Abstract
Hypothalamic kisspeptin neurons integrate and translate cues from the internal and external environments that regulate gonadotropin-releasing hormone (GnRH) secretion and maintain fertility in mammals. However, the intracellular signaling pathways utilized to translate such information into changes in kisspeptin expression, release, and ultimately activation of the kisspeptin-receptive GnRH network have not yet been identified. PI3K is an important signaling node common to many peripheral factors known to regulate kisspeptin expression and GnRH release. We investigated whether PI3K signaling regulates hypothalamic kisspeptin expression, pubertal development, and adult fertility in mice. We generated mice with a kisspeptin cell-specific deletion of the PI3K catalytic subunits p110α and p110β (kiss-p110α/β-KO). Using in situ hybridization, we examined Kiss1 mRNA expression in gonad-intact, gonadectomized (Gdx), and Gdx + steroid-replaced mice. Kiss1 cell number in the anteroventral periventricular hypothalamus (AVPV) was significantly reduced in intact females but not in males. In contrast, compared with WT and regardless of steroid hormone status, Kiss1 cell number was lower in the arcuate (ARC) of kiss-p110α/β-KO males, but it was unaffected in females. Both intact Kiss-p110α/β-KO males and females had reduced ARC kisspeptin-immunoreactive (IR) fibers compared with WT animals. Adult kiss-p110α/β-KO males had significantly lower circulating luteinizing hormone (LH) levels, whereas pubertal development and fertility were unaffected in males. Kiss-p110α/β-KO females exhibited a reduction in fertility despite normal pubertal development, LH levels, and estrous cyclicity. Our data show that PI3K signaling is important for the regulation of hypothalamic kisspeptin expression and contributes to normal fertility in females.
Keywords: phosphoinositide 3-kinase, kisspeptin, gonadotropins, fertility, hypothalamus
in mammals, successful reproduction depends on the intermittent release of gonadotropin-releasing hormone (GnRH) from hypothalamic neurons; these neurons form the final common pathway through which many factors influence gonadal activity. Thus, signals in the internal or external environment affecting fertility often do so by altering GnRH release. However, GnRH neurons lack many of the receptors responsible for conveying peripheral regulatory signals, including estrogens [estrogen receptor-α (ERα)] (75) and leptin [the leptin receptor (LepR)] (30). Therefore, GnRH neurons receive information from the periphery through multiple afferents. Among these, neurons expressing kisspeptin, the product of the Kiss1 gene, are pivotal for the maturation and function of the hypothalamic-pituitary-gonadal (HPG) axis (22, 72).
Release of kisspeptin, activation of the kisspeptin receptor (Kiss1R), and subsequent activation of GnRH neurons are necessary for puberty in mammals (15, 41, 78). In addition, the kisspeptin-Kiss1R signaling system integrates and translates cues from the internal and external environment to regulate GnRH release (14, 69). In rodents, mainly two hypothalamic populations of kisspeptin neurons exist, one in the anteroventral periventricular nucleus (AVPV) and the other in the arcuate nucleus (ARC) of the hypothalamus (16, 39, 48, 79).
Several peripheral signals regulate Kiss1 expression, release, and function. These include sex steroid hormones (79), growth factors (43, 83), and metabolic cues (56, 77). In females, estradiol (E2) acts through ERα in hypothalamic kisspeptin neurons to regulate pubertal development (58). Kisspeptin neurons may also represent a link between metabolic signals such as insulin and leptin and the central maintenance of reproductive function (4). Leptin and insulin receptors are expressed in subpopulations of kisspeptin neurons of the ARC, a major site for the metabolic control of the HPG axis (67, 77). Moreover, metabolic conditions such as undernutrition and obesity, which reduce GnRH release and reproductive competence, drastically alter Kiss1 expression (12, 13, 68, 71). However, few studies have addressed the intracellular mechanisms that regulate Kiss1 expression and function.
The phospholipid enzyme phosphoinositide 3-kinase (PI3K) is an important signaling node common to many peripheral factors that regulate kisspeptin expression and GnRH release. Examples include insulin, leptin, IGF-I, and gonadal steroid hormones, all of which modulate puberty and gonadotropin release (1). PI3Ks generate the second messenger phosphatidylinositol 3,4,5-triphosphate, which recruits and activates the serine/threonine kinase Akt. Akt then regulates multiple target proteins important for insulin signaling and glucose metabolism (32). Class IA PI3Ks, which are the major targets of insulin and leptin, are composed of a regulatory/adapter subunit (p85α, p85β, and p55γ) associated with a catalytic subunit (p110α, p110β, and p110δ) (11, 86).
The present study investigated whether PI3K signaling in kisspeptin cells is important for Kiss1 expression and the proper function of the HPG axis. We generated mice with a kisspeptin cell-specific deletion of PI3K catalytic subunits p110α and p110β (kiss-p110α/β KOs). Examining the reproductive phenotypes of mice with targeted disruption of PI3K catalytic subunits in kisspeptin cells provides evidence of a novel molecular mechanism that regulates kisspeptin expression in both sexes, gonadotropin release in males, and fertility in females.
MATERIALS AND METHODS
Animals
Animals were housed at Stony Brook University, Division of Laboratory Animal Resources, under a 12:12-h light-dark cycle and had access to water and rodent chow ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Stony Brook University Medical Center in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Generation of Kiss-p110α/β-Knockout Mice
All animals were of a 129Sv-C57BL/6/FVB/N mixed genetic background. To generate mice in which the genes for the PI3K catalytic subunits p110α (Pik3ca) and p110β (Pik3cb) are deleted specifically in kisspeptin cells, double-floxed mice, p110αflx/flx/p110βflx/flx were mated with kisspeptin-IRES-CRE mice (58). Double-floxed animals bear loxP sites flanking exon 1 of the PIK3CA gene and exons 3 and 4 of the PIK3CB gene (55). The resulting F1 animals were then intercrossed to generate KissIC−p110αflx/flx/p110βflx/flx and KissIC+p110αflx/flx/p110βflx/flx animals, referred to here as wild-type (WT) and kiss-p110α/β knockout (KO), respectively. Cre-negative littermates (p110αflx/flx/p110βflx/flx) served as WT controls. Where indicated, mice also carried Gt(ROSA)26Sir locus-inserted enhanced yellow fluorescent protein (EYFP) gene (stock no. 006148; Jackson Laboratories, Bar Harbor, ME), which served as a reporter under the control of Cre recombinase expression. Animals were screened for the presence of Cre and floxed p110α and p110β by PCR of isolated genomic tail DNA as described previously (3, 55, 58).
Fluorescence-Activated Cell Sorting
Fluorescence-activated cell sorting (FACS) and RNA extraction were done as described previously, with minor modifications (19, 67). To avoid contamination, dissociation of hypothalamic tissue and FACS for KissIC/R26-YFP and KissIC-p110α/β- KO/R26-YFP were done separately. We combined tissue from three males from each genotype (KissIC/R26-YFP or KissIC-p110α/β KO/R26-YFP) for these experiments. Using a brain matrix (Braintree Scientific, Braintree, MA), 1-mm slices were generated from the rostral preoptic area to the caudal mammillary bodies. After dissociation, cells were resuspended in FACS buffer containing 1% EDTA and processed to isolate EYFP-expressing cells via FACS ARIA (BD Biosciences, San Jose, CA) using green fluorescent protein (GFP) wavelength (488-nm laser). Neurons were collected in RNAlater solution. Total RNA was extracted using Arcturus PicoPure RNA isolation kit, and reverse transcription was done using Superscript VILO (both from Applied Biosystems, Foster City, CA). PCR was performed using primers for Kiss1 (agctgctgcttctcctctgt and gcataccgcgattcctttt) (56), p110α (atgcctccacgaccatcttc and ttctcgattgaggatcttttc, exon 1), and p110β (atgttatggaagcaagttcac and cttttcctataagaacccca, exon 4).
In Situ Hybridization
Tissue preparation.
Coronal sections (20 μm) of frozen brains were cut on a cryostat, thaw-mounted onto SuperFrost Plus slides (Fisher Scientific), and stored at −80°C. Sections were collected in series of four starting at the medial septum/diagonal band of Broca and continuing to the mammillary bodies. Tissues from intact females and from males of all treatment groups [intact, gonadectomized (Gdx) + vehicle (V), and Gdx + testosterone (T)] were assayed together. Because of limited numbers of females, tissues from females that were ovariectomized (Ovx) and replaced with V or E2 were collected later and processed separately.
Probe preparation.
35S-cRNA probes against Kiss1 were generated using a linearized mouse Kiss1 cDNA template (39, 52). Radiolabeled probes were synthesized in vitro, as described (39, 79). The riboprobes were separated from unincorporated nucleotides with ProQuant G-50 MicroColumns (Amersham).
Slides containing the hypothalamic regions of interest were processed before hybridization by fixing in paraformaldehyde, treating with triethanolamine and acetic anhydride, washing in saline-sodium citrate (SSC), and dehydrating in graded alcohol as, described previously (39, 79). Radiolabeled antisense Kiss1 riboprobe was denatured, added to hybridization solution (50% formamide, 10% dextran sulfate, 300 mM NaCl, 10 mM Tris, pH 8.0, 1 mM EDTA, 1× Denhardt's solution, 10 mM DTT, and tRNA) at a concentration of 5,000,000 counts·min−1·ml−1, and applied to slides. Slides were coverslipped and incubated in a humid chamber overnight at 55°C. The next day, slides were washed in 4× SSC and then incubated in RNase A in buffer (10 mM Tris, pH 8.0, 500 mM NaCl, and 1 mM EDTA), followed by washing with 2× SSC and 0.1× SSC at 65°C. Slides were washed at room temperature in 0.1× SSC and then dehydrated in 50 and 80% alcohol with ammonium acetate, followed by 100% alcohol. Slides were air-dried for at least 1 h and then dipped in NTB-2 liquid emulsion (VWR). Slides were stored at 4°C for 2 wk and then developed, counterstained with thionin, dehydrated, cleared with Citrisolve (Fisher Scientific), and coverslipped with Permount (VWR).
Kiss1 quantification and analysis.
The quantification of Kiss1 mRNA-expressing cells in emulsion-dipped sections was done using MCID image analysis software following capture of both bright-field and dark-field photomicrographs (50). Counts of cells per section and grains per cell were obtained bilaterally for the AVPV and the midrostrocaudal ARC in two and three sections per animal, respectively (50). Cells counted were considered kisspeptin positive when grain count was more than three times background.
Kiss1 Real-Time RT-PCR
To measure Kiss1 mRNA, the preoptic area (POA) and the mediobasal hypothalamus (MBH) were dissected manually. The POA was defined as the region ventral to the anterior commissure extending rostrally from the caudal limit of the medial septum to the optic chiasm (4, 68). The MBH was delineated rostrally by the posterior margin of the optic chiasm, laterally by the hypothalamic sulci, and caudally by the mammillary bodies (4, 68).
Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer's instructions. RNA (2 μg) was reverse-transcribed to cDNA using the SuperScript VILO cDNA synthesis kit (Applied Biosystems). Real-time PCR was performed at the Genomics Core Facility, Stony Brook University, with an ABI 7300 Real-Time PCR system, using TaqMan probe-based gene expression analysis and primer sequences specific for the Kiss1 gene (Id: Mm458301; Applied Biosystems). A 100-ng total RNA equivalent cDNA was used for Taqman PCR. Samples were run in duplicate to obtain an average threshold value (CT). Quantities of Kiss1 mRNA were normalized to 18S ribosomal RNA (Id: Mm03928990_g1). We used the standard curve method for relative quantification of the transcript level. Samples prepared without reverse transcriptase served as negative controls.
Western Blotting
After an overnight fast, WT and kiss-p110α/β KO mice were injected intraperitoneally with 0.9% saline or with insulin (1.5 U/kg body wt). Ten minutes later, animals were euthanized and the livers snap-frozen in liquid nitrogen. The livers were homogenized in RIPA buffer containing protease inhibitors (1:1,000 dilution) and converted vanadate (1:10 dilution). Aliquots of protein (50 μg) were subjected to SDS-PAGE and immunoblotting, as described previously (62). We quantified Akt phospho-Ser473 (Epitomics, Burlingame, CA) and total Akt (Santa Cruz Biotechnology, Dallas, TX) as control. Signals were visualized using horseradish peroxidase-linked secondary antibodies and chemiluminescence reagents. Densitometry was performed using ImageJ (NIH).
Perfusion and Immunohistochemistry
Mice were anesthetized using ketamine and xylazine (100 and 10 mg/kg, respectively) and transcardially perfused with 4% paraformaldehyde. Brains were removed and postfixed for 24 h and then allowed to saturate with 30% sucrose. Brains were cut into three sets of 30-μm coronal sections. Sections were treated with 3% hydrogen peroxide for 10 min to quench endogenous peroxidase activity and rinsed in PBS. To detect GnRH neurons, the tissue was incubated overnight at room temperature in PBST containing anti-GnRH antibody (LR-5, gift from Dr. R. Benoit, Montréal General Hospital; 1:20,000). To detect kisspeptin immunoreactivity (IR), sections were incubated for 48 h at 4°C in TBS-Tween 20 (0.3% Triton X-100, 0.25% BSA, and 2% normal goat serum) containing polyclonal rabbit anti-kisspeptin-10 antiserum (AC 566) (34). After washes, sections were incubated in buffer containing biotinylated goat anti-rabbit IgG (1:500; Vector Laboratories) for 1 h, followed by incubation in Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA) for 90 min at room temperature. Sections were then washed, and the IR was visualized using nickel-enhanced Vectastain DAB Peroxidase substrate kit (Vector Laboratories). Sections were washed, mounted on slides, dried overnight, and coverslipped.
GnRH and kisspeptin neurons were counted under an Olympus microscope (model BX 51). Kisspeptin IR neurons in the AVPV and GnRH-immunoreactive neurons were quantified as described previously (2, 68). To measure kisspeptin IR in the ARC, images (−1.5 mm relative to Bregma) were transformed to grayscale images of 8 bits, and the mean optical density was measured using ImageJ software (24, 64).
Pubertal Assessment and Estrous Cyclicity
Vaginal opening in females or balanopreputial separation in males was assessed daily. To determine of age of first estrus, vaginal cells were collected daily by saline lavage beginning on the day of vaginal opening. To examine the effects of genotype on estrous cyclicity, daily vaginal lavages from female mice (1.5–3 mo) were obtained between 0900 and 1000 for 14 days. A normal estrous cycle was defined as exhibiting vaginal histology that was leukocytic for 2 days, followed by 1 day of nucleated and 1–2 days of cornified cells. Wet testicular weights were determined in freshly dissected animals.
Breeding Studies
Control and kiss-p110α/β-KO females were paired with a proven fertile WT male for 7 days, and females were checked for plugs daily (8). The female was then removed and housed separately for 25–28 days. Four cycles of pairing were evaluated in which the males were alternated between pairings with WT and kiss-p110α/β-KO females. To assess male fertility, WT and kiss-p110α/β males were paired with two proven WT females for 7 days. After 7 days, females were removed and housed separately until a litter was born. Gestation time and litter size were recorded.
Gonad Histology
Ovaries were collected from mice and placed immediately in 4% paraformaldehyde. Testes were fixed in Bouin's reagent. Tissue was embedded in paraffin and cut into 5-μm sections at the Research Histology Core Laboratory, Stony Brook University. Gonads were stained with hematoxylin and eosin (2). The total number of corpora lutea (CL) was counted in a blinded fashion, choosing the largest cross-sectional area of the CL by comparing the section with the preceding one and following section to avoid double counting (2, 63, 81). Testis sections were examined for gross morphological effects.
Gonadectomy and Steroid Hormone Replacement
All gonadectomies were done under isoflurane anesthesia. Adult female mice from each genotype were randomly assigned to one of three groups: intact sham operated (controls), Ovx + V, or Ovx + E2. Immediately after surgery, animals received subcutaneous Silastic capsules containing sesame oil vehicle (Ovx + V) or 1 mg/ml E2 in sesame oil (Ovx + E2). Adult male mice of both genotypes were randomly assigned to one of three groups: intact sham operated (controls), Gdx + V, or Gdx + T. At the time of surgery, each animal received a subcutaneous Silastic capsule (1.02 × 2.16 mm od) 5 mm in length filled with crystalline T or left empty (3, 60). Ten days after surgeries, animals were euthanized between 0900 and 1030, and tissue and brains were obtained. Serum was frozen until assayed for LH, E2, or T levels.
Glucose Homeostasis
Glucose measurements from 3-mo-old mice were obtained from tail blood using a glucometer (One-Touch Ultra Mini; Lifescan, Milpitas, CA). For glucose tolerance tests (GTT), mice were fasted overnight, and blood glucose levels were measured immediately before (t = 0) and 30, 60, and 120 min after intraperitoneal (ip) injection with d-glucose (2 g/kg). For insulin tolerance tests (ITT), 4-h-fasted mice were injected ip with 1 mU/g (males) or 0.5 mU/g (females) human insulin. Blood glucose was measured before and 15, 30, 60, 90, and 120 min after insulin injection.
Hormone Assays
Serum measurements of LH from male and female animals were conducted at the University of Virginia Core Facility using RIA reagents obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD), including LH reference (RP-3) and antirat LH antibody (S-11). The assay had a lower limit of detection of 0.2 ng/ml. Intraassay and interassay coefficients of variance were 0.24 and 4.71%, respectively. Serum T levels were measured by ELISA (R & D Systems, Minneapolis, MN), with a sensitivity of 0.041 ng/ml. Serum E2 was measured by ELISA (Calbiotech, Spring Valley, CA), with sensitivity of <3 pg/ml.
Statistical Analyses
Data are expressed as means ± SE unless stated otherwise. Statistical analysis was done using Sigma Plot 10 (San Jose, CA). Repeated-measures ANOVA was used to analyze body weights. We calculated area under the curve to analyze glucose levels. Mating success rate between WT and kiss-p110α/β-KO mice was analyzed with Chi Square. For puberty onset, estrous cycle length, number of CL, litter size, kisspeptin-IR, and number of cells expressing Kiss1 mRNA in gonad-intact females, the differences between WT and kiss-p110α/β-KO mice were analyzed using unpaired Student's t-test. In experiments analyzing the effects of Gdx and steroid hormone replacement, data were analyzed using a two-way ANOVA (genotype × treatment), followed by the Holm-Sidak multiple-comparison method. For all statistical analyses, P < 0.05 was considered significant.
RESULTS
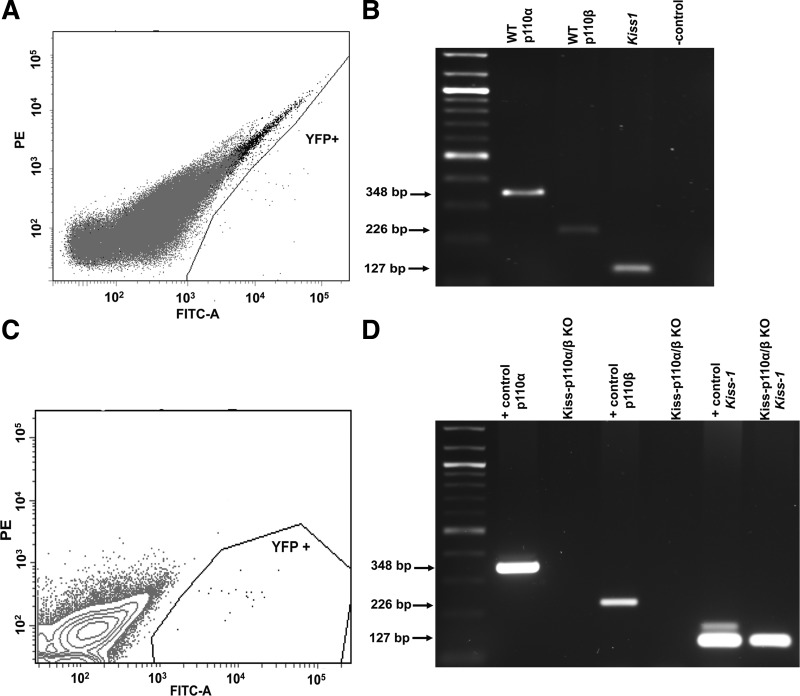
To confirm the deletion of p110α and p110β in hypothalamic kisspeptin cells, we isolated these neurons by FACS sorting from EYFP reporter mice (Fig. 1, A and C). RT-PCR showed bands for Kiss1, p110α, and p110β in kisspeptin-EYFP neurons from WT mice (Fig. 1B). In contrast, no amplification of p110α or p110β in neurons from kiss-p110α/β-KO mice was detected, demonstrating the successful deletion of PI3K catalytic subunits in kiss-p110α/β mice (Fig. 1D).
Fig. 1.
Validation of the kiss-p110α/β-knockout (KO) model. Fluorescence-activated sell sorting (FACS; A and C) and RT-PCR (B and D) of p110α, p110β, and Kiss1 genes from yellow fluorescent protein (YFP)-positive cells isolated from wild-type (WT) male reporter mice (A and B) (n = 3) and kiss-p110α/β-KO-YFP+ reporter males (C and D) (n = 3). D: mediobasal hypothalamus (MBH) from WT mice served as positive (+) control in the gel. PE, phycoerythrin.
Kiss-p110α/β-KO Mice Show Normal Tolerance to Glucose and Insulin
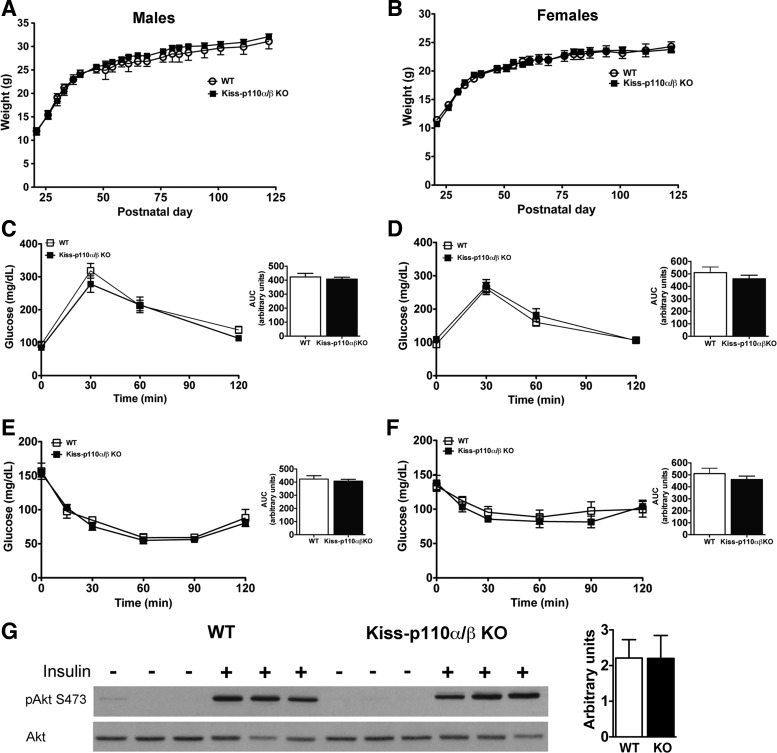
PI3K signaling plays a pivotal role in metabolism, including glucose homeostasis (32, 82), and kisspeptin-expressing cells are found in peripheral tissues, including the liver and pancreas (82). Hence, we determined whether deletion of p110α and p110β in kisspeptin cells affected body weight, glucose, and insulin sensitivity.
WT and kiss-p110α/β-KO mice of both sexes had comparable body weights from day 21 until 3 mo (Fig. 2, A and B). GTTs did not show differences between kiss-p110α/β-KO and WT animals of either sex (Fig. 2, C and D), and fasting glucose levels at the start of the GTTs did not differ. In addition, ITTs showed that adult kiss-p110α/β-KO mice display insulin sensitivity comparable with WT littermates (Fig. 2, E and F).
Fig. 2.
Postweaning body weight (BW), glucose, and insulin tolerance tests in kiss-p110α/β-KO and littermate control mice (n = 7–11). Deletion of p110α and p110β in kisspeptin cells did not affect postweaning BW in males (A) or females (B). WT and kiss-p110α/β-KO showed similar glucose (C and D) and insulin (E and F) sensitivity. Calculated areas under the curve (AUC) are shown as insets. Adult (2- to 3-mo-old) chow-fed mice were used for glucose and insulin tolerance tests. Results are expressed as mean glucose concentration ± SE. G: baseline and insulin-stimulated p-Akt in the liver of WT and kiss-p110α/β-KO males. Liver lysates from saline and insulin-stimulated male mice were blotted with antibodies against p-Akt (Ser473). The blots were then stripped and reprobed with antibodies to total Akt. Densitometry analysis (normalized to total Akt) is shown in the adjacent bar graph; the values are means ± SE expressed as arbitrary units.
To measure insulin responsiveness in the liver, male mice from each genotype were fasted overnight and treated with insulin or saline. Following insulin treatment, p-Akt levels increased to comparable levels in the livers of WT and kiss-p110α/β-KO males (Fig. 2G).
Normal Pubertal Onset But Decreased Reproductive Capacity in Kiss-p110α/β-KO Females
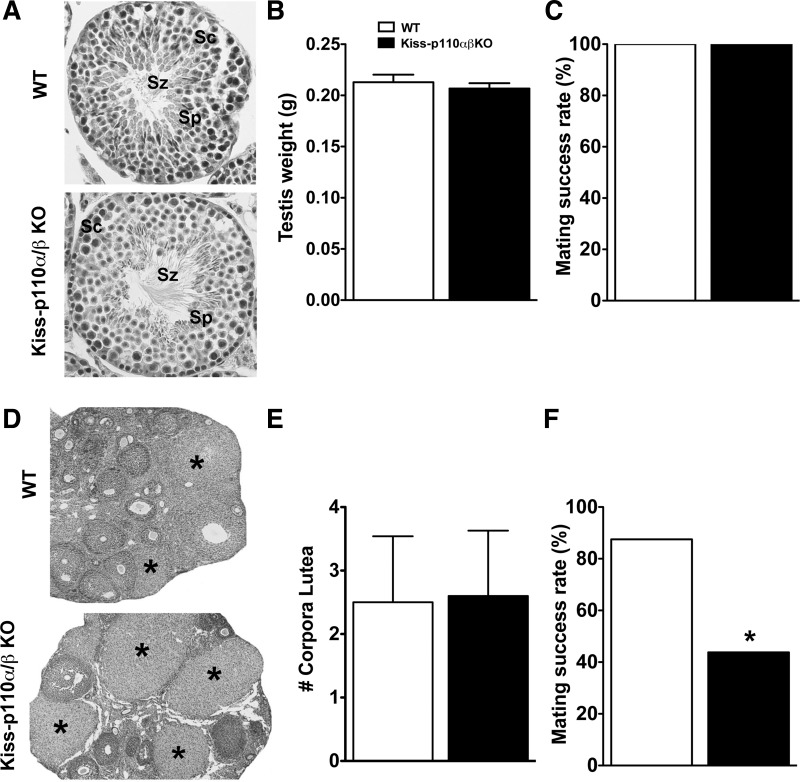
In males, no genotype effects were observed on the day of preputial separation, an external marker of puberty onset (Table 1), or on adult testicular weight (Fig. 3B). WT and kiss-p110α/β-KO testicular tissue showed normal seminiferous tubules with all types of spermatogenic cells and spermatozoa (Fig. 3A). WT and kiss-p110α/β KO males showed equal mating success rates, with all of the animals able to sire pups in proven fertile WT females (Fig. 3C).
Table 1.
Puberty onset in WT and Kiss-p110α/β-KO mice and serum T levels and pituitary LH content from adult WT and Kiss-p110α/β-KO males
| WT |
Kiss-p110α/β-KO |
|||||
|---|---|---|---|---|---|---|
| Puberty (Age of BS, days) | 30.5 ± 1.0 (n = 10) | 30.5 ± 0.8 (n = 12) | ||||
| Intact (n = 5–6) | Gdx + V (n = 6) | Gdx + T (n = 5–6) | Intact (n = 6–7) | Gdx + V (n = 5–6) | Gdx + T (n = 5–6) | |
| T, ng/ml | 15.0 ± 6.0a | 0.8 ± 0.1b | 4.9 ± 0.5b | 11.9 ± 4.1a | 1.4 ± 0.1b | 5.3 ± 0.7b |
| LH, ng/ml | 10.8 ± 0.8a | 5.9 ± 1.4b | 7.9 ± 1.5a,b | 10.3 ± 1.2a | 8.4 ± 0.9a,b | 7.9 ± 0.8a,b |
Data are expressed as means ± SEM.
WT, wild-type; KO, knockout; BS, balanopreputial separation; Gdx, gonadectomized; V, vehicle; T, testosterone.
There was a significant treatment effect on serum T levels (P < 0.001, 2-way ANOVA) and on pituitary LH levels (P < 0.05, 2-way ANOVA). Different superscripted letters denote significance (P < 0.05) after Holm-Sidak multiple comparison test.
Fig. 3.
Normal fertility in kiss-p110α/β-KO males but reduced fertility in kiss-p110α/β KO females. A: representative transverse sections of WT and kiss-p110α/β-KO mice showing normal seminiferous tubules with all types of spermatogonic elements (Sz, spermatozoa; Sc, spermatocytes; Sp, spermatids). B: testicular weight in WT and kiss-p110α/β-KO mice (n = 11). C: percent of litter-producing matings by WT and kiss-p110α/β-KO males (kiss-p110α/β-KO, n = 4; WT, n = 5). D: representative photomicrographs of adult ovaries from WT and kiss-p110α/β-KO mice, *Corpora lutea (CL). Average no. of CL obtained from 1 ovary/animal (n = 4–5). F: percent of litter-producing matings by WT and kiss-p110α/β-KO females (n = 4/genotype). Values are means ± SE. *P < 0.05.
In females, genotype had no effect on pubertal onset (Table 2). Compared with WT, kiss-p110α/β-KO mice tended to show earlier first estrus (P = 0.06; Table 2). In adults, estrous cycle length did not differ between WT and kiss-p110α/β-KO (4.4 ± 0.2 vs. 4.5 ± 0.2 days, respectively, P > 0.05), and the ovaries of WT and kiss-p110α/β-KO mice had similar numbers of CL (Fig. 3E). However, Fig. 3F shows that kiss-p110α/β KO females had a 50% reduction in fertility compared with WT littermates (Chi square = 6.8, P < 0.01). The number of pups/litter produced by kiss-p110α/β-KO females that became pregnant was also significantly lower than WT females (5.1 ± 0.3 vs. 6.4 ± 0.3, Kiss-p110α/β-KO and WT, respectively, P < 0.05).
Table 2.
Puberty onset in WT and Kiss-p110α/β-KO female mice and serum E2 levels from adult WT and Kiss-p110α/β-KO females
| WT |
Kiss-p110α/β-KO |
|||||
|---|---|---|---|---|---|---|
| Puberty | ||||||
| Age of VO, days | 30.6 ± 1.1 (n = 11) | 31.5 ± 0.8 (n = 19) | ||||
| First estrus, days | 46.6 ± 1.6 (n = 10) | 41.5 ± 1.7 (n = 19) | ||||
| Intact (n = 5) | Ovx + V (n = 5) | Ovx + E2 (n = 5) | Intact (n = 5) | Ovx + V (n = 7) | Ovx + E2 (n = 6) | |
| E2, pg/ml | 6.8 ± 1.1 | 10.3 ± 2.0 | 24.2 ± 2.4** | 7.22 ± 0.9 | 10.1 ± 1.5 | 25.4 ± 4.4** |
Data are expressed as means ± SE. VO, vaginal opening; Ovx, ovariectomized; E2, estradiol. There was a significant treatment effect on E2 levels (P < 0.0001, 2-way ANOVA);
P < 0.0001, Ovx + E2 compared with intact and to Ovx + V (Holm-Sidak multiple-comparison test).
Lower Serum LH Levels in Kisspeptin-p110α/β-KO Males
In males, LH levels varied with treatment and genotype (P = 0.048 and P <0.001, respectively), with no interaction between treatment and genotype (P > 0.05; Fig. 4A). LH levels were significantly lower in kiss-p110α/β-KO than in WT males. Gdx increased and T replacement reduced serum LH levels in animals of both genotypes. No genotype effect was observed on serum T levels in intact animals (Table 1). T capsules were effective in producing physiological T levels (P < 0.05; Table 1).
Fig. 4.
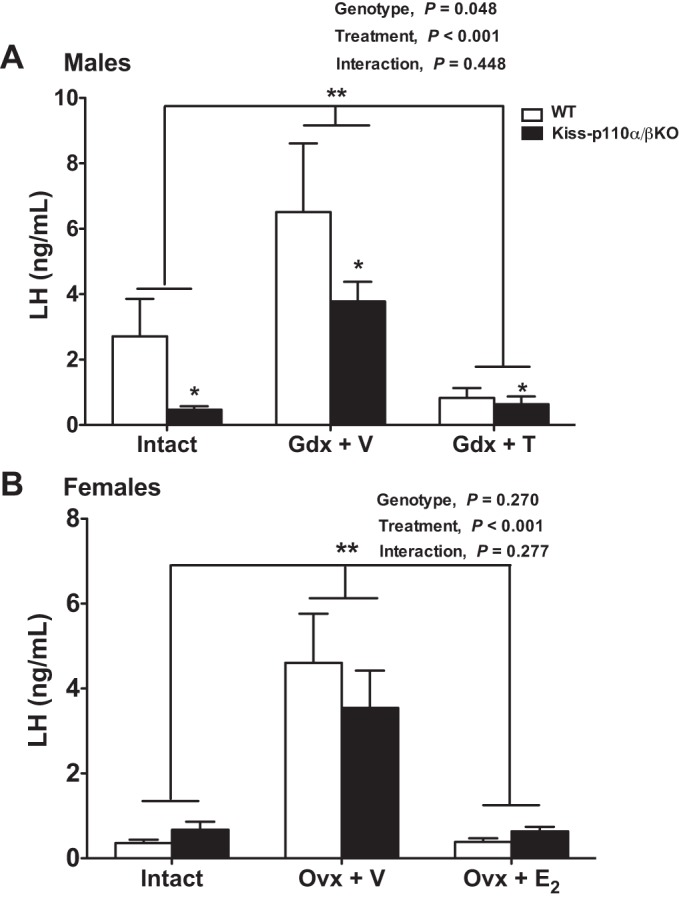
Reduced LH in kiss-p110α/β-KO males but normal negative steroid hormone feedback in kiss-p110α/β-KO males and females. Serum LH levels from WT and kiss-p110α/β-KO mice that were intact, gonadectomized (Gdx), and treated with vehicle [Gdx + V for males, ovariectomized (Ovx) + V for females] or Gdx and treated with steroid hormone [Gdx + testosterone (T) for males, Ovx + estradiol (E2) for females] for 10 days (n = 6–10 mice/treatment/genotype). A: a significant genotype and treatment effect on LH levels was observed in males (2-way ANOVA). B: a significant treatment effect but no genotype effect on LH levels was observed in females (2-way ANOVA). No genotype effect on LH levels was observed in females from each treatment group. Values are means ± SE. *P <0.05 vs. WT; **P <0.001 vs. Gdx + V.
To determine whether differences in serum LH levels between WT and kiss-p110α/β-KO males were due to changes in pituitary LH content, we analyzed pituitary LH levels. There was no effect of genotype on pituitary LH content (P > 0.05; Table 1). There was a significant treatment effect on pituitary LH (P = 0.02), with pituitary LH levels in intact animals being significantly higher than those in the Gdx + V males (Table 1).
Kisspeptin-p110α/β-KO Female Mice Have Normal E2 Negative Feedback
Basal LH levels from intact animals on the morning of diestrus did not differ significantly between kiss-p110α/β-KO and WT females (Fig. 4B). Ovx increased LH levels in both kiss-p110α/β-KO and WT littermates, and E2 suppressed LH levels in both groups (P <0.001). Serum E2 levels were significantly higher in mice receiving E2 (P < 0.01; Table 2). Serum E2 levels were not significantly different between WT and Kiss-p110α/β-KO mice at diestrus (P > 0.05; Table 2).
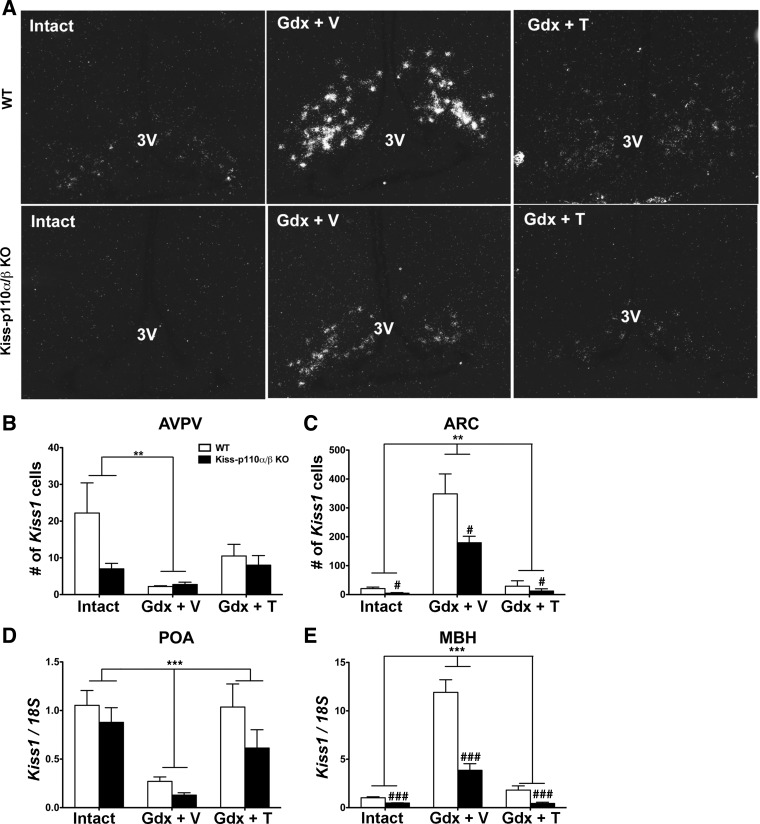
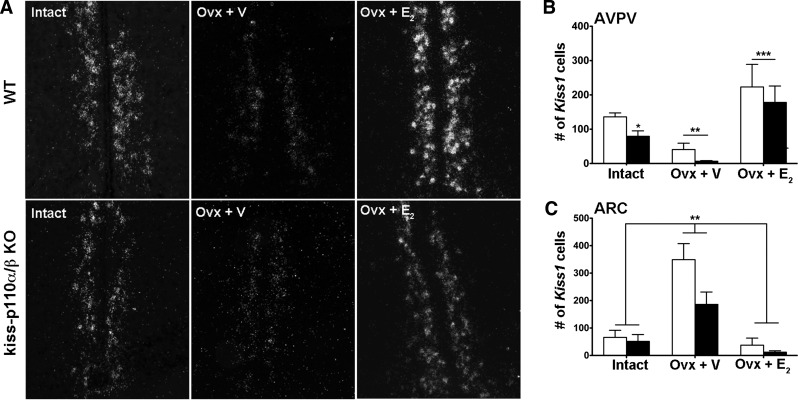
Deletion of PI3K Catalytic Subunits in Kisspeptin Cells Reduces Kiss1 Cell Number in the ARC of Adult Males and the AVPV of Intact Females
In the AVPV, the number of Kiss1 cells was not significantly different between WT and kiss-p110α/β-KO males (Fig. 5B). A significant treatment effect was observed, with the number of Kiss1 cells in Gdx + V-treated mice lower than that in intact animals (P < 0.05). No significant treatment effect on Kiss1 mRNA expression (grains/cell) was observed (P = 0.06; Table 3).
Fig. 5.
Deletion of phosphoinositide 3-kinase (PI3K) catalytic subunits in kisspeptin cells reduces Kiss1 cell number and Kiss1 mRNA levels in the arcuate nucleus (ARC) and MBH, respectively, of adult males. A: representative dark-field images showing Kiss1 mRNA-expressing cells in the ARC of WT and kiss-p110α/β KO males that were gonad intact, Gdx + V, and Gdx + T. B and C: no. of cells expressing Kiss1 mRNA in the anteroventral periventricular nucleus (AVPV; B) and the ARC (C) of WT and kiss-p110α/β-KO males that were gonad intact, Gdx + V, and Gdx + T. D and E: Kiss-1 mRNA levels in the preoptic area (POA; D) and the MBH (E) of intact, Gdx + V-treated, and Gdx + T-treated WT and kiss-p110α/β KO males. Data are expressed as means ± SEM. ISH data: #P < 0.05 vs. WT; **P < 0.001 vs. Gdx + V, 2-way ANOVA, n = 4–6/genotype/treatment. Real-time PCR data: ###P < 0.0001, genotype; ***P < 0.0001, treatment, 2-way ANOVA, n = 6/genotype/treatment. 3V, 3rd ventricle.
Table 3.
Kiss-1 mRNA expression (grains/cell) in the ARC and the AVPV from intact, Gdx, and Gdx + T-treated WT and kiss-p110α/β-KO male mice
| WT |
Kiss-p110α/β-KO |
|||||
|---|---|---|---|---|---|---|
| Males | Intact (n = 5–6) | Gdx + V (n = 5) | Gdx + T (n = 4) | Intact (n = 5–6) | Gdx + V (n = 4–5) | Gdx + T (n = 5) |
| Kiss-1 mRNA, grains/cell | ||||||
| ARC | 61.9 ± 2.2 | 81.0 ± 3.0** | 62.8 ± 5.3 | 44.0 ± 9.0 | 75.3 ± 1.5** | 62.6 ± 5.1 |
| AVPV | 63.4 ± 3.8 | 43.1 ± 4.5 | 52.9 ± 8.9 | 50.7 ± 7.4 | 41.5 ± 5.0 | 48.0 ± 5.1 |
Data are expressed as means ± SE for each treatment and genotype. ARC, arcuate nucleus; AVPV, anteroventral periventricular nucleus.
P < 0.001, 2-way ANOVA: Gdx + V treatment compared to intact and Gdx + steroid hormone-treated group.
Genotype significantly affected the number of Kiss1 mRNA-expressing cells in the ARC, with Kiss1 cell numbers in the ARC of kiss-p110α/β-KO males significantly lower than in WT males (P < 0.05; Fig. 5, A and C). In addition, there was a significant treatment effect on ARC Kiss1 cell number. Gdx and T treatment elevated and reduced Kiss1 expression, respectively (P < 0.001). In terms of cellular Kiss1 mRNA expression (grains/cell), a significant treatment effect was observed (P < 0.001) without a significant effect of genotype or a significant interaction between genotype and treatment (P > 0.05; Table 3).
Because we were concerned about low abundance of detectable Kiss1 mRNA-expressing cells in the AVPV of males, we quantified Kiss1 mRNA in the POA and the MBH by real-time RT-PCR (Fig. 5, D and E). There was a significant treatment effect (P < 0.001), but the effect of genotype missed statistical significance (P = 0.06; Fig. 5D). As expected, Kiss1 mRNA expression in the POA of Gdx + V males was significantly lower than in intact and Gdx + T-treated animals. In the MBH, the RT-PCR results using this approach mirrored what we found using in situ hybridization (Fig. 5E), with a significant genotype and treatment effect as well as a significant interaction (genotype: P < 0.0001; treatment: P < 0.0001; interaction: P < 0.0001).
The number of Kiss1 cells in the AVPV of intact female mice was significantly lower in kiss-p110α/β-KO than in WT females (t-test, P < 0.05; Fig. 6, A and B). Ovx + E2 treatment increased Kiss1 cell numbers above that of Ovx + V treatment in both genotypes (P <0.001; Fig. 6, A and B). Similarly, a significant treatment effect was found on AVPV grains/cell (P < 0.001; Table 4).
Fig. 6.
Deletion of PI3K catalytic subunits in kisspeptin significantly reduced Kiss1 cell number in the AVPV of intact females. A: representative photomicrographs showing Kiss1 mRNA-expressing cells in the AVPV of intact, Ovx + V-treated, and Ovx + E2-treated WT and kiss-p110α/β-KO females. B and C: no. of cells expressing Kiss1 mRNA in the AVPV (B) and the ARC (C) of intact, Ovx + V-treated, and Ovx + E2-treated WT and kiss-p110α/β-KO females. Values are means ± SE; n = 4–6/genotype/treatment. *P < 0.05 vs. WT, unpaired Student's t-test; **P < 0.001 vs. Ovx + V; ***P < 0.001 vs. intact and Ovx + V, 2-way ANOVA.
Table 4.
Kiss-1 mRNA expression (grains/cell) in the ARC and the AVPV from intact, Ovx, and Ovx + E2-treated WT and kiss-p110α/β-KO female mice
| WT |
Kiss-p110α/β-KO |
|||||
|---|---|---|---|---|---|---|
| Females | Intact (n = 6) | Ovx + V (n = 6) | Ovx + E2 (n = 6) | Intact (n = 6) | Ovx + V (n = 6) | Ovx + E2 (n = 6) |
| Kiss-1 mRNA, grains/cell | ||||||
| ARC | 65.0 ± 2.1 | 96.2 ± 3.1** | 63.3 ± 2.1 | 61.8 ± 3.3 | 85.1 ± 8.8** | 58.2 ± 5.1 |
| AVPV | 88.4 ± 3.0 | 62.1 ± 2.9** | 75.5 ± 4.1 | 74.1 ± 1.7 | 51.4 ± 11.1** | 75.9 ± 5.6 |
Data are expressed as the means ± SE for each treatment and genotype.
P < 0.001, 2-way ANOVA: Ovx + V treatment compared with intact and Ovx + steroid hormone-treated group.
In the ARC, no significant effect of genotype or interaction between genotype and treatment was found on Kiss1 cell number (P > 0.05; Fig. 6C). However, a significant treatment effect was observed (P < 0.001). Compared with Ovx + E2 and intact females, Ovx + V females had significantly higher Kiss1 cell numbers in both genotypes (P < 0.001). Similarly, the number of grains/cell was significantly higher in Ovx + V-treated animals than intact and Ovx + E2, without a significant genotype effect (P < 0.001; Table 4).
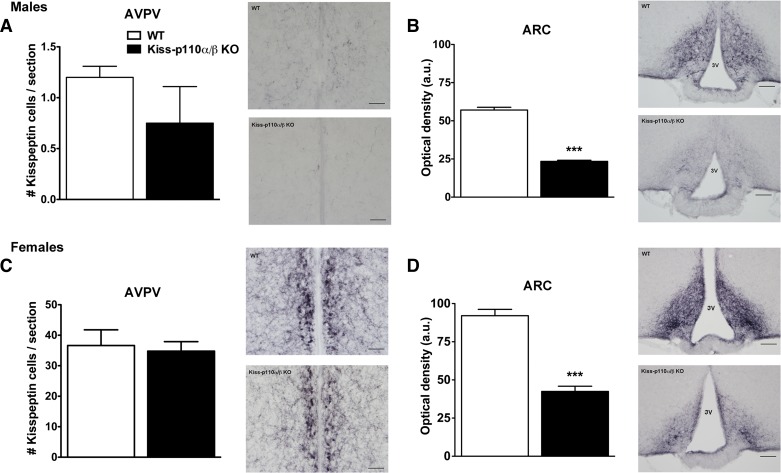
Kiss-p110α/β-KO Males and Females Show Decreased Kisspeptin-IR Fibers in the ARC
To investigate whether changes in Kiss1 mRNA expression observed in kiss-p110α/β-KO mice were accompanied by changes in the number of neurons expressing detectable kisspeptin IR, we performed kisspeptin immunohistochemistry in adult tissue. There was a slight decrease in the number of kisspeptin-IR cells in the AVPV of kiss-p110α/β-KO males, but this did not reach statistical significance (Fig. 7A). Similarly, cell counts in the AVPV from intact WT and Kiss-p110α/β-KO females did not reveal a significant effect of genotype (Fig. 7C). Compared with WT littermates, a significant reduction in kisspeptin-IR fiber density was observed in the ARC of intact adult kiss-p110α/β-KO males and females (***P < 0.0001; Fig. 7, B and D). We also investigated whether the changes in Kiss1 mRNA and kisspeptin-IR fibers were associated with altered GnRH cell numbers or distribution. Immunohistochemical analysis of GnRH-immunoreactive cells in WT and kiss-p110α/β males and females did not reveal any genotype effect on cell number or GnRH neuron distribution (data not shown).
Fig. 7.
The kisspeptin cell-specific ablation of PI3K catalytic subunits resulted in a significant reduction of kisspeptin fiber immunostaining in the ARC of adult male and female mice. A: quantification of kisspeptin-immunoreactivity (IR) cell number in the AVPV of adult males (n = 6). B: optical density measurements of kisspeptin-ir in the ARC of WT and kiss-p110α/β-KO males (n = 3). C: quantification of kisspeptin-IR cell number in the AVPV of adult females (kiss-p110α/β, n = 4; WT, n = 5). D: optical density measurements of kisspeptin-IR in the ARC of WT and kiss-p110α/β-KO females (n = 3). Adjacent to each graph are representative images showing kisspeptin-IR in the AVPV (A and C) and the ARC (B and D) of each genotype. Bar, 100 μm. ***P < 0.0001, Student's t-test.
DISCUSSION
By examining the reproductive phenotypes of male and female mice with targeted disruption of PI3K catalytic subunits in kisspeptin cells, we uncovered a new molecular mechanism involved in the regulation of hypothalamic kisspeptin expression, gonadotropin release in males, and fertility in females. The absence of p110α and p110β in kisspeptin cells decreased expression of Kiss1 mRNA in the AVPV of intact females and in the ARC of males and decreased kisspeptin-IR fibers in the ARC of males and females. Kiss-p110α/β-KO males had lower basal LH levels despite normal pituitary LH content but normal pubertal onset and fertility. Kiss-p110α/β-KO females showed a marked reduction in fertility despite normal puberty onset, estrous cycles, and ovarian histology. Importantly, the effects of the kisspeptin cell-specific deletion of p110α and p110β on hypothalamic kisspeptin expression and reproduction were not accompanied by metabolic abnormalities. Therefore, our results suggest that central PI3K signaling is important for the expression and function of a subpopulation of hypothalamic kisspeptin cells and that kisspeptin cell-specific PI3K signaling contributes to fertility in females.
Upstream Regulators of PI3K Signaling in Kisspeptin Neurons
PI3K is common to the signaling pathways of a number of peripheral metabolic cues, including insulin and leptin, which regulate both metabolism and reproduction (1). Other signals known to regulate PI3K include growth factors such as IGF-I and steroid hormones (43, 44, 79). However, identifying which upstream signal(s) is responsible for the effects of PI3K signaling in kisspeptin neurons is a very difficult task. Studies using genetically altered mouse strains have begun to elucidate the role of some of these signals in the regulation of kisspeptin neurons. Direct comparisons between ours and other models of cre-mediated deletion of genes in kisspeptin cells are problematic due to strain differences, but some generalizations can be made. For example, the phenotype of kiss-p110α/β-KO mice is very different from that of mice in which insulin or leptin receptors were deleted in kisspeptin cells. Male and females with kisspeptin cell-specific deletion of leptin receptors showed normal sexual maturation, fertility, and fecundity, suggesting that leptin action in Kiss1 neurons is not necessary for puberty and reproduction (25). The same group also demonstrated that leptin signaling in kisspeptin neurons arises only after sexual maturation (18). Cre-mediated deletion of the insulin receptor (IR) in kisspeptin cells delayed puberty in males and females, but AVPV kisspeptin-IR cells were reduced only in juvenile kiss1-Cre IRlox/lox females (67). In contrast, using a different kiss1-Cre model to delete insulin receptors in kisspeptin cells, Evans et al. (29) reported no reproductive phenotype in male or female mice with a kisspeptin cell-specific deletion of the insulin receptor. Hence, it is likely that not one but many factors converge on PI3K signaling to regulate kisspeptin expression and function (1).
Organizational vs. Activational Effects of PI3K Signaling in Kisspeptin Neurons
The PI3K signaling pathway is a pro-survival signaling system in neurons and controls a number of cellular processes in neuronal development. Examples include but are not limited to cellular proliferation, neuronal migration, and synapse formation (46, 88). Moreover, a broad range of input signals that play important functions in neural development can activate PI3K signaling; these include growth factors, insulin, cytokines, and G protein-coupled receptors (46, 88). Therefore, loss of PI3K might impair specification of kisspeptin neurons. Alternatively, Kiss1 neurons in the hypothalamus of kiss-p110α/β-KO mice might survive but lose their ability to express Kiss1 transcript at some point during development. In this case, transcriptional repression may explain the low kisspeptin expression observed in the hypothalamus of adult kiss-p110α/β-KO mice. A number of in vivo studies have identified peripheral cues contributing to early signaling processes controlling GnRH secretion and the timing of puberty in mammals. Examples include IGF-I, leptin, and insulin, all of which are known regulators of Kiss1 gene expression and are upstream regulators of PI3K signaling (1). For example, IGF-I stimulation of hypothalamic Kiss1 gene expression in female rats is mediated by Akt (43, 44). To differentiate between these two possibilities, lineage-tracing studies using kiss-CRE+/YFP+ and kiss-p110α/β KO-YFP mice combined with developmental gene expression analysis are being pursued in our laboratory.
Candidate Downstream PI3K Signaling Targets in Kisspeptin Neurons
Activation of PI3K affects cellular functions through specific signaling pathways, such as the serine/threonine protein kinase Akt. Intracellular proteins that are targets of the Akt pathway include glycogen synthase kinase-3β, the mammalian target of rapamycin, and members of the forkhead family of transcription factors such as FoxO1 (1, 11). Depending on the cell system, FoxO1 can augment or repress transcriptional activity (84). Because FoxO1 phosphorylation and exclusion from the nucleus are PI3K/Akt dependent, kisspeptin neurons lacking p110α and p110β are expected to have increased FoxO1 activity. Indeed, deletion of p110α in SF-1 neurons in the ventromedial nucleus of the hypothalamus (89) or in proopiomelanocortin neurons (74) results in increased FoxO1 localization in the nucleus. Furthermore, our own published studies have shown that FoxO1GFP nuclear exclusion in GALP neurons is abolished in GALP-p110α/β-KO mice (3). Therefore, activation of PI3K signaling in kisspeptin neurons might result in FoxO1 retention in the cytoplasm (i.e., nuclear exclusion) and disinhibition of target gene expression (i.e., Kiss1). A similar mechanism has been shown to take place in mice lacking Irs2 in LepR-b expressing neurons. In this model, concomitant deletion of FoxO1 from LepR-b-expressing neurons normalizes energy balance, glucose homeostasis, and ARC gene expression (70).
Kisspeptin Cell-Specific PI3K Signaling and Fertility
Kiss-p110α/β-KO females showed a significant reduction in mating success rate. However, when housed with a proven fertile WT male they displayed copulatory plugs, indicating that mating was not affected. In addition, estrous cyclicity and number of CL were not significantly affected in kiss-p110α/β-KO females. The young age at which we examined our animals (2.5–3 mo) might have contributed in failing to observe genotype effects on CL numbers. However, the apparent lack of effects on CL number is not unheard of in mouse models of infertility. For example, mice with mutations in leptin (Lepob/ob) or leptin receptors (Leprdb/db) are infertile (6, 47). CL are present in these models, and the ovaries of leptin signaling-deficient mice are functional (48). Impairment of afferent pathways controlling GnRH neurosecretory activity is thought to be the major mechanism underlying infertility in these animals (73).
The subfertility observed in kiss-p110α/β-KO females was accompanied by a significant reduction in AVPV Kiss1 mRNA expression and in ARC kisspeptin-IR. AVPV kisspeptin neurons play an important role in E2-positive feedback effects on GnRH neurons (17, 27). The majority of AVPV kisspeptin neurons express ERα, which mediates E2-positive feedback (17, 34, 58). In many cell systems, including hypothalamic neurons, E2 activates PI3K through interactions between ERα and p85 regulatory subunits of PI3K, which integrates membrane and nuclear ERα signaling (65, 76). Activation of PI3K can also phosphorylate ERα on specific residues to regulate its transcriptional activity and stability (10). Both ERα classical and nonclassical estrogen response element-independent mechanisms participate in the regulation of steroid feedback and hypothalamic Kiss1 expression (37, 40). PI3K may be part of the intracellular signaling mechanisms mediating the activation of kisspeptin neurons during the E2-induced LH surge. If so, ablation of p110α and p110β in AVPV kisspeptin neurons could interfere with the ability of E2 through ERα to activate these neurons during the afternoon LH surge, reducing GnRH activation and decreasing fertility. Alternatively, kisspeptin is also expressed in the ovary, oviduct, and uterus (9, 26, 35). Recent studies suggest that kisspeptin-Kiss1R signaling may regulate female fertility independent of its ability to regulate GnRH release. For example, Calder et al. (9) showed that gonadotropin and E2 replacement restores ovulation, mating, and fertilization in Kiss1−/− mice. However, infertility was still present due to defects in implantation. PI3K signaling also participates in oocyte maturation, preimplantation embryo development, embryo survival, and pregnancy outcome (54, 90). Recent studies with PI3K inhibitors have linked the PI3K/Akt signaling pathway to the processes that affect embryo implantation in rodents (90). Hence, deletion of PI3K catalytic subunits in uterine kisspeptin cells may impair implantation, thereby reducing fertility in kiss-p110α/β-KO females. Future studies will determine whether the subfertility observed is of hypothalamic or peripheral origin.
Possible Sex-Specific Role of PI3K Signaling in Kisspeptin Cells
The male-specific decrease in ARC kisspeptin expression and concomitant reduction in serum LH levels observed in kiss-p110α/β-KO did not affect male fertility. Thus kisspeptin neurons may have different roles in regulating fertility in the two sexes, and the mechanisms by which PI3K regulates kisspeptin function may also be sex specific. The sexual dimorphism in AVPV kisspeptin expression and the differential regulation of AVPV vs. ARC kisspeptin neurons by E2 are major mechanisms underlying the ability of females, but not males, to experience positive feedback (5, 7, 38). The development of AVPV kisspeptin neurons is highly dependent on gonadal hormones, but sex steroid-independent mechanisms participate in the regulation of ARC kisspeptin neurons (5, 7, 15, 36). Indeed, some studies suggest that steroid hormone-independent mechanisms may restrain ARC kisspeptin neuronal activation before puberty in males (51). In contrast, ERα signaling in ARC kisspeptin neurons of female mice is important for restraining GnRH activation before puberty onset (51). Kisspeptin cell-specific deletion of ERα impairs pubertal development in female mice, whereas males are mostly unaffected (58). Recent studies also demonstrate sex differences in ARC kisspeptin neurons. A larger proportion of ARC kisspeptin neurons is spontaneously active in males compared with females (21), and a higher density of ARC kisspeptin-IR is observed in diestrous females compared with males (64). Perhaps the apparent differential relevance of steroid hormone (AVPV) vs. nonsteroidal signals (ARC) controlling kisspeptin neurons of males and females explains the sex-specific effects observed after ablation of PI3K catalytic subunits in kisspeptin cells. Our results are also similar to those described in the GNR23−/− mice lacking a significant percentage of GnRH neurons in the brain (only 12%). In this model, females are markedly subfertile, whereas males are not (42). Pulsatile LH secretion is possible in males with only 70 GnRH neurons; however, it is thought that more GnRH neurons are needed for the complex control of the estrous cycle in females. Similarly, it has been speculated that a significant “boost” of kisspeptin is needed to activate the number of GnRH neurons necessary for the LH surge in female mice (66). As discussed previously, the kisspeptin system regulates female reproduction at various extrahypothalamic sites. However, only a modulatory role of kisspeptin on the fertilization capacity of mouse spermatozoa has been demonstrated (45). Therefore, the lack of an infertility phenotype in kiss-p110α/β-KO males might reflect the seemingly more complex and widespread involvement of kisspeptin signaling in other reproductive organs of the female. An additional possibility might involve sex-specific compensatory expression of stimulatory neuropeptides such as neurokinin B or the reduction in inhibitory neuropeptides such as dynorphin (61).
Despite detecting significant changes in hypothalamic kisspeptin of adults, we did not detect significant genotype effects on puberty in either sex or on fertility in males. Kisspeptin is the most potent LH secretagogue known, and minute quantities of kisspeptin may be sufficient to support reproduction. Adult males with a 95% reduction of kisspeptin transcript maintain virtually all their reproductive capacity (66). These findings are similar to ours in which kiss-p110α/β-KO males have normal puberty and fertility despite significant reduction of hypothalamic kisspeptin expression. In contrast, females with a 95% reduction of kisspeptin had reduced fertility and ovulation but normal cyclicity, serum LH levels at diestrus, and pubertal onset (73). Similarly, kiss-p110α/β-KO females showed a significant reduction in fertility but normal LH levels at diestrus, cyclicity, and puberty onset. Therefore, one might conclude that an “oversupply” of kisspeptin ensures reproductive success, particularly in males. Nonetheless, genetic ablation of 97% of kisspeptin neurons did not affect the timing of puberty or fertility in female mice (59). In contrast, conditional ablation of kisspeptin neurons in adult female mice produced abnormal cyclicity and infertility (59). It is possible that in these models and in kiss-p110α/β-KO mice, compensation for the chronic loss of kisspeptin occurs. A high degree of phenotypic variability is observed in kiss1−/− females, with a subgroup showing an increased LH responsiveness to administration of kisspeptin (53). Kiss1−/− mice also exhibit increased sensitivity to N-methyl-d-aspartate (20). Hence, the long-term absence of kisspeptin expression increases GnRH sensitivity to kisspeptin and to other nonkisspeptin stimulatory inputs. Studies to assess when the effects of kiss-p110α/β deletion on kisspeptin expression begin to manifest during development will help determine whether such compensatory mechanisms are possible.
Ever since the seminal findings by Seminara et al. (72) and de Roux et al. (22) describing hypogonadotropic hypogonadism in patients with inactivating mutations of Kiss1R, studies across many species, including humans, have supported the participation of kisspeptin-expressing neurons in the programming of puberty in mammals and in the feedback actions of E2 on GnRH/LH release (33). Clinical trials are underway to characterize kisspeptin analogs (agonists and antagonists) to treat pubertal and infertility disorders (23, 53, 57). However, without a clear understanding of the molecular mechanisms controlling kisspeptin expression and function during physiological and pathophysiological states, we may be left with unsuccessful trial outcomes, losing valuable time and resources. PI3K gene expression and function are disrupted in disorders that impair pubertal development and fertility, such as obesity and inflammation (1, 85). Indeed, a number of therapeutic strategies targeting PI3K isoforms are being developed for the treatment of cancer, cardiovascular, and metabolic diseases (28, 31, 87). Our model offers the opportunity to uncover additional roles of kisspeptin cell-specific PI3K signaling in the regulation of the HPG axis.
GRANTS
This work was supported by Eunice Kennedy Shriver National Institute of Child and Human Development Grant 5R00HD055446-04 and the Office of the Vice President for Research at Stony Brook.
DISCLOSURES
The authors have nothing to disclose
AUTHOR CONTRIBUTIONS
M.B., A.L.N., G.Y., C.M., R.Z.L., and U.B. performed experiments; M.B. and S.W. analyzed data; M.B. drafted manuscript; M.B., A.L.N., R.Z.L., U.B., and M.A.-M. edited and revised manuscript; M.B., A.L.N., G.Y., S.W., C.M., R.Z.L., U.B., and M.A.-M. approved final version of manuscript; M.A.-M. conception and design of research; M.A.-M. interpreted results of experiments; M.A.-M. prepared figures.
ACKNOWLEDGMENTS
We thank Drs. Todd Miller and Tom White for sharing resources, Dr. Andrew Wolfe for technical assistance, and Dr. Anne Etgen for comments on the manuscript.
REFERENCES
- 1.Acosta-Martinez M. PI3K: An Attractive Candidate for the Central Integration of Metabolism and Reproduction. Front Endocrinol (Lausanne) 2: 110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta-Martinez M, Luo J, Elias C, Wolfe A, Levine JE. Male-biased effects of gonadotropin-releasing hormone neuron-specific deletion of the phosphoinositide 3-kinase regulatory subunit p85alpha on the reproductive axis. Endocrinology 150: 4203–4212, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz R, Beymer M, Negrón AL, Newshan A, Yu G, Rosati B, McKinnon D, Fukuda M, Lin RZ, Mayer C, Boehm U, Acosta-Martínez M. Galanin-like peptide (GALP) neurone-specific phosphoinositide 3-kinase signalling regulates GALP mRNA levels in the hypothalamus of males and luteinising hormone levels in both sexes. J Neuroendocrinol 26: 426–438, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker H, Joh TH, Ruggiero DA, Reis DJ. Variations in number of dopamine neurons and tyrosine hydroxylase activity in hypothalamus of two mouse strains. J Neurosci 3: 832–843, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker J, Pierman S, Gonzalez-Martinez D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav 57: 390–395, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Boquist L, Hellman B, Lernmark A, Taljedal IB. Influence of the mutation “diabetes” on insulin release and islet morphology in mice of different genetic backgrounds. J Cell Biol 62: 77–89, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology 154: 2739–2749, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab 12: 295–305, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calder M, Chan YM, Raj R, Pampillo M, Elbert A, Noonan M, Gillio-Meina C, Caligioni C, Berube NG, Bhattacharya M, Watson AJ, Seminara SB, Babwah AV. Implantation failure in female kiss1(−/−) mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology 155: 3065–3078, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 276: 9817–9824, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci 114: 1439–1445, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Caron E, Ciofi P, Prevot V, Bouret SG. Alteration in neonatal nutrition causes perturbations in hypothalamic neural circuits controlling reproductive function. J Neurosci 32: 11486–11494, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146: 3917–3925, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Castellano JM, Roa J, Luque RM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. KiSS-1/kisspeptins and the metabolic control of reproduction: physiologic roles and putative physiopathological implications. Peptides 30: 139–145, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150: 3214–3220, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol 21: 673–682, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28: 8691–8697, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cravo RM, Frazao R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, Vianna C, Elias CF. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One 8: e58698, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr., Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173: 37–56, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.d'Anglemont de Tassigny X, Ackroyd KJ, Chatzidaki EE, Colledge WH. Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J Neurosci 30: 8581–8590, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology 153: 5384–5393, 2012. [DOI] [PubMed] [Google Scholar]
- 22.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100: 10972–10976, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 92: 3958–3966, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Ruiz O, Zapata A, Sha L, Zhang Y, Tomac AC, Malik N, de la Cruz F, Backman CM. Selective deletion of PTEN in dopamine neurons leads to trophic effects and adaptation of striatal medium spiny projecting neurons. PloS One 4: e7027, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donato J, Jr., Cravo RM, Frazao R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr., Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121: 355–368, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorfman MD, Garcia-Rudaz C, Alderman Z, Kerr B, Lomniczi A, Dissen GA, Castellano JM, Garcia-Galiano D, Gaytan F, Xu B, Tena-Sempere M, Ojeda SR. Loss of ntrk2/kiss1r signaling in oocytes causes premature ovarian failure. Endocrinology 155: 3098–3111, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod 88: 146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenreich A, Rauch U. PI3K inhibitors in cardiovascular disease. Cardiovasc Ther 29: 29–36, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Evans MC, Rizwan M, Mayer C, Boehm U, Anderson GM. Evidence that insulin signalling in gonadotrophin-releasing hormone and kisspeptin neurones does not play an essential role in metabolic regulation of fertility in mice. J Neuroendocrinol 26: 468–479, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 139: 4652–4662, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Foster JG, Blunt MD, Carter E, Ward SG. Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacol Rev 64: 1027–1054, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Foukas LC, Withers DJ. Phosphoinositide signalling pathways in metabolic regulation. Curr Top Microbiol Immunol 346: 115–141, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Franceschini I, Desroziers E. Development and Aging of the Kisspeptin-GPR54 System in the Mammalian Brain: What are the Impacts on Female Reproductive Function? Front Endocrinol (Lausanne) 4: 22, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett 401: 225–230, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Gaytan F, Gaytan M, Castellano JM, Romero M, Roa J, Aparicio B, Garrido N, Sanchez-Criado JE, Millar RP, Pellicer A, Fraser HM, Tena-Sempere M. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab 296: E520–E531, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One 5: e11911, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104: 8173–8177, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Martinez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of gonadotropin-releasing hormone 1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory luteinizing hormone surges. Endocrinology 149: 2333–2340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145: 4073–4077, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29: 9390–9395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25: 11349–11356, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149: 597–604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiney JK, Srivastava VK, Les Dees W. Insulin-like growth factor-1 stimulation of hypothalamic KiSS-1 gene expression is mediated by Akt: effect of alcohol. Neuroscience 166: 625–632, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiney JK, Srivastava VK, Pine MD, Les Dees W. Insulin-like growth factor-I activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology 150: 376–384, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu MC, Wang JY, Lee YJ, Jong DS, Tsui KH, Chiu CH. Kisspeptin modulates fertilization capacity of mouse spermatozoa. Reproduction 147: 835–845, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Hu Y, Poopalasundaram S, Graham A, Bouloux PM. GnRH neuronal migration and olfactory bulb neurite outgrowth are dependent on FGF receptor 1 signaling, specifically via the PI3K p110alpha isoform in chick embryo. Endocrinology 154: 388–399, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science 153: 1127–1128, 1966. [DOI] [PubMed] [Google Scholar]
- 48.Israel DD, Sheffer-Babila S, de Luca C, Jo YH, Liu SM, Xia Q, Spergel DJ, Dun SL, Dun NJ, Chua SC., Jr Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology 153: 2408–2419, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 94: 4315–4323, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol 20: 1089–1097, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297: E1212–E1221, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152: 2020–2030, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148: 4927–4936, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Wang Y, Yu Q. The PI3K/Akt signaling pathway exerts effects on the implantation of mouse embryos by regulating the expression of RhoA. Int J Mol Med 33: 1089–1096, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Z, Jiang YP, Wang W, Xu XH, Mathias RT, Entcheva E, Ballou LM, Cohen IS, Lin RZ. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation 120: 318–325, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology 148: 4601–4611, 2007. [DOI] [PubMed] [Google Scholar]
- 57.MacLean DB, Matsui H, Suri A, Neuwirth R, Colombel M. Sustained exposure to the investigational Kisspeptin analog, TAK-448, down-regulates testosterone into the castration range in healthy males and in patients with prostate cancer: results from two phase 1 studies. J Clin Endocrinol Metab 99: E1445–E1453, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107: 22693–22698, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci 14: 704–710, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Moger WH. Effect of testosterone implants on serum gonadotropin concentrations in the male rat. Biol Reprod 14: 665–669, 1976. [DOI] [PubMed] [Google Scholar]
- 61.Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne) 3: 48, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson VL, Jiang YP, Dickman KG, Ballou LM, Lin RZ. Adipose tissue insulin resistance due to loss of PI3K p110α leads to decreased energy expenditure and obesity. Am J Physiol Endocrinol Metab 306: E1205–E1216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novaira HJ, Yates M, Diaczok D, Kim H, Wolfe A, Radovick S. The gonadotropin-releasing hormone cell-specific element is required for normal puberty and estrous cyclicity. J Neurosci 31: 3336–3343, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Overgaard A, Tena-Sempere M, Franceschini I, Desroziers E, Simonneaux V, Mikkelsen JD. Comparative analysis of kisspeptin-immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides 45: 85–90, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Pasapera Limón AM, Herrera-Muñoz J, Gutiérrez-Sagal R, Ulloa-Aguirre A. The phosphatidylinositol 3-kinase inhibitor LY294002 binds the estrogen receptor and inhibits 17beta-estradiol-induced transcriptional activity of an estrogen sensitive reporter gene. Mol Cell Endocrinol 200: 199–202, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Popa SM, Moriyama RM, Caligioni CS, Yang JJ, Cho CM, Concepcion TL, Oakley AE, Lee IH, Sanz E, Amieux PS, Caraty A, Palmiter RD, Navarro VM, Chan YM, Seminara SB, Clifton DK, Steiner RA. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology 154: 2784–2794, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Bruning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology 154: 1337–1348, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology 152: 1541–1550, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 150: 3664–3671, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadagurski M, Leshan RL, Patterson C, Rozzo A, Kuznetsova A, Skorupski J, Jones JC, Depinho RA, Myers MG, Jr., White MF. IRS2 signaling in LepR-b neurons suppresses FoxO1 to control energy balance independently of leptin action. Cell Metab 15: 703–712, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sánchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Garcia-Galiano D, Castaño JP, Luque RM, Romero-Ruiz A, Castellano JM, Diéguez C, Pinilla L, Tena-Sempere M. Obesity-induced hypogonadism in the male: premature reproductive neuroendocrine senescence and contribution of Kiss1-mediated mechanisms. Endocrinology 155: 1067–1079, 2014. [DOI] [PubMed] [Google Scholar]
- 72.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr., Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 349: 1614–1627, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Sheffer-Babila S, Sun Y, Israel DD, Liu SM, Neal-Perry G, Chua SC., Jr Agouti-related peptide plays a critical role in leptin's effects on female puberty and reproduction. Am J Physiol Endocrinol Metab 305: E1512–E1520, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi X, Zhou F, Li X, Chang B, Li D, Wang Y, Tong Q, Xu Y, Fukuda M, Zhao JJ, Li D, Burrin DG, Chan L, Guan X. Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab 18: 86–98, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 304: 345–347, 1983. [DOI] [PubMed] [Google Scholar]
- 76.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407: 538–541, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 18: 298–303, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Smith JT, Clarke IJ. Kisspeptin expression in the brain: catalyst for the initiation of puberty. Rev Endocr Metab Disord 8: 1–9, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146: 3686–3692, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Song WJ, Mondal P, Wolfe A, Alonso LC, Stamateris R, Ong BW, Lim OC, Yang KS, Radovick S, Novaira HJ, Farber EA, Farber CR, Turner SD, Hussain MA. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab 19: 667–681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spanel-Borowski K, Schäfer I, Zimmermann S, Engel W, Adham IM. Increase in final stages of follicular atresia and premature decay of corpora lutea in Insl3-deficient mice. Mol Reprod Dev 58: 281–286, 2001. [DOI] [PubMed] [Google Scholar]
- 82.Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose, and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab 3: 343–353, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Tata BK, Chung WC, Brooks LR, Kavanaugh SI, Tsai PS. Fibroblast growth factor signaling deficiencies impact female reproduction and kisspeptin neurons in mice. Biol Reprod 86: 119, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thackray VG. Fox tales: regulation of gonadotropin gene expression by forkhead transcription factors. Mol Cell Endocrinol 385: 62–70, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151: 4109–4115, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci 30: 194–204, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Vogt PK, Bader AG, Kang S. Phosphoinositide 3-kinase: from viral oncoprotein to drug target. Virology 344: 131–138, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Waite K, Eickholt BJ. The neurodevelopmental implications of PI3K signaling. Curr Top Microbiol Immunol 346: 245–265, 2010. [DOI] [PubMed] [Google Scholar]
- 89.Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, Lowell BB, Zigman JM, Zhao JJ, Elmquist JK. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab 12: 88–95, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeng X, Huang Z, Mao X, Wang J, Wu G, Qiao S. N-carbamylglutamate enhances pregnancy outcome in rats through activation of the PI3K/PKB/mTOR signaling pathway. PLoS One 7: e41192, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeng X, Mao X, Huang Z, Wang F, Wu G, Qiao S. Arginine enhances embryo implantation in rats through PI3K/PKB/mTOR/NO signaling pathway during early pregnancy. Reproduction 145: 1–7, 2013. [DOI] [PubMed] [Google Scholar]