RVSP) measured by direct right ventricle (RV) puncture in KO and CO mice exposed to 35 days of chronic hypobaric hypoxia (Hypo) (395 Torr, equivalent to 18,000 ft or 10% oxygen). Time-matched normoxic mice (Norm) were maintained at Denver ambient altitude for 35 days. Both strains completed tamoxifen treatments 2 wk before the start of the environmental exposure. B: right ventricular hypertrophy (RVH), measured by the ratio of right ventricle/left ventricle + septum weight (RV/LV + septum). There was a significant difference in RVSP and RVH between strains and treatment, determined by 2-way ANOVA. *P < 0.05 vs. CO Norm; #P < 0.05 vs. CO Hypo, n = 7–13.
Conditional depletion of SMC-derived EC-SOD worsened hypoxia-induced pulmonary artery remodeling.
Chronic hypoxia-induced vascular remodeling within the medial wall was determined in lung sections stained for α-SMA by counting the number of small muscularized vessels in a high-powered field and measuring the MWT of midsized pulmonary arteries. The number of small muscularized vessels increased in both strains after 35 days of chronic hypoxia, with a significantly greater increase in SMC EC-SOD KO mice compared with EC-SOD controls (Fig. 3, A and B). MWT was increased in the SMC EC-SOD KO mice under normoxic conditions compared with the EC-SOD control mice. However, chronic hypoxia did not increase MWT in either strain (Fig. 3, C and D).
Fig. 3.
Conditional depletion of SMC EC-SOD augments chronic hypoxia-induced vascular medial wall remodeling. A: representative lung α-smooth muscle actin (α-SMA) immunostaining (brown signal) with counterstain by hematoxylin (blue) after 35 days of hypoxia or normoxia in CO and KO mice. Bar = 200 μm. Arrows indicate muscularized small vessels identified by positive α-SMA immunostaining. B: quantitation of muscularized small vessels (<50 μm) in a ×10 high-power field (HPF) in normoxia and after 35 days of hypoxia. Data are expressed as no. vessels/HPF, n = 6 mice/group. *P < 0.05 vs. CO Norm; #P < 0.05 vs. CO Hypo. C: representative lung α-SMA immunostaining of a midsized pulmonary artery used to determine medial wall thickening. Bar = 60 μm. D: quantitation of medial wall thickness (MWT) of pulmonary arteries adjacent to terminal bronchioles (<200 μm). Data expressed as no. of vessels/HPF, n = 5–11 mice/group and 4–9 vessels/mouse. *P < 0.05 vs. CO Norm.
Vascular remodeling within the adventitia was evaluated by chronic hypoxia-induced collagen deposition, measured by two photon microscopy-SHG. There was no difference in collagen content at baseline. Both strains showed an increase in collagen deposition around the pulmonary arteries after 35 days of chronic hypoxia, and the collagen content was significantly increased in the SMC EC-SOD KO mice (Fig. 4, A and B).
Fig. 4.

Conditional depletion of SMC EC-SOD augments chronic hypoxia-induced collagen deposition. A: collagen visualized by 2 photon excitation-second harmonic generation in representative unstained tissue sections from EC-SOD control and SMC EC-SOD KO mice exposed to normoxia or 35 days of hypoxia. Red signal, collagen; green signal, tissue autofluorescence. B: quantification of collagen standardized for vessel size (n = 6 for all groups, 5 vessels analyzed/lung). *P < 0.05 vs. CO Norm; #P < 0.05 vs. CO Hypo.
GC-cGMP-PDE5 signaling axis was similar in SMC EC-SOD KO and EC-SOD control mice.
Because PH in the setting of oxidative stress has been associated with disruption of cGMP signaling, we examined the GC/cGMP/PDE5 signaling pathway. There was a trend toward increased sGC protein expression in both EC-SOD control and SMC EC-SOD KO mice following exposure to chronic hypoxia relative to normoxic controls, which was similar between strains (Fig. 5, A and B). There was no difference in sGC enzyme activity in SMC EC-SOD KO mice vs. EC-SOD control mice either at baseline or following chronic hypoxia (Fig. 5C). Chronic hypoxia significantly increased total lung cGMP with no difference between EC-SOD control mice and SMC EC-SOD KO mice. Post hoc analysis further identified a statistically significant increase in cGMP content in response to hypoxia in the EC-SOD control mice (Fig. 5D). PDE5 protein expression tended to decrease with chronic hypoxia in both EC-SOD control and SMC EC-SOD KO mice relative to room air controls, although there was no difference between strains (Fig. 6, A and B). Furthermore, lung PDE5 enzyme activity was similar with normoxia and chronic hypoxia in both strains (Fig. 6C).
Fig. 5.
Conditional depletion of SMC EC-SOD did not impact lung soluble guanylate cyclase (sGC) or cGMP. A: densitometry for sGC α- and β-isoforms relative to β-actin in total lung homogenates from CO and KO mice exposed to normoxia and 35 days of hypoxia; n = 5–6. B: representative Western blot analysis for sGC subunits. C: sGC activity in lung homogenates from CO and KO mice exposed to normoxia or 35 days of hypoxia; n = 5–6. D: cGMP content in lung homogenates from CO and KO mice exposed to normoxia or 35 days of hypoxia. *P < 0.05 vs. CO Norm by 2-way ANOVA, n = 5–6.
Fig. 6.
Conditional depletion of SMC EC-SOD did not impact lung phosphodiesterase 5 (PDE5) expression or activity. A: densitometry for PDE5 relative to β-actin in total lung homogenates from CO and KO mice exposed to normoxia and 35 days of hypoxia; n = 5–6. B: representative Western blot analysis for PDE5. C: PDE5 activity in lung homogenates from CO and KO mice exposed to normoxia or 35 days of hypoxia; n = 5–6.
Active eNOS was impaired in SMC EC-SOD KO mice.
Total eNOS expression was similar between the SMC EC-SOD KO and EC-SOD control mice in normoxia and hypoxia. In contrast, active phosphorylated eNOS, shown both by the relative amount of phosphorylated eNOS and the ratio of active phosphorylated eNOS to total eNOS, decreased only in the chronic hypoxia-exposed SMC EC-SOD KO mice (Fig. 7, A–D). GTPCH-1, the rate-limiting enzyme responsible for synthesis of tetrahydrobiopterin (BH4), also decreased only in the chronic hypoxia-exposed SMC EC-SOD KO mice (Fig. 7, A and E).
Fig. 7.
Active eNOS and GTP cyclohydrolase-1 (GTPCH-1) expression in lung decreased in hypoxia-exposed KO mice. A: representative Western blots for total eNOS (eNOS), phosphorylated active eNOS (ph-eNOS), GTPCH-1, and β-actin in total lung homogenates from CO and KO mice exposed to normoxia and 35 days of hypoxia. Western blot analysis for eNOS/β-actin relative to CO Norm (B), ph-eNOS/β-actin relative to CO Norm (C), ph-eNOS/total eNOS (D), and GTPCH-1/β-actin relative to CO Norm (E). *P < 0.05 vs. CO Norm by 2-way ANOVA, n = 3–6.
DISCUSSION
In this study, we demonstrate that the conditional SMC knockdown of EC-SOD depleted pulmonary vascular EC-SOD without changing total lung EC-SOD content or activity and worsened chronic hypoxic PH. Loss of the EC-SOD would be expected to increase ambient levels of O2·−, which is known to react with NO· at a near-diffusion limited rate and thereby reduce bioactive NO·. Our current data suggest that the loss of EC-SOD also impacts other aspects of the NO· pathway in a deleterious fashion, by diminishing activation of the eNOS and reducing the enzyme responsible for synthesis of the eNOS cofactor BH4.
Our first observation was that the conditional knockdown of SMC-derived EC-SOD markedly depleted EC-SOD in the pulmonary artery and aortic homogenates but did not impact total lung EC-SOD content. This is consistent with the initial report using this mouse strain, in which the activation of Cre led to loss of EC-SOD in the aorta and mesenteric arteries but not the lung, heart, or kidney (23). Our results indicate that, despite production and secretion of active EC-SOD by other cells in the lung, the vascular SMC is the predominant source of pulmonary artery EC-SOD. We did not detect any differences in baseline pulmonary artery pressures in the EC-SOD control strain compared with the SMC EC-SOD KO mice. Similarly, these mice previously were shown to have normal baseline systemic blood pressures compared with the control strain (23). This mouse strain is useful to interrogate the impact of insufficient vascular EC-SOD on the pathogenesis of disease in models of PH.
The conditional depletion of SMC-derived EC-SOD significantly augmented the severity of chronic hypoxia-induced PH, shown by elevated RVSP and RV hypertrophy, as well as pulmonary vascular remodeling, shown by medial wall thickening, muscularization of small pulmonary vessels, and perivascular collagen deposition. Loss of EC-SOD augments the severity of disease in a wide range of lung and vascular diseases, including PH. For example, Xu et al. reported that mice lacking total body EC-SOD develop worsened chronic hypoxic PH (36). Our laboratory and others have convincingly demonstrated that total lung overexpression of EC-SOD, either in genetically engineered mice overexpressing lung EC-SOD or rodents treated with adenoviral EC-SOD expression vectors, protects against chronic hypoxic-, bleomycin-, or monocrotaline-induced PH (3, 17, 19, 27, 32, 36). We recently tested a novel strain of mice lacking vascular EC-SOD due to the knockin of a common human single nucleotide polymorphism (R213G EC-SOD KI) that lowers the heparin binding affinity of EC-SOD. Similar to the SMC EC-SOD KO mice used in this study, both strains have decreased vascular EC-SOD. However, the R213G EC-SOD KI mice differ since they have an allele-dependent decrease in total lung EC-SOD content along with a concurrent increase in plasma and bronchoalveolar lavage fluid EC-SOD. This model reflects not a loss of synthesis but instead a redistribution of EC-SOD from its bound state to the extracellular fluids. These R213G EC-SOD KI mice also show exaggerated chronic hypoxic PH and pulmonary vascular remodeling, similar to the SMC EC-SOD KO mice (16). These results indicate that insufficient vascular EC-SOD due to either decreased production by the SMC or decreased binding to the vasculature both increase the severity of chronic hypoxic PH, and this response is independent of stable lung EC-SOD, as in the SMC EC-SOD KO, or increased plasma EC-SOD, as in the R213G EC-SOD KI. The localization of EC-SOD depletion specifically within the pulmonary artery dictates the vascular response. Two other published studies provide strong evidence that loss of EC-SOD within a specific local environment impacts disease progression. First, while SMC KO of EC-SOD did not augment angiotensin II-induced systemic hypertension, selective knockdown of central EC-SOD expressed by the circumventricular organ raises systemic blood pressure and enhances angiotensin II-induced hypertension, showing the importance of the specific site of localization of EC-SOD (23). Second, the conditional knockdown of EC-SOD in lung mesenchymal stem cells augmented chronic hypoxic PH, through activation of canonical Wnt signaling that caused a loss of stemness with a transition to a contractile pericyte/myofibroblast phenotype (5). Our findings using this novel mouse strain have broader implications to the progression of PH because EC-SOD expression and activity are decreased in a number of models of PH across species as well as a human study of idiopathic PAH (25). Collectively, we propose these findings confirm a critical role for vascular EC-SOD in protection against PH and vascular remodeling.
NO· can directly bind to and activate sGC, leading to cGMP-dependent vasodilation. In PH, loss of endothelial-derived NO· can decrease activation of sGC and thus decrease cGMP-dependent vasodilation. In addition, increased PDE5 activity can enhance the degradation of cGMP, also impairing vasodilation (11, 12, 14, 34). Vascular EC-SOD can preserve the bioavailability of NO· by preventing the rapid inactivation of NO· by O2·−, and prior studies showed increased vascular O2·− and decreased vascular NO· in SMC EC-SOD KO mice (23, 28). We therefore tested whether disruption of the sGC-cGMP-PDE5 signaling axis could account for the exaggerated PH in chronically hypoxic SMC EC-SOD KO mice. Interestingly, we note that hypoxia increased cGMP levels, with no overall difference between strains. On post hoc analysis, we could only demonstrate an increase in cGMP in the EC-SOD control mice, potentially suggesting that the ability to increase cGMP-dependent signaling in the SMC EC-SOD KO was in fact impaired. Although the finding that hypoxia increased lung cGMP differed from results reported in some studies of PH, it was consistent with published data by others demonstrating that hypoxic exposures ranging from 6 days to 5 wk increased total lung cGMP (21, 31). The hypoxia-induced increase in cGMP has been proposed to be a compensatory response to the ongoing vascular remodeling and vessel pruning. We did not note any significant changes in either sGC expression or activity or PDE5 expression or activity in either EC-SOD control or SMC EC-SOD KO mice in hypoxia, suggesting that the increased cGMP may be regulated by other pathways such the natriuretic peptide pathway and particulate GC. These studies are particularly interesting in light of studies suggesting that different sources of oxidative stress may initiate different intracellular signaling pathways. For example, we previously demonstrated that hyperoxia increases ROS production within the mitochondrial matrix in isolated PASMC, which upregulates PDE5 enzyme activity and decreases cGMP levels in these cells (13, 14). Thus, it is possible that we did not see changes in PDE5 and impaired cGMP signaling in the present study because different sources of ROS are activated by hypoxia, and vascular EC-SOD targets extracellular O2·−. Overall, the augmented chronic hypoxic PH and vascular remodeling in SMC EC-SOD KO mice could not be conclusively attributed to loss of cGMP-dependent signaling.
Our final experiment demonstrated that the EC-SOD control and SMC EC-SOD KO mice expressed similar total eNOS, whereas only the chronically hypoxic SMC EC-SOD KO mice showed a marked decrease in the active phosphorylated form of eNOS as well as the redox-sensitive enzyme GTPCH-1, responsible for BH4 synthesis, an essential cofactor for eNOS. These data indicate that the augmented chronic hypoxic PH in the SMC EC-SOD KO mice was due, at least in part, to disrupted eNOS activity. This seemed to be multifactorial, with both loss of phosphorylation of eNOS and uncoupling due to loss of substrate for eNOS activity. These data are consistent with studies from the ductal ligation model of neonatal PH that also suggests that decreased EC-SOD activity is associated with decreased eNOS function and eNOS uncoupling. Delivery of recombinant SOD to neonatal lambs with PH reversed PH, increased GTPCH-1 expression, and increased BH4 levels, indicating that GTPCH-1 is a redox-regulated enzyme and loss of extracellular SOD also impairs its expression and activity in this model of PH (4, 9, 11, 12, 34, 35). Future studies may determine the specific cGMP-independent targets responsible for worsened PH due to increased production of O2·− and decreased production of NO· following the disruption of eNOS activity.
We recognize several limitations with this study. The mouse model of chronic hypoxia-induced PH, while reproducible and well-characterized, does not reflect the marked degree of pulmonary vascular remodeling or inflammation that is observed in human disease. Although we demonstrated worsened medial and adventitial wall remodeling, this model does not enable us to assess the impact of insufficient vascular EC-SOD on intimal changes, even though this is an important feature of pulmonary artery remodeling in the clinical setting. In the future, we plan to test more robust models of PH associated with remodeling in all three layers of the vessel wall as well as inflammation. Another limitation is that, since we used lung tissue to evaluate the sGC-cGMP-PDE5 axis, we may miss more subtle changes in pulmonary artery expression or activity. The lung changes may not fully reflect the changes observed in the pulmonary artery, as has been shown in large animal models (10, 11). Finally, one possible hypothesis is that the SMC EC-SOD KO mice have uncoupled eNOS, which further amplifies oxidative stress in the vasculature. Measuring and quantifying in vivo oxidant stress, while not impossible, is challenging, particularly when considering the effects of reoxygenation on ROS and NO· production. Future studies are needed to more fully understand the mechanisms responsible for the exaggerated injury in the SMC EC-SOD KO mice in response to chronic hypoxia.
In summary, we demonstrate that, despite no change in total lung EC-SOD content, the selective depletion of SMC-derived pulmonary vascular EC-SOD worsened chronic hypoxic PH. The augmented PH was shown by measures of hemodynamics as well as analysis of pulmonary vascular remodeling. We did not find evidence that the enhanced PH in the setting of low vascular EC-SOD was due to impaired cGMP-dependent signaling. Instead, we found that there was decreased activation of eNOS and decrease in GTPCH-1, indicating that impaired eNOS activity could account for the exaggerated PH in the mice with insufficient pulmonary vascular EC-SOD. These studies demonstrate the necessity to consider the compartmentalization of oxidant production and antioxidant activity and the importance of extracellular oxidant/antioxidant imbalance on specific redox-sensitive signaling pathways.
GRANTS
The work was funded in part by National Heart, Lung, and Blood Institute Grants RO1-HL-086680 and HL-086680 S1 (E. Nozik-Grayck), and R01 HL109478 (K. N. Farrow); an American Heart Association Postdoctoral training grant (L. R. Villegas); Program Project Grant 5P01 HL-014985-39, National Institutes of Health (NIH) Axis Grant 1R01-HL-114887-02, and Training Grant 2T32 HL07171-36 (K. R. Stenmark). Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by NIH/NCRR Colorado CTSI Grant No. UL1-TR-001082.
DISCLOSURES
Contents are the authors' sole responsibility and do not necessarily represent official NIH views.
AUTHOR CONTRIBUTIONS
E.N.-G. and K.N.F. conception and design of research; E.N.-G., C.W., J.M.T., R.K.B., R.D.J., L.R.V., D.C.I., and K.N.F. performed experiments; E.N.-G., C.W., J.M.T., R.K.B., R.D.J., L.R.V., D.C.I., and K.N.F. analyzed data; E.N.-G., J.M.T., R.K.B., L.R.V., K.R.S., D.G.H., S.M.M., D.C.I., and K.N.F. interpreted results of experiments; E.N.-G., C.W., J.M.T., R.D.J., and K.N.F. prepared figures; E.N.-G. and K.N.F. drafted manuscript; E.N.-G., C.W., J.M.T., R.K.B., R.D.J., L.R.V., K.R.S., D.G.H., S.M.M., D.C.I., and K.N.F. edited and revised manuscript; E.N.-G., C.W., J.M.T., R.K.B., R.D.J., L.R.V., K.R.S., D.G.H., S.M.M., D.C.I., and K.N.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marcia McGowan for assistant formatting the manuscript and Dr. Russell Bowler for helpful discussions.
REFERENCES
- 1.Adachi T, Yamada H, Yamada Y, Morihara N, Yamazaki N, Murakami T, Futenma A, Kato K, Hirano K. Substitution of glycine for arginine-213 in extracellular-superoxide dismutase impairs affinity for heparin and endothelial cell surface. Biochem J 313: 235–239, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal S, Gross CM, Sharma S, Fineman JR, Black SM. Reactive oxygen species in pulmonary vascular remodeling. Comp Physiol 3: 1011–1034, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed MN, Zhang Y, Codipilly C, Zaghloul N, Patel D, Wolin M, Miller EJ. Extracellular superoxide dismutase overexpression can reverse the course of hypoxia-induced pulmonary hypertension. Mol Med 18: 38–46, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belik J, McIntyre BA, Enomoto M, Pan J, Grasemann H, Vasquez-Vivar J. Pulmonary hypertension in the newborn GTP cyclohydrolase I-deficient mouse. Free Radic Biol Med 51: 2227–2233, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow K, Fessel JP, Kaoriihida S, Schmidt EP, Gaskill C, Alvarez D, Graham B, Harrison DG, Wagner DH, Jr, Nozik-Grayck E, West JD, Klemm DJ, Majka SM. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm Circ 3: 31–49, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu Y, Alwahdani A, Iida S, Lund DD, Faraci FM, Heistad DD. Vascular effects of the human extracellular superoxide dismutase R213G variant. Circulation 112: 1047–1053, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Crosswhite P, Sun Z. Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. J Hypertens 28: 201–212, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demarco VG, Whaley-Connell AT, Sowers JR, Habibi J, Dellsperger KC. Contribution of oxidative stress to pulmonary arterial hypertension. World J Cardiol 2: 316–324, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois M, Delannoy E, Duluc L, Closs E, Li H, Toussaint C, Gadeau AP, Godecke A, Freund-Michel V, Courtois A, Marthan R, Savineau JP, Muller B. Biopterin metabolism and eNOS expression during hypoxic pulmonary hypertension in mice. PloS one 8: e82594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 102: 226–233, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, Russell JA, Steinhorn RH. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 299: L109–L116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrow KN, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, Smith CL, Steinhorn RH, Schumacker PT. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxid Redox Signal 17: 460–470, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrow KN, Wedgwood S, Lee KJ, Czech L, Gugino SF, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 174: 272–281, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund-Michel V, Guibert C, Dubois M, Courtois A, Marthan R, Savineau JP, Muller B. Reactive oxygen species as therapeutic targets in pulmonary hypertension. Ther Advances Respir Dis 7: 175–200, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Hartney JM, Stidham T, Goldstrohm DA, Oberley-Deegan RE, Weaver MR, Valnickova-Hansen Z, Scavenius C, Benninger RK, Leahy KF, Johnson R, Gally F, Kosmider B, Zimmermann AK, Enghild JJ, Nozik-Grayck E, Bowler RP. A common polymorphism in EC-SOD affects cardiopulmonary disease risk by altering protein distribution. Circulation Cardiovasc Genet In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartney T, Birari R, Venkataraman S, Villegas L, Martinez M, Black SM, Stenmark KR, Nozik-Grayck E. Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PLoS One 6: e27531, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10–S19, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med 177: 219–226, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Lee KJ, Berkelhamer SK, Kim GA, Taylor JM, O'Shea KM, Steinhorn RH, Farrow KN. Disrupted pulmonary artery cyclic guanosine monophosphate signaling in mice with hyperoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol 50: 369–378, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Laubach VE, Johns RA. Upregulation of lung soluble guanylate cyclase during chronic hypoxia is prevented by deletion of eNOS. Am J Physiol Lung Cell Mol Physiol 281: L369–L376, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Lim RS, Kratzer A, Barry NP, Miyazaki-Anzai S, Miyazaki M, Mantulin WW, Levi M, Potma EO, Tromberg BJ. Multimodal CARS microscopy determination of the impact of diet on macrophage infiltration and lipid accumulation on plaque formation in ApoE-deficient mice. J Lipid Res 51: 1729–1737, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lob HE, Vinh A, Li L, Blinder Y, Offermanns S, Harrison DG. Role of vascular extracellular superoxide dismutase in hypertension. Hypertension 58: 232–239, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci USA 79: 7634–7638, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masri FA, Comhair SA, Dostanic-Larson I, Kaneko FT, Dweik RA, Arroliga AC, Erzurum SC. Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clin Transl Sci 1: 99–106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van Rheen Z, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 295: L422–L430, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase: a regulator of nitric oxide bioavailability. Lab Invest 75: 617–636, 1996. [PubMed] [Google Scholar]
- 29.Stenmark KR, Rabinovitch M. Emerging therapies for the treatment of pulmonary hypertension. Pediatr Crit Care Med 11: S85–S90, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Stralin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arteriosclerosis Thromb Vasc Biol 15: 2032–2036, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Sun JZ, Chen SJ, Li G, Chen YF. Hypoxia reduces atrial natriuretic peptide clearance receptor gene expression in ANP knockout mice. Am J Physiol Lung Cell Mol Physiol 279: L511–L519, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Van Rheen Z, Fattman C, Domarski S, Majka S, Klemm D, Stenmark KR, Nozik-Grayck E. Lung extracellular superoxide dismutase overexpression lessens bleomycin-induced pulmonary hypertension and vascular remodeling. Am J Respir Cell Mol Biol 44: 500–508, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wedgwood S, Black SM. Role of reactive oxygen species in vascular remodeling associated with pulmonary hypertension. Antioxid Redox Signal 5: 759–769, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH. Increased p22(phox)/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn. Antioxidants Redox Signal 18: 1765–1776, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widder JD, Chen W, Li L, Dikalov S, Thony B, Hatakeyama K, Harrison DG. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res 101: 830–838, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Xu D, Guo H, Xu X, Lu Z, Fassett J, Hu X, Xu Y, Tang Q, Hu D, Somani A, Geurts AM, Ostertag E, Bache RJ, Weir EK, Chen Y. Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension 58: 303–309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]