Abstract
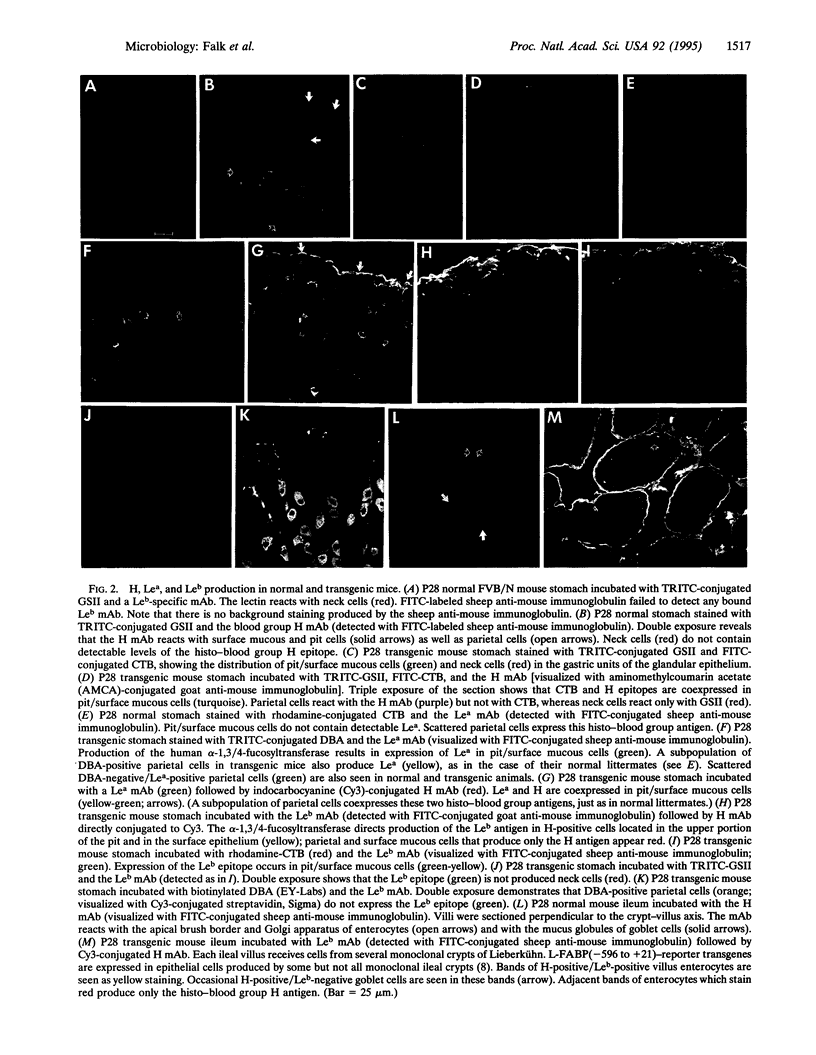
Helicobacter pylori is a human pathogen associated with the development of gastric and duodenal ulcers and gastric adenocarcinoma. To test the hypothesis that the human Lewis(b) blood group antigen (Le(b)) functions as a receptor for the bacteria's adhesins and mediates its attachment to gastric pit and surface mucous cells, a human alpha-1,3/4-fucosyltransferase was expressed in these cell lineages in FVB/N transgenic mice. The fucosyltransferase directed production of the Leb epitope without any apparent effect on the proliferation and differentiation programs of this lineage. Moreover, clinical isolates of H. pylori bound to these cells in transgenic mice but not in their normal littermates. Binding was blocked by pretreatment of the bacteria with soluble Le(b). This mouse model could be useful for examining the molecular pathogenesis of diseases caused by H. pylori infection. Creating novel pathways for production of specific oligosaccharides in selected cell lineages of transgenic animals represents an approach for examining the role of complex carbohydrates in regulating cellular differentiation and host-microbe interactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J. Helicobacter pylori: microbiology of a 'slow' bacterial infection. Trends Microbiol. 1993 Oct;1(7):255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- Borén T., Falk P., Roth K. A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993 Dec 17;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- CLARKE C. A., EDWARDS J. W., HADDOCK D. R., HOWEL-EVANS A. W., MCCONNELL R. B., SHEPPARD P. M. ABO blood groups and secretor character in duodenal ulcer; population and sibship studies. Br Med J. 1956 Sep 29;2(4995):725–731. doi: 10.1136/bmj.2.4995.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman M. W., Hauft S. M., Gordon J. I. The mouse ileal lipid-binding protein gene: a model for studying axial patterning during gut morphogenesis. J Cell Biol. 1994 Sep;126(6):1547–1564. doi: 10.1083/jcb.126.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk P., Roth K. A., Gordon J. I. Lectins are sensitive tools for defining the differentiation programs of mouse gut epithelial cell lineages. Am J Physiol. 1994 Jun;266(6 Pt 1):G987–1003. doi: 10.1152/ajpgi.1994.266.6.G987. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Hermiston M. L. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994 Dec;6(6):795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Karam S. M. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993 Jun;236(2):314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- Karam S. M., Leblond C. P. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993 Jun;236(2):280–296. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- Karam S. M., Leblond C. P. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993 Jun;236(2):297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- Kukowska-Latallo J. F., Larsen R. D., Nair R. P., Lowe J. B. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes Dev. 1990 Aug;4(8):1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]
- Lorenz R. G., Gordon J. I. Use of transgenic mice to study regulation of gene expression in the parietal cell lineage of gastric units. J Biol Chem. 1993 Dec 15;268(35):26559–26570. [PubMed] [Google Scholar]
- Roth K. A., Cohn S. M., Rubin D. C., Trahair J. F., Neutra M. R., Gordon J. I. Regulation of gene expression in gastric epithelial cell populations of fetal, neonatal, and adult transgenic mice. Am J Physiol. 1992 Aug;263(2 Pt 1):G186–G197. doi: 10.1152/ajpgi.1992.263.2.G186. [DOI] [PubMed] [Google Scholar]
- Weston B. W., Smith P. L., Kelly R. J., Lowe J. B. Molecular cloning of a fourth member of a human alpha (1,3)fucosyltransferase gene family. Multiple homologous sequences that determine expression of the Lewis x, sialyl Lewis x, and difucosyl sialyl Lewis x epitopes. J Biol Chem. 1992 Dec 5;267(34):24575–24584. [PubMed] [Google Scholar]