Abstract
Dietary carotenoids like β-carotene are converted within the body either to retinoid, via β-carotene-15,15′-dioxygenase (BCO1), or to β-apo-carotenoids, via β-carotene-9′,10′-oxygenase 2. Some β-apo-carotenoids are potent antagonists of retinoic acid receptor (RAR)-mediated transcriptional regulation, which is required to ensure normal heart development and functions. We established liquid chromatography tandem mass spectrometery methods for measuring concentrations of 10 β-apo-carotenoids in mouse plasma, liver, and heart and assessed how these are influenced by Bco1 deficiency and β-carotene intake. Surprisingly, Bco1−/− mice had an increase in heart levels of retinol, nonesterified fatty acids, and ceramides and a decrease in heart triglycerides. These lipid changes were accompanied by elevations in levels of genes important to retinoid metabolism, specifically retinol dehydrogenase 10 and retinol-binding protein 4, as well as genes involved in lipid metabolism, including peroxisome proliferator-activated receptor-γ, lipoprotein lipase, Cd36, stearoyl-CoA desaturase 1, and fatty acid synthase. We also obtained evidence of compromised heart function, as assessed by two-dimensional echocardiography, in Bco1−/− mice. However, the total absence of Bco1 did not substantially affect β-apo-carotenoid concentrations in the heart. β-Carotene administration to matched Bco1−/− and wild-type mice elevated total β-apo-carotenal levels in the heart, liver, and plasma and total β-apo-carotenoic acid levels in the liver. Thus, BCO1 modulates heart metabolism and function, possibly by altering levels of cofactors required for the actions of nuclear hormone receptors.
Keywords: retinoid, retinoic acid, β-carotene, liquid chromatography tandem mass spectrometry, heart
retinoids (vitamin A and its natural and synthetic analogs) regulate cell proliferation, differentiation, and apoptosis (36, 39, 41). These actions of retinoids are mediated primarily by all-trans-retinoic acid (ATRA), which is a potent transcriptional regulator and a natural ligand for the retinoic acid receptor (RAR) family of nuclear hormone transcription factors that regulate lipid metabolism (1, 24, 41). Nongenomic effects of ATRA in the regulation of cell signaling pathways have also been identified and studied (1, 19, 46).
All retinoids must be acquired from the diet either as preformed vitamin A, primarily as retinol or retinyl ester, or as a provitamin A carotenoid, such as β-carotene (7, 16, 29). Most tissues maintain some retinoid stores, primarily as retinyl esters, but for healthy well-nourished organisms, the liver accounts for 80–90% of all retinoid present in the body (7, 29). Liver retinoid stores are used physiologically to maintain blood retinoid levels constant (7, 29). This involves the secretion of retinol bound to its specific circulating binding protein, retinol-binding protein 4 (RBP4), which delivers retinol to tissues, where it is taken up and either stored or enzymatically oxidized to ATRA (34, 38). Provitamin A carotenoids like β-carotene are taken up by the intestine and cleaved in situ by the enzyme β-carotene-15,15′-dioxygenase (BCO1) to retinoid, which is then metabolically indistinguishable from dietary preformed vitamin A (6, 11, 43). In humans, some dietary provitamin A carotenoid is absorbed intact into the body, along with other dietary lipids, in nascent chylomicrons and distributed to tissues throughout the body (6, 11, 43). There are no specific tissue sites for carotenoid storage per se, but some tissues, like adipose tissue, accumulate higher concentrations of carotenoids than others. Moreover, many tissues express Bco1 mRNA and protein, and this has been proposed to allow for the cleavage of provitamin A carotenoids to retinoids within these tissues (31, 35, 44, 48, 49). In addition to BCO1, one other mammalian enzyme is known to be able to metabolize carotenoids: β-carotene-9′,10′-oxygenase 2 (BCO2) (15, 16, 21, 22). Unlike BCO1, which catalyzes β-carotene cleavage about its central 15–15′ double bond, BCO2 catalyzes the asymmetric cleavage of β-carotene at the 9′-10′ double bond, forming β-apo-10′-carotenal, one of the structurally distinct products that are collectively known as β-apo-carotenals (11, 18, 21, 44). These β-apo-carotenals can then either undergo enzymatic oxidation to corresponding β-apo-carotenoic acids or a reduction to corresponding β-apo-carotenols, which can subsequently undergo esterification or conversion to retinoid (4, 11, 18, 21, 44). Some β-apo-carotenoids are also formed nonenzymatically.
It has been known for several decades that the asymmetric β-apo-carotenoid cleavage products of β-carotene are present in human and animal blood and tissues (37, 45). To some degree, these compounds are destined for elimination from the body (3), but the extent of this has not been established. In 2007, Plutsky and colleagues (52, 53) reported that one of these compounds, β-apo-14′-carotenal, interacts with retinoid X receptors (RXRs) and both peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ to oppose known effects of both synthetic and natural ligands of these receptors, thereby preventing target gene induction in vitro and functional cellular responses in vivo. More recently, Eroglu et al. (11, 13) reported that some apo-β-carotenoids, specifically β-apo-13-carotenone, β-apo-14′-carotenal, and β-apo-14′-carotenonic acid, are very potent RAR antagonists, with binding affinities in the low nanomolar range. These binding affinities are similar in magnitude to that of ATRA for RARs (13). In addition, β-apo-13-carotenone is a potent antagonist of RXRα transactivation (12).
To gain better understanding of the metabolism and actions of β-apo-carotenoids within the body, we established liquid chromatography tandem mass spectrometry (LC/MS/MS) protocols for measuring β-apo-carotenoid levels in the mouse plasma, liver, and heart. We were interested in understanding if the lack of Bco1 expression might influence tissue β-apo-carotenoid levels. To this end, we used matched male wild-type (WT) and Bco1-deficient (Bco1−/−) mice in our investigations and studied one tissue that normally expresses Bco1, the liver, and one tissue that does not, the heart. Adult Bco1−/− mice reproduce normally but have been reported to develop hepatic steatosis more readily than WT mice (17), and female Bco1−/− mice fed a control diet display increased inflammation in their lungs compared with WT mice (42). By studying mice with a genetic ablation of Bco1, we discovered that the absence of Bco1 affects cardiac retinoid and lipid homeostasis and heart function.
MATERIALS AND METHODS
Animals, husbandry, and experimental diets.
Experiments involving animals were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (27) and were approved by the Institutional Animal Care and Use Committee of Columbia University. For all of our experiments, we used male WT C57BL/6J mice (Jackson Laboratory) and Bco1−/− mice congenic in the C57BL/6J genetic background. Bco1−/− mice, which were originally described for the mixed C57BL/6;129svJ genetic background (14), have undergone 10 backcrosses with C57BL/6J mice to render them congenic in this inbred background. Genotypes of Bco1−/− mice were determined by PCR using a previously described protocol (14). Mice were housed in a pathogen-free animal facility under 12:12-h light-dark cycles at constant temperature and humidity and had ad libitum access to chow diet (5053 PicoLab Rodent Diet 20, Purina Mills) containing 15 IU vitamin A/g diet. For β-carotene supplementation, we ised water-soluble β-carotene beadlets (10% CWS, DSM Nutritional Products) dispersed in PBS at a final concentration of 1 mg/100 μl. β-Carotene was administered to mice by intraperitoneal injection in a single dose given 3 days before euthanization. All manipulations involving β-carotene were carried out under red light.
LC/MS/MS analyses of β-apo-carotenoids, retinoids, and lipids.
For analysis of β-apo-carotenoids and β-carotene levels in tissues, livers and hearts were homogenized in 4 volumes of PBS to render them 20% (wt/vol) homogenates. Internal standards (retinyl acetate and 13C40-β-carotene) were then added in 1 ml of ethanol containing 0.1% (wt/vol) butylated hydroxytoluene as an antioxidant. Subsequently, 6 volumes of 10:6:7:7 hexane-ethanol-acetone-toluene (HEAT) were added along with saturated NaCl solution (200 μl) to facilitate phase separation. Samples were vortexed for 60 s and centrifuged at 5,000 g for 5 min at 4°C. The upper organic layer was removed, and the aqueous phase was extracted two more times by adding HEAT-NaCl. The three organic phases were combined and dried under a stream of nitrogen. The residue was reconstituted in 40 μl of 1:1 (vol/vol) methyl t-butyl ether-methanol before LC/MS/MS. An aliquot of whole plasma was extracted using the same protocol but scaled for the plasma volume.
LC/MS/MS analyses of plasma and tissue concentrations of β-apo-carotenoids and retinoic acid were conducted using a Waters Xevo TQ MS ACQUITY UPLC system controlled by MassLynx software (version 4.1). Samples were maintained at 4°C in the autosampler, and 5 μl were loaded onto a Waters ACQUITY UPLC BEH Phenyl column (3.0 × 100 mm, 1.7-μm particle size) for analysis of β-apo-carotenals or β-apo-carotenoic acids or an ACQUITY UPLC BEH HSS column (3.0 × 100 mm, 1.7-μm particle size) for analysis of β-apo-13-carotenone and ATRA. The running columns were preceded by a 2.1 × 5-mm guard column containing the same packing material. Columns were maintained at 40°C. Separations were achieved using water/acetonitrile gradients in the presence of formic acid for β-apo-13-carotenone and ATRA or water/methanol gradients in the presence of formic acid and ammonium formate for β-apo-carotenals or β-apo-carotenoic acids. Positive ESI-MS in the multiple reaction monitoring mode was performed. Concentrations of β-apo-carotenoids in serum and tissues were determined by comparing integrated peak areas for each compound against those of known amounts of purified standards that were chemically synthesized as previously described (11, 13). Loss of β-apo-carotenoids during extraction was accounted for by adjusting for the recovery of the internal standard retinyl acetate added before solvent extraction.
Retinoic acid concentrations were determined as we have previously reported (47). Penta-deuterated ATRA (ATRA-d5) was used as an internal standard and was purchased from Toronto Research Chemicals (North York, ON, Canada).
Free fatty acid (FFA), ceramide, endocannabinoid, and N-acylethanolamine (NAE) concentrations were determined by LC/MS/MS according to protocols we have previously described in detail (9).
mRNA isolation and quantitative RT-PCR analysis.
Total RNA was isolated from the heart and liver using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA samples were DNase I digested using the RNeasy mini kit (Qiagen) to remove residual genomic DNA and subsequently quantified using a NanoDrop spectrophotometer. For cDNA synthesis, 1 μg of total RNA was reverse transcribed using the High Capacity Reverse Transcription Kit (Applied Biosystems). cDNA synthesis was carried out for 10 min at 20°C and 120 min at 37°C. The reaction was stopped by incubation at 85°C for 5 min using a thermal cycler (Eppendorf). Sequences for the primers used in this study have been previously published (9). Quantitative RT-PCR was performed in a total volume of 25 μl containing diluted cDNA template (1:40), forward and reverse primers (1 μM each), and LightCycler 480 SYBR Green I Master Mix (Roche) on a LightCycler 480 II instrument (Roche). Expression levels of target genes are presented relative to the reference gene 18S. A dissociation curve program was used after each reaction to verify the purity of the PCR products.
Immunoblot analysis.
Dissected perfused livers and hearts were homogenized in RIPA buffer containing protease inhibitor cocktails (Sigma) with 50 mM Tris·HCl (pH 8), 150 mM NaCl, 0.1% (wt/vol) SDS, 0.5% (wt/vol) sodium deoxycholate, 1% (vol/vol) Triton X-100, and 1 mM EDTA. Cell lysates were obtained after centrifugation at 15,000 g for 15 min at 4°C. Total protein extract (30 μg) was applied for SDS-PAGE and transferred onto polyvinylidene difluoride membranes for subsequent probing with polyclonal antiserum against mouse BCO1 (1:2,000 dilution) (31) in conjugation with horseradish peroxidase-conjugated secondary antibody and an ECL detection system (Thermo Scientific). Protein loading was normalized using horseradish peroxidase-conjugated monoclonal α-tubulin antibody (1:1,000, Santa Cruz Biotechnology).
Plasma and tissue triglyceride and total cholesterol measurements.
Blood was collected from mice for measurements of plasma triglycerides (TGs), total cholesterol (TC), and nonesterified fatty acids (FFAs). TGs and TC from plasma and pieces of the liver and myocardium were measured enzymatically using Infinity kits (ThermoFisher Scientific) according to the manufacturer's instructions. FFAs were measured using a NEFA kit (Wako Pure Chemical Industries) according to the manufacturer's instructions. For measurements of tissue lipids, livers and hearts were first perfused with PBS and then homogenized at 4°C in PBS. Lipids were extracted from ∼50 mg tissue in 6 volumes of 2:1 (vol/vol) choloroform-methanol. Chloroform-extractable lipids were dried under a gentle stream of N2 and subsequently solubilized in water containing 2% (vol/vol) Triton X-100, and aliquots taken for assay.
Echocardiography.
Two-dimensional echocardiography was performed using a high-resolution imaging system with a 30-MHz imaging transducer (Vevo 770, VisualSonics) in unconscious 19-wk-old male mice (n = 5–8 mice/group). Mice were anesthetized with 1.5–2% isoflurane and thereafter maintained on 1–1.5% isoflurane throughout the procedure. Two-dimensional echocardiographic images were obtained and recorded in a digital format. Images were then analyzed offline by a single observer blinded to the murine genotype. Left ventricular (LV) end-diastolic dimension (LVDd) and LV end-systolic dimension (LVDs) were measured. The ejection fraction (EF) was calculated as follows: EF (in %) = [(LVDd3 − LVDs3)/LVDd3] × 100%. Fractional shortening (FS), which quantifies contraction of the ventricular wall and is an indication of muscle function, was calculated as follows: FS (%) = [(LVDd − LVDs)/LVDd] × 100%.
Statistical analysis.
Data are presented as means ± SE. Student's t-test was used to identify differences between control and knockout strains. P values of <0.05 were considered statistically significant.
RESULTS
To understand how Bco1 expression affects concentrations of β-apo-carotenals and β-apo-carotenoic acids within tissues and blood, we set up LC/MS/MS methods for assessing β-apo-carotenoid levels. We developed a method that allowed us to chromatographically separate and detect chemically synthesized standards for β-apo-14′-, β-apo-12′-, β-apo-10′-, and β-apo-8′-carotenals and -carotenoic acids as well as β-ionone and β-apo-13-carotenone. Using this LC/MS/MS protocol, we established that we could detect each of these β-carotene metabolites in extracts prepared from the plasma, liver, and heart of chow-fed adult mice. We did not observe matrix effects for these tissues, as assessed by determinations of recoveries and separations for liver, heart, and plasma extracts spiked with different concentrations of authentic β-apo-carotenoid standards.
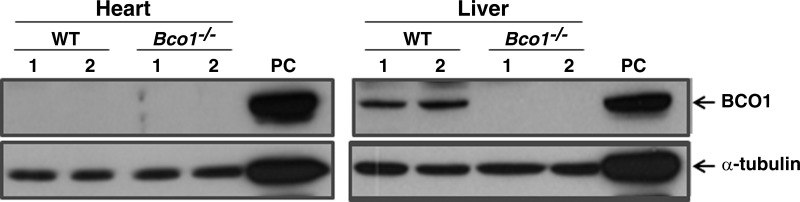
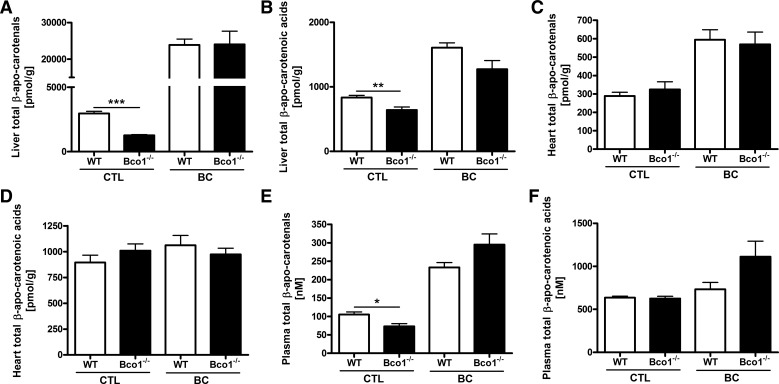
We focused on the liver and heart since the adult liver but not the adult heart expresses BCO1 protein, whereas the adult heart does not (Fig. 1). The liver and heart have both been reported to express BCO2 (18). All 10 β-apo-carotenoids were present in the liver, heart, and plasma of 4- to 5-mo-old male chow-fed WT and Bco1−/− mice. To facilitate understanding of these data, we summed individual tissue levels (as shown in Fig. 2) as total β-apo-carotenal and total β-apo-carotenoic acid levels. As shown in Fig. 2, A and B, levels of both total β-apo-carotenals and β-apo-carotenoic acids were significantly lower in livers of Bco1−/− compared with WT mice, whereas, no statistically significant differences in these levels were observed for the heart (Fig. 2, C and D). Plasma β-apo-carotenal levels were significantly lower in Bco1−/− mice compared with WT (Fig. 2E), but no effect of genotype on plasma β-apo-carotenoic acid levels was observed (Fig. 2F). No statistically significant differences in liver, heart, or plasma levels of β-apo-13-carotenone were detected for Bco1−/− and WT mice fed a chow diet (data not shown). Tissue levels of individual β-apo-carotenals and β-apo-carotenoic acids are shown in Tables 1–3.
Fig. 1.
β-Carotene-15,15′-dioxygenase (BCO1) protein is not expressed in adult hearts of male wild-type (WT) mice. Western blot analysis of BCO1 and α-tubulin protein levels in extracts prepared from hearts and livers of matched 19-wk-old WT and Bco1-deficient (Bco1−/−) mice is shown. As a positive control (PC), we used homogenates prepared from Chinese hamster ovary cells overexpressing mouse BCO1.
Fig. 2.
Effects of Bco1 deficiency and β-carotene supplementation on total β-apo-carotenal and total β-apo-carotenoic acid levels in the liver, heart, and plasma. The total β-apo-carotenal level was the sum of β-apo-14′-, β-apo-12′-, β-apo-10′-, and β-apo-8′-carotenal levels determined individually for each tissue. The total β-apo-carotenoic acid level was the sum of β-apo-14′-, β-apo-12′-, β-apo-10′-, and β-apo-8′-carotenoic acid levels determined individually for each tissue. Liver total β-apo-carotenal (A) and total β-apo-carotenoic acid (B) levels were statistically different for chow-fed [control (CTL)] WT and Bco1−/− mice but not for mice that received a 1-mg dose of β-carotene (BC) 3 days before euthanization. Heart total β-apo-carotenal (C) and total β-apo-carotenoic acid (D) levels were not different for WT or Bco1−/− mice that received either a chow diet (CTL) or were supplemented with a 1-mg dose of β-carotene (BC) 3 days before euthanization. Plasma levels of total β-apo-carotenals (E) but not total β-apo-carotenoic acids (F) were statistically different in Bco1−/− mice compared WT mice maintained on a chow diet (CTL). Administration of a 1-mg dose of β-carotene (BC) 3 days before euthanization abolished the effect of genotype on plasma total β-apo-carotenal levels (E). Values are means ± SE; n = 5–8. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 1.
β-Apo-carotenoid levels in livers of WT mice versus Bco1−/− mice
| Chow |
BC (1 mg) |
|||||
|---|---|---|---|---|---|---|
| WT mice, pmol/g tissue | Bco1−/− mice, pmol/g tissue | P value | WT mice, pmol/g tissue | Bco1−/− mice, pmol/g tissue | P value | |
| β-13-Carotenone | 11.4 ± 7.2 | 4.6 ± 4.0 | 0.12 | 47.5 ± 63.7 | 59.9 ± 36.0 | 0.72 |
| β-Apo-14′-carotenal | 2,870 ± 390 | 1,178 ± 137 | 0.0002*** | 17,720 ± 3,575 | 18,010 ± 7,483 | 0.95 |
| β-Apo-14′-carotenoic acid | 504 ± 87 | 392 ± 115 | 0.17 | 580 ± 149 | 438 ± 214 | 0.31 |
| β-Apo-12′-carotenal | 16.3 ± 2.4 | 17.1 ± 3.0 | 0.70 | 3,210 ± 682 | 3,456 ± 582 | 0.58 |
| β-Apo-12′-carotenoic acid | 253 ± 29 | 179 ± 19 | 0.002** | 200 ± 27 | 204 ± 41 | 0.84 |
| β-Apo-10′-carotenal | 15.6 ± 4.0 | 19.4 ± 5.9 | 0.31 | 2,489 ± 593 | 2,203 ± 407 | 0.42 |
| β-Apo-10′-carotenoic acid | 22.2 ± 5.9 | 28.9 ± 5.7 | 0.13 | 337 ± 83 | 242 ± 69 | 0.11 |
| β-Apo-8′-carotenal | 57.7 ± 8.5 | 49.6 ± 6.5 | 0.15 | 487 ± 117 | 378 ± 90 | 0.16 |
| β-Apo-8′-carotenoic acid | 56.3 ± 12 | 41.9 ± 13.7 | 0.17 | 491 ± 73 | 388 ± 154 | 0.28 |
Concentrations of compounds are provided as means ± SD.
WT mice, wild-type mice; Bco1−/− mice, β-carotene-15,15′-dioxygenase-deficient mice; BC, β-carotene.
Significantly different from the corresponding concentration in WT mice:
P < 0.01, and
P < 0.005.
Table 3.
β-Apo-carotenoid levels in plasma of WT mice versus Bco1−/− mice
| Chow |
BC (1 mg) |
|||||
|---|---|---|---|---|---|---|
| WT mice, nM | Bco1−/− mice, nM | P value | WT mice, nM | Bco1−/− mice, nM | P value | |
| β-13-Carotenone | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.01* | 0.6 ± 0.3 | 1.4 ± 0.7 | 0.1 |
| β-Apo-14′-carotenal | 101 ± 17 | 69 ± 15 | 0.02* | 217 ± 29 | 263 ± 57 | 0.22 |
| β-Apo-14′-carotenoic acid | 472 ± 24 | 455 ± 34 | 0.45 | 635 ± 186 | 991 ± 359 | 0.14 |
| β-Apo-12′-carotenal | 2.9 ± 0.6 | 3.2 ± 0.4 | 0.40 | 15 ± 4.6 | 31 ± 5.1 | 0.003*** |
| β-Apo-12′-carotenoic acid | 131 ± 29 | 119 ± 18 | 0.46 | 43 ± 9.1 | 44 ± 12 | 0.95 |
| β-Apo-10′-carotenal | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.45 | 0.3 ± 0.1 | 1.2 ± 0.2 | 0.001*** |
| β-Apo-10′-carotenoic acid | 34 ± 5.0 | 51 ± 7.2 | 0.008** | 50 ± 6.7 | 73 ± 13 | 0.03* |
| β-Apo-8′-carotenal | 0.4 ± 0.1 | 0.6 ± 0.2 | 0.21 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.46 |
| β-Apo-8′-carotenoic acid | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.89 | 4.4 ± 0.6 | 3.6 ± 0.9 | 0.18 |
Concentrations of compounds are provided as means ± SD. Significantly different from the corresponding concentration in WT mice:
P < 0.05,
P < 0.01, and
P < 0.005.
Table 2.
β-Apo-carotenoid levels in hearts of WT mice versus Bco1−/− mice
| Chow |
BC (1 mg) |
|||||
|---|---|---|---|---|---|---|
| WT mice, pmol/g tissue | Bco1−/− mice, pmol/g tissue | P value | WT mice, pmol/g tissue | Bco1−/− mice, pmol/g tissue | P value | |
| β-13-Carotenone | 1.9 ± 0.3 | 2.0 ± 0.3 | 0.82 | 2 ± 0.9 | 2.3 ± 0.6 | 0.91 |
| β-Apo-14′-carotenal | 257 ± 43 | 289 ± 75 | 0.49 | 494 ± 114 | 349 ± 120 | 0.11 |
| β-Apo-14′-carotenoic acid | 463 ± 127 | 574 ± 82 | 0.16 | 564 ± 159 | 489 ± 70 | 0.38 |
| β-Apo-12′-carotenal | 12 ± 8.8 | 12 ± 4.1 | 0.94 | 58 ± 10 | 103 ± 23 | 0.02* |
| β-Apo-12′-carotenoic acid | 404 ± 61 | 405 ± 62 | 0.99 | 390 ± 74 | 358 ± 76 | 0.55 |
| β-Apo-10′-carotenal | 17 ± 3.5 | 19 ± 8.0 | 0.61 | 33 ± 8.0 | 110 ± 28 | 0.009** |
| β-Apo-10′-carotenoic acid | 18 ± 5.0 | 10 ± 3.5 | 0.04* | 56 ± 16 | 85 ± 8.9 | 0.01* |
| β-Apo-8′-carotenal | 3 ± 0.8 | 4.3 ± 1.2 | 0.20 | 10 ± 1.6 | 8.2 ± 1.6 | 0.17 |
| β-Apo-8′-carotenoic acid | 10 ± 4.1 | 21 ± 6.2 | 0.03* | 52 ± 13 | 41 ± 7.6 | 0.19 |
Concentrations of compounds are provided as means ± SD. Significantly different from the corresponding concentration in WT mice:
P < 0.05;
P < 0.01.
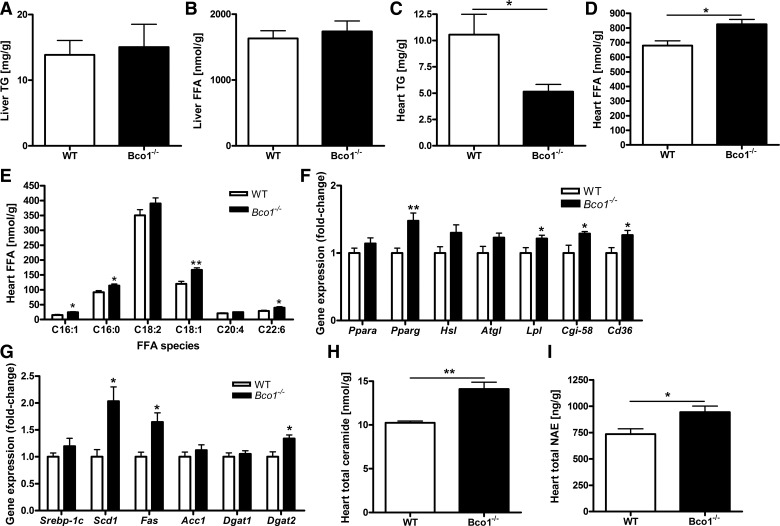
Hessel et al. (17) reported an elevation of hepatic total lipids and TGs in Bco1−/− mice compared with WT mice. To understand whether there are relationships between plasma or tissue β-apo-carotenoid concentrations and hepatic lipids, we measured TGs, FFAs, TC, and free cholesterol levels in livers, hearts, and plasma of our chow-fed experimental mice. In contrast to Hessel et al. (17), we did not observe differences in hepatic TG or FFA levels (Fig. 3, A and B) or hepatic TC levels (data not shown) between matched Bco1−/− and WT mice. Nor did we observe differences between knockout and WT mice in plasma levels of these lipids (data not shown). Surprisingly, though, heart TG levels were significantly lower in Bco1−/− mice than in matched WT mice (Fig. 3C). This was accompanied by a significant elevation in heart levels of FFAs (Fig. 3D). We further analyzed, by LC/MS/MS, the acyl composition of the FFA pool within the heart. Many individual FFA species were modestly but significantly elevated in Bco1−/− hearts, including C16:0, C16:1, C18:1, C18:2, and C22:6 (Fig. 3E).
Fig. 3.
Liver and heart levels of lipids for age- and genetic background-matched chow-fed male WT and Bco1−/− mice. Liver triglyceride (TG; A) and total nonesterified fatty acids [free fatty acid (FFA); B] concentrations were not different for WT and Bco1−/− mice, whereas heart TG (C) concentrations were diminished and total FFAs (D) were elevated in Bco1−/− mice compared with WT mice. A number of relatively abundant individual FFA species were also significantly elevated in Bco1−/− hearts (E). Expression of peroxisome proliferator-activated receptor (PPAR)-γ (Pparg), lipoprotein lipase (Lpl), comparative gene identification 58 (Cgi-58), and Cd36 mRNAs but not PPAR-α (Ppara), hormone-sensitive lipase (Hsl), or adipocyte triglyceride lipase (Atgl) were significantly elevated in Bco1−/− hearts (F). Elevated expression for genes involved in FFA synthesis and metabolism, including stearoyl-CoA desaturase 1 (Scd1), fatty acid synthase (Fas), and diacylglycerol O-acyltransferase 2 (Dgat2), were also elevated in Bco1−/− hearts (G). Total ceramide (H) and total N-acylethanolamine (NAE; I) concentrations were also significantly elevated in hearts of Bco1−/− mice. Values are means ± SE; n = 5–8. *P < 0.05; **P < 0.01.
These lipid changes could suggest that intracardiac TG lipolysis is increased in the Bco1−/− heart. However, we did not detect any differences in intracellular lipase expression for either hormone-sensitive lipase or adipocyte triglyceride lipase (Fig. 3F). Surprisingly, mRNA levels for lipoprotein lipase (Lpl) and the FFA uptake transporter Cd36 were increased (Fig. 3F), suggesting that there is increased FFA uptake from the circulation by the Bco1−/− heart. We also observed increased expression of genes important for FFA synthesis and metabolism in the Bco1−/− heart, including Pparg (Fig. 3F) as well as stearoyl-CoA desaturase 1 (Scd1) and fatty acid synthase (Fas) (Fig. 3G). Interestingly, expression of diacylglycerol O-acyltransferase 2 (Dgat2), which encodes an enzyme important for TG synthesis, was also found to be elevated in Bco1−/− hearts, although TG levels were significantly lower in these hearts.
Increased heart FFAs with decreased TG storage might have led to the incorporation of FFAs into other lipids. For this reason, we assessed levels of ceramides and found that these too were elevated in hearts of Bco1−/− mice compared with WT mice (Fig. 3H). We also identified a statistically significant increase in total NAE species (the sum of arachidonoyl, myristoyl, palmitoyl, lineoloyl, oleoyl, and stearoyl ethanolamide levels measured individually) in hearts of Bco1−/− mice (Fig. 3I). However, levels of the two canonical endocannabinoids, arachidonoyl ethanolamide and 2-arachidonoyl glycerol, were not significantly different in mutant hearts (data not shown).
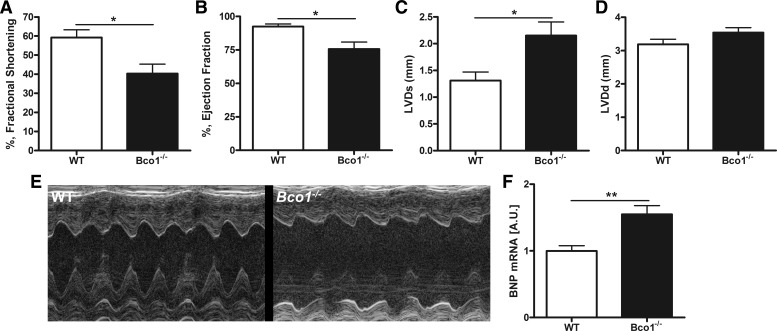
Since we observed marked differences in Bco1−/− heart lipid levels, including potentially toxic ceramides, we carried out an experiment to determine whether heart functions may be compromised in Bco1−/− mice. We identified differences in a number of parameters associated with heart function by two-dimensional echocardiography analysis of Bco1−/− and WT mice (Fig. 4, A–E). Hearts from Bco1−/− mice had decreased FS, decreased EF, and increased LVDs. Whereas LVDd was not affected (WT vs. Bco1−/− mice: 3.19 ± 0.34 vs. 3.54 ± 0.41, P = not significant), LVDs was significantly increased (WT vs. Bco1−/− mice: 1.31 ± 0.35 vs. 2.15 ± 0.72, P < 0.05), suggesting that Bco1−/− mice have reduced cardiac contractibility. FS {FS (%) = [(LVDd − LVDs)/LVDd] × 100%} observed for Bco1−/− mice clearly indicated that systolic dysfunction (decreased FS due to increased systolic diameter) was the primary cause of the impaired cardiac contractility.
Fig. 4.
Heart functions are impaired in Bco1−/− mice as determined by two-dimensional echocardiography. A–D: percent fractional shortening (A), percent ejection fraction (B), left ventricular (LV) systolic diameter (LVDs; C), and LV diastolic diameter (LVDd; D) as measured by two-dimensional echocardiography for 19-wk-old chow-fed male WT and Bco1−/− mice in the C57BL/6 genetic background. E: photographs of representative echocardiograms for matched WT and Bco1−/− mice. F: heart brain natriuretic peptide (Bnp) mRNA levels for WT and Bco1−/− mice as determined by quantitative RT-PCR analysis. AU, arbitrary units. Values are means ± SE; n = 5–8. *P < 0.05; **P < 0.01.
We also investigated possible gene expression changes that might be associated with heart functions. We observed a significant increase in mRNA expression levels for brain natriuretic peptide (BNP) in Bco1−/− mice (Fig. 4F). No differences in heart mRNA expression levels for glucose transporter 4, pyruvate dehydrogenase kinase 4, α-myosin heavy chain, or β-myosin heavy chain were observed. Histological analysis of heart slices did not show differences between Bco1−/− and WT mice with regards to overall structure (data not shown).
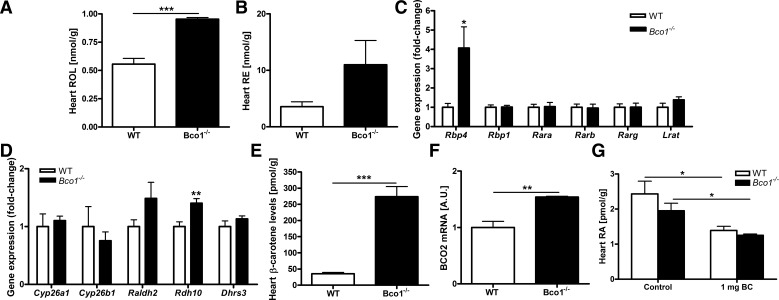
Heart levels of retinol but not retinyl esters were significantly elevated in Bco1−/− mice (Fig. 5, A and B). This was accompanied by increased mRNA expression of Rbp4 but not Rbp1, Rara, Rarb, Rarg, or lecithin retinol acyltransferase (Fig. 5C). Interestingly, heart expression of retinol dehydrogenase 10, a gene that encodes the enzyme responsible for catalyzing the first oxidative step needed for forming ATRA from all-trans-retinol, was significantly elevated. Expression of other genes involved in ATRA synthesis and catabolism [the cytochrome P-450 (Cyp) family members Cyp26a1 and Cyp26b1, aldehyde dehydrogenase 1 family member A2, or dehydrogenase/reductase (SDR family) member 3] were not different (Fig. 5D). Heart β-carotene levels were also elevated in Bco1−/− mice (Fig. 5E), as were mRNA levels for Bco2 (Fig. 5F). Bco2 mRNA levels were not different in the liver (data not shown). Although heart ATRA levels tended to be lower for chow-fed Bco1−/− mice than in WT mice, this did not reach statistical significance (Fig. 5G).
Fig. 5.
Heart retinoid and β-carotene levels as well as expression levels for genes associated with retinoid physiology. Heart levels of retinol (ROL; A) and β-carotene (E) were significantly elevated in Bco1−/− mice compared with WT mice. Although heart retinyl ester (RE) levels tended to be higher for Bco1−/− mice, this did not reach statistical significance (B). Of the genes encoding enzymes and binding proteins involved in retinoid metabolism, only retinol-binding protein 4 (Rbp4) (C) and retinol dehydrogenase 10 (Rdh10; D) were expressed at significantly different levels in hearts of WT and Bco1−/− mice. Heart levels of all-trans-retinoic acid (RA) were not significantly different for Bco1−/− versus WT mice fed a chow diet (G). For both Bco1−/− and WT mice, heart all-trans-retinoic acid levels were significantly diminished upon administration of a single dose of β-carotene (1 mg) given 3 days before euthanization. Gene expression data established that only levels for Rbp4 mRNA (E) and β-carotene-9′,10′-oxygenase 2 (Bco2) mRNA (F) were significantly different for Bco1−/− mice versus matched WT mice. Rara, Rarb, and Rarg, retinoic acid receptor-α, -β, and -γ, respectively; Lrat, lecithin retinol acyltransferase; Cyp, cytochrome P-450; Raldh2, aldehyde dehydrogenase 1 family member A2; Dhrs3, dehydrogenase/reductase (SDR family) member 3. Values are means ± SE; n = 5–8. *P < 0.05; **P < 0.01; ***P < 0.001.
We then tested whether a large dose of water-soluble β-carotene (1 mg), given 3 days before euthanization of the mice, might affect plasma and tissue β-apo-carotenoids. As shown in Fig. 2, β-carotene supplementation resulted in elevated liver total β-apo-carotenal and total β-apo-carotenoic acid concentrations and in elevated heart and plasma β-apo-carotenal levels for both Bco1−/− and WT mice. β-Carotene supplementation did not markedly affect the relative rank orders of the concentrations of individual β-apo-carotenoids in the three tissues (Tables 1–3). For mice that received this dose (1 mg) of β-carotene 3 days before euthanization, we also observed statistically significant decreases in heart ATRA concentrations for both Bco1−/− and WT mice (Fig. 5G).
DISCUSSION
Eroglu et al. (11, 13) established that some eccentric cleavage products of β-carotene are potent antagonists of ATRA and RAR transcriptional regulation. This finding raises the possibility that some eccentric cleavage products of β-carotene may act physiologically to modulate retinoid transcriptional activity. To better understand the physiology of β-carotene cleavage products, we established very sensitive LC/MS/MS methods that allowed us to assess tissue levels of 10 eccentric β-carotene cleavage products, including the potent antagonists β-apo-14′-carotenal and β-apo-13-carotenone. To our knowledge, no systematic studies of tissue levels of β-apo-carotenal or β-apo-carotenoic acid levels have been reported in the literature. Thus, we determined the levels of these compounds in the liver, heart, and plasma of 4- to 5-mo-old chow-fed male mice. We note that a rodent chow diet contains carotenoids that are added as plant materials to the diet, for instance, dehydrated alfalfa meal in the case of the chow diet we used in our study. As shown in Fig. 2 and Tables 1–3, compared with the heart, the liver possesses higher concentrations of a majority of the individual β-apo-carotenoids as well as total β-apo-carotenals and total β-apo-carotenoic acids. Plasma levels for each of the β-apo-carotenoids were either lower or at the same level as those found in the liver and/or heart. The distributions and levels of these metabolites in the liver, heart, and plasma appear to be both tissue and metabolite dependent. However, metabolite levels do not segregate into distinct biochemical (structural) patterns that readily lend themselves to inferences.
Administration of a 1-mg bolus dose of β-carotene (per body weight, equivalent to administering 2.8 g β-carotene to a 70-kg human) 3 days before euthanization resulted in a very marked elevation in hepatic total β-apo-carotenal levels and increased hepatic total β-apo-carotenoic acid levels. Although this doubled heart total β-apo-carotenal levels, there was no affect on heart total β-apo-carotenoic acid concentrations. Plasma total β-apo-carotenal levels were also doubled in β-carotene-supplemented mice compared with unsupplemented mice. These data establish that tissue β-apo-carotenoid levels are responsive to β-carotene supplementation. Surprisingly, though, unlike mice fed a chow diet, we observed no differences for the three tissues examined in total β-apo-carotenal or total β-apo-carotenoic acid levels for Bco1−/− versus WT mice. This could be explained if the metabolic capacity of the liver, heart, and other tissues to catabolize/eliminate these β-carotene metabolites is saturated owing to the large amount of β-carotene administered to the animals.
Most impressively, we found that Bco1−/− mice develop a cardiac phenotype. We unexpectedly observed significantly diminished TG levels in hearts of Bco1−/− mice, and this was accompanied by an elevation in heart FFA, ceramide, and NAE levels. Analysis of the acyl composition of the FFAs in Bco1−/− hearts showed that most FFA species were elevated, suggesting that the elevation in FFAs was not due to a block in a specific arm of fatty acid metabolism, i.e., de novo lipogenesis or fatty acid elongation. We observed elevations in heart mRNA levels for both Lpl and Cd36. We take this finding to indicate that Bco1−/− hearts are taking up more FFAs from the circulation. We also observed elevated expression of Pparg, Fas, and Scd1, genes involved in FFA synthesis and desaturation. Collectively, these findings suggest that elevated FFA levels in Bco1−/− hearts arise through both increased endogenous FFA synthesis and increased FFA uptake from the circulation. Paradoxically, given the significantly lower TG levels in Bco1−/− hearts, we observed elevated expression of Dgat2, one of two enzymes that catalyze the final step of TG synthesis. This is possibly a futile attempt aimed at allowing greater TG accumulation in the heart. Our observations are consistent with findings from Dixon et al. (10), who reported similar differences in lipids present in whole day 14.5 postcoitum Bco1−/− embryos. These authors found lower levels of TGs, cholesteryl esters, and phospholipid species in Bco1−/− embryos and suggested that BCO1 may have a direct role in regulating esterification (formation) of these lipid species (10).
Since FFAs comprise the major fuel for the adult heart and ceramides are known to be potentially toxic to the heart (inducing apoptosis) (33), we wondered whether differences in heart TG, FFA, and ceramide levels might be associated with other differences in heart physiology. To assess this possibility, we undertook high-resolution echocardiography experiments to assess heart functions for 4- to 5-mo-old chow-fed male Bco1−/− and WT mice. This analysis revealed altered cardiac parameters (decreased FS and increased LVDs) in hearts of Bco1−/− mice. These findings indicate that Bco1−/− mice have compromised heart function, characterized by reduced cardiac contractibility.
What is the molecular basis for the altered cardiac contractibility associated with Bco1 deficiency? We suggest there are at least three possible explanations, each invoking a retinoid-dependent mechanism. It is well established that ATRA and RAR signaling are required for ensuring normal heart development in utero and for maintaining a healthy heart after birth. Developmental cardiovascular defects have been reported for animal models that received either retinoid-deficient or retinoid-excess diets and in retinoid receptor knockout embryos (20, 25, 28, 32). These developmental defects include ventricular septal defects, atrial septal defects, abnormal aortic arch patterning, atrioventricular canal defects, and ventricular chamber hypoplasia (25, 28, 31, 32). Interestingly, Osuala et al. (30) reported that targeted disruption of the gene for dopamine β-hydroxylase results in embryonic lethality due to heart failure. This was associated with a marked downregulation of Bco1 mRNA expression in the embryonic heart and effects on ATRA levels. Possibly, the differences we observed for 4- to 5-mo-old mice reflect congenital effects arising from altered retinoid availability in utero. To verify this possibility will require a systematic study of heart development in Bco1−/− mice.
In the adult, ATRA-mediated signaling pathways have an important role in regulating cardiac remodeling, suppressing cardiac hypertrophic features, including increased total protein content, protein synthesis, cell size, and myofibrillar reorganization (32). ATRA signaling has been reported by Bilbija et al. (5) to be activated in the mouse heart after permanent coronary artery ligation. These investigators further proposed that ATRA signaling may play a role in regulating damage and repair during heart remodeling (5). Minicucci et al. (26) reported that local heart retinoid insufficiency is associated with intensified ventricular remodeling after experimental myocardial infarction, worsening diastolic dysfunction. These differences were accompanied by an increase in BNP expression. Our data indicate that BNP expression is elevated in the Bco1−/− heart (Fig. 4F). BNP has been proposed to be a cardioprotective hormone that prevents pathological hypertrophy, fibrosis, and apoptosis of cardiomyocytes, and its expression has recently been shown to be induced by retinoids (23, 40). Thus, it is possible that an alteration in retinoid homeostasis in the Bco1−/− adult heart may lead to effects on downstream signaling pathways important for regulating heart remodeling, including the oxytocin-natriuretic peptide system, which is known to be responsive to ATRA. This possibly contributes to the cardiac phenotype observed in Bco1−/− mice.
Differences in lipid accumulation and metabolism could also directly contribute to the observed impairments in Bco1−/− heart function. Young et al. (50) established that increased fatty acid availability is associated with lipid accumulation and cardiac contractile dysfunction in obese Zucker rats. This was extended by Zhou et al. (51), who demonstrated that increased fatty acid availability was associated with elevated heart ceramide levels. Finck et al. (15) reached the same conclusion studying mouse models. We observed elevated levels of both FFAs and ceramides in the hearts of Bco1−/− mice. Interestingly, ATRA treatment of cultured human SK-N-SK and SK-N-AS neuroblastoma cells or MCF-7 mammary epithelial cells results in increased cellular ceramide levels (8, 22). For these cell model systems, this caused an inhibition of cell growth. Thus, there is precedence in the literature for ATRA modulation of endogenous ceramide concentrations, which then affects cell growth. Collectively, this leads to the hypothesis that the altered retinoid homeostasis in the Bco1−/− heart affects tissue FFA availability and ceramide concentrations, which contribute to the observed impaired cardiac contractility. Although this hypothesis, and/or the other two hypotheses discussed above, could explain the metabolic and contractility phenotypes we observed for hearts of Bco1−/− mice, this will need to be established through future studies aimed specifically at understanding better these possibilities.
GRANTS
This work was supported by National Institutes of Health Grants R01 DK068437 (to W. S. Blaner), R21 AA021366 (to W. S. Blaner), R01 HL049879 (to E. H. Harrison), R01 HL45095 (to I. J. Goldberg), and R01 HL073029 (to I. J. Goldberg). C. M. Trent was supported by a predoctoral grant from the American Heart Association (Heritage Affiliate).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.-A.L., H.J., C.M.T., R.W.C., E.H.H., I.J.G., M.S.M., and W.S.B. conception and design of research; S.-A.L., H.J., C.M.T., J.J.Y., and S.N. performed experiments; S.-A.L., H.J., C.M.T., J.J.Y., E.H.H., I.J.G., and W.S.B. analyzed data; S.-A.L., H.J., C.M.T., J.J.Y., R.W.C., E.H.H., I.J.G., M.S.M., and W.S.B. interpreted results of experiments; S.-A.L., H.J., I.J.G., and W.S.B. drafted manuscript; S.-A.L., H.J., C.M.T., J.J.Y., R.W.C., E.H.H., I.J.G., and W.S.B. approved final version of manuscript; S.-A.L., H.J. and C.M.T. prepared figures; C.M.T., R.W.C., E.H.H., I.J.G., M.S.M., and W.S.B. edited and revised manuscript.
REFERENCES
- 1.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 54: 1761–1775, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amerngual J, Gouranton E, van Helden YGJ, Hessel S, Ribot J, Kramer E, Kiec-Wilk B, Razny U, Lietz G, Wyss A, Dembinska-Kiec A, Palou A, Keijer J, Landrier JF, Bonet ML, von Lintig J. Beta-carotene reduces body adiposity of mice via BCO1. PLOS ONE 6: e20644, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 25: 948–959, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amengual J, Widjaja-Adhi AK, Rodriguez-Santiago S, Hessel S, Golczak M, Palczewski K, von Lintig J. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J Biol Chem 288: 3408–34096, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilbija D, Haugen R, Sagave J, Baysa A, Bastani N, Levy FO, Sirsjö A, Blomhoff R, Valen G. Retinoic acid signaling is activated in the postischemic heart and may influence remodelling. PLOS ONE 7: e44740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaner WS, Olson JA. Retinol and retinoic acid metabolism. In: The Retinoids: Biology, Chemistry, and Medicine, edited by Sporn MB, Roberts AB, Goodman DS. New York: Raven, 1994, p. 229–256. [Google Scholar]
- 7.Blomhoff R, Green MH, Green JB, Berg T, Norum KR. Vitamin A metabolism: new perspectives on absorption, transport, and storage. Physiol Rev 71: 951–990, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Clarke CJ, Mediwala K, Jenkins RW, Sutton CA, Tholanikunnel BG, Hannun YA. Neutral sphingomyelinase-2 mediates growth arrest by retinoic acid through modulation of ribosomal S6 kinase. J Biol Chem 286: 21565–21576, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clugston RD, Jiang H, Lee MX, Piantedosi R, Yuen JJ, Ramakrishnan R, Lewis MJ, Gottesman ME, Huang LS, Goldberg IJ, Berk PD, Blaner WS. Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic and gene expression study. J Lipid Res 52: 2021–2031, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon JL, Kim YK, Brinker A, Quadro L. Loss of β-carotene 15,15′-oxygenase in developing mouse tissues alters esterification of retinol, cholesterol and diacylglycerols. Biochim Biophys Acta 1841: 34–43, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eroglu A, Harrison EH. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J Lipid Res 54: 1719–1730, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eroglu A, Hruszkewycz DP, Curley RW, Jr., Harrison EH. The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch Biochem Biophys 504: 11–16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW, Jr., Harrison EH. Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J Biol Chem 287: 15886–15895, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fierce Y, de Morais Vieira M, Piantedosi R, Wyss A, Blaner WS, Paik J. In vitro and in vivo characterization of retinoid synthesis from β-carotene. Arch Biochem Biophys 472: 126–138, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finck BN, Han X, Courtois M, Almond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA 100: 1226–1231, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta 1821: 70–77, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice. J Biol Chem 282: 33553–33561, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalzying cleavage of carotenoids in vitro and in vivo. J Biol Chem 281: 19327–19338, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane MA, Folias AE, Pingitore A, Perri M, Obrochta KM, Krois CR, Cione E, Ryu JY, Napoli JI. Identification of 9-cis-retinoic acid as a pancreas-specific autocoid that attenuates glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 107: 21884–21889, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keegan BR, Feldman JL, Bergemann G, Ingham PW, Yelon D. Retinoic acid signaling restricts the cardiac progenitor pool. Science 307: 247–249, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 276: 14110–14116, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Kraveka JM, Li L, Bielawski J, Obeid LM, Ogretmen B. Involvement of endogenous ceramide in the inhibition of telomerase activity and induction of morphologic differentiation in response to all-trans-retinoic acid in human neuroblastoma cells. Arch Biochem Biophys 419: 110–119, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Manolescu DC, Jankowski M, Danalache BA, Wang D, Broderick TL, Chaisson JL, Gutkowska J. All-trans retinoic acid stimulates gene expression of the cardio-protective natriuretic peptide system, prevents fibrosis and apoptosis of cardiomyocytes in obese mice. Appl Physiol Nutr Metab; 10.1139/apnm-2014-0005. [DOI] [PubMed] [Google Scholar]
- 24.McKenna N. EMBO Retinoids 2011: mechanisms, biology, and pathology of signaling by retinoic acid and retinoic acid receptors. Nucl Recept Signal 10: e003, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 129: 2271–2282, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minicucci MF, Azevedo PS, Oliveria SA, Jr., Martinez PF, Chiuso-Minicucci F, Polegato BR, Justulin LA, Jr., Matsubara LS, Matsubara BB, Paiva SAR, Zornoff LAM. Tissue vitamin A insufficiency results in adverse ventricular remodeling after experimental myocardial infarction. Cell Physiol Biochem 26: 523–530, 2010. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council. Guide for the Care and Use of Laboratory Animals (8th ed.). Washington, DC: National Academies, 2011. [Google Scholar]
- 28.Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dollé P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128: 1019–1031, 2001. [DOI] [PubMed] [Google Scholar]
- 29.O'Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res 54: 1731–1743, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osuala K, Baker CN, Nguyen HL, Martinez C, Weinshenker D, Ebert SN. Physiological and genomic consequences of adrenergic deficiency during embryonic/fetal development in mice: impact on retinoic acid metabolism. Physiol Genomics 44: 934–947, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave β-carotene. The formation of retinoids. J Biol Chem 276: 32160–32168, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Pan J, Baker KM. Retinoic acid and the heart. Vitam Horm 75: 257–283, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Park TS, Yamashita H, Blaner WS, Goldberg IJ. Lipids in the heart: a source of fuel and a source of toxins. Curr Opin Lipidol 18: 277–282, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 18: 4633–4644, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr. Identification, expression, and substrate specificity of a mammalian β-carotene 15,15′-dioxygenase. J Biol Chem 276: 6560–6565, 2001. [DOI] [PubMed] [Google Scholar]
- 36. Vitamin A and carotenoids. In: Modern Nutrition in Health and Disease, edited by Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ. Philadelphia, PA: Lippincott, Williams & Wilkins, 2006, p. 351–375. [Google Scholar]
- 37.Sharma RV, Mathur SN, Dmitrowskii AA, Das RC, Ganguly J. Studies on the metabolism of β-carotene and apo-β-carotenoids in rats and chickens. Biochim Biophys Acta 486: 183–194, 1976. [DOI] [PubMed] [Google Scholar]
- 38.Soprano DR, Blaner WS. Plasma retinol-binding protein. In: The Retinoids: Biology, Chemistry, and Medicine, edited by Sporn MB, Roberts AB, Goodman DS. New York: Raven, 1994, p. 229–256. [Google Scholar]
- 39.Sporn MB, Roberts AB, Goodman DS. The Retinoids: Biology, Chemistry, and Medicine (2nd ed.). New York: Raven, 1994. [Google Scholar]
- 40.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA 97: 4239–4244, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol 6: 345–364, 2011. [DOI] [PubMed] [Google Scholar]
- 42.van Helden YG, Heil SG, van Schooten FJ, Kramer E, Hessel S, Amengual J, Ribot J, Teerds K, Wyss A, Lietz G, Bonet ML, von Lintig J, Godschalk RW, Keijer J. Knockout of the Bcmo1 gene results in an inflammatory response in female lung, which is suppressed by dietary β-carotene. Cell Mol Life Sci 67: 2039–2056, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr 30: 35–56, 2010. [DOI] [PubMed] [Google Scholar]
- 44.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving β-carotene to retinal. J Biol Chem 275: 11915–11920, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Wang XD, Tang GW, Fox G, Krinsky NI, Russell RM. Enzymatic conversion of β-carotene into β-apo-carotenals, and retinoids by human, monkey, ferret and rat tissues. Arch Biochem Biophys 285: 8–16, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Wei LN. Non-canonical activity of retinoic acid in epigenetic control of embryonic stem cells. Transcription; 10.4161/trns.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wongsiriroj N, Jiang H, Piantedosi R, Yang KJ, Kluwe J, Schwabe RF, Ginsberg H, Goldberg IJ, Blaner WS. Genetic dissection of retinoid esterification and accumulation in the liver and adipose tissue. J Lipid Res 55: 104–114, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Riss G, Bachmann H, Hunziker W. Expression pattern and localization of β,β-carotene 15,15′-dioxygenase in different tissues. Biochem J 354: 521–529, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan W, Jang GF, Haeseleer F, Esumi N, Chang J, Kerrigan M, Compochiaro M, Compochiaro P, Palczewski K, Zack DJ. Cloning and characterization of a human β,β-carotene-15,15′-dioxygenase that is highly experssed in the retinal pigment epithelium. Genomics 72: 193–202, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes 51: 2587–2595, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97: 1784–1789, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutsky J. Asymmetric cleavage of β-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol 21: 77–88, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: new insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett 582: 32–38, 2008. [DOI] [PubMed] [Google Scholar]