Abstract
Early deficiency of the methyl donors folate and vitamin B12 produces hyperhomocysteinemia and cognitive and motor disorders in 21-day-old rat pups from dams fed a diet deficient in methyl donors during gestation and lactation. These disorders are associated with impaired neurogenesis and altered synaptic plasticity in cerebellum. We aimed to investigate whether these disorders could be related to impaired expression of neurosteroidogenesis-associated proteins, key regulator receptors, and some steroid content in the cerebellum. The methyl donor deficiency produced a decreased concentration of folate and vitamin B12, along with accumulation of homocysteine in Purkinje cells in both sexes, whereas the S-adenosylmethionine/S-adenosylhomocysteine ratio was reduced only in females. The transcription level and protein expression of StAR, aromatase, ERα, ERβ, and LH receptors were decreased only in females, with a marked effect in Purkinje cells, as shown by immunohistochemistry. Consistently, reduced levels of estradiol and pregnenolone were measured in cerebellar extracts of females only. The decreased expression levels of the transcriptional factors CREB, phospho-CREB, and SF-1, the lesser increase of cAMP concentration, and the lower level of phospho-PKC in the cerebellum of deficient females suggest that the activation of neurosteroidogenesis via cAMP-mediated signaling pathways associated with LHR activation would be altered. In conclusion, a gestational methyl donor deficiency impairs neurosteroidogenesis in cerebellum in a sex-dependent manner.
Keywords: early methyl donor deficiency, neurosteroids, cerebellum development, cyclic AMP signaling cascade
deficiency of methyl donors (folate and vitamin B12) involved in the one-carbon metabolism and the consecutive accumulation of the neurotoxic amino acid homocysteine (HCY) have been associated with various neurological diseases (33). A loss of cerebellar granule cells along with HCY accumulation was reported in a transgenic model of mice deficient in methylenetetrahydrofolate reductase, a key enzyme of the one-carbon metabolism. This observation is of particular interest since the accumulation of HCY in the brain appeared to be heterogeneous, with the cerebellum displaying a higher amount than other cerebral regions (6).
In a validated animal model based on rats born to dams fed a diet deficient in methyl donors during the gestational and lactating periods (2–5), the 21-day-old progeny showed a marked accumulation of HCY, along with increased apoptosis in selective brain structures, notably the cerebellum (5). At the time of weaning, animals exhibited cognitive and motor defects, and it has been reported that methyl donor privation during early life is associated with alteration of synaptic plasticity and impaired neurogenesis (9), processes that are closely related to an increased neurosteroidogenesis during postnatal age in the cerebellum (36). Evidence that neurosteroids play a critical role during fetal, postnatal, and adolescent development has been provided (16). The cerebellum contains the whole enzymatic machinery allowing neurosteroid synthesis, with Purkinje cells being a major site for neurosteroid formation (36). The first rate-limiting step implies the recruitment of the steroidogenic acute regulatory (StAR) protein, which transfers cholesterol into the mitochondrial inner membrane, thus allowing its further conversion into pregnenolone via the cholesterol side chain cleavage enzyme (CYP11A1) activity (19, 35). StAR is regulated during cerebellar development, and the highest mRNA levels are detected at early postnatal age (21). The final step of neurosteroidogenesis occurs via the key enzyme aromatase (CYP19A1), which converts testosterone into estradiol. Aromatase expression in the Purkinje cells confers to this enzyme a role during the cerebellum postnatal development (1, 36). Estradiol synthesized de novo in the cerebellum acts locally on Purkinje cell dendritic growth and synapse formation. These effects are mediated through the ERα and ERβ estrogen receptors, which play distinct roles throughout the development of the cerebellum (13). ERβ receptors are required for controlling the maturation of Purkinje cells, where they are present around 3 wk after birth in the rat. In contrast to ERβ, which remains detectable during adulthood, ERα is expressed specifically during development and participates in Purkinje cell differentiation, dendritic growth, and synapse formation (17).
The expression level of the StAR gene is induced by the binding of the luteinizing hormone (LH) to the LH receptor (LHR), and its presence has been shown in the cerebellum (26, 27). The binding of LH allows the transcriptional regulation of the StAR gene via a G protein/adenylate cyclase pathway with cAMP formation, which in turn activates protein kinase A (PKA). This results in the phosphorylation of various transcription factors that bind to the StAR gene promoter region (28), notably the cAMP response element (CRE)-binding protein (CREB) and the steroidogenic factor-1 (SF-1; NR5A1) (Fig. 1). The protein kinase C (PKC) also participates to the regulation of StAR by reinforcing its cAMP-mediated expression (30), which has been reported in neural cells (19, 23).
Fig. 1.
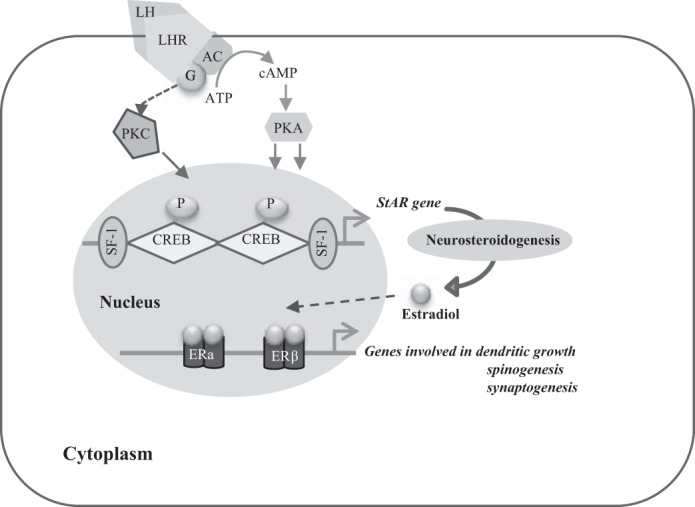
Schematic representation of the signaling cascade regulating steroidogenic acute regulatory (StAR) gene transcription. The binding of the luteinizing hormone (LH) to its receptor allows the transcriptional regulation of the StAR gene via a G protein/adenylate cyclase pathway with cAMP formation, which in turn activates protein kinase A (PKA). This results in the phosphorylation of the transcription factors binding to the StAR gene promoter region. The protein kinase C (PKC) also participates in the regulation of StAR. LHR, luteinizing hormone receptor; CREB, cAMP response element-binding protein; SF-1, steroidogenic factor-1; ERα and -β, estrogen receptor-α and -β, respectively. Adapted with permission from Manna et al. (28).
The orphan nuclear receptor SF-1 is widely distributed in the steroidogenic tissues and plays a central role in steroid synthesis regulation through binding to its response element, which is present in the promoter regions of the genes encoding for StAR and LHR as well as cytochrome P450 (CYP) enzymes (31). SF-1 is itself under the regulation of various factors at the transcriptional level, including the cAMP pathway (25).
To our knowledge, nothing is known about perinatal alterations of the one-carbon metabolism on local steroid synthesis in the cerebellum despite their determinant role during cerebellar development. Therefore, we investigated the expression levels of aromatase, StAR, LHR, and ERα and ERβ receptors as well as the nuclear factor SF-1 in the cerebellum of 21-day-old rats born to dams subjected to a methyl donor-deficient diet (3, 5). In addition, the protein levels of the key regulators of SF-1 and StAR gene transcriptional activity, i.e., PKA, PKC, CREB, and p-CREB, were monitored. The cAMP, pregnenolone, and estradiol concentrations were also measured.
METHODS
Animals.
Animal experiments were performed on Wistar rats (Charles River Laboratories, l'Arbresle, France) and conducted in accordance with the internal guidelines (Lorraine-University Ethic Comity) for animal care and housing, and they met all applicable standards for the ethics of experimentation and research integrity. Adult female rats were maintained under standard laboratory conditions on a 12:12-h light-dark cycle, with food and water available ad libitum. One month before pregnancy, they were fed either with standard food (n = 4, maintenance diet M20; Scientific Animal Food and Engineering, Villemoisson-sur-Orge, France) or with a lowered choline diet lacking methyl donors, as described previously (n = 4; Special Diet Service, Saint-Gratien, France) (12). The assigned diet was maintained until weaning of the offspring on postnatal day 21.
Tissue collection.
Rat pups were euthanized at postnatal day 21 by an excess of isoflurane. Intracardiac blood samples were drawn for the measurement of HCY plasma concentrations. The cerebellum was rapidly harvested. For immunohistochemical analyses, one-half of the cerebellum was immediately frozen in methylbutane previously chilled to −30°C and stored at −80°C. The other half was rapidly dissected before freezing in liquid nitrogen and storage at −80°C until biochemical analyses. Gonads, lungs, and hypothalamus were also collected as positive and negative controls for protein expression.
Liquid chromatography-mass spectrometry/mass spectrometry analysis of neurosteroids.
Lipids of cerebellar tissues were extracted according to the method of Folch et al. (14). Briefly, samples were added with internal standards and homogenized in chloroform-methanol solution (2:1 vol/vol) using a tissue lyser (Qiagen). After 30-min incubation at ambient temperature under ultrasonication, the former mix was added to a water-methanol solution (1:1, vol/vol) and centrifuged at 760 g for 1 min. The organic phases were added to methanol-water-chloroform solution (48:47:3, vol/vol/vol) and dried under a nitrogen stream after ultrasonication. The extracts were dissolved in a 1-ml methanol-acetic acid solution (99:1, vol/vol), with 1 ml of water added, and passed through Strata-X cartridges (60 mg adsorbent; Phenomenex, Torrance, CA) conditioned prior to use. The purified steroids were evaporated under nitrogen atmosphere, and the dried extracts were dissolved in methanol-formate solution (60:40) (Sigma-Aldrich) and stirred for 15 min (PST Thermoshaker, Biosan, France).
Pregnenolone and estradiol were quantified by a single, fast liquid chromatography-mass spectrometry/mass spectrometry procedure with a run time of 12 min according to Higashi et al. (18). Briefly, 10 μl of the sample was injected in the Acquity UPLC HSST3 column (1.8 μm, 2.1 × 100 mm; Waters, St. Quentin en Yvelines, France). D3-testosterone was used as an internal standard (Accros Organics, Geel, Belgium). The analysis of the steroids was performed using a triple-quadripole mass spectrometer 4000 QTRAP (ABSciex, Courtaboeuf, France) equipped with an APCI ionization source used in positive. Quantification was made using calibration curves with correction against internal standard peak area.
Preparation of mitochondrial and microsomal fractions.
The tissue samples were homogenized (1 g tissue/3-ml buffer) in the isolation medium (0.32 M sucrose, 10 mM Tris·HCl, and 1 mM EDTA, pH 7.4) and first centrifuged at 2,000 g for 3 min at 4°C (TLX ultracentrifuge Optima, Beckman, France). The supernatant was then centrifuged at 12,500 g for 10 min at 4°C, resulting in a new supernatant retained for the isolation of microsomes. The pellet was resuspended in the isolation medium and centrifuged at 11,000 g for 10 min. The mitochondrial fraction was pelleted by additional centrifugation at 10,000 g for 10 min and suspended in RIPA buffer [136 mM NaCl, 1.7 mM KH2PO4, 10 mM Na2HPO4, 1% Tergitol (vol/vol), 0.5% deoxycholate (wt/vol), 0.1% sodium dodecyl sulphate (wt/vol), 100 mM Na3VO4, 57 mM phenylmethylsulfonylfluoride, and 0.14% protease inhibitor] so that the mitochondrial fraction extracted from 0.1 g of tissue was suspended in 0.5 ml of RIPA buffer.
For the microsomal fractions, the previously collected supernatant was centrifuged at 15,000 g for 20 min at 4°C. The pellet was discarded, and the supernatant was centrifuged once more at 105,000 g for 90 min at 4°C. The microsomal pellet was resuspended in the storage buffer [80 mM K2HPO4, 19 mM KH2PO4, 0.2 mM EDTA, and 20% glycerol (vol/vol), pH 7.4] so that 1 ml of suspension contained the microsomal fraction extracted from 3 g of tissue.
The protein concentration in subcellular fractions was assessed by a bicinchoninic acid (BCA) protein determination kit (Interchim), using BSA as a standard.
Measurement of plasma homocysteine and cerebellar vitamin B12, folate, S-adenosylmethionine, S-adenosylhomocysteine, and cAMP concentration.
Plasma concentrations of HCY were measured as described previously (5). Vitamin B12 and folate concentrations were measured by radiodilution isotope assay (simulTRAC-SNB; ICN Pharmaceuticals, Versailles, France) (7). The S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) concentrations were measured in cerebellar tissue homogenates using high-performance liquid chromatography adapted from Delabar et al. (11). Proteins were precipitated with 0.2 N HClO4 before injection on the column (Lichrospher, 100 RP-C18, 5 μm, 250 × 4 mm id). The mobile phase, consisting of 50 mmol/l sodium phosphate (pH 3.2), 10 mmol/l heptane sulfonate, and acetonitrile (10–20% from 0 to 20 min), was applied at a 0.75 ml/min flow rate. Amounts of SAM and SAH were quantified using a UV detector set at 254 nm.
Twenty milligrams of cerebellar extracts was homogenized in 200 μl of 0.1 M HCl solution, and cAMP level was measured using the direct cAMP ELISA kit (Enzo Life Sciences, Villeurbanne, France), following the manufacturer's recommendations.
Immunohistochemistry.
Immunohistochemical analyses were performed on cryostat-generated, 20-μm sagittal brain sections mounted onto glass slides. For immunostaining, tissue sections previously fixed in 4% paraformaldehyde (Sigma-Aldrich) for 15 min at room temperature were permeabilized for 10 min in PBS-0.1% Triton. After three washes in PBS of 5 min each, nonspecific binding was blocked by incubation in PBS-BSA 10% (wt/vol) for 1 h. Tissue sections were further incubated for 72 h at 4°C with the different primary antibodies (given in Table 1) used at a 1:200 dilution. Brain slices were then incubated for 1 h at room temperature in the presence of the secondary antibody conjugated to Alexa Fluor (1:2,000; Molecular Probes, Cergy Pontoise, France).
Table 1.
List of antibodies used in the study
| Target | Source | Dilution for Western Blot Analyses |
|---|---|---|
| HCY | Millipore | NA |
| StAR protein | D. Stocco, Texas Tech University | 1:1,000 |
| Aromatase | Biovision Research Products | 1:1,000 |
| SF-1 | Abcam | 1:250 |
| Estradiol | US Biological | NA |
| ERα | Santa Cruz Biotechnology | 1:200 |
| ERβ | Abcam | 1:1,000 |
| LHR | Santa Cruz Biotechnology | 1:100 |
| PKA | Cell Signaling Technology | 1:700 |
| PKCα | Abcam | 1:700 |
| p-PKCα | Abcam | 1:700 |
| CREB | Cell Signaling Technology | 1:1,000 |
| p-CREB | Cell Signaling Technology | 1:1,000 |
HCY, homocysteine; StAR, steroidogenic acute regulatory; SF-1, steroidogenic factor-1; ERα and -β; LHR, luteinizing hormone receptor; CREB, cAMP response element-binding protein; p-PKCα and p-CREB, phosphorylated PKCα and CREB, respectively; NA, not applicable. The above antibodies were used to detect the proteins of interest in the cerebellum extracts and were used at the indicated concentrations. Some of them were also used at a 1:200 dilution to perform immunostaining on brain sections.
The cell nuclei were stained by the fluorescent dye 4,6-diamidino-2-phenylindole (DAPI; 5 μg/ml in distilled water). Slices were then washed three times in PBS, coverslipped using mounting medium Fluoromount (Sigma, St. Louis, MO), and kept in the dark until fluorescence analysis. The sections were observed using a fluorescence microscope (Olympus, Rungis, France) at ×20 magnification. The pictures were digitized with a monochromatic camera and analyzed using Cell F (Olympus, Rungis, France). Specific labeling was absent in the control sections incubated without the primary antibodies.
Western immunoblot analyses.
The dissected cerebral regions were solubilized in RIPA lysis buffer, lysed by three cycles of freezing/thawing, and finally centrifuged at 4°C for 30 min at 15,000 g. The supernatant protein concentration was determined using the BCA protein assay kit. Samples were mixed with an equal volume of 2× Laemmli buffer and denatured by heating the mixture for 5 min at 100°C.
Thirty micrograms of cerebellar mitochondrial fractions (for StAR detection) or microsomal fractions (for aromatase detection) and 30–50 μg of total proteins were separated on SDS-PAGE (10–12%; Bio-Rad Mini Protean 3) (24) and transferred onto polyvinylidene difluoride membranes (Immobilon-PS2; Millipore, Bedford, MA) in Tris-glycine transfer buffer with 20% ethanol in a miniblotter (Bio-Rad). After being blocked in 5% nonfat dry milk in Tris phosphate-buffered saline solution (200 mM Tris, 1.5 M NaCl, pH 7.4)-0.1% Tween 20 for 1 h under shaking, membranes were incubated with various primary antibodies at 4°C overnight (given in Table 1). Secondary peroxidase-labeled antibodies were purchased from Santa Cruz Biotechnology and used at a 1:2,000 dilution. Immunoreactivity was detected with a chemiluminescence kit (ECL Plus, Amersham BioSciences, Piscataway, NJ) and a chemiluminescence detector (Fusion FX7; Thermo Fisher). To normalize the total amount of protein per lane, membranes were stripped and incubated with a monoclonal goat antibody against β-actin (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) or a monoclonal mouse antibody against GAPDH (1:1,000; Millipore). Densitometric analysis of the Western blot band intensity was performed using ImageJ 1.44p.
Quantitative RT-PCR analysis.
After rat brain tissues were grinded in liquid nitrogen, total RNA was isolated using the RNeasy Lipid Tissue Mini Kit (Qiagen, Courtaboeuf, France), following the manufacturer's instructions. cDNA was reverse-transcribed from total RNA (1 μg) using the QuantiTect Reverse Transcription Kit (Qiagen), following the manufacturer's specifications. Real-time PCR was performed on the Light Cycler 2.0 instrument (Roche Diagnostics, Manheim, Germany) using the QuantiTect SYBR Green PCR kit (Qiagen). The validated couples of primers are indicated in Table 2. Temperature cycling consisted of one cycle to activate the enzyme (95°C, 15 min) followed by 43 cycles, each one consisting of denaturation (95°C, 10 s), annealing (53°C for StAR, 56°C for both aromatase and SF-1; 15 s), and extension (72°C; 10 s). Melting curve analyses were performed by increasing temperature from 65 to 95°C. Cycle threshold (CT) was determined for each sample, and real-time PCR amplification efficiencies were expressed by calculating the ratio of crossing points of amplification curves, using the RelQuant software (Roche Diagnostics). The expression of the genes of interest was normalized to that of polII using the 2-ΔΔCT method.
Table 2.
Sequences of primers used for quantitative PCR
| Target | Forward Primer | Reverse Primer |
|---|---|---|
| StAR | AGGAAAGCCAGCAGGAGAAT | CTGTCCATGGGCTGGTCTA |
| Aromatase | TTGATTTTCGCTGAGAGACG | ACAGAGTGACGGACATGGTG |
| SF-1 | GTCCAGAACAACAAGCATTACAC | ATCAGCACGCACAGCTTC |
| Polymerase II | GCATTAACATCAGGAACAATAAAGGC | GATCTCTCTAAAGTTGACCTCATTGG |
Sequences are given in the 5′ to 3′ orientation.
Statistical analysis.
Data were prospectively collected and analyzed with SAS software (SAS Institute, Berkley, CA). Raw data reported as means ± SD were compared by using one-way analysis of variance (ANOVA) with Fisher's test. A P value of <0.05 was considered to indicate statistical significance.
RESULTS
Folate and vitamin B12 concentrations are reduced in the cerebellum of deficient rat pups.
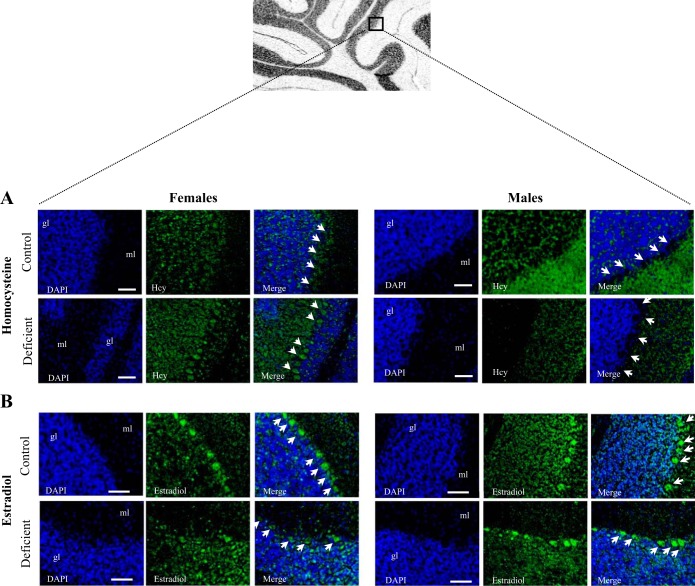
To validate the experimental protocol, concentrations of folate and vitamin B12 were measured in cerebellum extracts prepared from pups fed by dams receiving the control or the methyl donor (folate and vitamin B12)-deficient diet. Both male and female animals exhibited substantial lower folate and vitamin B12 concentrations in the cerebellum compared with the controls (Table 3). As reported previously in the same animal model, HCY plasma levels increased significantly in the 21-day-old offspring from methyl donor deprived mothers (Table 3). HCY detection was higher in brain sections of deficient female pups, with a marked accumulation in the cerebellum Purkinje cells, compared with control animals (Fig. 2A). However, this sustained accumulation of HCY was not observed in males.
Table 3.
Circulating HCY and cerebellar concentration of folate, vitamin B12, SAM, and SAH measured in 21-day-old rats from controls and dams fed the methyl donor-deficient diet during pregnancy and lactation periods
| Females |
Male |
|||
|---|---|---|---|---|
| Normal diet | Deficient diet | Normal diet | Deficient diet | |
| HCY, μmol/l | 9.27 ± 0.91 | 39.24 ± 21.78** | 9.45 ± 1.03 | 30.24 ± 14.11** |
| Vitamin B9, nmol/g tissue | 0.92 ± 0.12 | 0.32 ± 0.19** | 0.89 ± 0.24 | 0.32 ± 0.19* |
| Vitamin B12, pmol/g tissue | 44.8 ± 11.34 | 21.76 ± 2.05** | 26.63 ± 6.43 | 18.00 ± 2.12* |
| SAM, nmol/g tissue | 26.12 ± 9.00 | 26.23 ± 9.94 | 31.77 ± 4.24 | 31.13 ± 9.92 |
| SAH, nmol/g tissue | 4.55 ± 1.05 | 8.33 ± 2.02** | 4.88 ± 0.84 | 6.38 ± 1.96 |
| SAM/SAH ratio | 5.82 ± 1.92 | 3.36 ± 1.63* | 6.70 ± 1.62 | 5.30 ± 2.30 |
Data are means ± SD; n = 5 in each group. SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine. Statistically significant differences between control and deficient animals for each sex (ANOVA).
P < 0.05 and
P < 0.01 (ANOVA).
Fig. 2.
Immunostaining of homocysteine (HCY) and estradiol in the 21-day-old rat cerebellum. A: distribution of HCY (green) in the cerebellum of 21-day-old female rats. Immunostaining of HCY-positive cells was performed on sagittal brain sections by means of a rabbit polyclonal antibody against HCY (n = 5/group). The cell nuclei were counterstained by the fluorescent dye 4,6-diamidino-2-phenylindole (DAPI; blue). In the deficient group, strong accumulation of HCY was observed in the cerebellar Purkinje cells. B: localization of estradiol (green) in the Purkinje cells of cerebellum counterstained with DAPI (blue) (n = 5/group). ml, Molecular layer; gl, granular layer. Calibration bars, 200 μm. Original magnifications, ×20.
The concentrations of the classical determinants of HCY metabolism were also measured in cerebellar homogenates. The universal methyl donor SAM, generated through the methionine cycle, is demethylated to form SAH, which is finally hydrolyzed into HCY. As illustrated in Table 3, SAM concentration remained unchanged, whereas SAH concentration was higher solely in the cerebellum of deficient compared with control females. Therefore, the SAM/SAH ratio was lower in the deficient female cerebellum (Table 3), with no significant change between control and deficient males.
Neurosteroidogenesis is altered in female cerebellum subjected to methyl donor deficiency.
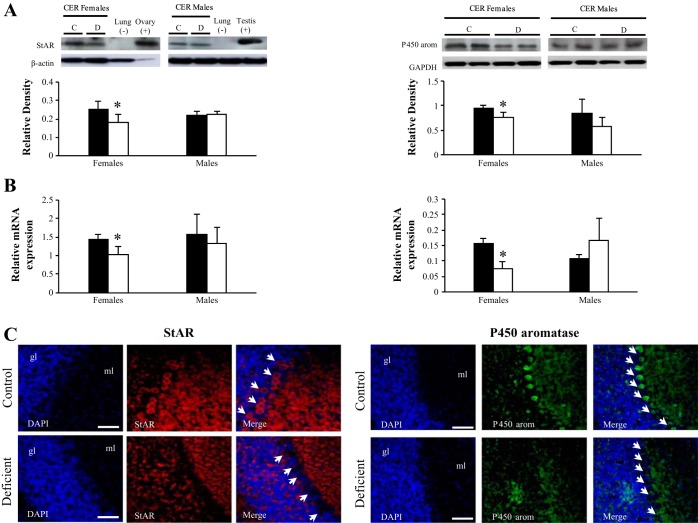
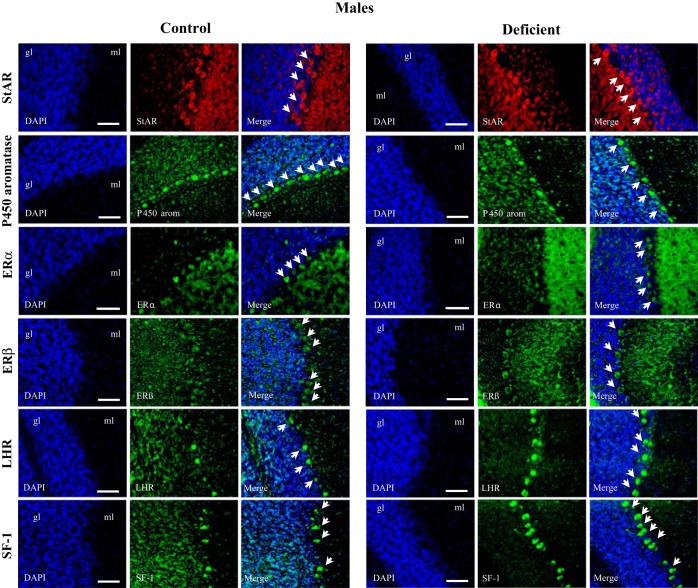
We first investigated the expression of the two key step proteins of neurosteroidogenesis, StAR and aromatase. Western blot analyses on cerebellum extracts showed a decreased expression of StAR and aromatase in the deficient female pups (Fig. 3A, left and right). The low levels of StAR and aromatase in deficient females were mirrored by the diminution of the corresponding mRNAs, as determined by quantitative (q)RT-PCR (Fig. 3B). This result suggests that the deficiency in methyl donors affects the transcriptional or posttranscriptional regulation of both genes. Immunoreactivity for StAR (Fig. 3A, left) and aromatase (Fig. 3A, right) was detected mostly in Purkinje cells in control but not in deficient female pups. The pattern of expression of both proteins was not altered in males (Fig. 4).
Fig. 3.
Expression of StAR protein and aromatase in the cerebellum of 21-day-old female rats. A: Western-blot analysis. Left: representative set of a Western blot showing immunodetectable StAR protein. From left to right, the lanes contain mitochondria from cerebellum (CER; 30 μg protein) of control (C) and deficient (D) 21-day-old rats. Lung of adult rat (30 μg of protein) and ovary or testis of adult rats (5 or 30 μg of protein) were used as negative or positive controls, respectively. Expression of StAR (30 kDa) was quantified and normalized against β-actin (43 kDa). Right: representative set of Western blots showing immunodetectable P450 aromatase protein. The lanes contain microsomes isolated from CER (30 μg of protein) of C and D 21-day-old rats. Expression of aromatase (55 kDa) was quantified and normalized against GAPDH (38 kDa). Densitometric data were obtained from 5 separate experiments. Results are presented as means ± SD. Statistically significant differences between the 2 experimental groups. B: RT-quantitative (q)PCR analysis of the effect of the deficient diet on StAR and aromatase mRNA expression in the cerebellum of 21-day-old rats. Arbitrary unit refers to an internal standard in the CER of C and D animals (n = 5/group, run in duplicate). Statistically significant differences between the 2 experimental groups: C: immunohistochemical analysis. Observations were restricted to Purkinje cells of 21-day-old females exposed early to the deficient diet compared with control rats (n = 5/group). Localization of StAR (red) and aromatase (green) in the Purkinje cells of cerebellum counterstained with DAPI (blue). Calibration bars, 200 μm. Original magnifications, ×20. *P < 0.05 (ANOVA).
Fig. 4.
Immunostaining of key proteins of neurosteroidogenesis in the 21-day-old rat male CER. Immunostaining of StAR, aromatase, ER receptor, LHR, and SF-1-positive cells was performed on sagital brain sections (n = 5/group). The cell nuclei were counterstained by the fluorescent dye DAPI (blue). No significant difference of expression was observed between control and deficient pups from dams fed the deficient diet.
We next determined the consequences of the diminished StAR and aromatase expression levels. Therefore, we measured the levels of pregnenolone and estradiol, the two metabolites directly affected by an alteration of StAR and aromatase activity, respectively. In agreement with the diminished expression level of the two enzymes, the amounts of pregnenolone and estradiol were reduced in deficient compared with control female cerebellar extracts, but not in males (Table 4).
Table 4.
Pregnenolone and estradiol concentrations measured in the cerebellums of 21-day-old rats
| Females |
Males |
|||
|---|---|---|---|---|
| Normal diet | Deficient diet | Normal diet | Deficient diet | |
| Pregnenolone, ng/g tissue | 38.25 ± 5.70 | 27.55 ± 2.17** | 39.35 ± 3.12 | 40.15 ± 10.44 |
| Estradiol, ng/g tissue | 0.91 ± 0.35 | 0.31 ± 0.06* | 1.47 ± 0.52 | 0.84 ± 0.21 |
Data are means ± SD; n = 5 in each group. Statistically significant differences between control and deficient animals for each sex (ANOVA).
P < 0.05 and
P < 0.01 (ANOVA).
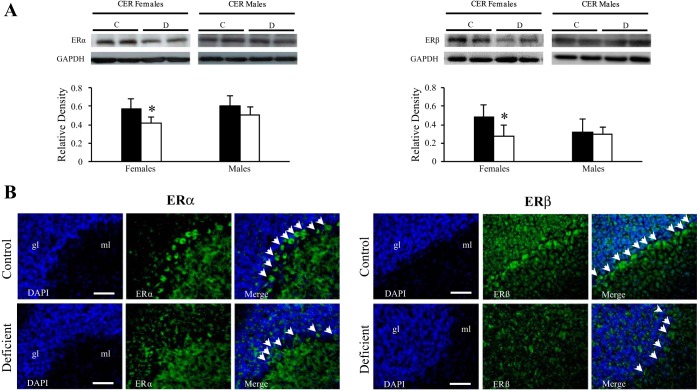
The presence of the ERα and ERβ receptors was assessed by Western immunoblot analysis of cerebellar fractions (Fig. 5A). A single band located at 66 kDa (Fig. 5A, left) and at 55 kDa (Fig. 5A, right) was observed for ERα and ERβ, respectively. Densitometric analysis showed a strong decline for both receptors in the cerebellum fractions of female pups from deficient dams compared with controls. No significant differences were observed in males. Immunohistochemical analysis showed the presence of ERα and ERβ receptors (Fig. 5B, left and right, respectively) in the cerebellum granular layer, with no immunostaining detected in the Purkinje cells from deficient female pups (Fig. 5B).
Fig. 5.
Localization of ERα and ERβ receptors in the CER of 21-day-old female rats. A: Western-blot analysis. Left: representative set of Western blots showing immunodetectable ERα protein. From left to right, the lanes contain total proteins isolated from CER (50 μg protein) of C and D 21-day-old rats. Right: representative set of Western blots showing immunodetectable ERβ protein. The lanes contain total proteins isolated from CER (50 μg of protein). Expression of both ERα (66 kDa) and ERβ (55 kDa) was quantified and normalized against GAPDH (38 kDa). Densitometric data were obtained from 5 separate experiments. Results are presented as means ± SD. Statistically significant differences between the 2 experimental groups: *P < 0.05 (ANOVA). B: immunohistochemical analysis. Observations were restricted in the cerebellar Purkinje cells of 21-day-old females exposed early to the deficient diet compared with control rats (n = 5/group). Localization of ERα (green; left) and ERβ (green; right) in the Purkinje cells of CER counterstained with DAPI (blue). Calibration bars, 200 μm. Original magnifications, ×20.
Taken together, these results show that neurosteroidogenesis is altered in female cerebellum following exposure to methyl donor deficiency.
The signaling cascade regulating neurosteroidogenesis is altered in the cerebellum of females subjected to methyl donor deficiency.
To understand the events linking the methyl donor deficiency to altered neurosteroidogenesis, we investigated key proteins involved in the signaling cascade in steroidogenic tissues.
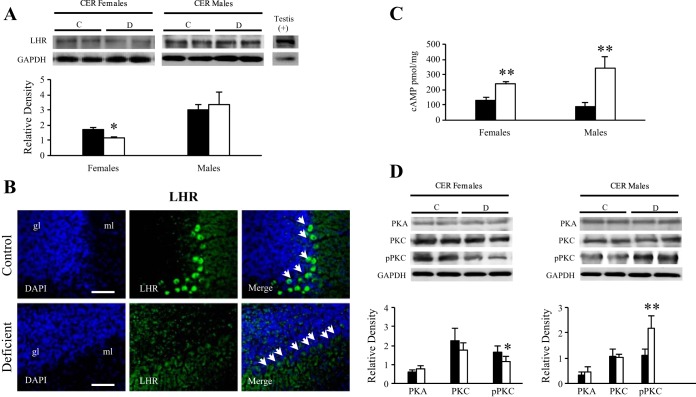
Densitometric analysis of Western blot performed against LHR showed a significant fall in the expression level of the receptor in the cerebellum fractions of female pups from deficient dams compared with controls. No significant difference was observed in males (Fig. 6A). This result was confirmed by immunostaining of LHR that was reduced in the Purkinje cells from deficient compared with control females (Fig. 6B), but not in males (Fig. 4).
Fig. 6.
Analysis of the signaling cascade proteins and cAMP concentration in the cerebellum of the 21-day-old rats. Western blot analysis of LHR. Representative set of Western blots showing immunodetectable LHR protein. Expression of LHR (80 kDa) was quantified and normalized against GAPDH (38 kDa). Testis (5 μg of protein) was used as a positive control. Densitometric data were obtained from 5 separate experiments. Results are presented as means ± SD. Statistically significant differences between the 2 experimental groups. B: immunostaining of LHR. Observations were restricted in the cerebellar Purkinje cells of 21-day-old females exposed early to the deficient diet compared with control rats (n = 5/group). Localization of LHR (green) in the Purkinje cells of CER counterstained with DAPI (blue). Calibration bars, 200 μm. Original magnifications, ×20. C: cAMP levels in cerebellar extracts of control and deficient 21-day-old rats. Results are presented as means ± SD. Statistically significant differences between C and D pups in each sex. D: Western blot analysis of PKA, PKC, and phospho-PKC (pPKC) of female (left) and male (right) cerebellar extracts. The lanes contain total proteins (40 μg) isolated from C and D 21-day-old rats. Expression of each protein of interest was quantified and normalized against GAPDH (38 kDa). Densitometric data were obtained from 5 separate experiments. Results are presented as means ± SD. Statistically significant differences between the 2 experimental groups. *P < 0.05 and **P < 0.01 (ANOVA).
Ligand binding on the LHR leads to the production of cAMP, which serves as a secondary messenger for activating PKA that is responsible for the phosphorylation of transcriptional factors and regulators as well as the StAR protein itself. Therefore, we measured cAMP levels in the cerebellum of male and female pups (Fig. 6C). An increase in cAMP levels was found in pups of both sexes fed the deficient diet compared with controls. However, the increase was smaller in females than in males (P < 0.05) (Fig. 6C), suggesting a less efficient activation of neurosteroidogenesis in females. The level of PKA was not significantly affected in male or female deficient rats (Fig. 6D). However, the level of phospho-PKC, which was involved in a parallel signaling pathway, was significantly decreased in deficient females and increased in deficient males. Taken together, these results suggest that the activation of neurosteroidogenesis is altered and less efficient in deficient females.
A specific dysregulation of the transcription factors CREB and SF-1 occurs in deficient females.
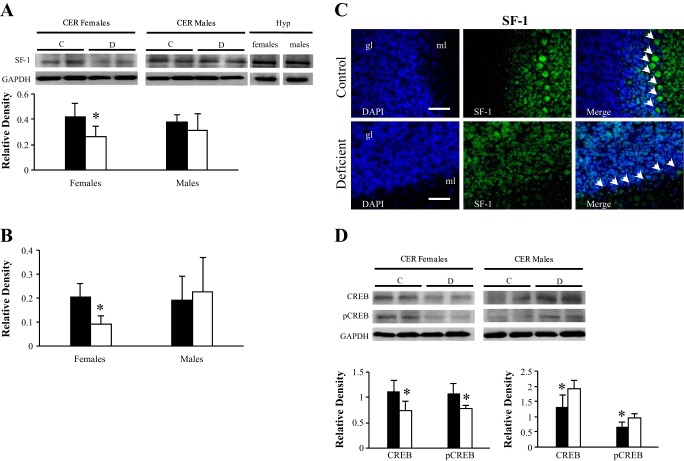
In addition to the signaling cascade, the expression of the ultimate activators SF-1 and CREB was assessed in the cerebellar tissues. By Western blot analysis, a single band of 46 kDa was detected for SF-1, and its intensity was significantly reduced in deficient females compared with controls or males (Fig. 7A). This diminution of protein expression was mirrored by a fall of mRNA levels in deficient females, as measured by qRT-PCR (Fig. 7B). An immunopositive signal was detected in the Purkinje cells of control animals (Fig. 7C). In line with previous observations regarding StAR and aromatase, the absence of SF-1 immunostaining of Purkinje cells was observed only in the case of deficient females and not deficient males (Fig. 4). In parallel, a diminution of CREB and phospho-CREB protein levels was also observed in the cerebellum of deficient females, whereas the expression of both proteins tended to increase in deficient males (Fig. 7D).
Fig. 7.
Expression levels of the transcription factors CREB and SF-1 in the cerebellum of the 21-day-old rats. A: representative set of Western blots showing immunodetectable SF-1 protein. The lanes contain total proteins isolated from CER (30 μg of protein) of C and D 21-day-old rats. Expression of SF-1 (55 kDa) was quantified and normalized against GAPDH (38 kDa). Two additional lanes contain proteins extracted from female and male hypothalamus (30 μg) used as positive controls. Densitometric data obtained from 5 separate experiments. Results are presented as means ± SD. Statistically significant differences between the 2 experimental groups. B: RT-qPCR analysis of the effect of the deficient diet on SF-1 mRNA expression in the cerebellum of 21-day-old rats. Arbitrary unit refers to an internal standard in the cerebellum of C and D animals (n = 5/group, run in duplicate). Statistically significant differences between the 2 experimental groups. C: immunohistochemical staining of SF-1. Observations were restricted to Purkinje cells of 21-day-old females exposed early to the deficient diet compared with control rats (n = 5/group). Localization of SF-1 in the Purkinje cells of CER counterstained with DAPI (blue). Calibration bars, 200 μm. Original magnifications, ×20. D: CREB and phospho-CREB (p-CREB) Western blot analysis. The lanes contain total proteins isolated from cerebellum (40 μg of protein) of C and D 21-day-old rats. Expression of CREB and p-CREB was quantified and normalized against GAPDH (38 kDa). Densitometric data obtained from 5 separate experiments. Results are presented as means ± SD. Statistically significant differences between the 2 experimental groups. *P < 0.05 (ANOVA).
DISCUSSION
The postnatal period is determinant for the cerebellum development since neurogenesis is achieved around the third postnatal week in the rat (13, 22), which corresponds to the age of the pups assessed in our study. Our data demonstrate for the first time that early methyl donor deficiency impaired neurosteroidogenesis and consecutively the content of pregnenolone and estradiol in female cerebellum in association with decreased protein expression levels of StAR, aromatase, and estrogen receptors ERα and ERβ as well as LHR. This was accompanied by impairment of the cAMP-dependent signaling pathway and of the expression levels of the ultimate activators CREB and SF-1 in deficient female pups.
As expected, exposure to the methyl donor-deficient diet until weaning markedly reduced tissue levels of folate and vitamin B12, with a subsequent accumulation of HCY, notably in Purkinje cells, in agreement with previous reports (4, 5). SAH concentration was increased only in deficient females, resulting in a decreased index of methylation, as reflected by the SAM/SAH ratio. This may affect epigenetic regulation of gene expression in accord with the previously reported lower amounts of methylated DNA in the olfactory bulbs of 21-day-old deficient females (12) as well as in the embryonic brains of the progeny from dams fed the deficient diet (20). Regarding steroidogenesis, epigenetic regulation occurs principally through SF-1, which is the major transcriptional regulator of the StAR and aromatase genes (29, 38). A decreased SF-1 expression associated with impaired steroidogenesis has been reported previously in mouse adrenocortical cells (15). This nuclear factor plays a pivotal role in endocrine development but also in female sexual behavior and in energy balance (22, 34). According to the literature, the cerebral localization of SF-1 would be restricted to the ventromedial hypothalamic neurons, except for one paper describing the presence of SF-1 in the pyramidal cells of rat hippocampus (37). We report herein for the first time the expression of SF-1 in the cerebellar Purkinje cells. This suggests that SF-1 could play additional roles by regulating locally the synthesis of neurosteroids at the postnatal age in the cerebellum. Thus, the decreased expression levels of SF-1 could explain the impairment of StAR and aromatase expression measured in the cerebellum of female pups born to dams fed the deficient diet.
The reduced expression of StAR in the cerebellum of 21-day-old females is particularly relevant due to its involvement in pregnenolone synthesis as well as in the control of its expression levels via the LHR. Accordingly, both pregnenolone tissue concentration and LHR protein level were altered in deficient female pups, with a strong decrease in LHR immunostaining in the Purkinje cells.
Concerning the key step of aromatase-mediated conversion of testosterone to estradiol, both tissue content and Purkinje cell immunostaining of aromatase and estradiol were strongly reduced in deficient females. This could dramatically compromise the building of the neuronal circuits and the sexual maturation of the cerebellum since they act on the density of Purkinje dendritic spines and synapses through both ERα and ERβ receptors, whose protein levels were also strongly reduced in the cerebellum of the 21-day-old deficient females.
The exploration of the cAMP-mediated signaling cascade associated with LHR activation allowed us to refine the molecular mechanisms accounting for the observed deleterious effects of methyl donor deficiency on neurosteroidogenesis. Indeed, the lesser increase of cAMP concentration and the lower level of phospho-PKC suggest that the activation of neurosteroidogenesis via phosphorylation mechanisms may be altered. Moreover, the expression levels of the transcriptional factors CREB, phospho-CREB, and SF-1 were also decreased specifically in deficient females.
Because all of these observations occurred specifically in female pups, it appears that the gestational methyl donor privation affects the cerebellum in a sex-dependent manner. Indeed, the effect of sex difference appears not only in the consequences on the neurosteroid synthesis pathway but also on HCY metabolism, since a reduced SAM/SAH ratio was measured only in female cerebellum. This is in accord with the findings of da Silva et al. (8), who recently reported sex differences in the alteration of methyl metabolism in newborn mouse brains after exposure to an early vitamin B-deficient diet.
Other previous reports about the pivotal role of the neurosteroidogenesis machinery in cerebellar development may contribute to explain the effect of sex difference observed in the present study. According to Dean and McCarthy (10), the sex-dependent differences are not related to the basal amounts of neurosteroids or receptors but to the differential regulation of their levels following environmental insults. Moreover, perinatal exposure to chemical toxins affects neurodevelopment, motor coordination, and behavior differently in males and females, in line with sex-dependent changes in cerebellar protein expression (32). Such dimorphic variations could account for the sex-dependent prevalence of some neuropsychological disorders, like depression or schizophrenia, in adulthood.
In summary, our results provide the first evidence of the dramatic effects of an early methyl donor deficiency on the cerebellar neurosteroidogenic pathway at the postnatal stage in female rats, with a decreased expression in major components of this pathway. This is in good accord with our previous report of methyl donor deficiency-associated alteration of neurosteroidogenesis in the olfactory bulbs, in association with impaired olfactory performances in female rat progeny (12). In the present study, we also provide the first evidence of a cerebellar expression of SF-1, the main regulator of steroidogenic gene expression, the expression levels of which were also impaired in the cerebellum of females born to dams fed a deficient diet. These alterations of the neurosteroidogenesis-related proteins may participate in the previously documented impaired locomotor coordination involving cerebellar functions in the same animal model of methyl donor deficiency (5). In addition, the cerebellum is also involved in cognitive tasks through the coordination of the cortico-thalamic-cerebellar-cortical circuit (10), and our observations may also account for the impaired learning capacities and memory documented previously in deficient rat pups (5).
Early privation of folate also has long-term effects in adulthood, and these observations are in accord with the hypothesis of “fetal programming” on life-long health. This suggests that the influence of an early deficiency in folate and vitamin B12 on brain neurosteroidogenesis and brain development and maturation may prove to be a potential mechanism accounting for neurologic disorders in the early and later steps of life.
GRANTS
Institutional grants were received from the French National Agency for Research (ANR Nutrivigene Project) and the Region of Lorraine (France).
DISCLOSURES
The authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
S.E.H.C., N.D., J.W., J.-L.D., J.-L.G., and B.L.-M. conception and design of research; S.E.H.C., N.D., J.W., L.C.-R., E.J., and J.-M.A. performed experiments; S.E.H.C., N.D., J.W., L.C.-R., E.J., J.-M.A., J.-L.D., J.-L.G., and B.L.-M. analyzed data; S.E.H.C., N.D., J.W., E.J., J.-M.A., and B.L.-M. interpreted results of experiments; S.E.H.C. and N.D. prepared figures; S.E.H.C., N.D., J.-L.D., and B.L.-M. drafted manuscript; S.E.H.C., N.D., J.W., L.C.-R., E.J., J.-M.A., J.-L.D., J.-L.G., and B.L.-M. approved final version of manuscript; N.D., J.-L.D., J.-L.G., and B.L.-M. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank D. Stocco (Texas Tech University) for providing antibodies against StAR protein and members of the laboratory for helpful comments. We also thank Rémy Umoret and Marilyn Schwartz for technical assistance.
REFERENCES
- 1.Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience 191: 139–147, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Blaise S, Alberto JM, Nedelec E, Ayav A, Pourie G, Bronowicki JP, Gueant JL, Daval JL. Mild neonatal hypoxia exacerbates the effects of vitamin-deficient diet on homocysteine metabolism in rats. Pediatr Res 57: 777–782, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Blaise SA, Alberto JM, Audonnet-Blaise S, Gueant JL, Daval JL. Influence of preconditioning-like hypoxia on the liver of developing methyl-deficient rats. Am J Physiol Endocrinol Metab 293: E1492–E1502, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Blaise SA, Nedelec E, Alberto JM, Schroeder H, Audonnet S, Bossenmeyer-Pourie C, Gueant JL, Daval JL. Short hypoxia could attenuate the adverse effects of hyperhomocysteinemia on the developing rat brain by inducing neurogenesis. Exp Neurol 216: 231–238, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Blaise SA, Nedelec E, Schroeder H, Alberto JM, Bossenmeyer-Pourie C, Gueant JL, Daval JL. Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats. Am J Pathol 170: 667–679, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Schwahn BC, Wu Q, He X, Rozen R. Postnatal cerebellar defects in mice deficient in methylenetetrahydrofolate reductase. Int J Dev Neurosci 23: 465–474, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chery C, Barbe F, Lequere C, Abdelmouttaleb I, Gerard P, Barbarino P, Boutroy JL, Gueant JL. Hyperhomocysteinemia is related to a decreased blood level of vitamin B12 in the second and third trimester of normal pregnancy. Clin Chem Lab Med 40: 1105–1108, 2002. [DOI] [PubMed] [Google Scholar]
- 8.da Silva VC, Fernandes L, Haseyama EJ, Agamme AL, Guerra Shinohara EM, Muniz MT, D'Almeida V. Effect of vitamin B deprivation during pregnancy and lactation on homocysteine metabolism and related metabolites in brain and plasma of mice offspring. PLoS One 9: e92683, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daval JL, Blaise S, Gueant JL. Vitamin B deficiency causes neural cell loss and cognitive impairment in the developing rat. Proc Natl Acad Sci USA 106: E1; author reply E2, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean SL, McCarthy MM. Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum 7: 38–47, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delabar U, Kloor D, Luippold G, Muhlbauer B. Simultaneous determination of adenosine, S-adenosylhomocysteine and S-adenosylmethionine in biological samples using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 724: 231–238, 1999. [DOI] [PubMed] [Google Scholar]
- 12.El Hajj Chehadeh S, Pourié G, Martin N, Alberto JM, Daval JL, Guéant JL, Leininger-Muller B. Gestational methyl donor deficiency alters key proteins involved in neurosteroidogenesis in the olfactory bulbs of newborn female rats and is associated with impaired olfactory performance. Br J Nutr 111: 1021–1031, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Fan X, Xu H, Warner M, Gustafsson JA. ERbeta in CNS: new roles in development and function. Prog Brain Res 181: 233–250, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 15.Frigeri C, Tsao J, Czerwinski W, Schimmer BP. Impaired steroidogenic factor 1 (NR5A1) activity in mutant Y1 mouse adrenocortical tumor cells. Mol Endocrinol 14: 535–544, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Frye CA, Hirst JJ, Brunton PJ, Russell JA. Neurosteroids for a successful pregnancy. Stress 14: 1–5, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Guo XZ, Su JD, Sun QW, Jiao BH. Expression of estrogen receptor (ER) -alpha and -beta transcripts in the neonatal and adult rat cerebral cortex, cerebellum, and olfactory bulb. Cell Res 11: 321–324, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Higashi T, Yokoi H, Nagura Y, Nishio T, Shimada K. Studies on neurosteroids XXIV. Determination of neuroactive androgens, androsterone and 5alpha-androstane-3alpha,17beta-diol, in rat brain and serum using liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 22: 1434–1441, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Karri S, Dertien JS, Stocco DM, Syapin PJ. Steroidogenic acute regulatory protein expression and pregnenolone synthesis in rat astrocyte cultures. J Neuroendocrinol 19: 860–869, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kerek R, Geoffroy A, Bison A, Martin N, Akchiche N, Pourié G, Helle D, Guéant JL, Bossenmeyer-Pourié C, Daval JL. Early methyl donor deficiency may induce persistent brain defects by reducing Stat3 signaling targeted by miR-124. Cell Death Dis 4: e755, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HJ, Park CH, Roh GS, Kang SS, Cho GJ, Choi WS. Changes of steroidogenic acute regulatory protein mRNA expression in postnatal rat development. Brain Res Dev Brain Res 139: 247–254, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Kim KW, Sohn JW, Kohno D, Xu Y, Williams K, Elmquist JK. SF-1 in the ventral medial hypothalamic nucleus: a key regulator of homeostasis. Mol Cell Endocrinol 336: 219–223, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci 22: 10613–10620, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 25.Lalli E, Doghman M, Latre de Late P, El Wakil A, Mus-Veteau I. Beyond steroidogenesis: novel target genes for SF-1 discovered by genomics. Mol Cell Endocrinol 371: 154–159, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology 132: 2262–2270, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Liu T, Wimalasena J, Bowen RL, Atwood CS. Luteinizing hormone receptor mediates neuronal pregnenolone production via up-regulation of steroidogenic acute regulatory protein expression. J Neurochem 100: 1329–1339, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod 15: 321–333, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM. Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol 30: 381–397, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Manna PR, Stocco DM. The role of specific mitogen-activated protein kinase signaling cascades in the regulation of steroidogenesis. J Signal Transduct 2011: 821615, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Arguelles DB, Papadopoulos V. Epigenetic regulation of the expression of genes involved in steroid hormone biosynthesis and action. Steroids 75: 467–476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguon K, Ladd B, Baxter MG, Sajdel-Sulkowska EM. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog Brain Res 148: 341–351, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Obeid R, Schlundt J, Umanskaya N, Herrmann W, Herrmann M. Folate is related to phosphorylated neurofilament-H and P-tau (Ser396) in rat brain. J Neurochem 117: 1047–1054, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res 57: 19–36, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Stocco DM. The role of the StAR protein in steroidogenesis: challenges for the future. J Endocrinol 164: 247–253, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsui K. Biosynthesis, mode of action and functional significance of neurosteroids in the developing Purkinje cell. J Steroid Biochem Mol Biol 102: 187–194, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J Neurochem 76: 1879–1886, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Young M, McPhaul MJ. A steroidogenic factor-1-binding site and cyclic adenosine 3′,5′-monophosphate response element-like elements are required for the activity of the rat aromatase promoter in rat Leydig tumor cell lines. Endocrinology 139: 5082–5093, 1998. [DOI] [PubMed] [Google Scholar]