Abstract
Nitric oxide (NO) receptor soluble guanylyl cyclase (sGC) is a key regulator of several important vascular functions and is important for maintaining cardiovascular homeostasis and vascular plasticity. Diminished sGC expression and function contributes to pathogenesis of several cardiovascular diseases. However, the processes that control sGC expression in vascular tissue remain poorly understood. Previous work in animal and cell models revealed the complexity of alternative splicing of sGC genes and demonstrated its importance in modulation of sGC function. The aim of this study was to examine the role of alternative splicing of α1 and β1 sGC in healthy and diseased human vascular tissue. Our study found a variety of α1 and β1 sGC splice forms expressed in human aorta. Their composition and abundance were different between samples of aortic tissue removed during surgical repair of aortic aneurysm and samples of aortas without aneurysm. Aortas with aneurysm demonstrated decreased sGC activity, which correlated with increased expression of dysfunctional sGC splice variants. In addition, the expression of 55-kDa oxidation-resistant α1 isoform B sGC (α1-IsoB) was significantly lower in aortic samples with aneurysm. The α1-IsoB splice variant was demonstrated to support sGC activity in aortic lysates. Together, our results suggest that alternative splicing contributes to diminished sGC function in vascular dysfunction. Precise understanding of sGC splicing regulation could help to design new therapeutic interventions and to personalize sGC-targeting therapies in treatments of vascular disease.
Keywords: nitric oxide, soluble guanylyl cyclase, alternative splicing, aorta
vascular stiffness and endothelial dysfunction are two important factors that precede and contribute to the development of major cardiovascular disorders, such as coronary artery disease, atherosclerosis, and hypertension (15, 19, 49). Reduction of nitric oxide signaling is considered to be a hallmark of endothelial dysfunction (12). The majority of beneficial effects of NO in blood vessels, including vasodilation, reduction of platelet aggregation, inhibition of smooth muscle cell proliferation, and leukocyte adhesion and infiltration, is mediated by activation of the enzyme soluble guanylyl cyclase (sGC) (29). Several cardiovascular diseases are accompanied by downregulation of sGC protein levels and activity (2, 21, 36). Diminished sGC function contributes to elevated arterial stiffness, hypertension (11, 22), and atherosclerosis (25, 31, 48).
Catalytically active sGC is an obligatory heterodimer composed of α and β subunits. Each subunit type is encoded by two independent genes: GUCY1a3 (α1) and GUCY1a2 (α2); GUCY1b3 (β1) and GUCY1b2 (β2). The α1/β1 sGC is the most highly expressed heterodimer in vascular tissues (20). Several molecular mechanisms were implicated in downregulation of sGC expression and activity in vascular disease, including inhibition of transcription (1, 33, 34, 39, 47), destabilization of mRNA (22), and protein degradation in increased oxidative environment (3, 9, 14). Systematic analysis of the NCBI nucleotide database identifies seven alternatively spliced transcripts for GUCY1A3 gene of the α1 subunit (α1-Tr1 to α1-Tr7) and six splice transcripts for the GUCY1B3 gene of the β1 subunit (β1-Tr1 to β1-Tr6) (42). In addition to canonical full-length α1 (isoform A) and β1 (isoform 2) subunits, three more α1 protein isoforms (isoforms B, C, D) and five more β1 isoforms (isoforms 1, 3, 4, 5, 6) are predicted to be encoded by these transcripts. Our and other laboratories identified and characterized several sGC splice forms and demonstrated that their expression can modulate sGC function and enzymatic properties in vitro (4, 5, 7, 8, 43). These and other studies strongly suggested that alternative splicing of sGC transcripts may be important in regulation of sGC function in vivo. However, splicing regulation of sGC and its biological role in vascular tissue was never previously examined.
In this study, we investigated the alternative splicing of α1 and β1 sGC genes in healthy human aortas and aortas with aneurysm. Our results indicate that altered splicing contributes to sGC dysfunction in aortas and may be a factor in vascular dysfunctions. Examination of expression of individual transcripts may be a useful tool in predicting changes in sGC function and activity in vascular tissue and may serve as a potential diagnostic tool for optimizing personalized sGC-targeted treatments.
MATERIALS AND METHODS
Collection of human aortic tissue samples.
De-identified patient tissue samples were collected at the Department of Clinical Cardiothoracic Surgery at Herman-Memorial Hospital according to the protocol HSC-MS-10-0267 approved by Institutional Review Board Committee for the Protection of Human Subjects and conforming to the principles outlined in the Declaration of Helsinki and with patients' informed consent. Aortic samples were submerged in prechilled DMEM media right after the tissue removal during surgical repair of aortic aneurysm and stored on ice (no longer than 4 h) before snap freezing in liquid nitrogen. According to supplier's (Capital Biosciences) protocol, the samples from donors that succumbed to causes unrelated to aortic aneurysm were harvested within 2 h of death and frozen in liquid nitrogen. All samples were shipped on dry ice. For processing, each sample was thawed on ice and dissected in cold media to remove adventitia. Pieces of whole thickness of aortic wall, containing both intima and media layers, were snap frozen in liquid nitrogen and pulverized by pestle in a prechilled mortar. Aortic tissue powder was split for protein lysate preparation and RNA purification. Total RNA was purified using RiboPure kit (Life Technologies) according to manufacturer's instruction. For preparation of protein lysates, tissue powder was resuspended in cold 50 mM TEA buffer (pH 7.4) containing protease inhibitor cocktail (Sigma-Aldrich) and homogenized by Polytrone. Debris was discarded by centrifugation, whereas supernatant was sonicated. Cleared supernatants prepared by 100,000-g centrifugation for 1 h at 4°C were used for Western blotting and immunoprecipitation.
Quantitative and semi-quantitative reverse transcriptase-polymerase chain reactions.
cDNA for analysis was prepared using high-capacity cDNA kit (Applied Biosystems). The semi-quantitative reverse transcriptase (qRT)-PCR assay for detection of α1 and β1 splice variants was performed as described previously (10). Primer sets used for reverse transcriptase (RT)-PCR detection of individual splice transcripts, and amplicon length are described in Table 1. Amplified PCR products were separated on 3% agarose gel and visualized by ethidium bromide. Electrophoretograms were quantified by densitometry using QuantityOne software (Bio-Rad). Identity of PCR bands was confirmed by direct sequencing. GAPDH levels were used as internal controls for normalization in quantitation of RT-PCR results. The following primers were used for GAPDH amplification: forward: 5′agaaggctggggctcatttg-3′; reverse: 5′-gtgatggcatggactgtggt-3′. Titration with different cDNA amounts was performed to ensure that quantification was used in the linear range of amplification. For qPCR analysis of sGC transcripts, commercial Hs01015574_m1 and Hs00168336_m1 assays (Applied Biosystems) were used to quantify the total levels of α1 and β1 sGC transcripts, respectively. Ribosomal 18S RNA (4308329, Applied Biosystems) was used as an endogenous control for normalization of qPCR results and calculation of ΔCT values (comparative Ct values, where Ct is the PCR cycle when detection threshold is achieved). TaqMan reactions (Applied Biosystems) were performed according to the manufacturer's protocol. To estimate the fold changes in the level of transcripts between two groups, the ΔΔCT value was calculated as ΔCT1 − ΔCT2. The fold difference in the expression was calculated as 2ΔΔCT.
Table 1.
Human samples used in studies
| Sample No. and Group | Age, yr | Gender | Cause of Death/Diagnosis/Notes |
|---|---|---|---|
| Donors without aortic abnormalities* | |||
| 1. CVH | 85 | F | Breast cancer |
| 2. CVH | 61 | M | Alzheimer's disease |
| 3. CVH | 64 | F | Appendiceal cancer |
| 4. CVH | 64 | M | Colon cancer |
| 5. CVH | 81 | F | Acute stroke |
| 6. CVH | 62 | M | Acute stroke |
| 7. CVH | 83 | F | Gastric cancer |
| 8. CVH | 71 | F | Colon and rectal cancer |
| 9. CVH | 80 | F | Brain tumor |
| 10. CVH | 75 | F | Colon and rectal cancer |
| 11. CVH | 47 | M | Colon and rectal cancer |
| Donors with aortic aneurisms† | |||
| 13. AA | 67 | M | Descending thoracic aortic aneurysm, right-sided arch; aneurysm of proximal left subclavian artery; coagulopathy |
| 14. AA | 39 | M | Thoracoabdominal aortic aneurysm, extent IV; chronic aortic dissection; Marfan syndrome |
| 15. AA | 66 | F | Thoracoabdominal aortic aneurysm; myocardial infarction; coronary artery disease |
| 16. AA | 71 | F | Descending thoracic aneurysm; acute respiratory failure; post tracheostomy, acute renal failure |
| 17. AA | 66 | M | Thoracoabdominal aortic aneurysm; carotid stenosis; acute renal failure |
| 18. AA | 49 | M | Infrarenal abdominal aortic aneurysm; hypertension |
| 19. AA | 59 | F | Descending thoracic aortic aneurysm; chronic repaired type A aortic dissection; hypertension |
| 20. AA | 57 | M | Descending thoracic aortic aneurysm extent C; chronic repaired type A aortic dissection; hypertension |
| 21. AA | 25 | F | Thoracoabdominal aortic aneurysm; hypertension |
| 22. AA | 75 | F | Ascending aortic aneurysm; moderate-to-severe aortic insufficiency; transverse arch aneurysm |
| 23. AA | 62 | M | Acute type A aortic dissection; hypertension |
| 24. AA | 66 | M | Ascending aortic aneurysm; aneurysm of the proximal transverse aortic arch (hemiarch); aortic stenosis, insufficiency |
| 25. AA | 64 | F | Ascending aortic aneurysm; cardiomyopathy; hypertension |
| 26. AA | 63 | M | Aneurysm of the ascending aorta, the tubular portion |
| 27. AA | 75 | M | Ascending aortic aneurysm; chronic obstructive pulmonary disease |
| 28. AA | 44 | F | Ascending aorta and proximal transverse arch aneurysm |
| 29. AA | 68 | F | Ascending aortic aneurysm; coronary artery disease, ischemic colitis |
| 30. AA | 75 | M | Ascending aortic aneurysm |
| 31. AA | 76 | M | Ascending aorta and proximal transverse arch aneurysm; coronary artery disease, hypertension, hyperlipidemia, Diabetes mellitus Type 2 |
| 32. AA | 61 | M | Ascending aorta and proximal transverse arch aneurysm; hypertension |
F, female; M, male.
Total n = 11; mean age 70.2 ± 11.7; 7 F, 4 M.
Total n = 20; mean age 61.3 ± 13.2 yr; 8 F, 12 M.
Western blot analysis and immunoprecipitation.
Western blot analysis was performed as described previously (20). Cleared supernatant fractions of protein lysates were separated by SDS-PAGE and transferred on PVDF membranes. For α1 sGC detection, primary monoclonal anti-α1 antibodies (30) that recognize an epitope in the middle portion of the α1 subunit (data not shown) were used. To detect β1 sGC, polyclonal anti-β1 antibodies (26) targeting the COOH terminus were used. Equal sample loading was tested by anti-α-actin antibodies (Abcam). Antibodies conjugated to horseradish peroxidase (Sigma) were used to visualize the signal by enhanced chemiluminescence (ECL Plus, Amersham). For immunoprecipitation, 250 μg of protein from cleared aortic homogenate were incubated with 25 μg of polyclonal anti-β1sGC antibodies (43) overnight at 4°C in 400 μl PBS with protease inhibitors. The samples were then combined with 50 μl of preequilibrated protein G magnetic beads (Fisher Scientific) and further incubated for 1.5 h at room temperature. The supernatant was removed and saved for Western blots, whereas the beads were washed three times with 40 mM TEA, 200 mM NaCl, 1% Nonidet P-40, pH 7.4. Bound proteins were eluted by boiling in 100 μl of Laemmli buffer.
Subcloning and recombinant expression of α1-IsoD (α1-Tr7) in Cos7 cells.
A premature stop codon was introduced by QuickChange site-directed mutagenesis (Stratagene) in the α1 sGC ORF cloned in pcDNA3.1 (Invitrogen) (26) to generate α1-IsoD with a COOH-terminal 66 amino acid deletion. Cos-7 cells were grown in DMEM media supplemented with 10% FBS, 0.1 mM MEM non-essential amino acids, and penicillin-streptomycin mixture (50 U/ml and 50 μg/ml) at 37°C and 5% CO2. A 10-cm tissue culture dish of 80% confluent Cos-7 cells was transfected with 30 μg of pcDNA3.1-α1 or pcDNA3.1-α1isoD and 15 μg of pcDNA3.1-β1 plasmids using Lipofectamine LTX/PLUS reagents (Invitrogen), according to manufacturer's instructions. Used plasmid ratio provides equal expression of both subunits. After 48 h, the cells were collected and sonicated in 500 μl of 50 mM triethanolamine, pH 7.4, 1 mM DTT, 1 mM EGTA, 1 mM EDTA, protease inhibitors, and 0.1 mM IBMX. Supernatants from 15,000-g centrifugation (15 min at 4°C) were tested for sGC activity.
Assay of sGC activity.
Enzymatic activity was assayed using the [32P]GTP-[32P]cGMP conversion assay as described previously (31). For activity in lysates from transfected Cos7 cells, 100 μg of protein lysate was used in a 10-min assay. For activity in aortic tissue homogenates, 250 μg of protein were used in a 25-min assay in the presence of 10 μM DEA-NO or 1 μM BAY58-2667.
Data analysis.
Two-way ANOVA was used to analyze the difference between the control group and groups with descending and ascending aortic aneurysm. Since no statistical difference was detected between the data from aneurysm groups, they were combined and compared with data from the control group. Statistical comparisons between groups were performed by unpaired, two-tailed t-test. P < 0.05 was considered statistically significant. Data are expressed as means ± SD.
RESULTS
Expression of α1 and β1 sGC splice forms in human aorta.
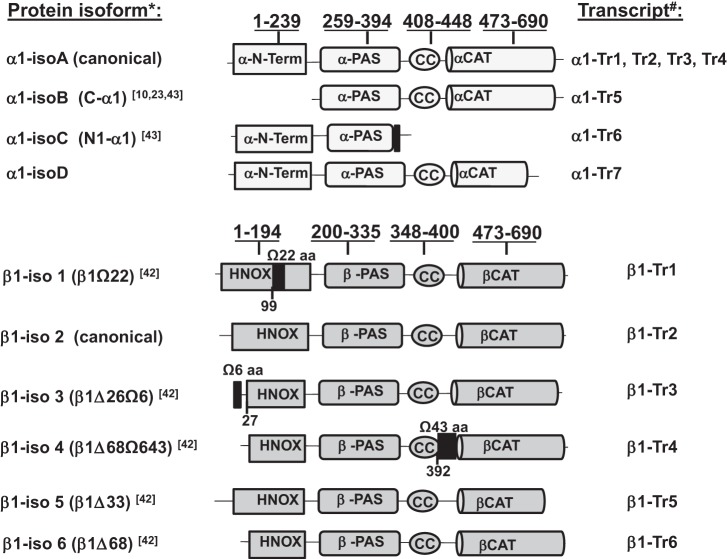
Proper expression of sGC genes is vital for maintaining vascular function. Alternative splicing of α1 and β1 sGC can potentially affect the composition, abundance, and functional properties of sGC protein isoforms expressed in blood vessels. Previous studies demonstrated the expression of human sGC splice forms in cell culture models (42). To investigate whether α1 and β1 sGC splice forms are expressed in human vascular tissue, we obtained aortic specimens from donors deceased from causes unrelated to aortic dysfunction (Capital Biosciences). Samples were collected from middle-age or older (70.2 ± 11.7 yr old) donors of both genders (Table 1). Using RT-PCR analysis with primers targeted to individual α1 or β1 sGC transcripts, we identified eight different splice forms expressed in full thickness (media and intima layers) of human aorta (Table 2). The current nomenclature of α1 and β1 sGC splice transcripts and domain organization of corresponding splice protein variants are summarized in Fig. 1. These data demonstrate that α1 and β1 sGC genes undergo alternative splicing in human aortic tissue.
Table 2.
Functionality and detection of human α1 sGC (GUCY1a3) and β1 (GUCY1b3) sGC transcripts
| Transcript, NCBI Acc. No. | Detected in Aorta | Isoform/functional | Primers | Targeted Sequence | Product Length, bp |
|---|---|---|---|---|---|
| α1 sGC splice isoforms | |||||
| Transcript 1 NM000856 | + | Isoform A/Yes | Fr1: GAAAGCGTGAGCAGGGGGCC | Unique exon 3 | Tr1: 174 |
| Rv1: ACCCTGCTGCGATCTCCTCCT | |||||
| Transcript 2 NM001130682 | − | Isoform A/Yes | No specific sequences, no primers | ||
| Transcript 3 NM001130683 | − | Isoform A/Yes | Fr3: ACACCTGGAACAAGAGCAGCAAA | Exon 1 shared with Tr 5 | Tr3: 155 |
| Rv3: AGGGAAGACGGCAGCTCACTGT | |||||
| Transcript 4 NM001130684 | − | Isoform A/Yes | Fr4: TGGGCCCCAGAGCCCATCAG | Unique exon 1 | Tr4: 259 |
| Fr4: ACCCTGCTGCGATCTCCTCCT | |||||
| Transcript 5 NM001130685 | + | Isoform B//Yes# | Fr5: CAGCCCCGAGGTGTGCGAAG | Deletion in first coding exon | Tr 2,3,6,7,8: 273 Tr 5: 94 |
| Rv5: GGCACGGTTGCTTTGCAGCT | |||||
| Transcript 6 NM001130686 | + | Isoform C/Dominant negative | Fr6: ACCAGACGTTTAGCGGGATCA | Rv primer in unique 3′ end of last exon | Tr6: 192 |
| Rv6: ATCAAACATTTGAAGTGATGCATATA | |||||
| Transcript 7 NM001130687 | + | Isoform D/No activation# | Fr7: AGTTATGTCTCCCCATGGAGAACCT | Rv primer in unique 3′ end of last exon | Tr7: 208 |
| Rv7: AGGTGTTTAACATAATTACTACCTGTAAG | |||||
| β1 sGC splice isoforms | |||||
| Transcript 1 AK300296 | + | Isoform 1/TBD, likely no activation | Fr10: ACAATCTTGCGTGTCCTGGGCTC | In frame insertion of exon 3′ | Tr2: 217 |
| Rv10: GTGCCACTGTTTTGATGATGCCCT | Tr1: 151 | ||||
| Transcript 2 NM000857 | + | Isoform 2/Yes | Fr8: CCTCTGCATGATGCCACGCG | Sequence in exon 10 | Tr2: 300 |
| Rv8: TCTGGTGTAGAGGTCGTTGAGGAGG | |||||
| Transcript 3 AK296680 | − | Isoform 3/TBD, likely no activation | Fr9: CGTACCTCTGCGTGGGGGCT | In frame unique exon 4′ insertion | Tr2: 226 |
| Rv9: TTGAGGACTTTGCTTGCAGCAGCA | Tr3: 378 | ||||
| Transcript 4 AK307838 | + | Isoform 4/TBD, likely no activation | Fr11: GTACAAACGACACTGATGCTGTGTG | Insertion in exon 10 | Tr4: 300 |
| Rv11: CAAATGGGTTTTTCCGGGAATCAGTC | |||||
| Transcript 5 AF020340 | − | Isoform 5/TBD, likely no activation | Fr8: CCTCTGCATGATGCCACGCG | Deletion in exon 10 | Tr2: 300 |
| Rv8: TCTGGTGTAGAGGTCGTTGAGGAGG | Tr5: 240 | ||||
| Transcript 6 AK299916 | + | Isoform 6/TBD, likely no activation | Fr12: GCCAGCGGAAGGGAAGGCTCC | Unique alternative exon 1 | Tr6: 150 |
| Rv12: TTGATGTCTTCCCACACCTCGGGGC | |||||
Fig. 1.
Domain organization of α1 and β1 soluble guanylyl cyclase (sGC) splice variants. The underlined numbers above bars are domain boundaries. The numbering is in respect to canonical variants of human α1 and β1 sGC. The size and positions of splice form-specific inserts (black boxes) are indicated. The names of splice form transcripts and proteins are in accordance with an updated NCBI nomenclature. References and original names for splice forms are indicated in parenthesis. HNOX, domain binding heme/NO/oxygen; PAS, Per/Arnt/Sim domain; CC, coiled coil domain; CAT, catalytic domain.
Impairment of NO/cGMP signaling is one of the prominent features of vascular dysfunction, systemic arterial hypertension, and atherosclerosis (32, 37). The expression and activity of sGC is diminished in these conditions (11, 25, 31, 48). To determine whether sGC splicing and expression is altered in human tissue at late stages of vascular disease, we collected aortic samples from patients who underwent aortic repair surgery due to aortic aneurysm. As presented in Table 1, samples from 20 middle-age patients (61.3 ± 13.2 yr old) of both sexes were collected for these studies.
The expression of α1 and β1 sGC transcripts and proteins is altered in aortas with aneurysm.
First, we assessed whether total α1 or β1 sGC mRNA levels differ in samples from control aortas and aortas with aneurysm. Aortic tissue containing both intima and media layers were used for analysis. We performed a qPCR assays using commercial primers (Applied Biosystems) that target amplicons of α1 or β1 mRNA shared by all of the gene transcripts. Analysis with this pan-specific primers employing comparative ΔCt method demonstrated 3.2 and 2.3 times increase of, respectively, α1 and β1 mRNA in samples with aneurysm (Fig. 2).
Fig. 2.
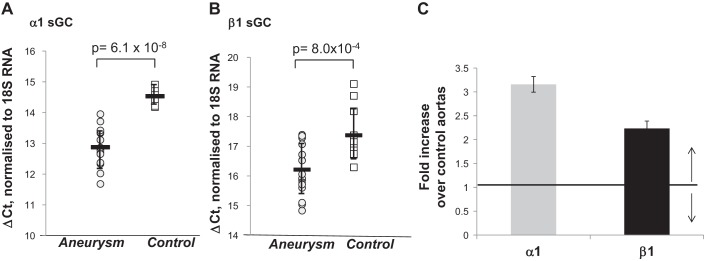
The level of total α1 and β1 sGC transcripts is increased in human aortas with aneurysm. The levels of α1 and β1 transcripts in control aortas and aortas with aneurysm were determined by qRT-PCR using commercial pan-specific α1 (A) and β1 (B) primers and probes. Circles and squares represent the ΔCt value for each individual aortic sample normalized to 18S RNA. These values are inversely proportional to the level of sGC transcripts. Means (horizontal bars) ± SD are shown. C: the fold overexpression for α1 and β1 sGC transcripts in samples with aneurysm over control samples was determined using ΔΔCt method as detailed in methods. P values for diseased vs. control samples are indicated.
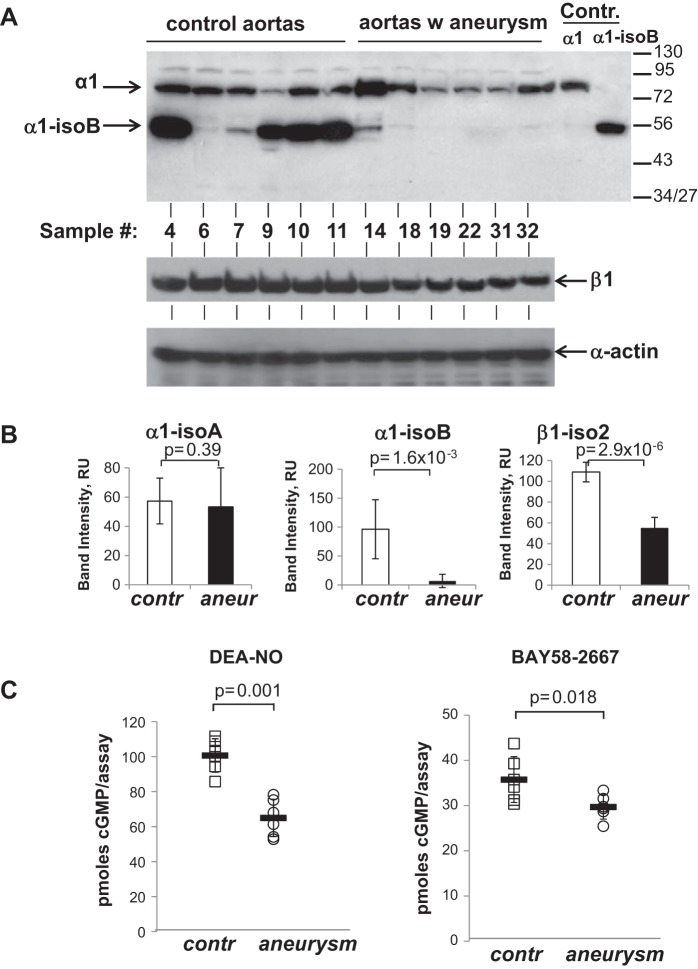
However, Western blot analysis of aortic lysates suggested a more complex pattern of expression, as shown in Fig. 3, A and B. Although the level of canonical full-length α1 (isoform α1-isoA) sGC protein varied significantly among individuals (Fig. 3A), overall difference between control and diseased groups (Fig. 3B) was not statistically significant. Opposite to mRNA data, the level of β1 sGC protein was generally higher in control groups than in those with aneurysm (Fig. 3, A and B). In addition to canonical subunit proteins, we detected a ∼55-kDa α1-immunoreactive band (Fig. 3A). This band was present primarily in control samples. Out of 11 control samples, 8 samples expressed this band. In five samples, this 55-kDa band was the major α1 immunoreactive signal. On the contrary, only 8 of 20 samples with aneurysm expressed this band and at a significantly lower level than in control group.
Fig. 3.
sGC protein level, subunit composition, and activity are altered in aortas with aneurysm. A: 50 μg of protein lysates from aortic samples prepared as described in material and methods were tested by Western blotting using antibodies against α1 (top) or β1 (middle) sGC subunits. Top also contains recombinant α1 and α1-isoB proteins as positive controls (Contr.). Equal protein loading was applied for α1 and β1 test and confirmed by anti-α-actin Western blotting (bottom). Six representative samples for control and aneurysm aortas are shown. The numbers are sample code numbers as presented in Table 1. B: densitometry analysis of α1 and β1 sGC immunoreactive bands normalized to α-actin. C: cGMP-forming activity in 250 μg of aortic lysates was determined in the presence of 10 μM DEA-NO or 1 μM BAY58-2667 activator. Circles and squares represent sGC activity determined for individual samples. Means (horizontal bars) ± SD for each group are shown. The P values for aneurysm vs. control samples are indicated.
sGC activity is diminished in aortic samples with aneurysm.
Next, we evaluated whether the observed changes in sGC proteins expression are reflected in sGC activity. We measured the cGMP-forming activity in aortic lysates obtained from control and aneurysm groups. As demonstrated in Fig. 3C, NO-induced cGMP-forming activity was higher by 36 ± 3.8% in control samples than in aneurysm samples, indicating that sGC function is diminished in diseased aortic tissue. sGC activity induced by NO-independent heme-replacing sGC activator BAY58-2667 was also lower, albeit only by 14 ± 6%, in samples with aneurysm. BAY58-2667 is a heme-independent sGC activator that has a strong preference toward oxidized or heme-deficient sGC (38). Together, these data demonstrate that sGC enzymatic activity is decreased in aortas with aneurysm. This decrease in NO-induced activity is most likely associated with a higher content of sGC with dysfunctional heme.
The 55-kDa immunoreactive band is a constituent part of active sGC in human aorta.
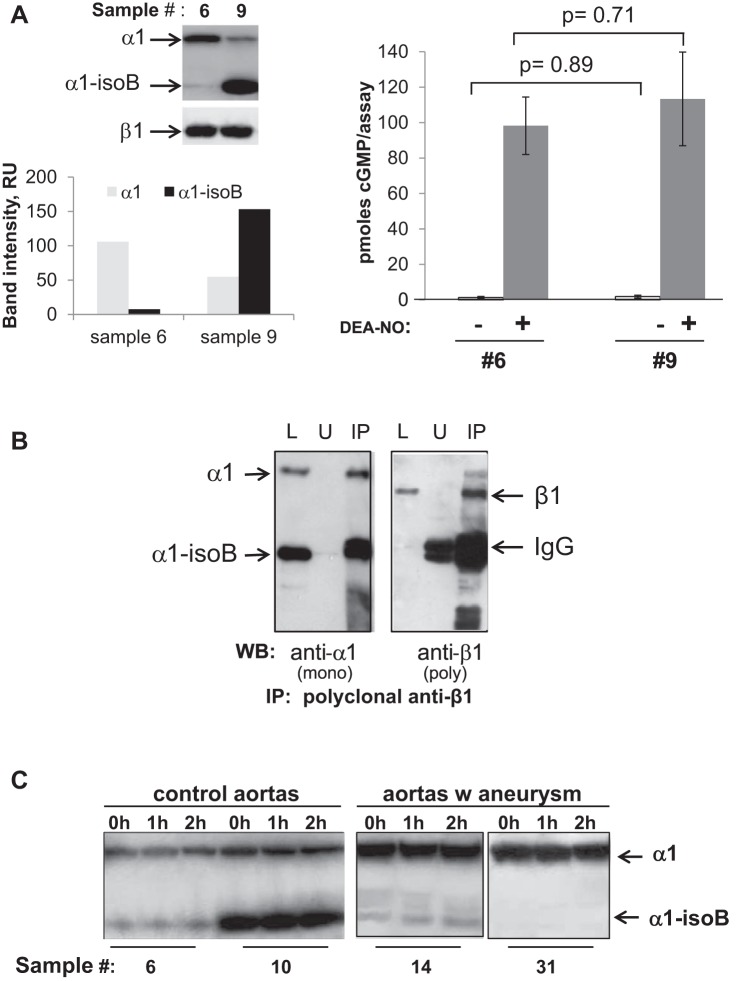
The 55-kDa band is recognized by highly specific anti-α1 monoclonal antibodies and displays the same mobility as the recombinant α1-isoB sGC (Fig. 3A). To evaluate the activity of the α1-isoB/β1 heterodimer, we compared the cGMP-forming activity of control samples from patients 6 and 9, who predominantly express, respectively, either α1/β1 or α1-isoB/β1 heterodimer (Fig. 4A, left). We found that, when normalized to the amount of β1 sGC protein, NO-induced sGC activity in lysates was comparable, regardless of whether α1-isoA or α1-isoB was predominant (Fig. 4A, right).
Fig. 4.
The 55-kDa immunoreactive band is a constituent part of active sGC in human aorta. A: top left: Western blotting of lysates from control samples 6 and 9 that express predominantly α1 or α1-isoB proteins. Bottom left: densitometry data showing the ratio of α1 and α1-isoB isoforms in samples 6 and 9. Right: the expression of α1-isoB compensates for sGC activity in samples with low level of canonical α1 subunit. The volume of lysates from control samples 6 and 9 containing equal level of β1 subunit was used to determine cGMP-forming activity under basal conditions or in the presence of 10 μM DEA-NO. Data are means ± SD (n = 4).The P values are indicated. B: the α1-isoB variant co-precipitates with β1 sGC subunit; lysate from sample 11 (Fig. 3) was incubated with polyclonal anti-β1 sGC antibodies and pooled down with protein G magnetic beads, as detailed in material and methods. The lysate (L), immunopellet (IP), and unbound samples (U) were probed by Western blotting using monoclonal anti-α1 or polyclonal anti-β1 antibodies. C: the immunoreactive ∼55-kDa α1-isoB band is not a degradation product of canonical α1 sGC protein; the intensity of α1 and α1-isoB bands in aortic lysates does not change even after 2 h of incubation at room temperature.
Next, we investigated whether α1-isoB sGC associates with β1 sGC subunit. Using anti-β1 antibodies, we immunoprecipitated the β1 subunit from a sample that expresses both α1 and α1-isoB sGC. The immunoprecipitation was optimized to quantitatively capture the β1 subunit in the immunopellet (fraction IP in Fig. 4B). No β1 signal was detected in the unbound fraction (fraction U in Fig. 4B). Under these conditions, both α1-isoA and α1-isoB were completely captured in the immunopellet (Fig. 4B). These results strongly indicate that α1-isoB forms a stable heterodimer β1 sGC in aortic lysates.
In addition, we considered whether α1-immunoreactive 55-kDa band is the product of degradation of canonical α1 sGC subunit. We tested whether incubation of aortic lysates at room temperature diminishes the α1-isoA amount or increases the α1-isoB amount. As shown in Fig. 4C, we did not observe any changes in protein levels of α1-isoA or α1-isoB sGC neither in control nor in aneurysm samples over a period of 2 h. All samples used in these studies were collected within this time window and were kept at <4°C before being frozen in liquid nitrogen. Separately, we confirmed that the patterns of α1 and β1 sGC immunoreactive bands do not change with prolong storage of aortic tissue at −80°C (data not shown). These data allow us to conclude that the ∼55-kDa band is not a degradation product but the α1-isoB sGC isoform. Together, stability, immunoprecipitation, and activity data argue that the α1-isoB/β1 heterodimer is expressed in human aorta and supports sGC activity.
The expression of nonfunctional splice forms of α1 and β1 transcripts is elevated in aortas with aneurysm.
Since control aortic samples contained various alternative splice variants of α1 and β1 sGC subunits, we investigated whether the expression of individual splice form transcripts is altered in aortas with aneurysm. Using a semi-quantitative RT-PCR analysis with splice form-specific primers, we observed that the patterns of expression of individual sGC splice transcripts do not always coincide with the overall increased of total sGC mRNA in aneurysm group (Figs. 5 and 6).
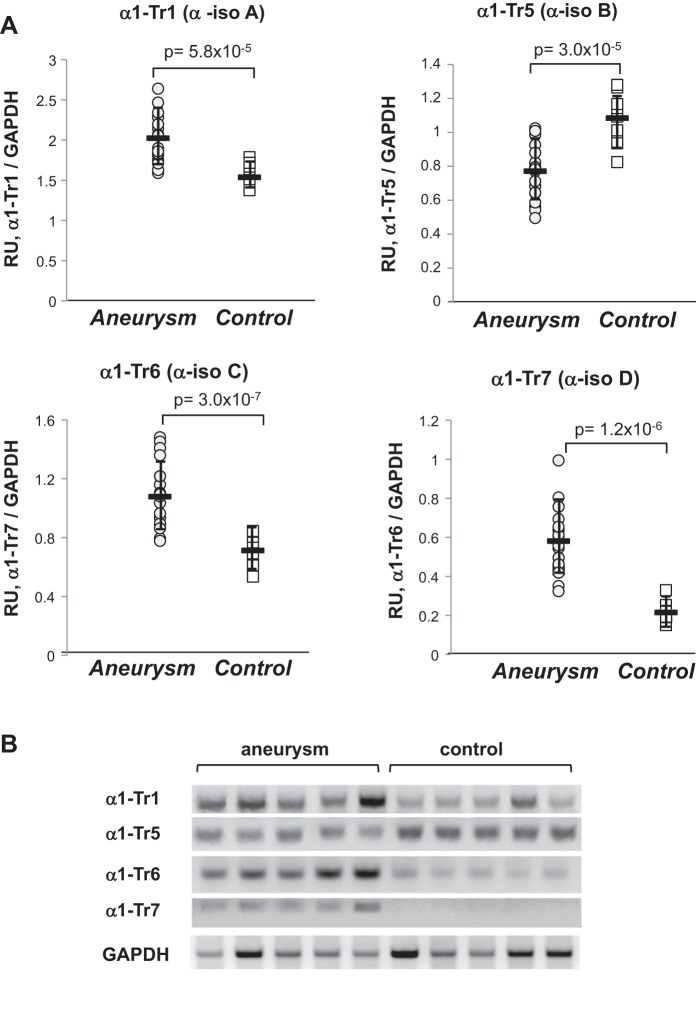
Fig. 5.
The expression of α1 sGC splice transcripts is altered in aortas with aneurysm. A: the relative abundance of α1 splice transcripts detected in human aorta was determined by semi-quantitative RT-PCR and quantified by densitometry. The intensity of transcripts α1-Tr1, α1-Tr5, α1-Tr6, and α1-Tr7 bands was normalized to GAPDH. Results are means ± SD (n = 20 for aortas with aneurysm and n = 11 for control group). The P values for aneurysm vs. control samples are indicated. B: representative electrophoretogram for five samples with aneurysm and five control aortas.
Fig. 6.
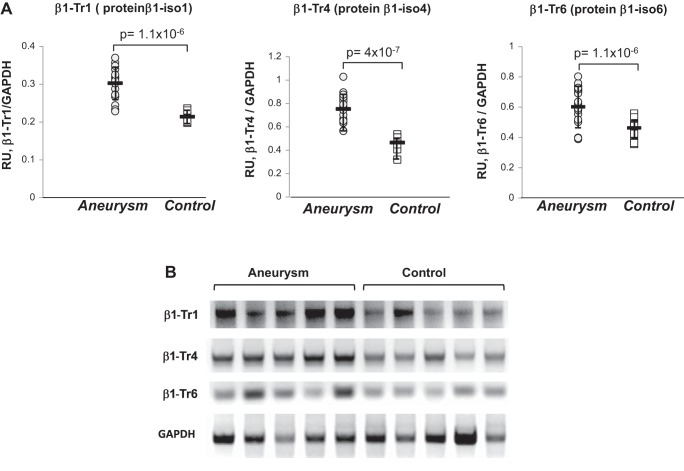
The expression of individual β1 sGC transcripts is altered in aortas with aneurysm. A: the relative abundance of β1 splice transcripts detected in human aorta was determined by semi-quantitative RT-PCR, quantified by densitometry, and normalized to GAPDH. Means ± SD are shown (n = 20 for aortas with aneurysm and n = 11 for control group). The P values for aneurysm vs. control samples are indicated. B: representative electrophoretogram for five samples with aneurysm and five control aortas.
Consistent with pan-specific qRT-PCR, the level of transcripts α1-Tr1 (protein isoform α1-isoA), α1-Tr6 (protein isoform α1-isoC), and α1-Tr7 (protein isoform α1-isoD) were higher in diseased aortas (Fig. 5). The α1-isoC isoform lacks the COOH-terminal catalytic domain (Fig. 1) and is catalytically inactive. In fact, previous in vitro studies demonstrated that the α1-isoC variant is inhibitory when co-expressed with canonical α1-isoA/β1 sGC (43). The level of α1-Tr7, encoding isoform α1-isoD, was several times higher in aneurysm samples. Isoform α1-isoD lacks 66 COOH-terminal residues (Fig. 1) and has impaired enzymatic activity (see below). On the contrary, the level of α1-Tr5, encoding oxidation-resistant α1-isoB, was lower in aneurysm samples. Thus, despite the overall increase in α1 sGC mRNA in aneurysm samples, the transcripts coding for dysfunctional α1-isoC and α1-isoD variant were also increased, whereas the transcript coding for functional α1-isoB was diminished.
For β1 sGC splice variants, the difference between control and aneurysm groups was less pronounced. The lack of a unique sequence specific for canonical transcript 2 β1 sGC prevented us from evaluating the level of β1-Tr2 alone without the input of other splice variants. However, we were able to assess the level of other β1 splice transcripts. We found that the expression of β1-Tr1, β1-Tr4, and β1-Tr6 transcripts was higher in aneurysm group (Fig. 6). The β1-Tr1 transcript encodes the β1-isoform 1 protein, an isoform that contains a 22-amino acid insertion in the HNOX domain (see Fig. 1). The HNOX domain of sGC is responsible for heme and NO binding. Therefore, the insertion in that area should negatively affect the binding of heme in β1-iso1 protein. The β1-Tr6 transcript encodes the β1-isoform 6 protein with a large 68-amino acid deletion in HNOX domain (see Fig. 1). This β1-isoform 6 sGC also should have an impaired heme function. The β1-isoform 4 sGC, encoded by the β1-Tr4 transcript, not only carries the same 68-residue deletion but also a 43-amino acid insertion close to the catalytic domain (see Fig. 1). Although β1 sGC splice forms are not yet biochemically characterized, alterations in proteins they encode suggest that respective sGC heterodimers would have impaired NO stimulation and diminished cGMP-forming activity.
α1-IsoD/β1 heterodimer has a diminished activation response.
RT-PCR analysis indicated that α1-Tr7 splice transcript is barely detectable in control aortas but is well represented in aneurysm samples (Fig. 5). The α1-Tr7 transcript encodes the α1-isoD protein isoform, which lacks 66 residues at the COOH terminus. To understand how the presence of α1-isoD might affect the overall sGC function in aorta, we evaluated enzymatic properties of recombinant α1-IsoD subunit. The plasmid that expresses α1-IsoD variant was generated and transfected into Cos7 cells together with the plasmid expressing canonical β1 sGC subunit. Cos7 cells lack endogenous sGC expression and are widely used to evaluate the properties of recombinant sGC (27). The lysates from Cos7 cells expressing α1-IsoD and β1 sGC displayed a detectable basal cGMP-forming activity, confirming the formation of a catalytically competent α1-isoD/β1 heterodimer (Fig. 7). However, the activity of α1-IsoD/β1 sGC was only marginally induced by 1 μM NO treatment or by a combination of NO and allosteric sGC stimulator BAY41–2272, in contrast to strong activation of canonical α1/β1 sGC. Another allosteric sGC stimulator dicyanocobinamide CN2-Cbi, which activate sGC independently of heme status (41), induced only a marginal increase of the basal activity in α1-IsoD/β1-containing lysates. These data demonstrate that α1-IsoD/β1 heterodimer has a significantly impaired activation capacity, suggesting that increased expresson of α1-IsoD should interfere with sGC function in vascular cells.
Fig. 7.
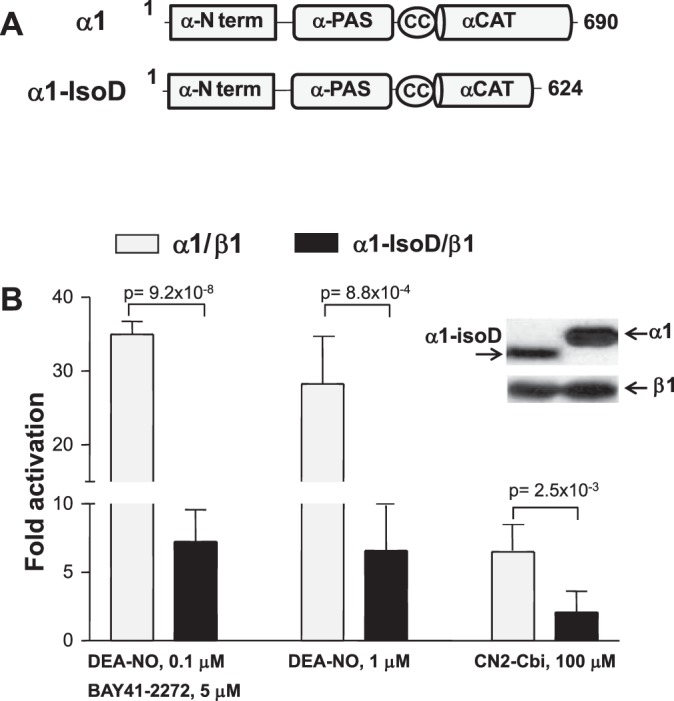
sGC heterodimer containing α1-isoD has a diminished activation capacity. A: domain organization of canonical α1 and α1-isoD variants. α-N term., NH2-terminal region; PAS, Per/Arnt/Sim domain; CC, coiled coil domain; CAT, catalytic domain. B: fold activation of cGMP-forming activity in lysates of COS7 cells ectopically expressing α1/β1 and α1-isoD/β1 heterodimers. Data are means ± SD from three independent experiments performed in triplicates. The P values for α1/β1 vs. α1-isoD/β1 sGC activities for each treatment are indicated.
DISCUSSION
Diminished sGC function is associated with elevated arterial stiffness, progression of hypertension, and atherosclerosis. Therefore, it is important to understand the processes affecting expression of functional sGC in normal and diseased human vasculature. It has been reported that expression of sGC protein is regulated by a combination of transcriptional, posttranscriptional, translational, and protein stability processes (35, 42). The heterodimeric sGC structure adds a higher level of complexity, since changes in expression of even one subunit affect sGC activity. In this report, we demonstrate that α1 and β1 sGC genes undergo extensive alternative splicing in human aortic tissue. We found that in human aorta each gene is represented by four different spliced forms, which encode unique protein variants (Fig. 1; Table 2). Moreover, we report that the abundance and composition of sGC splice forms differs between aortas with and without aneurysm. Interestingly, the diversity of mammalian sGC splice forms seems to be restricted to humans. Presently, there are very few alternative splice variants of sGC detected in rodents, most of which do not have direct human orthologs (NCBI database, http://www.ncbi.nlm.nih.gov/).
An unexpected finding was that qPCR analysis with pan-specific primers demonstrated a two- to threefold increase of total amount of α1 and β1 sGC transcripts in diseased samples. However, this increase in total sGC transcripts did not result in higher level of functional sGC protein. On the contrary, aortas with aneurysm had the same expression of canonical full-size α1-IsoA sGC and a lower level of β1 sGC than control aortas. We also found that most control aortas highly express the α1-IsoB splice form, which was the major α1 protein in some samples. The expression of 55-kDa α1-isoB immunoreactive band was reported previously in human brain (18). Without the benefit of current bioinformatics data, the band was considered the product of a degradation process that occurs in vivo but not in vitro. Immunoprecipitation studies and activity evaluation presented in this report clearly demonstrate that α1-IsoB forms a functional heterodimer with β1 subunit in aorta in vivo, validating previous in vitro observations (23, 43). However, aortas with aneurysm had only traces of the α1-IsoB protein and a much lower level of the respective α1-Tr 5 transcript. We have previously reported that ectopic expression of α1-IsoB protects β1 sGC subunit from oxidative protein degradation (43). Slightly higher levels of β1 protein observed in control samples are perhaps the result of this protective function.
Expression of α1-Tr6 and α1-Tr7 transcripts, coding for α1-isoC and α1-IsoD protein isoforms, was elevated in aortas with aneurysm. Previous studies demonstrated that α1-IsoC inhibits sGC activity (43), whereas data presented in this report demonstrate that α1-IsoD does not support sGC activation (Fig. 7). The levels of β1-Tr1, β1-Tr4, and β1-Tr6 splice transcripts, encoding protein isoforms β1-Iso1, β1-Iso4, and β1-Iso6 sGC, were also elevated in aortas with aneurysm. Current understanding of sGC structure and function (13) predicts with a high degree of confidence that insertions and deletions in the heme-binding domain of these β1 sGC splice isoforms will result in heterodimers with significantly impaired heme content and activation by NO. Therefore, analysis of expression of individual α1 and β1 sGC splice forms indicates that the composition of sGC transcripts in aortas with aneurysm is altered in favor of nonfunctional or partially functional sGC splice variants. These changes predict a diminished sGC function in vasculature. This conclusion is directly confirmed by sGC activity measurements. NO-dependent sGC activity was lower by 36% in aortas with aneurysm, suggesting impaired sGC function in these samples. Even more revealing is that the difference between sGC activity in control aortas and aortas with aneurysm was less pronounced when it was probed by BAY58-2667, an activator that preferentially targets heme-deficient sGC, or sGC with oxidized heme (45). These data indicate that aortas with aneurysm have a higher content sGC with deficient/oxidized heme.
sGC is expressed in smooth muscle and endothelial cells of both vascular cell types. Considering that the monolayer of endothelial cells has a relatively small contribution to the total amount of protein or RNA material isolated from investigated aortic samples, our findings most likely reflect the changes in sGC splicing occurring in the more abundant vascular smooth muscle cells.
It is important to note that multiple risk factors contribute to the development of aortic aneurysm. Only 20% of cases are considered to have genetic predisposition (28). Other risk factors include alterations in the direct mechanical forces on the aortic wall (i.e., hypertension, hypervolemia, derangements of aortic flow) and abnormalities in intrinsic properties of aortic tissue (connective tissue disorders or direct chemical damage) (17). Uncontrolled hypertension is present in 70% of individuals with thoracic aortic diseases (6). Both atherosclerosis and hypertension have been associated with diminished sGC expression and function (11, 25, 31, 48). As represented in Table 1, diseased samples were collected from patients with descending or ascending aortic aneurysm, which have different etiologies. Nevertheless, when the groups with descending or ascending aortic aneurysm were analyzed separately, the changes in the composition and abundance of sGC splice transcripts between these two groups were not statistically significant (results not shown). Many donors in disease group had preexisting cardiovascular conditions before the development of aneurysm; most samples had clear signs of atherosclerosis (not shown), and a significant number of donors had been diagnosed with hypertension (Table 1). Individuals from the control group, although not diagnosed with aortic dysfunctions, deceased from a variety of causes. It is revealing that, despite the great variability between donors' preexisting cardiovascular abnormalities, age, and sex differences, differences in sGC splicing were well pronounced between healthy and diseased aortas. These data argue that sGC splicing is an important, previously unrecognized factor contributing to the development of vascular disease.
It has been demonstrated that inflammatory conditions, including exposure to several cytokines and increased concentrations of reactive oxygen species (ROS), decrease the expression of sGC (42). In cell culture models, when oxidative stress was mimicked by treatments with H2O2 (10) or pharmacological oxidation of sGC heme (43), the apparent half-life of canonical sGC was ∼5 h. Co-expression of α1-isoB splice form, which has a much longer half-life, resulted in significant stabilization of sGC. Oxidative stress is known to persist in vascular disorders and to increase with age, which may explain accumulation of α1-IsoB in control aortas. Therefore, increased α1-isoB expression observed in control aortas in the present report may be a positive factor contributing to the maintenance of sGC function under oxidative stress conditions. On the contrary, diminished expression of α1-isoB compounded by increased expression of dysfunctional sGC splice forms should diminish sGC function, further exacerbating the dysfunction of NO/cGMP signaling in diseased vasculature. This should negatively affect not only vascular relaxation but also cellular differentiation and vascular remodeling (24, 30, 40). Further studies are needed to understand the contribution of individual sGC splice forms to the development of aortic aneurysm.
The patients recovering after aortic repair surgeries are under a complex drug regimen, which in most cases includes sGC-targeting nitrovasodilators. Within the last decade, a number of NO-independent sGC regulators have been developed and are undergoing clinical trials or have been approved for treatment (44, 46). The efficacy of sGC-targeting drugs is strongly affected by the level and functional state of sGC enzyme. Therefore, determining the composition and ratio of sGC isoforms in surgically removed aortic samples may provide necessary information for optimizing personalized sGC-targeting therapy in postsurgical patients. Although more extensive validation is needed, the levels for transcripts α1-Tr6 and α1-Tr7 and β1-Tr4 may be important indicators of impaired NO/cGMP signaling.
Emerging new strategies allow for targeted manipulations of RNA splicing (50). Stable interfering morpholino RNA oligonucleotides are currently being developed to alter and/or control splicing of selected genes, e.g, splicing correction using this method is presently under development for the treatment of β-thalassemia (26) and cystic fibrosis (16), among other things. This technology potentially provides new therapeutic tools modulating sGC function in cardiovascular diseases to restore the composition of sGC splice transcripts featured in healthy blood vessels.
In summary, our report provides the first clear evidence for alternative splicing of α1 and β1 sGC in human vasculature. The data presented here indicate that alternative splicing may contribute to impairment of sGC function in aortic aneurysm. Precise understanding of sGC splicing regulation should help to develop new approaches to treatment of vascular dysfunction and assist in optimizing personalized sGC-targeting treatments.
GRANTS
This work was supported by departmental start-up funds (I.G.S.), National Institute of Heart, Lung, and Blood Institute (grant 5R01 HL-088128, E.S.M.), and American Heart Association (grant 12GRNT11930007, E.S.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.S.M., E.B.G., A.E., and I.G.S. performed experiments; E.S.M., E.B.G., S.T.L., and I.G.S. analyzed data; E.S.M., S.T.L., A.E., and I.G.S. interpreted results of experiments; E.S.M., E.B.G., and I.G.S. prepared figures; E.S.M., S.T.L., A.E., and I.G.S. edited and revised manuscript; E.S.M. and I.G.S. approved final version of manuscript; S.T.L. and I.G.S. conception and design of research; I.G.S. drafted manuscript.
ACKNOWLEDGMENTS
We are thankful to Drs. Gilbert Cote, Heinrich Taegtmeyer, and Susan Tybur for critical reading of the manuscript.
REFERENCES
- 1.Baltrons MA, Garcia A. Nitric oxide-independent down-regulation of soluble guanylyl cyclase by bacterial endotoxin in astroglial cells. J Neurochem 73: 2149–2157, 1999. [PubMed] [Google Scholar]
- 2.Bauersachs J, Bouloumie A, Mulsch A, Wiemer G, Fleming I, Busse R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc Res 37: 772–779, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep 60: 119–126, 2008. [PubMed] [Google Scholar]
- 4.Behrends S, Harteneck C, Schultz G, Koesling D. A variant of the alpha 2 subunit of soluble guanylyl cyclase contains an insert homologous to a region within adenylyl cyclases and functions as a dominant negative protein. J Biol Chem 270: 21109–21113, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Behrends S, Steenpass A, Porst H, Scholz H. Expression of nitric oxide-sensitive guanylyl cyclase subunits in human corpus cavernosum. Biochem Pharmacol 59: 713–717, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Booher AM, Isselbacher EM, Nienaber CA, Froehlich JB, Trimarchi S, Cooper JV, Demertzis S, Ramanath VS, Januzzi JL, Harris KM, O'Gara PT, Sundt TM, 3rd, Pyeritz RE, Eagle KA, International Registry of Acute Aortic Dissection I. Ascending thoracic aorta dimension and outcomes in acute type B dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am J Cardiol 107: 315–320, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Cabilla JP, Ronchetti SA, Nudler SI, Miler EA, Quinteros FA, Duvilanski BH. Nitric oxide sensitive-guanylyl cyclase subunit expression changes during estrous cycle in anterior pituitary glands. Am J Physiol Endocrinol Metab 296: E731–E737, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Chhajlani V, Frandberg PA, Ahlner J, Axelsson KL, Wikberg JE. Heterogeneity in human soluble guanylate cyclase due to alternative splicing. FEBS Lett 290: 157–158, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Costell MH, Ancellin N, Bernard RE, Zhao S, Upson JJ, Morgan LA, Maniscalco K, Olzinski AR, Ballard VL, Herry K, Grondin P, Dodic N, Mirguet O, Bouillot A, Gellibert F, Coatney RW, Lepore JJ, Jucker BM, Jolivette LJ, Willette RN, Schnackenberg CG, Behm DJ. Comparison of soluble guanylate cyclase stimulators and activators in models of cardiovascular disease associated with oxidative stress. Front Pharmacol 3: 128, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cote GJ, Zhu W, Thomas A, Martin E, Murad F, Sharina IG. Hydrogen peroxide alters splicing of soluble guanylyl cyclase and selectively modulates expression of splicing regulators in human cancer cells. PLos One 7: e41099, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dangel O, Mergia E, Karlisch K, Groneberg D, Koesling D, Friebe A. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J Thromb Haemost 8: 1343–1352, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 109: 27–32, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Ann Rev Biochem 81: 533–559, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nature Rev Drug Disc 5: 755–768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation 126: 753–767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman KJ, Kole J, Cohn JA, Knowles MR, Silverman LM, Kole R. Correction of aberrant splicing of the cystic fibrosis transmembrane conductance regulator (CFTR) gene by antisense oligonucleotides. J Biol Chem 274: 36193–36199, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Green KIL, Ri G. Aortic dissection. In: Cardiac Surgery in the Adult, edited by Cohn L. New York: McGraw-Hill, 2003, p. 1095–1122. [Google Scholar]
- 18.Ibarra C, Nedvetsky PI, Gerlach M, Riederer P, Schmidt HH. Regional and age-dependent expression of the nitric oxide receptor, soluble guanylyl cyclase, in the human brain. Brain Res 907: 54–60, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamisaki Y, Saheki S, Nakane M, Palmieri JA, Kuno T, Chang BY, Waldman SA, Murad F. Soluble guanylate cyclase from rat lung exists as a heterodimer. J Biol Chem 261: 7236–7241, 1986. [PubMed] [Google Scholar]
- 21.Kloss S, Bouloumie A, Mulsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension 35: 43–47, 2000. [PubMed] [Google Scholar]
- 22.Kloss S, Rodenbach D, Bordel R, Mulsch A. Human-antigen R (HuR) expression in hypertension: downregulation of the mRNA stabilizing protein HuR in genetic hypertension. Hypertension 45: 1200–1206, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kraehling JR, Busker M, Haase T, Haase N, Koglin M, Linnenbaum M, Behrends S. The amino-terminus of nitric oxide sensitive guanylyl cyclase alpha(1) does not affect dimerization but influences subcellular localization. PLos One 6: e25772, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88: 4651–4655, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Lacerra G, Sierakowska H, Carestia C, Fucharoen S, Summerton J, Weller D, Kole R. Restoration of hemoglobin A synthesis in erythroid cells from peripheral blood of thalassemic patients. Proc Natl Acad Sci USA 97: 9591–9596, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamothe M, Chang FJ, Balashova N, Shirokov R, Beuve A. Functional characterization of nitric oxide and YC-1 activation of soluble guanylyl cyclase: structural implication for the YC-1 binding site? Biochemistry 43: 3039–3048, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Ann Rev Genomics Human Genetics 9: 283–302, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Noiri E, Lee E, Testa J, Quigley J, Colflesh D, Keese CR, Giaever I, Goligorsky MS. Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol Cell Physiol 274: C236–C244, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Luscher TF. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation 97: 2494–2498, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papapetropoulos A, Marczin N, Mora G, Milici A, Murad F, Catravas JD. Regulation of vascular smooth muscle soluble guanylate cyclase activity, mRNA, and protein levels by cAMP-elevating agents. Hypertension 26: 696–704, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Pedraza CE, Baltrons MA, Heneka MT, Garcia A. Interleukin-1 beta and lipopolysaccharide decrease soluble guanylyl cyclase in brain cells: NO-independent destabilization of protein and NO-dependent decrease of mRNA. J Neuroimmunol 144: 80–90, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Pyriochou A, Papapetropoulos A. Soluble guanylyl cyclase: more secrets revealed. Cell Signal 17: 407–413, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Ruetten H, Zabel U, Linz W, Schmidt HH. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res 85: 534–541, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, Kaye DM. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation 110: 3680–3686, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handbook Exp Pharmacol 309–339, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Scott WS, Nakayama DK. Escherichia coli lipopolysaccharide downregulates soluble guanylate cyclase in pulmonary artery smooth muscle. J Surg Res 80: 309–314, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Seki J, Nishio M, Kato Y, Motoyama Y, Yoshida K. FK409, a new nitric-oxide donor, suppresses smooth muscle proliferation in the rat model of balloon angioplasty. Atherosclerosis 117: 97–106, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Sharina I, Sobolevsky M, Doursout MF, Gryko D, Martin E. Cobinamides are novel coactivators of nitric oxide receptor that target soluble guanylyl cyclase catalytic domain. J Pharmacol Exp Ther 340: 723–732, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharina IG, Cote GJ, Martin E, Doursout MF, Murad F. RNA splicing in regulation of nitric oxide receptor soluble guanylyl cyclase. Nitric Oxide 25: 265–274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharina IG, Jelen F, Bogatenkova EP, Thomas A, Martin E, Murad F. Alpha1 soluble guanylyl cyclase (sGC) splice forms as potential regulators of human sGC activity. J Biol Chem 283: 15104–15113, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Hand Exp Pharmacol 218: 279–313, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handbook Exp Pharmacol 277–308, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takata M, Filippov G, Liu H, Ichinose F, Janssens S, Bloch DB, Bloch KD. Cytokines decrease sGC in pulmonary artery smooth muscle cells via NO-dependent and NO-independent mechanisms. Am J Physiol Lung Cell Mol Physiol 280: L272–L278, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Verbeuren TJ, Jordaens FH, Van Hove CE, Van Hoydonck AE, Herman AG. Release and vascular activity of endothelium-derived relaxing factor in atherosclerotic rabbit aorta. Eur J Pharmacol 191: 173–184, 1990. [DOI] [PubMed] [Google Scholar]
- 49.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation 105: 546–549, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Wilton SD, Fletcher S. RNA splicing manipulation: strategies to modify gene expression for a variety of therapeutic outcomes. Curr Gene Ther 11: 259–275, 2011. [DOI] [PubMed] [Google Scholar]