Abstract
It is unclear whether physical activity changes following long-term overfeeding and in response to different dietary protein intakes. Twenty-five (16 males, 9 females) healthy adults (18–35 yr) with BMI ranging from 19 to 30 kg/m2 enrolled in this inpatient study. In a parallel group design, participants were fed 140% of energy needs, with 5, 15, or 25% of energy from protein, for 56 days. Participants wore an RT3 accelerometer for at least 59 days throughout baseline and during overfeeding and completed 24-h whole room metabolic chamber assessments at baseline and on days 1, 14, and 56 of overfeeding and on day 57, when the baseline energy intake was consumed, to measure percent of time active and spontaneous physical activity (SPA; kcal/day). Changes in activity were also assessed by doubly labeled water (DLW). From accelerometry, vector magnitude (VM), a weight-independent measure of activity, and activity energy expenditure (AEE) increased with weight gain during overfeeding. AEE remained increased after adjusting for changes in body composition. Activity-related energy expenditure (AREE) from DLW and percent activity and SPA in the metabolic chamber increased with overfeeding, but SPA was no longer significant after adjusting for change in body composition. Change in VM and AEE were positively correlated with weight gain; however, change in activity was not affected by protein intake. Overfeeding produces an increase in physical activity and in energy expended in physical activity after adjusting for changes in body composition, suggesting that increased activity in response to weight gain might be one mechanism to support adaptive thermogenesis.
Keywords: obesity, spontaneous physical activity, energy expenditure, doubly labeled water, metabolic chamber
energy imbalance occurs when energy intake does not equate to energy expenditure. Much is known about the effects of weight loss on energy expenditure, but little is known about the effects of overfeeding on activity-related energy expenditure (AREE). It is thought that when body weight increases, energy expenditure is increased as a potential mechanism to dissipate a portion of the excess energy consumed. This hypothesized increase in energy expenditure above what is expected on the basis of weight gain is described as adaptive thermogenesis (34). Several mechanisms, including increased spontaneous physical activity (SPA) and increased sympathetic outflow of the nervous system, are believed to support adaptive thermogenesis in response to overfeeding. In our recent overfeeding study, Bray et al. (5) observed adaptive thermogenesis in individuals consuming the high- and normal-protein diets (25 and 15% of energy from protein, respectively) but not individuals consuming a low-protein diet (5% energy from protein). This followup analysis explores the role of changes in physical activity and activity energy expenditure (AEE) as potential mechanisms for adaptive thermogenesis in response to overfeeding.
Animal and human studies suggest an inverse and dose dependent response between physical activity and body weight (10–14, 31). Findings with respect to overfeeding and physical activity however, are less clear with reports suggesting that physical activity is increased (14), decreased (19) or maintained (7, 24). These discrepant observations could be explained by differences in diet composition which is a plausible explanation since diets varying in protein and fat can have profound effects on many components of daily energy expenditure (5, 15, 18). Changes in physical activity during the overfeeding of different diets remains largely unknown and has not been tested in a randomized controlled trial.
The aim of this study was to examine, for what is thought to be the first time, the daily changes in activity during overfeeding with diets that differed in dietary protein as an attempt to understand the potential role of physical activity on adaptive thermogenesis in response to overfeeding in healthy subjects. Since we previously observed differences in adaptive thermogenesis between the low-protein (5%) diet group and the normal- (15%) and high-protein (25%) groups (5), we hypothesized that changes in physical activity would also differ among the dietary protein treatment groups, with the low-protein diet having smaller increases in physical activity than the normal- and high-protein diet groups.
METHODS
This study was conducted according to the guidelines of the Declaration of Helsinki. All participants were given verbal and written explanations about the study and provided signed informed consent, and they received a monetary stipend. The study was approved by the Pennington Biomedical Research Center Institutional Review Board. The clinical trials registration number is NCT 00565149.
Experimental design.
Twenty-five weight-stable volunteers (males, n = 16; females, n = 9) with a BMI of 19–30 kg/m2 were recruited from the greater Baton Rouge, LA, area from advertisements. The study has been described previously (5). Briefly, this was a randomized, controlled, parallel arm trial that involved housing participants on an inpatient unit, with all meals provided by the metabolic kitchen during the baseline weight stabilization period (2–4 wk) and throughout 8 wk of overfeeding. Participants were enrolled from June 2005 through October 2007 on a rolling basis and not in cohorts. The study had three diet groups differentiated by protein composition: low-protein diet (5% of energy from protein), normal-protein diet (15% protein), and high-protein diet (25% of energy from protein). All diets provided ∼41% carbohydrates. Participants were overfed 40% above the individual baseline energy requirement (∼954 kcal/day).
Participants resided on the inpatient unit for the entire study (except for when certain procedures required temporary discharge or when they were given passes for a short duration, where the participant left the inpatient unit). Participants consumed all meals at the Center at designated times and while supervised by study dieticians. Lights out was designated as the time between 2300 and 0700 daily. Participants remained confined to the inpatient unit, and therefore, activity can be defined as extremely sedentary. No calisthenics or exercise were permitted during the study.
Vector magnitude and activity energy expenditure.
The RT3 accelerometer (Stayhealthy, Monrovia, CA) was worn at the waist during baseline (21, 26) and for the duration of the overfeeding study, except during certain procedures [dual-energy X-ray absorptiometry (DEXA), computed tomography, clamp etc.] and in infrequent cases when a participant was provided with a pass to leave the inpatient unit. Days on which the participant left the inpatient unit were excluded from the accelerometry data analysis. The device quantifies motion in three orthogonal planes [vertical (V), anterior-posterior (AP), and medial-lateral (ML)], which is called vector magnitude (VM) and is independent of body weight. Thus VM is a composite of activity counts from these three planes of motion. The equation is VM = square root (V2 + AP2 + ML2). All parameters were entered at baseline, and body weight was updated if there was a change of ≥0.5 kg compared with the previously used body weight. The RT3 calculates nonmoving energy expenditure from a proprietary equation on the basis of height, weight, age, sex, and VM.
Percent activity and SPA.
Participants also spent 24 h in a whole room metabolic chamber on five separate occasions. These were performed at baseline while on a weight maintenance diet, on days 1, 14, and 56 of overfeeding, and on the next day (day 57), when participants consumed their baseline energy intake. Volunteers entered the metabolic chamber prior to breakfast at ∼8 AM and stayed in the chamber until ∼7:30 AM the following morning. Radar motion detectors (model D9/50; Microwave Sensors, Ann Arbor, MI) continuously monitored the subject's movement. Oxygen and carbon dioxide levels in the chambers were measured using a Magnos 4G magneto-pneumatic oxygen analyzer (Magnos 4G; Hartmann and Braun, Frankfurt, Germany) and an infrared CO2 analyzer (Uras 3G; Hartmann and Braun), both of which sample O2 and CO2 concentrations 60 times/s. Calculations of O2 consumption and CO2 production were done from 10-s averaging and plots of the average values at 15-min intervals. Energy expenditure data are calculated from O2 consumption, CO2 production, and 24 h urinary nitrogen excretion (16).
The two measurements of physical activity were quantified from the metabolic chamber, i.e., percent activity or the percent of time that the participant was active throughout the 24-h period and SPA, defined as the energy expended in spontaneous activities (kcal/day). Both variables were computed using previously described methods (16, 22). Briefly, the motion detectors estimated the amount of activity. Two radar motion detectors (model D9/50; Microwave Sensors, Ann Arbor, MI) mounted in the chamber monitored SPA. Both units, placed at the two ends of the respiratory chamber, continuously emitted a signal (two separate wave lengths) that was reflected by the walls. When these signals hit a moving object or body, the frequency of the signal was changed (Doppler effect) and measured by a transceiver. The sensitivity of the system was set to detect any movement greater than chest movement in breathing. The measured SPA was irrespective of work intensity. The radar method, furthermore, did not interfere with the volunteer's behavior. It was also found to be more reliable and also better correlated to energy expenditure than the wrist motion sensor (29). The output of both radars was analyzed by our data acquisition system and averaged over 15-min periods.
A participant may be active for 15% of the time spent in the metabolic chamber. This would represent 1,440 min/day × 0.15 = 216 min of activity in that chamber stay. The amount of energy required from activity was calculated by plotting the energy expenditure data on the y-axis against the percent activity on the x-axis, and the slope is determined to calculate the energy requirements of activity of the 24-h chamber stay.
Activity-related energy expenditure.
Participants were dosed with doubly labeled water (DLW) at baseline and during week 7 for a 9-day DLW study. Total daily energy expenditure (TDEE) was calculated as described previously (6, 28). The TDEE results were described previously (5), and here we calculated AREE as TDEE − {[sleeping metabolic rate] + [thermic effect of food (TEF) from day 1 of metabolic chamber stay]}. These calculations are similar to what was described previously (23) but incorporate the thermic effect of food to better account for changes with overfeeding. The metabolic chamber TEF calculation is {[resting metabolic rate intercept] − [(sleeping metabolic rate/1,440)/EI] × (15 × 60) × 100}.
Body composition.
DEXA (Hologics QDR 4500A whole body scanner) was performed at baseline and at weeks 2, 4, 6, 7, and 8, as described previously (5). These data were used to adjust measurements of activity energy expenditure for change in body composition (fat and fat-free mass).
Peak oxygen consumption.
Aerobic fitness was tested during baseline by measuring peak oxygen consumption (V̇o2 peak) during treadmill exercise testing that had 1-min stages and an increase in speed and grade until exhaustion. No post-study test was performed. At least two out of three criteria needed to be met for the peak oxygen consumption test: 1) plateau in V̇o2, 2) respiratory exchange ratio >1.05, and 3) heart rate within ±10 beats/min of maximal heart rate.
Statistical analysis.
Data were distributed normally and thus did not require transformation or nonparametric statistical methods. Data were reported means ± SE. Subject characteristics used a one-way analysis of variance, and differences between groups were compared using the Tukey-Kramer method. Accelerometry data were averaged for each study week. A mixed-model analysis of variance with repeated measurements was used to test whether activity changed during overfeeding and whether changes in activity differed by diet, time, and diet by time interaction, with time being the repeated factor. For each variable, the baseline data were included as a covariate in the model. Sex, race, and age were tested as potential covariates. Terms with P > 0.25 were dropped for the final model unless they were treatment, time, and their interaction. Planned a priori comparisons were conducted to determine whether the change in activity differed between the dietary treatments and throughout overfeeding as well as a priori least squares means for change from baseline for dietary treatment and time.
The following procedures were used to determine whether activity changed during overfeeding after adjusting for change in body composition. Activity parameters that were expressed in kilocalories per 24 h (i.e., AEE, SPA, and AREE) were regressed against fat-free mass and fat mass from DEXA at baseline. These linear regression equations were then used to calculate expected values of AEE, SPA, and AREE at followup time points based on measured fat-free mass and fat mass at those time points. The observed values of AEE, SPA, and AREE were then subtracted from the expected values, and these values were analyzed to determine whether activity increased or decreased during overfeeding, independent of changes in body composition, and whether intake of protein affected change in activity.
Previously, energy efficiency has been implicated in body weight gain with overfeeding (2, 25); thus we tested whether V̇o2 was correlated with any physical activity parameter. The correlations between V̇o2 peak and change in physical activity measurements were established using a Pearson bivariate correlations procedure. Also, the correlations between baseline physical activity parameters and baseline body weight and change in physical activity parameters and change in body weight were established using a Pearson bivariate correlation procedure, and change in fat mass was controlled when a Pearson partial correlation was performed. Also, correlations between the change in physical activity parameters and the residual for TDEE using a Pearson bivariate correlation procedure were performed. Alpha was set at 0.05, and the Tukey-Kramer adjustment was used for pairwise comparisons. All analyses were conducted with the SAS version 9.2 software package (SAS Institute, Cary, NC) by biostatistician (HH).
RESULTS
Descriptive characteristics of the study sample.
Baseline characteristics by group are reported in Tables 1 and 2. As reported previously (5), individuals (16 males and 9 females) were 24 ± 1 yr of age, 74.4 ± 2.7 kg, and slightly overweight at 25.2 ± 0.6 kg/m2. Participants gained 5.3 ± 0.4 kg over 8 wk, with a 3.5 ± 0.2 kg increase in fat mass and a 1.8 ± 0.4 kg increase in fat-free mass. During the baseline period, physical activity level was 1.47 ± 0.06, which increased by 0.09 ± 0.04 during overfeeding.
Table 1.
Subject characteristics
| Protein Diet Group |
Change From Baseline |
|||||
|---|---|---|---|---|---|---|
| Low (5%) | Normal (15%) | High (25%) | Low (5%) | Normal (15%) | High (25%) | |
| Age, yr | 23 ± 1 | 23 ± 2 | 27 ± 1 | |||
| Sex (males/females) | 5/3 | 6/3 | 5/3 | |||
| Race | ||||||
| White | 4 | 0 | 3 | |||
| Black | 3 | 8 | 5 | |||
| Asian | 1 | 1 | 0 | |||
| BMI, kg/m2 | 24.4 ± 1.3 | 25.6 ± 1.1 | 25.7 ± 1.1 | |||
| Body weight, kg | 69.1 ± 4.4 | 77.7 ± 4.6 | 76.1 ± 5.8 | 3.2 ± 0.5a | 6.1 ± 0.8b | 6.5 ± 0.5b |
| Fat mass, kg | 16.6 ± 1.9 | 18.3 ± 2.6 | 19.6 ± 2.4 | 3.7 ± 0.3a | 3.6 ± 0.6a | 3.4 ± 0.3a |
| Lean body mass, kg | 53.2 ± 3.9 | 59.9 ± 4.4 | 57.1 ± 5.1 | −0.7 ± 0.3a | 2.9 ± 0.5b | 3.2 ± 0.3b |
| Physical activity level | 1.48 ± 0.12 | 1.48 ± 0.10 | 1.43 ± 0.10 | 0.02 ± 0.08a | 0.17 ± 0.09a | 0.08 ± 0.06a |
Data are means ± SE. Physical activity level is calculated as total energy expenditure divided by resting energy expenditure. Values with a different superscripted letter are different at P ≤ 0.05.
Table 2.
Baseline physical activity parameters
| Protein Diet Group | Low (5%) | Normal (15%) | High (25%) | Overall |
|---|---|---|---|---|
| Accelerometry | ||||
| VM (counts) | 83,963 ± 7,363 | 106,559 ± 11,669 | 115,193 ± 17,417 | 102,605 ± 7,973 |
| Activity, kcal/day | 218 ± 31 | 289 ± 34 | 327 ± 67 | 281 ± 29 |
| Chamber | ||||
| %Activity | 16.3 ± 1.8 | 16.5 ± 1.9 | 13.6 ± 1.0 | 15.4 ± 0.9% |
| SPA, kcal/day | 171 ± 65 | 203 ± 49 | 164 ± 36 | 180 ± 21 |
| DLW (AREE, kcal/day) | 548 ± 154 | 536 ± 166 | 387 ± 96 | 417 ± 81 |
Data are means ± SE.
VM, vector magnitude; SPA, spontaneous physical activity; DLW, doubly labeled water (9-day doubly labeled water period); AREE, activity-related energy expenditure.
Treatment by time and treatment effects.
Change in activity for each diet group is reported in Table 3. There were no significant dietary treatment by time interactions or dietary treatment differences for any of the variables examined (i.e., AEE, AREE, %time active in the metabolic chamber, and SPA are expressed as kcal/day). The figures present data by treatment and time, and the results below and also in the figures represent overall change in activity collapsed across the three protein diet groups (Figs. 1, A, C, and E, 2, A, C, and E, and 3, A and C).
Table 3.
Change from baseline activity for all 3 dietary protein treatments
| 5% | 15% | 25% | F Value | P Value | |
|---|---|---|---|---|---|
| Accelerometry | |||||
| VM (counts) | 4,358 ± 8,443 | 18,805 ± 8,109* | 17,692 ± 8,585* | 1.03 | 0.37 |
| 95% CI | −13,127, 21,843 | 2,005, 35,605 | −80, 35,463 | ||
| AEE, kcal/day | 39 ± 26 | 71 ± 25* | 61 ± 27* | 0.42 | 0.66 |
| 95% CI | −14, 93 | 18, 124 | 4, 117 | ||
| AEE, kcal/day† | 35 ± 41 | 67 ± 37 | −8 ± 38 | 1.05 | 0.37 |
| 95% CI | −51, 120 | −11, 144 | −87, 71 | ||
| Chamber | |||||
| Activity (%) | 0.3 ± 1.1 | 1.9 ± 1.2 | 1.6 ± 1.2 | 0.58 | 0.57 |
| 95% CI | −2.1, 2.7 | −0.6, 4.4 | −0.9, 4.1 | ||
| SPA, kcal/24 h | −1 ± 17 | 54 ± 18* | 11 ± 18 | 2.71 | 0.09 |
| 95% CI | −37, 36 | 17, 92 | −27, 49 | ||
| SPA, kcal/24 h† | 20 ± 24 | 17 ± 23 | −41 ± 25 | 2.0 | 0.16 |
| 95% CI | −29, 70 | −30, 64 | −92, 10 | ||
| DLW | |||||
| AREE, kcal/day | 180 ± 98 | 383 ± 98* | 228 ± 125 | 1.16 | 0.35 |
| 95% CI | −34, 394 | 170, 596 | −44, 500 | ||
| AREE, kcal/day† | 194 ± 143 | 371 ± 125* | 224 ± 146 | 0.48 | 0.63 |
| 95% CI | −124, 512 | 92, 650 | −101, 550 |
Data are means ± SE and 95% confidence intervals (CI). AEE, activity energy expenditure.
Adjusted for change in body composition (energy stores; fat and fat-free mass);
change values that are significantly different from 0 at P ≤ 0.05.
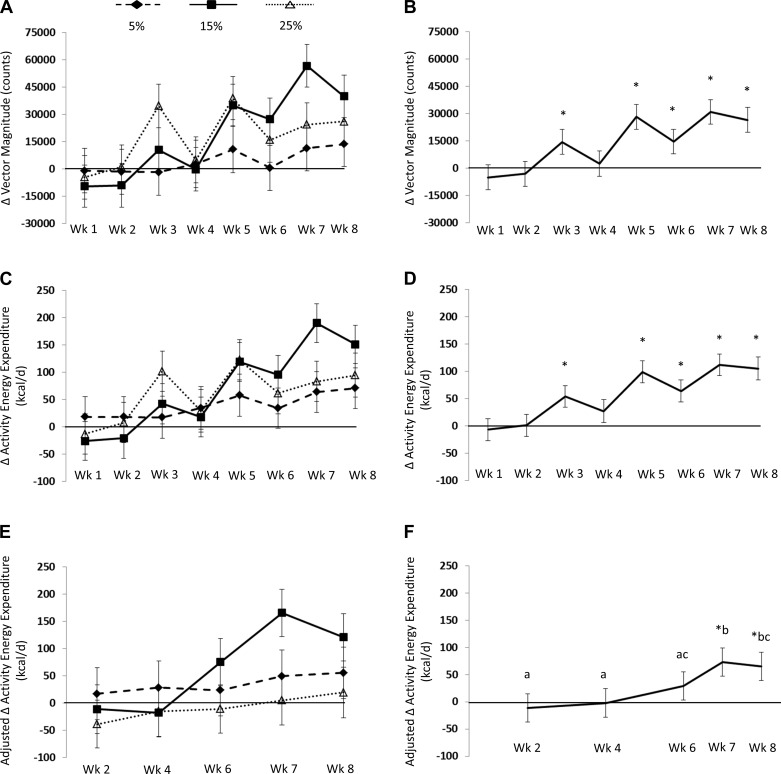
Fig. 1.
A: change in vector magnitude (VM), activity energy expenditure (AEE), and AEE adjusted for change in body composition measured by accelerometry. Values are means ± SE represented by vertical bars; VM counts based on treatment group from baseline. The low-protein diet (5%) VM did not increase from baseline. The normal-protein diet (15%) VM increased from baseline at weeks 5–8. The high-protein diet (25%) VM increased from baseline at weeks 3, 5, 7, and 8. B: collapsed across groups, VM counts increased from baseline. C: AEE based on treatment group from baseline. Low-protein diet (5%) AEE did not increase from baseline. Normal-protein diet (15%) AEE increased from baseline at weeks 5–8. High-protein diet (25%) AEE increased from baseline at weeks 5, 7, and 8. D: AEE, collapsed across groups, increased during overeating. E: after adjusting for change in body composition, AEE based on treatment group from baseline. Low-protein-adjusted AEE (5%) did not increase from baseline. Normal-protein-adjusted AEE (15%) increased at weeks 5–8. High-protein-adjusted (25%) AEE increased at weeks 3, 5, 7, and 8. F: AEE collapsed across groups increased from baseline during weeks 7 and 8, which were were higher than weeks 2, 4, and 6, after adjusting for change in body composition. Values with a different letter are different at P < 0.05. *Change values that are significantly different from 0 at P ≤ 0.05.
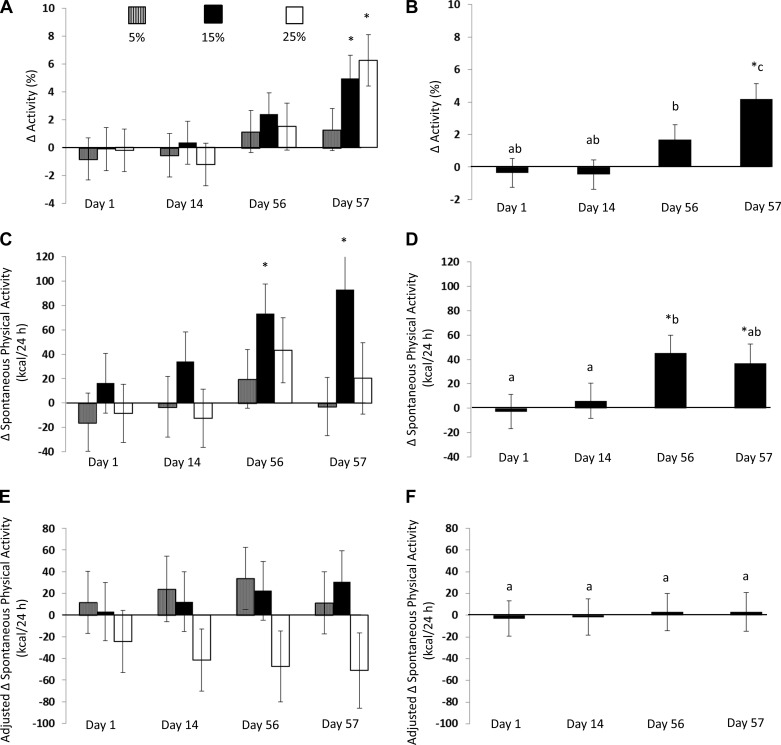
Fig. 2.
Change in %activity, spontaneous physical activity (SPA), and SPA adjusted for change in body composition measured in a metabolic chamber. Values are means ± SE represented by vertical bars. A: %activity based on treatment group from baseline. B: %activity increased from baseline following overeating while on the baseline weight maintenance diet (day 57). Also, %activity differed across time points. C: SPA based on treatment group from baseline. D: SPA increased from baseline on days 56 and 57, and day 56 was different from days 1 and 14 but not different from day 57. E: SPA based on treatment group from baseline after adjusting for change in body composition. F: after adjusting for change in body composition, SPA was not increased from baseline or different across time points. *Change values that are significantly different from 0 at P ≤ 0.05. Values with a different letter are different at P < 0.05.
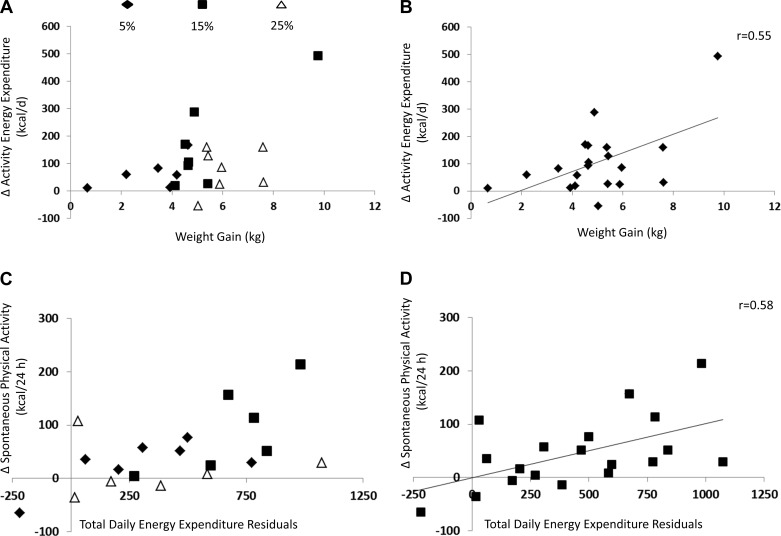
Fig. 3.
A: change in AEE correlated with body weight gain based on treatment group. B: change in AEE was positively correlated with weight gain (r = 0.55, P = 0.011). C: change in SPA correlated with change in total daily energy expenditure by treatment group. D: change in SPA was positively correlated with change in TDEE residuals (r = 0.58, P = 0.007).
AEE by accelerometry.
VM by accelerometry increased by a significant 30% during overfeeding (F = 6.72, P < 0.0001; Fig. 1B). AEE by accelerometry increased significantly during overfeeding both before (F = 6.84, P < 0.0001; Fig. 1D) and after (F = 3.48, P = 0.01; Fig. 1F) adjusting for change in body composition (fat and fat-free mass).
Percent activity and SPA in the metabolic chamber.
Overall, percent activity while confined to the metabolic chamber increased significantly during overfeeding (F = 6.14, P = 0.001; Fig. 2B). Percent activity did not change significantly between days 1 and 14. Percent activity was increased on day 57 compared with days 1 and 14. Percent activity increased from day 14 to day 56. The increase in percent activity on day 57 tended to be larger than day 56.
SPA increased significantly during overfeeding (F = 3.15, P = 0.03; Fig. 2D), although the change in SPA was no longer significant after changes in body composition (fat and fat-free mass) were considered (F = 0.10, P = 0.96; Fig. 2F).
AREE by DLW.
AREE by doubly labeled water increased by 206 ± 78 kcal/day during overfeeding (t = 2.66, P = 0.015) after adjusting for changes in body composition (fat and fat-free mass).
Body weight, physical activity, and V̇o2 peak.
Baseline body weight was positively correlated with baseline AEE and SPA (AEE: r = 0.68, P = 0.001; SPA: r = 0.74, P < 0.0001), but baseline body weight was not correlated with baseline VM or percent activity (data not shown).
At week 8, change in VM and change in AEE were positively correlated with weight gain (VM: r = 0.42, P = 0.06; AEE: r = 0.55, P = 0.011; Fig. 3B). When change in fat mass was controlled, change in VM and change in AEE remained positively correlated with weight gain (VM: r = 0.59, P = 0.016; AEE: r = 0.71, P = 0.002). However, changes in percent activity, SPA, and AREE were not correlated with weight change even when controlling for change in fat mass. Finally, residuals from TDEE were positively correlated with SPA (r = 0.58, P = 0.007; Fig. 3D). AEE was not significantly correlated with residuals from TDEE (P = 0.30).
V̇o2 peak at baseline did not significantly correlate with change in any measure of physical activity (data not shown).
DISCUSSION
This study indicates that during overfeeding in the confinement of an inpatient unit, activity increases, but the changes in activity are not influenced by different levels of dietary protein. It is notable there were differences in adaptive thermogenesis among the three diet groups, as reported in the primary analysis (5), but no differences in movement, physical activity, or energy expenditure were found between the diet groups within the present analyses. VM as well as AEE and AREE were significantly increased by overfeeding even after adjusting for the changes in body composition. Though slightly less evident, although activity was restricted to the metabolic chamber, percent activity and SPA were significantly increased with overfeeding. Weight gain was positively associated with change in activity measured daily with accelerometry (VM and AEE) with and without controlling for change in fat mass. These results support the hypothesis that weight gain is associated with an increase in activity, but in the present overfeeding study the increase in activity is not likely effective at attenuating weight gain. These data also suggest that changes in sleeping metabolic rate (SMR), resting metabolic rate (RMR), and/or TEF are presumably accounting for the treatment differences in total energy expenditure found in the main outcomes study by Bray et al. (5).
Our results are similar to Levine et al. (14), who found that SPA (or NEAT, as they described it) increased with 1,000 kcal overfeeding for 8 wk with a diet comprised of 40% carbohydrate, 40% fat, and 20% protein. The similarities between our findings are not surprising since the calculations that we used to compute AREE were similar. Levine utilized RMR and estimated TEF with DLW, whereas we utilized SMR and actual TEF from the chamber for our evaluation to include the important differences in TEF associated with the macronutrient composition of our three study diets. Levine et al. (14) did not detect a significant change in activity with accelerometry, although in our study we did find that VM and AEE by accelerometry increased during overfeeding. It is possible that the Caltrac accelerometers used in the Levine et al. (14) study were not sensitive enough to determine changes in physical activity from baseline to week 8 (20). In addition, the participants in study by Levine et al. (14) were free-living and not confined to an inpatient unit or a metabolic chamber. The results of the present study also indicate that increased activity was also detected in the confines of a respiratory chamber.
Hamilton (8) proposed that ∼20% dietary protein was the most metabolically efficient, with 20% dietary protein requiring the lowest heat increment. Originally, it was proposed that lower dietary protein intake decreases metabolic efficiency (8) by increasing diet-induced thermogenesis (through futile cycles) and nonshivering thermogenesis (30). As stated, we did not detect differing levels of physical activity among the dietary protein treatments. However, based on least squares means from accelerometry and chamber data, the normal-protein diet (15%) increased from baseline. The lack of a treatment difference was not hypothesized, since total energy expenditure was increased in the normal- and high-protein diet groups but not the low-protein diet group, and similarly, we observed differences in adaptive thermogenesis between the diet groups in our primary analysis (5). Furthermore whereas there was no differential increase in fat mass among treatment groups, lean body mass was increased in the normal- and high-protein diet groups but not the low-protein diet group. This finding suggests that the body mass differences are likely not traditional healthy lean body mass (i.e., muscle and water) but instead are comprised of extracellular matrix and other structural proteins supporting adipose tissue expansion (32) and even possibly liver proteins. Our results are similar to others. For example, Joosen et al. (9) found no differences in physical activity TDEE when healthy females were overfed 150% of energy requirements with 7% dietary protein for 2 wk, and there were no differences in metabolic efficiency. Thus, in adult humans the hypothesis of greater activity with low dietary protein has not been supported, as originally hypothesized by Stock (30).
The short-term physical activity estimates during and following overfeeding from accelerometry and the metabolic chamber relate to previous shorter-term overfeeding studies. After overfeeding 140% of energy for 2 days, when energy expenditure was clamped, participants reduced their step count and increased lying time and sleep on the next day of ad libitum feeding, during which time energy expenditure and activity were free to vary (1). Similar results have been found in other studies in individuals prone to obesity (27). These results suggest that participants may actually decrease AEE following short-term overfeeding, as was found with the nonsignificant reduction in VM during the first 2 wk in our study. Our findings with longitudinal measures of activity show that it may take weeks to detect increased activity in response to overfeeding. However, not all studies are in agreement since a decrease in physical activity has been observed after 6 wk of overfeeding (19).
SPA, before being adjusted for change in body composition, increased during overfeeding, as expected. However, after adjustment, SPA did not change significantly during overfeeding. SPA accounted for ∼30% of the variability in adaptive thermogenesis in the main study demonstrated by TDEE residuals (5). The study by Diaz et al. (7) that found an increase in TDEE in a chamber was not adjusted for change in body composition. To our knowledge, this was the only other long-term study that measured physical activity in a metabolic chamber. Energy expended in activity measured by both accelerometry and DLW increased in response to overfeeding. Tremblay et al. (33) reported from the Bouchard overfeeding study that increased energy intake in young men for more than 100 days resulted in a ∼345 kcal/day (131 MJ over 84 days) increase in total energy expenditure. However, not all studies have found similar results. As referenced previously, in a ≥60-day study by Pasquet et al. (19), physical activity (based on time allocation survey, movement recording electric device, and AREE by DLW) decreased according to all three metrics. Also, Norgan and Durnin (17) found no increase in total energy expenditure with 42 days of overfeeding in six young men. Most but not all previous studies have found that SPA increases during overfeeding.
Physical activity is influenced by genetics, lifestyle, and the environment. Black et al. (3) have shown that physical activity levels varied two- to threefold in humans through DLW analysis, but this was not during overfeeding. Bouchard et al. (4) found high intraclass correlation coefficients in body weight gain in identical male twins. The variance in physical activity among subjects may help account for the disparate results of the present and past studies since individuals have a high variability in intraindividual energy expenditure.
This study had numerous strengths, including a randomized distribution of participants to an inpatient unit with a controlled diet provided by a research kitchen. Furthermore, we have five 24-h metabolic chamber assessments, daily accelerometry, and DLW to examine the effects of dietary protein content during overfeeding on physical activity. One possible study limitation was that muscle or exercise efficiency was not measured. Finally, the methods used to calculate SPA in the metabolic chamber may affect results for the calculation of AEE.
In conclusion, we observed that overfeeding produces an increase in physical activity and an increase in calories expended in physical activity after adjusting for changes in body composition. This effect is less robust when habitual movement is further confined to the metabolic chamber. Increased SPA explains part of the adaptive thermogenesis in individuals during overfeeding.
GRANTS
This study was funded in part by US Department of Agriculture Grant 2010-34323-21052 and National Institutes of Health Grants K23-DK-068052, K99-HD-060762, P30-DK-072476, and U54-GM-104940.
DISCLOSURES
The authors have no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
J.W.A. and H.H. analyzed data; J.W.A., G.A.B., L.M.R., and C.K.M. interpreted results of experiments; J.W.A. prepared figures; J.W.A. drafted manuscript; J.W.A., G.A.B., L.M.R., and C.K.M. edited and revised manuscript; J.W.A., G.A.B., S.R.S., L.d.J., J.R., H.H., L.M.R., and C.K.M. approved final version of manuscript; G.A.B., S.R.S., L.d.J., and J.R. conception and design of research; G.A.B., S.R.S., L.d.J., and J.R. performed experiments.
ACKNOWLEDGMENTS
We thank the volunteers who made this study possible as well as Susan Mancuso and the clinic and Dr. Marlene Most, Courtney Brock, and the research kitchen staff.
Present affiliation of S. R. Smith: Translational Research Institute for Metabolism and Diabetes, Florida Hospital and Sanford Burnham Medical Research Institute, Orlando, FL 32804. Present affiliation of L. de Jonge: Department of Nutrition and Food Studies, George Mason University, Fairfax, VA 22030.
REFERENCES
- 1.Apolzan JW, Bray GA, Hamilton MT, Zderic TW, Han H, Champagne CM, Shepard D, Martin CK. Short-term overeating results in incomplete energy intake compensation regardless of energy density or macronutrient composition. Obesity (Silver Spring) 22: 119–130, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin KM, Joanisse DR, Haddad F, Goldsmith RL, Gallagher D, Pavlovich KH, Shamoon EL, Leibel RL, Rosenbaum M. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol Regul Integr Comp Physiol 301: R1259–R1266, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr 50: 72–92, 1996. [PubMed] [Google Scholar]
- 4.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med 322: 1477–1482, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, Most M, Brock C, Mancuso S, Redman LM. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 307: 47–55, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA. Field use of D2 18O to measure energy expenditure of soldiers at different energy intakes. J Appl Physiol 67: 1922–1929, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr 56: 641–655, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton JS. Heat increments of diets balanced and unbalanced with respect to protein. J Nutr 17: 583–599, 1939. [Google Scholar]
- 9.Joosen AM, Bakker AH, Westerterp KR. Metabolic efficiency and energy expenditure during short-term overfeeding. Physiol Behav 85: 593–597, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab 286: E551–E559, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol 294: R699–R710, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, Levine JA. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience 142: 29–36, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Levin BE. Spontaneous motor activity during the development and maintenance of diet-induced obesity in the rat. Physiol Behav 50: 573–581, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283: 212–214, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Mikkelsen PB, Toubro S, Astrup A. Effect of fat-reduced diets on 24-h energy expenditure: comparisons between animal protein, vegetable protein, and carbohydrate. Am J Clin Nutr 72: 1135–1141, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput 41: 572–578, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Norgan NG, Durnin JV. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Am J Clin Nutr 33: 978–988, 1980. [DOI] [PubMed] [Google Scholar]
- 18.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr 87: 1558S–1561S, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Pasquet P, Brigant L, Froment A, Koppert GA, Bard D, de Garine I, Apfelbaum M. Massive overfeeding and energy balance in men: the Guru Walla model. Am J Clin Nutr 56: 483–490, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity (Silver Spring) 15: 2371–2379, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Powell SM, Rowlands AV. Intermonitor variability of the RT3 accelerometer during typical physical activities. Med Sci Sport Exerc 36: 324–330, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 78: 1568–1578, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 4: e4377, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts SB, Young VR, Fuss P, Fiatarone MA, Richard B, Rasmussen H, Wagner D, Joseph L, Holehouse E, Evans WJ. Energy expenditure and subsequent nutrient intakes in overfed young men. Am J Physiol Regul Integr Comp Physiol 259: R461–R469, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Rothney MP, Schaefer EV, Neumann MM, Choi L, Chen KY. Validity of physical activity intensity predictions by ActiGraph, Actical, and RT3 accelerometers. Obesity (Silver Spring) 16: 1946–1952, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity (Silver Spring) 20: 2186–2193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr 118: 1278–1289, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Schutz Y, Ravussin E, Diethelm R, Jequier E. Spontaneous physical activity measured by radar in obese and control subject studied in a respiration chamber. Int J Obes 6: 23–28, 1982. [PubMed] [Google Scholar]
- 30.Stock MJ. Gluttony and thermogenesis revisited. Int J Obes 23: 1105–1117, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Stubbs RJ, Sepp A, Hughes DA, Johnstone AM, Horgan GW, King N, Blundell J. The effect of graded levels of exercise on energy intake and balance in free-living men, consuming their normal diet. Eur J Clin Nutr 56: 129–140, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Tam CS, Covington JD, Bajpeyi S, Tchoukalova Y, Burk D, Johannsen DL, Zingaretti CM, Cinti S, Ravussin E. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J Clin Endocrinol Metab 99: 1749–1757, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremblay A, Despres JP, Theriault G, Fournier G, Bouchard C. Overfeeding and energy expenditure in humans. Am J Clinical Nutr 56: 857–862, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Westerterp KR. Metabolic adaptations to over-and underfeeding—still a matter of debate? Eur J Clin Nutr 67: 443–445, 2013. [DOI] [PubMed] [Google Scholar]