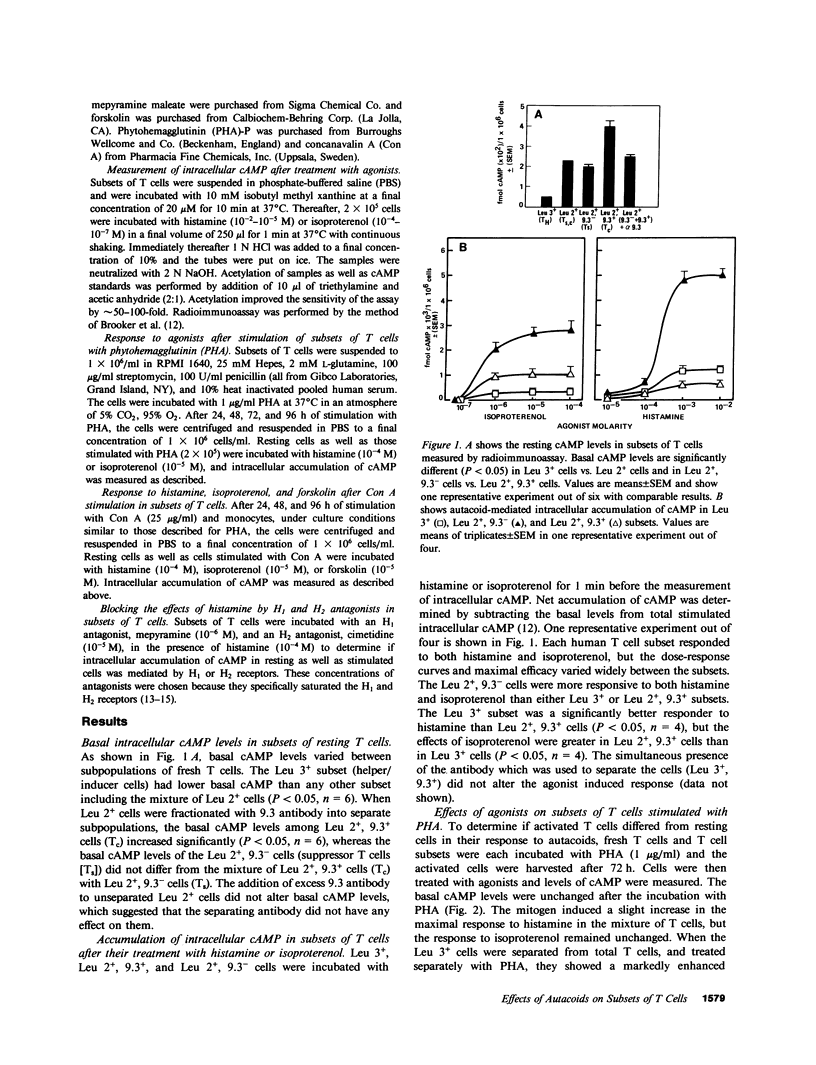
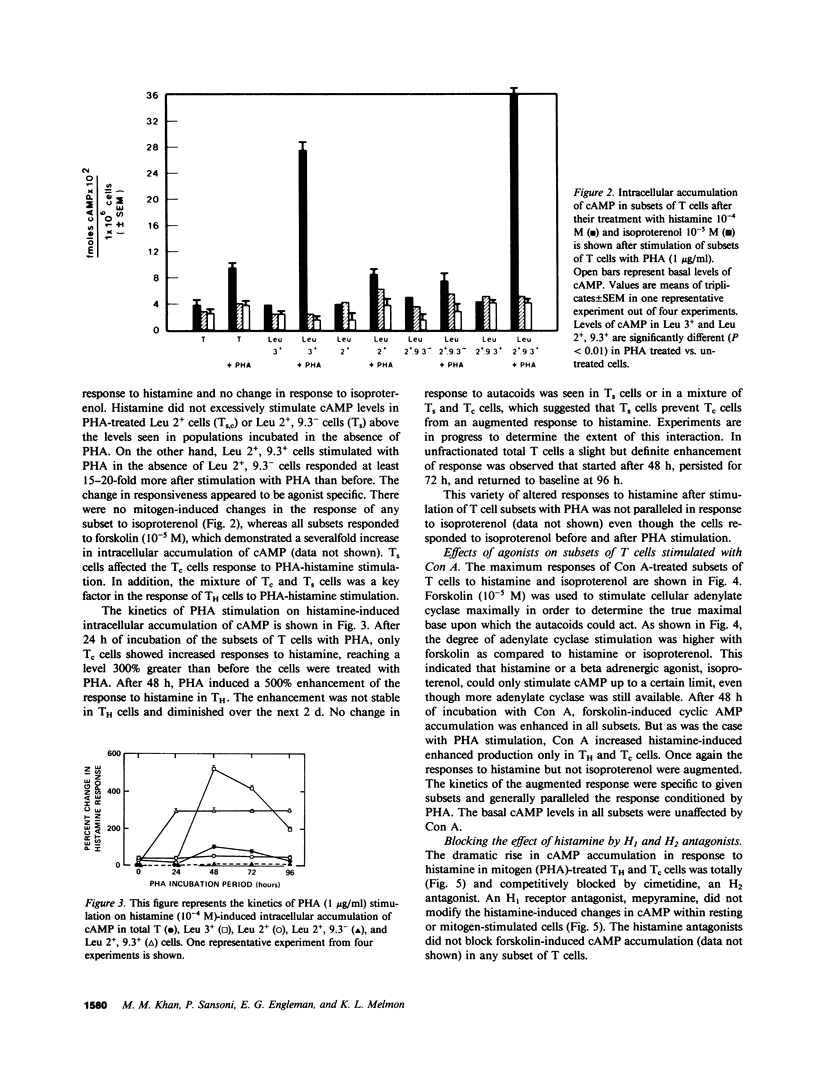
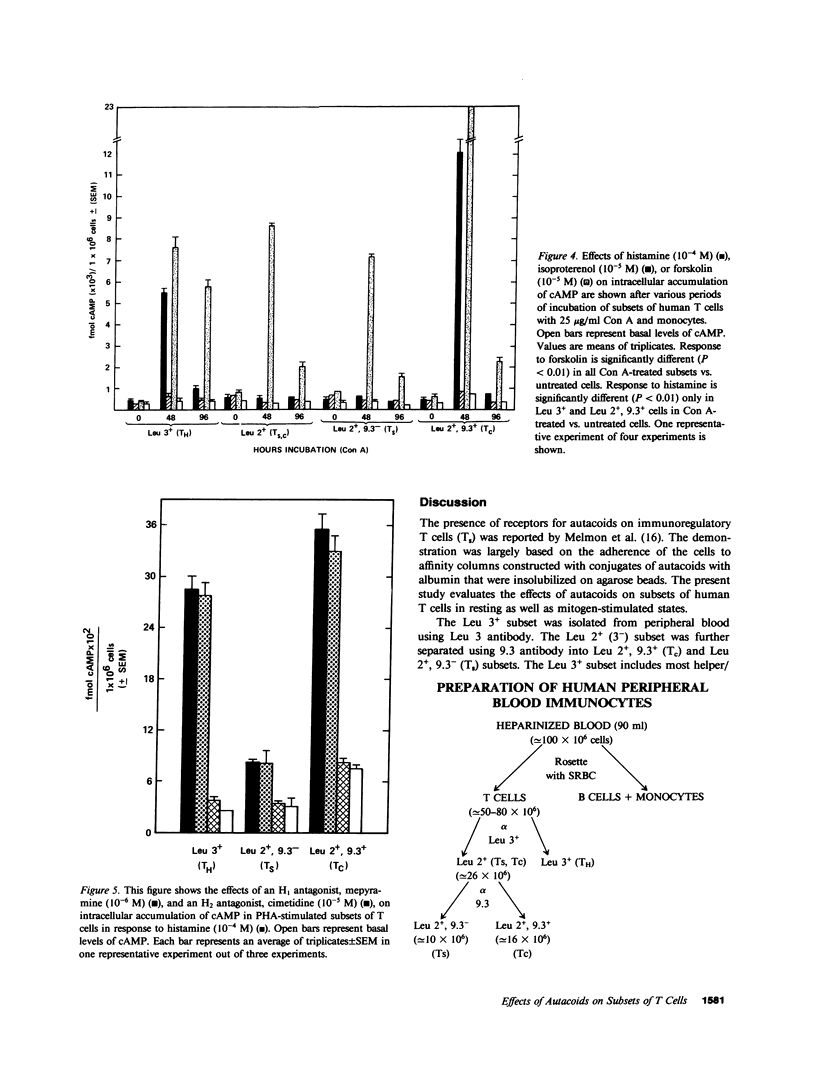
Abstract
Autacoids (principally histamine, beta adrenergic catecholamines, and prostaglandins E and A) have only recently been recognized as substantive moderators of a number of immune functions. If autacoids are to be considered as potential therapeutic immunomodulators, it is necessary to understand their effects on subsets of T cells while they are and are not in contact with each other. This report demonstrates that autacoid receptors are nonrandomly distributed on phenotypically and functionally distinct subsets of human T cells. Each human T cell subset responded to both histamine and isoproterenol, but the dose response curve and maximal efficacy varied widely between the subsets. The suppressor T cells were more responsive to both histamine and isoproterenol than helper/inducer T cells (TH) or cytotoxic T cells (Tc). We found that after mitogenic stimulation the response to histamine, but not isoproterenol, was greatly increased only in TH (Leu 3+) and Tc (Leu 2+, 9.3+) subsets, and that this effect may be regulated by suppressor T cells (Leu 2+, 9.3-). The dramatic rise in cAMP accumulation in response to histamine in mitogen-treated TH and Tc was totally blocked by an H2 antagonist (cimetidine), but not by an H1 antagonist (mepyramine). These findings indicate interdependence of (a) immunologically uncommitted subsets in their response to selected drugs, and (b) control of basal- and autacoid-induced cAMP production, as well as (c) increased qualitative and quantitative selectivity, which is caused by mitogen. If we had performed these experiments only on unseparated cells we would not have observed the remarkable selectivity of autacoid effects on subsets of T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A. Differences in Cyclic AMP Changes after Stimulation by Prostaglandins and Isoproterenol in Lymphocyte Subpopulations. J Clin Invest. 1975 May;55(5):1074–1081. doi: 10.1172/JCI108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Burchiel S. W., Melmon K. L. Augmentation of the in vitro humoral immune response by pharmacologic agents. I: An explanation for the differential enhancement of humoral immunity via agents that elevate cAMP. Immunopharmacology. 1979 Mar;1(2):137–150. doi: 10.1016/0162-3109(79)90050-x. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Mohagheghpour N., Hansen J. A., Engleman E. G. Alloantigen-specific cytotoxic and suppressor T lymphocytes are derived from phenotypically distinct precursors. J Immunol. 1983 Nov;131(5):2296–2300. [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Garovoy M. R., Reddish M. A., Rocklin R. E. Histamine-induced suppressor factor (HSF): inhibition of helper T cell generation and function. J Immunol. 1983 Jan;130(1):357–361. [PubMed] [Google Scholar]
- Gespach C., Saal F., Cost H., Abita J. P. Identification and characterization of surface receptors for histamine in the human promyelocytic leukemia cell line HL-60. Comparison with human peripheral neutrophils. Mol Pharmacol. 1982 Nov;22(3):547–553. [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Johnson C. L., Weinstein H., Green J. P. Studies on histamine H2 receptors coupled to cardiac adenylate cyclase. Blockade by H2 and H1 receptor antagonists. Mol Pharmacol. 1979 Sep;16(2):417–428. [PubMed] [Google Scholar]
- Kotzin B. L., Benike C. J., Engleman E. G. Induction of immunoglobulin-secreting cells in the allogeneic mixed leukocyte reaction: regulation by helper and suppressor lymphocyte subsets in man. J Immunol. 1981 Sep;127(3):931–935. [PubMed] [Google Scholar]
- Melmon K. L., Insel P. A. Inflammatory and immune responses: cell individuality amidst ubiquitous hormonal signals. Johns Hopkins Med J. 1977 Jul;141(1):15–22. [PubMed] [Google Scholar]
- Melmon K. L., Rocklin R. E., Rosenkranz R. P. Autacoids as modulators of the inflammatory and immune response. Am J Med. 1981 Jul;71(1):100–106. doi: 10.1016/0002-9343(81)90264-3. [DOI] [PubMed] [Google Scholar]
- Niaudet P., Beaurain G., Bach M. A. Differences in effect of isoproterenol stimulation on levels of cyclic AMP in human B and T lymphocytes. Eur J Immunol. 1976 Nov;6(11):834–836. doi: 10.1002/eji.1830061117. [DOI] [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Henney C. S. Increase in histamine receptors on thymus-derived effector lymphocytes during the primary immune response to alloantigens. Nature. 1973 Aug 3;244(5414):284–287. doi: 10.1038/244284a0. [DOI] [PubMed] [Google Scholar]
- Plescia O. J., Yamamoto I., Shimamura T. Cyclic AMP and immune responses: changes in the splenic level of cyclic AMP during the response of mice to antigen. Proc Natl Acad Sci U S A. 1975 Mar;72(3):888–891. doi: 10.1073/pnas.72.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E., Greineder D. K., Melmon K. L. Histamine-induced suppressor factor (HSF): further studies on the nature of the stimulus and the cell which produces it. Cell Immunol. 1979 May;44(2):404–415. doi: 10.1016/0008-8749(79)90015-7. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E. Modulation of cellular-immune responses in vivo and in vitro by histamine receptor-bearing lymphocytes. J Clin Invest. 1976 Apr;57(4):1051–1058. doi: 10.1172/JCI108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowski W., Plaut M., Lichtenstein L. M. Selective display of histamine receptors on lymphocytes. Science. 1977 Feb 18;195(4279):683–685. doi: 10.1126/science.190677. [DOI] [PubMed] [Google Scholar]
- Sansoni P., Silverman E. D., Khan M. M., Melmon K. L., Engleman E. G. Immunoregulatory T cells in man. Histamine-induced suppressor T cells are derived from a Leu 2+ (T8+) subpopulation distinct from that which gives rise to cytotoxic T cells. J Clin Invest. 1985 Feb;75(2):650–656. doi: 10.1172/JCI111743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Paetkau V. Biphasic effect of cyclic AMP on an immune response. Nature. 1974 Aug 9;250(5466):505–507. doi: 10.1038/250505a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Webb D. R. Antigen-stimulated changes in cyclic nucleotide levels in the mouse. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2320–2324. doi: 10.1073/pnas.72.6.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]



