Abstract
Mature cardiac myocytes are terminally differentiated, and the heart has limited capacity to replace lost myocytes. Thus adaptation of myocyte size plays an important role in the determination of cardiac function. The hypothesis tested is that regulation of the dynamic exchange of actin leads to cardiac hypertrophy. ANG II was used as a hypertrophic stimulant in mouse heart and neonatal rat ventricular myocytes (NRVMs) in culture for assessment of a mechanism for regulation of actin dynamics by phosphatidylinositol 4,5-bisphosphate (PIP2). Actin dynamics in NRVMs rapidly increased in a PIP2-dependent manner, measured by imaging and fluorescence recovery after photobleaching (FRAP). A significant increase in PIP2 levels was found by immunoblotting in both adult mouse heart tissue and cultured NRVMs. Inhibition of phosphatase and tensin homolog (PTEN) in NRVMs markedly blunted ANG II-induced increases in actin dynamics, the PIP2 level, and cell size. Furthermore, PTEN activity was dramatically upregulated in ANG II-treated NRVMs but downregulated when PTEN inhibitors were used. The time course of the rise in the PIP2 level was inversely related to the fall in the PIP3 level, which was significant by 30 min in ANG II-treated NRVMs. However, significant translocation of PTEN to the plasma membrane occurred by 10 min, suggesting a crucial initial step for PTEN for the cellular responses to ANG II. In conclusion, PTEN and PIP2 signaling may play an important role in myocyte hypertrophy by the regulation of actin filament dynamics, which is induced by ANG II stimulation.
Keywords: sarcomere remodeling, lipid signaling, membrane translocation, G protein receptors
the addition of sarcomeric units to cardiomyocytes requires filaments to be added for cardiac hypertrophy. Live imaging of actin in striated muscle has revealed that actin subunits in the thin filaments are dynamically exchanged (32). One of the regulators for actin dynamics is the actin capping protein Z (CapZ), and malfunction of CapZ resulted in disorganization of myofibril structures or disease (29). Both enhancers and stabilizers of actin dynamics are important for myocyte hypertrophy. However, the mechanisms for actin filament addition are not yet well understood.
PIP2 is a lipid messenger in its own right, instead of simply being a precursor of messengers. Direct interaction of PIP2 with the actin accessory proteins profilin and gelsolin promoted or inhibited assembly of F-actin filaments (10, 12). Recently, our group showed that PIP2 binding to CapZβ1 altered sarcomeric actin dynamics in cultured cardiomyocytes (8, 14). Many PIP2 effector proteins function in other organelles, for example in focal adhesion formation, vesicle trafficking via integrin, or E-cadherin, and at sites of mRNA processing in the nucleus (18, 24, 19, 36). PIP2 was localized to the Z-disc, which suggested its role in sarcomeric reorganization (14). Furthermore, PIP2 is implicated in ANG II-induced cardiac hypertrophy in an animal model (40).
The phosphatase and tensin homolog (PTEN) has numerous roles but was originally established as one of the most frequently mutated tumor suppressor genes in human cancer. PTEN is a 3′-lipid phosphatase, which comprises an NH2-terminal phosphatase domain, an NH2-terminal PIP2-binding polybasic tail, a C2 domain, and a COOH-terminal tail region that contains multiple phosphorylation sites (13, 23). Much is known about PTEN chemistry and its action for the control of cell number. However, less is understood about cell size regulation even though PTEN is widely expressed in cardiomyocytes (4, 34). The mechanisms involving PTEN in cardiac hypertrophy are controversial. The inactivation of PTEN increased cell size and growth by amplifying PI3K signaling, whereas overexpression of PTEN inhibited PI3K signaling, resulting in decreased cell size (11). However, a recent study identified that the loss of PTEN could prevent the development of maladaptive ventricular remodeling in response to pressure overload but not in response to ANG II, suggesting that additional complex regulatory pathways may exist (27).
The main physiological substrate of PTEN is membrane-bound PIP3, and PTEN is activated when recruited to the plasma membrane (5). PTEN converted the lipid second messenger phosphatidylinositol (3,4,5)-trisphosphate (PIP3) to PIP2 (22), as a negative regulator of phosphoinositide 3-kinase (PI3K) signaling. Thus the balance between PTEN and PI3K controls the basal levels of PIP3 in the plasma membrane, which in turn regulates cell survival and proliferation. Therefore, the activity and intracellular distribution of PTEN after treatment with the ANG II stimulus were measured in this study to test the hypothesis that PTEN plays a key role via PIP2 in regulating actin dynamics in cardiomyocytes. Our findings show that stimulation by ANG II increased the activity of PTEN, the level of PIP2, and dynamics of actin filament, resulting in increased cell size. Therefore, PTEN and PIP2 signaling may play an important role in myocyte hypertrophy by the regulation of actin filament dynamics, which is induced by ANG II stimulation.
MATERIALS AND METHODS
Ethical approval.
All experiments were conducted following National Institutes of Health guidelines. All experiments were approved by the University Committee on the Use and Care of Animals at the University of Illinois at Chicago and the Biologic Resources Laboratory. All euthanasia was performed following the recommendations of the University Committee on the Use and Care of Animals and the Biologic Resources Laboratory and the Committee for the Humane Use of Animals.
Animals.
Eight 8-wk-old C57B6/J mice were randomly divided into two groups of four animals. ANG II (Sigma-Aldrich) was administered with osmotic mini-pumps (Alzet model 1002, 1 μg·g−1·day−1) for 2 wk. In the control group, mice received vehicle (saline solution) for 2 wk. The dosage of ANG II was chosen from previous studies (20), which induced left ventricle hypertrophy in mice as confirmed by heart and weight measurements.
NRVM culture.
Hearts were removed and cells isolated from 1- to 2-day-old Sprague-Dawley rats with collagenase type II (Worthington, Lakewood, NJ) as previously described (3). NRVMs were resuspended, filtered through a metal sieve to remove large material, and plated at high density (1,000 cells/mm2) in PC-1 medium (Lonza Group) on fibronectin-coated (25 μg/ml) 6-well plates (200,000 cells/cm2). Cells were left undisturbed for 24 h in a 5% CO2 incubator. Unattached cells were removed by aspiration, and PC-1 media was replenished. Myocytes were incubated for another 24 h for beating to be reestablished before use. ANG II (1 μM; Catalog No. 091M5065; Sigma-Aldrich), neomycin (500 μM; Catalog No. N6386; Sigma-Aldrich), and PTEN pharmacological inhibitor dipotassium bisperoxo oxovanadate V (bpV) (1 μM; Catalog No. ALX-270-206; Enzo Life Sciences, Farmingdale, NY) and SF1670 (200 nM; Catalog No. B-0350; Echelon Biosciences, Salt Lake City, UT) were used in NRVMs for all figures. A new ANG II control group was used in every experiment, and responses were tested simultaneously.
Immunostaining and microscopy.
NRVMs were washed with PBS, fixed with 4% paraformaldehyde (Sigma-Aldrich) for 10 min, placed in cold 70% ethanol, and stored at −20°C until immunostaining. Primary anti-α-actinin antibody (Catalog No. ab9465, mouse IgG; Abcam, Cambridge, MA) was diluted (1:200) in 1% BSA in PBS (with 0.1% Triton X-100) and allowed to incubate on a shaker table at 4°C overnight. Secondary antibody (Catalog No. A-21202, Alexa Fluor 488 Goat anti-mouse IgG; Invitrogen, Grand Island, NY) was diluted at a ratio of 1:200 in 1% BSA in PBS and incubated for 1 h at 25°C. α-Actinin-positive mature cardiomyocytes were observed by Zeiss confocal microscopy. Cell surface areas were measured by ImageJ software. In each case, three independent experiments were performed, median values were calculated, and 20 cells from each condition were randomly chosen. These values were then used to calculate mean cell areas.
Dot blots for PIP2 and PIP3 levels.
NRVMs were stimulated with ANG II or inhibited with neomycin or bpV or SF1670 for the indicated times from 5 to 60 min. Whole cell lysates were then extracted from NRVMs in each experimental condition, spotted onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). These were probed with PIP2 or PIP3 antibody (PIP2 antibody: Catalog No. ab2335, mouse IgG; Abcam, Cambridge, MA; PIP3 antibody: Catalog No. Z-P345b, mouse IgG; Echelon Biosciences) at a 1:500 dilution and detected using a horseradish peroxidase conjugated secondary antibody (Catalog No. 7076, anti-mouse, HRP; Cell Signaling Technology, Boston, MA) and ECL. Experiments were repeated at least three times.
Subcellular fractionation and localization of PTEN.
For subcellular fractionation of myocytes, the Calbiochem ProteoExtract Subcellular Proteome Extraction Kit was used (Catalog No. 539790; EMD Millipore, Billerica, MA), following a previously described detergent-based protocol (2). Cellular proteins were sequentially extracted into four compartments: cytosolic, membrane/organelles, nuclei, and cytoskeleton. In this study, only cytosol and membrane were needed. Digitonin-EDTA is used to remove the cytosol. Triton-EDTA is used to remove the membrane-organelle fraction. Cells were briefly washed three times in PBS between each extraction fraction to prevent cross-contamination. After each fraction, cells were observed by microscopy to ensure that they were still attached to the dish. Cell integrity is maintained throughout the fractionation process. The accuracy of the fractionation method was verified with antibodies to well-documented subcellular distribution markers [heat shock protein (Hsp)70 for cytostol, β-integrin for membrane; anti-Hsp70 from Catalog No. sc-24, Santa Cruz Biotechnology, Dallas, TX; anti-β-integrin from Catalog No. MAB1900, EMD Millipore]. Experiments were repeated at least three times.
PTEN lipid phosphatase activity.
For the measurement of in vitro PTEN lipid phosphatase activity, the ELISA phosphatase assay kit (Echelon Biosciences) was used according to the manufacturer's instructions. Briefly, 500 μg of cell lysate was subjected to PTEN immunoprecipitation by the addition of 4 μl anti-PTEN mAb (Catalog No. 04-035; EMD Millipore), and the immunocomplex formed was captured by incubation with 20 μl protein A/G beads with gentle rotation at 4°C overnight. The beads were then washed twice in lysis buffer and once in enzyme reaction buffer (ERB) containing (in mM) 50 Tris·HCl (pH 8.0), 50 NaCl, 10 DTT, and 10 MgCl2 and distributed in triplicates of 30 μl in a 96-well flat-bottom plate (Echelon). The reaction was initiated by adding 30 μl of ERB containing the substrate dioctanoyl phosphatidylinositol 3,4,5-trisphosphate (PIP3-DiC8) (P-3908; Echelon) to 8 μM final concentration. After 1 h at 37°C, the reaction was stopped by 60 μl ERB. An additional detector and stop solution were added for 30 min, and then the absorbance was read at 450 nm after 30 min. A PIP3-only blank was used in parallel to correct for potential nonspecific phosphate release. The remaining beads were used for SDS-PAGE, Western blotting, and densitometric quantification to confirm that equivalent amounts of PTEN were immunoprecipitated from all samples. A standard curve was made by using the phosphate solution provided with the kit. The PTEN activity was expressed as of control group.
Fluorescence recovery after photobleaching for actin dynamics.
Recently, several microscopic techniques, such as fluorescence recovery after photobleaching (FRAP) (31) have yielded qualitative and quantitative information about the processes that regulate actin polymerization in living myocytes. The methods and analysis for FRAP of actin-GFP were described by our laboratory (17). In the present study, five myocytes were analyzed per culture and at least three separate cultures were studied per experimental condition.
Statistics.
Data are presented as means ± SE. Statistical significance was determined by one-way ANOVA. Significance was taken as P < 0.05.
RESULTS
Increased actin dynamics and cardiomyocyte hypertrophy induced by ang II are dependent on the PIP2 pathway.
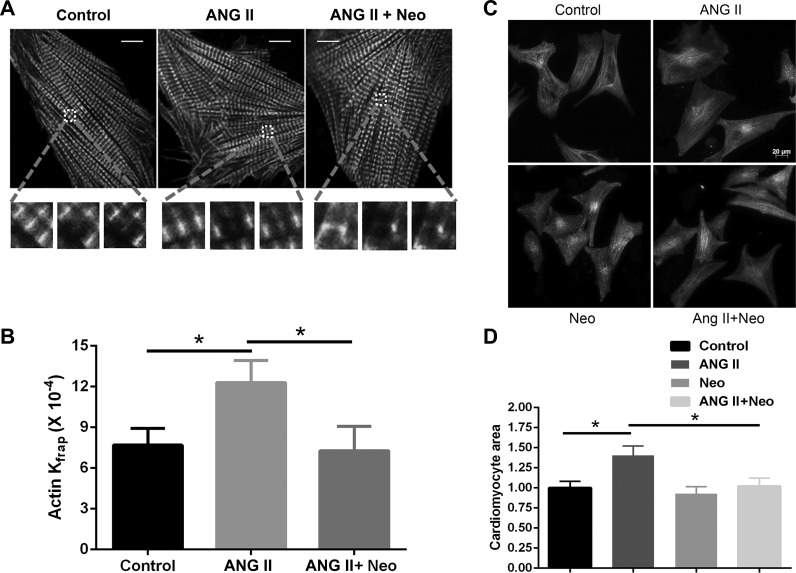
The FRAP experiments revealed differences after ANG II treatment (Fig. 1). After 1 h of ANG II treatment, the actin-GFP had a faster dynamic protein exchange in ANG II-treated myocytes than the vehicle group (12.30 ± 1.62 vs. 7.70 ± 1.23, × 10−4 s−1, P < 0.05; Fig. 1, A and B and Table 1). In NRVMs stimulated by ANG II and the PIP2 scavenger neomycin, the increased dynamics of actin-GFP were markedly reduced compared with ANG II treatment alone (7.28 ± 1.19 vs. 12.30 ± 1.62, ×10−4 s−1, P < 0.05; Table 1), demonstrating that dynamic exchange of actin-GFP is dependent on the PIP2 pathway after ANG II treatment.
Fig. 1.
Actin dynamics and cardiomyocyte hypertrophy with ANG II treatment. A and B: neomycin blunted the increased dynamics of actin detected by fluorescence recovery after photobleaching (FRAP) after 1-h treatment by ANG II in neonatal rat ventricular myocytes (NRVMs). A: microscopic images of living NRVMs infected with actin-GFP in control, ANG II, and neomycin-ANG II (ANG II + Neo)-treated cells. FRAP of the region of interest (ROI) for actin-GFP before, immediately after, and 8 min after photobleaching is shown. Scale bar = 10 μm. B: Kfrap of actin-GFP in the ANG II group had increased kinetic rates, which were significantly reduced by neomycin treatment. Means ± SE; *P < 0.05, n = 15. C and D: NRVMs treated with ANG II for 48 h had increased area, which was attenuated by neomycin treatment. Means ± SE; *P < 0.05 compared with untreated control group, n = 20. Scale bar = 20 μm.
Table 1.
Recovery kinetics (Kfrap) for actin under experimental conditions
| Experimental Conditions | Actin Kfrap, 10−4 s−1 |
|---|---|
| Control | 7.70 ± 2.23 |
| ANG II | 12.30 ± 2.62* |
| ANG II + neomycin | 7.28 ± 2.19# |
| bpV | 7.72 ± 2.19 |
| ANG II + bpV | 7.45 ± 2.12# |
| SF1670 | 7.81 ± 2.17 |
| ANG II + SF1670 | 6.34 ± 0.53# |
Values are means ± SE.
bpV, bisperoxo oxovanadate V.
P < 0.05 vs. control neonatal rat ventricular myocytes (NRVMs);
P < 0.05 vs. ANG II-treated NRVMs.
To determine whether PIP2 is involved in cardiac hypertrophy, NRVMs were treated with neomycin and ANG II for 48 h. Neomycin alone had no effects on myocardial size or phenotype, indicating that the effect of neomycin was not secondary to a toxic cellular effect. ANG II induced approximately a 40% increase in myocyte size, which was inhibited by neomycin treatment (27%; Fig. 1, C and D). These results suggest that neomycin attenuates ANG II induced cardiac hypertrophy and PIP2 plays an important role in it.
PIP2 increases in ang II-induced cardiac hypertrophy.
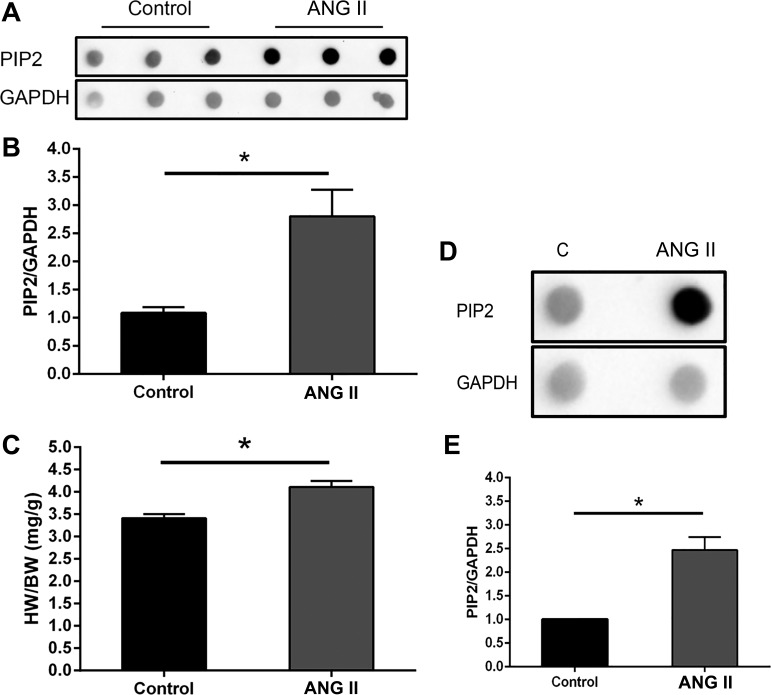
ANG II treatment in the mouse was used to test the hypothesis that PIP2 has a role in cardiac hypertrophy in vivo. The heart weight-to-body weight ratio (HW/BW) showed a significant increase in the ANG II-induced cardiac hypertrophy (Fig. 2C and Table 2). The PIP2 level increased significantly (P < 0.05) in ANG II-induced hypertrophic heart (Fig. 2, A and B). The PIP2 level increased in ANG II-stimulated NRVMs (Fig. 2, D and E), indicating that PIP2 may be involved in the ANG II-stimulated NRVM hypertrophic response.
Fig. 2.
Increased phosphatidylinositol 4,5-bisphosphate (PIP2) production in mouse heart and in cultured NRVMs by ANG II treatment. A and B: dot blot analysis of PIP2 was significantly increased in the heart after 2 wk of ANG II delivery by mini-pump. GAPDH was used to normalize the dot blot density. Means ± SE; *P < 0.05, n = 3. The increased heart weight (HW)-to-body weight (BW) ratio (C) in ANG II-treated mice showed significant cardiac hypertrophy. *P < 0.05, n = 3. D and E: after 1 h stimulation by ANG II, PIP2 production dramatically increased in the ANG II-treated group. Means ± SE. *P < 0.05, n = 3. C, control.
Table 2.
Heart weight and body weight in ANG II-induced heart-hypertrophy mice
| Body Weight, g |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | Before | After | Heart Weight, mg | Heart Weight-to-Body Weight Ratio, mg/g | Average | SE | t-test |
| Control | |||||||
| 1 | 24.00 | 29.96 | 99.60 | 3.32 | |||
| 2 | 24.00 | 28.72 | 95.20 | 3.31 | |||
| 3 | 24.86 | 29.84 | 107.3 | 3.60 | 3.41 | 0.095 | |
| ANG II | |||||||
| 1 | 28.00 | 32.83 | 127.40 | 4.00 | |||
| 2 | 25.00 | 29.30 | 128.50 | 4.39 | |||
| 3 | 24.00 | 29.62 | 116.70 | 3.94 | 4.11 | 0.141 | 0.0147 |
Increased PIP2 production by ANG II is dependent on the PTEN pathway.
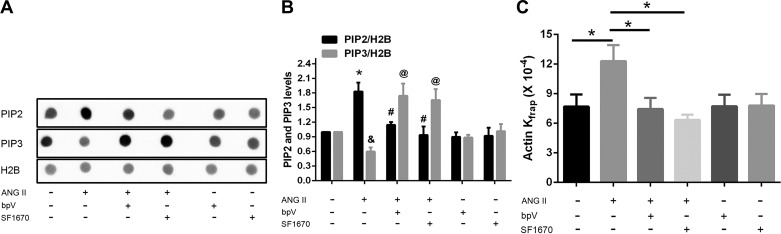
The PIP2 level was significantly increased in NRVMs after a 1-h treatment of ANG II, and PIP3 level was markedly decreased (Fig. 3, A and B). By prevention of PTEN activity through pharmacological inhibition with bpV (1 μM), ANG II-induced PIP2 was markedly reduced. In contrast, the reduced PIP3 in ANG II group was strikingly increased with inhibition of activity of PTEN by bpV (Fig. 3, A and B). To confirm the PTEN inhibition study, a specific PTEN inhibitor SF1670 originally used in neutrophils (15) and was confirmed here in cardiomyocytes. ANG II increased PIP2, which was significantly reduced by SF1670 treatment while also dramatically increasing PIP3 (Fig. 3, A and B). Thus, these data strongly suggest the role for PTEN in regulating ANG II-induced PIP2 production in cardiac myocytes.
Fig. 3.
Phosphatase and tensin homolog (PTEN) inhibitors blunt PIP2 and PIP3 production and actin dynamics after ANG II treatment. NRVMs were treated with either the PTEN inhibitor bisperoxo oxovanadate V (bpV; 1 μM) or with SF1670 (200 nM) for 30 min and then stimulated with 1 μM ANG II for 1 h. A and B: dot blotting performed with anti-PIP2 or anti-PIP3 antibodies, quantified by densitometry of immunoblots, expressed as fold changes normalized to the control group treated with the vehicle only. Means ± SE; *P < 0.05, &P < 0.05 vs. control groups; #P < 0.05, @P < 0.05 vs. ANG II groups; n = 3 independent experiments. C: significant increased kinetic rate of actin-GFP measured by Kfrap in NRVM with ANG II stimulation was significantly reduced by bpV or SF1670 inhibitors. Means ± SE; *P < 0.05, n = 15.
Inhibition of PTEN activity attenuates ang II-induced increased actin dynamics.
To determine whether the effects of PIP2 on actin dynamics could be attenuated, NRVMs were pretreated with bpV or SF1670 for 30 min and then subjected to ANG II for 1 h. The marked increase in actin-GFP dynamics induced by ANG II was significantly inhibited by bpV (7.45 ± 1.12 vs. 12.30 ± 1.62, ×10−4 s−1, P < 0.05) or by SF1670 (6.34 ± 0.53 vs. 12.30 ± 1.62, ×10−4 s−1, P < 0.05; Fig. 3C and Table 1). bpV or SF1670 alone had no effects on actin dynamics, indicating that the effect of bpV or SF1670 was not secondary to a toxic cellular effect. These results suggest that the activity of PTEN plays an important role in ANG II-induced cardiac hypertrophy.
ANG II stimuli activate PTEN.
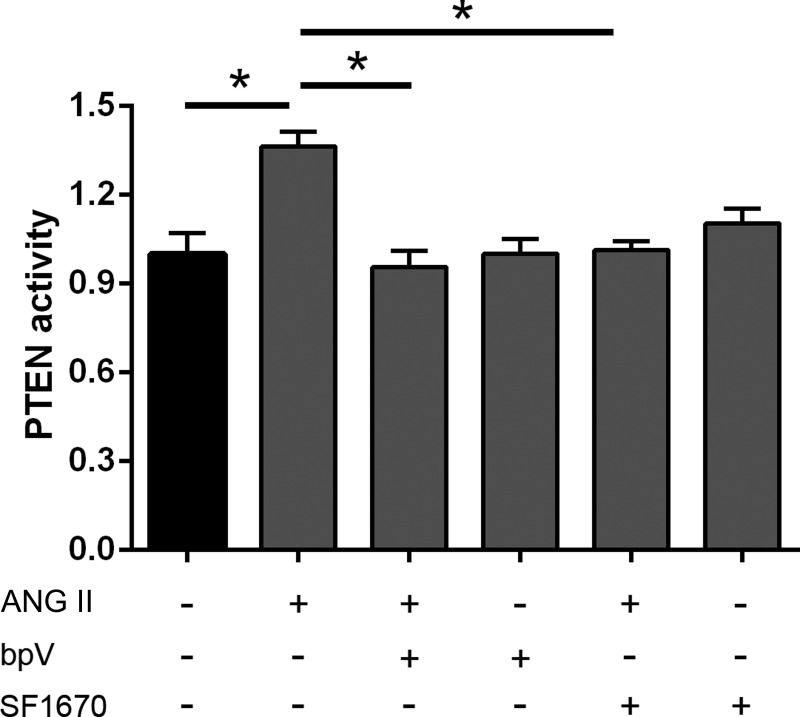
PTEN activation might be important since the PTEN inhibition blunted PIP2 and PIP3 levels and actin dynamics by ANG II. Therefore, NRVMs were treated with ANG II and PTEN inhibitors (bpV or SF1670), and PTEN activity was measured by ELISA PTEN activity kit (Echelon Biosciences). Assessment of PTEN activity showed that ANG II stimulation in NRVMs resulted in significantly increased lipid phosphatase activity of PTEN compared with the control group, and it was blunted by the inhibitors of PTEN bpV or SF1670 (Fig. 4). This suggests that activity of PTEN may be involved in the PIP2 production by ANG II, since PTEN converts PIP3 to PIP2.
Fig. 4.
PTEN activation increased with ANG II stimulation and decreased with PTEN inhibitors. NRVMs were treated with either the PTEN inhibitor bpV (1 μM) or with SF1670 (200 nM) for 30 min and then stimulated with 1 μM ANG II for 1 h. The fold change in PTEN phosphatase activity was increased by ANG II stimulation but decreased by bpV or SF1670 compared with the vehicle control group. Means ± SE; *P < 0.05, n = 3.
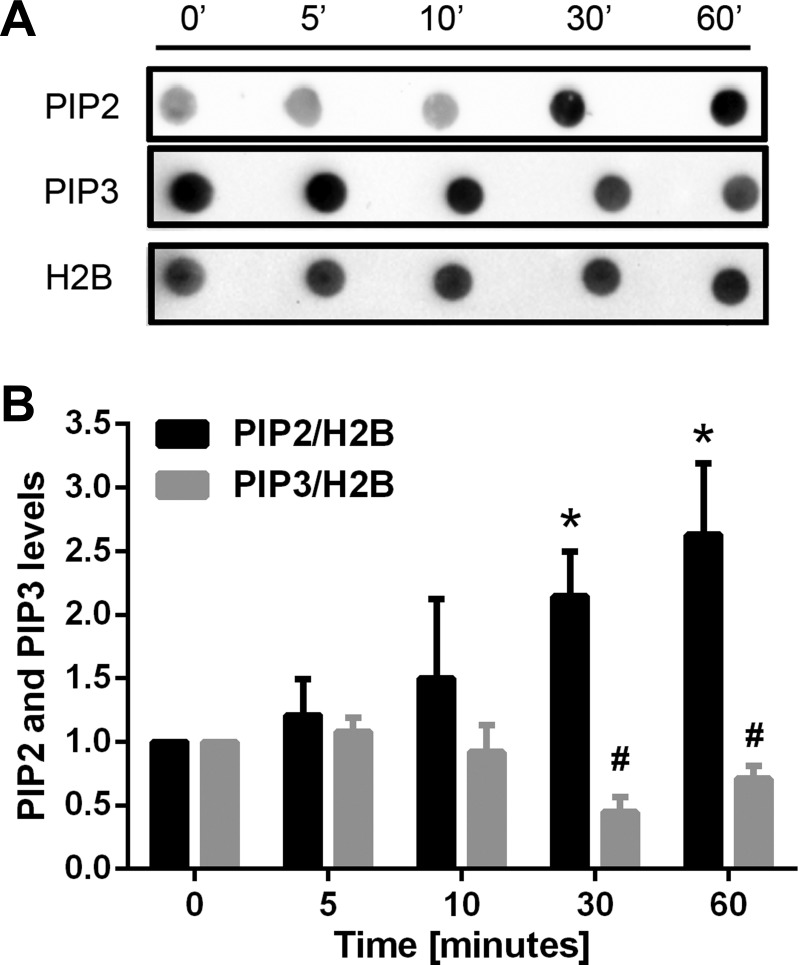
Time course of PIP2 and PIP3 levels and the translocation of PTEN by ang II.
The relative time courses of levels of PIP2 and PIP3 with ANG II are shown in Fig. 5. ANG II induced an increase of PIP2, which was detected at 30 min and sustained at 60 min. In contrast, PIP3 was decreased by ANG II, beginning at 30 min and staying low at 60 min.
Fig. 5.
Time course of production of PIP2 and PIP3 after ANG II treatment in NRVMs. A and B: NRVMs were stimulated with ANG II for the indicated times. The PIP2 level increased significantly at 30 min with ANG II treatment and stayed high at 60 min, whereas the PIP3 level decreased at 30 min and stayed low. H2B was used to normalize the dot blot density. Means ± SE. *P < 0.05 (for PIP2 level) vs. control group; #P < 0.05 (for PIP3 level) vs. control group; n = 3.
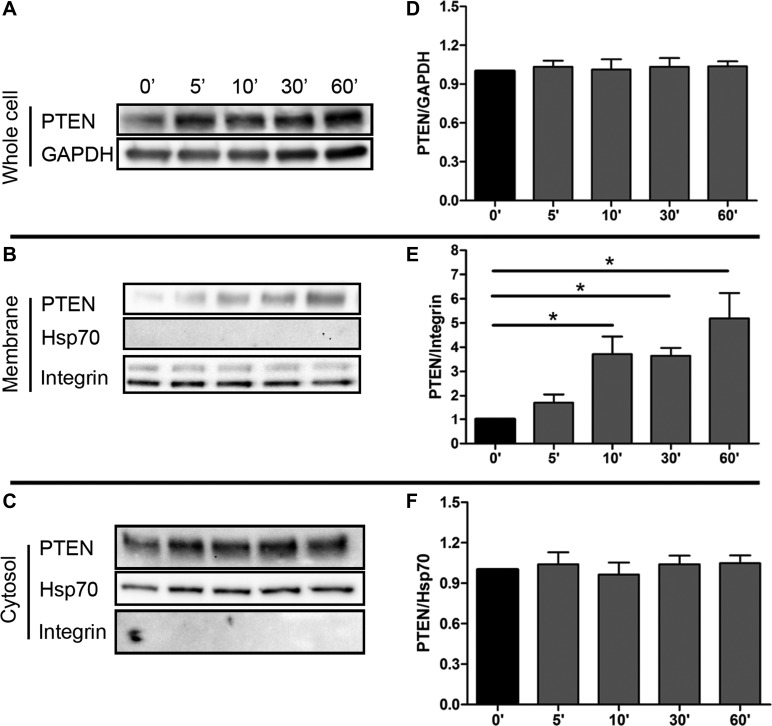
The PTEN level in ANG II-induced cardiomyocytes was examined. The protein level of PTEN in ANG II-treated NRVMs was not significantly changed at different time points up to 60 min (Fig. 6, A and D). The plasma membrane fraction of PTEN was significantly increased by 10 min (Fig. 6, B and E), although the total amount of PTEN and cytosolic fraction was not altered (Fig. 6, C and F). Because PIP2 production was increased at 30 min, which is later than the translocation of PTEN to plasma membrane by 10 min, these results suggest that the translocation of PTEN precedes the production of PIP2.
Fig. 6.
PTEN translocates to the plasma membrane in response to ANG II stimulation. Cytosol and plasma membrane levels of PTEN in NRVM at 0, 5, 10, 30, and 60 min after treatment with 1 μM ANG II. The amount of cytosol or whole cell PTEN was not altered (A and D, C and F); membrane PTEN was significantly increased with ANG II treatment at 10, 30, and 60 min (B and E). Means ± SE. *P < 0.05, n = 3. Hsp70, heat shock protein 70.
DISCUSSION
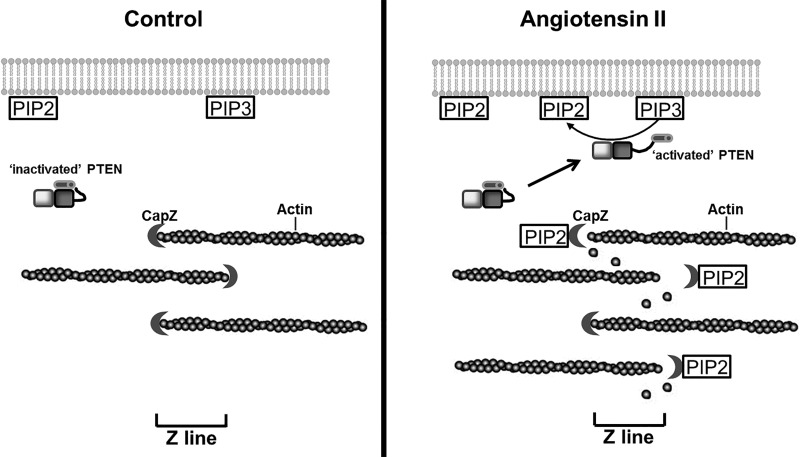
The present study demonstrates that actin dynamics and cell size were increased in NRVMs by stimulation with ANG II, which were tightly correlated to changes in the PTEN and PIP2 pathways. Furthermore, the PIP2 and PIP3 production by ANG II treatment was dependent on the PTEN pathway. The model proposed is that PTEN redistributes to the plasma membrane where it converts PIP3 to PIP2 (Fig. 7). Our results suggest that a possible mechanism for thin filament assembly partly relies on this increased level of PIP2.
Fig. 7.
Diagram of a proposed model for the mechanism of PTEN and PIP2 signaling linking actin dynamics to additional thin filament assembly. Left: in unstimulated spontaneously beating NRVMs, posttranslational modifications induce PTEN to assume an inactivated conformation (36). The production of PIP2 and PIP3 is at a basal level, and most of the inactive PTEN is in the cytosol. A functional, activated subpopulation of PTEN might only represent a small portion of the total cellular PTEN. Right: with hypertrophic stimulation, some inactive PTEN translocates from the cytosol to the plasma membrane and opens to become active. The active PTEN converts PIP3 to PIP2, which increases the PIP2 level. More PIP2 binds to CapZ resulting in an increase in the kinetics of actin. This suggests a possible mechanism for cell hypertrophy triggered partially by the increased PIP2 level resulting from activated PTEN at the plasma membrane, which leads to additional thin filament assembly.
The regulation of organization of the actin cytoskeleton upon G protein-coupled receptors (GPCRs) signaling has rarely been addressed. The angiotensin receptor (ATR) is one of the GPCRs, but a mechanism for the direct regulation of sarcomeric actin dynamics by the ATR has not yet been reported. However, there are some complicated clues that suggest that the ATR might regulate actin assembly through inhibiting PI3K pathways via β-arrestin (6, 28, 37) Some other GPCRs have been shown to regulate nonsarcomeric actin dynamics through different mechanisms. For example, serotonin receptors affected F-actin reorganization through cAMP signaling (7), endothelin receptors activated and induced association of paxillin with a cytoskeleton-enriched membrane fraction in vascular tissue (26), and ACh receptors played a role in cytoskeletal remodeling during ACh-induced contraction of smooth muscle through tyrosine phosphorylation of paxillin (35). Therefore, a mechanism involving the angiotensin receptor regulation of sarcomeric actin dynamics in cardiomyocytes is a possibility. Hypertrophy, resulting from chronic demands, requires addition of new sarcomeres. In the present study, we focused on the initial changes to sarcomeric actin dynamics after 1 h of ANG II treatment. Cell sizes in NRVMs and the PIP2 level in ANG II-induced hypertrophic mouse heart were shown after 2 days and 1 wk, respectively. Thus the acute parameters of actin dynamics precede actual cardiac hypertrophy by an interval in which many additional steps could occur, rather than being a direct linkage.
PIP2, has a direct role in regulating actin assembly by interaction with partnering proteins in many cells. In platelets, the half-life for a capped filament was 28 min, whereas the half-life to remain uncapped was only 0.2 s. Interestingly, the addition of PIP2 in these platelets reduced the half-life of the capped filament to 46 s, suggesting PIP2 regulation of actin filament capping dynamics (33). Our previous study clearly showed that PIP2 localized to the Z-disc in cardiomyocytes and regulated actin filament dynamics (14). In the present study, the increased actin dynamics by ANG II was in a PIP2-dependent manner (Fig. 1), suggesting PIP2 may play a critical role in increased actin assembly by ANG II induction.
The next question is how PIP2 is produced by ANG II. Some signaling pathways, such as PKC and PI4K, were involved in producing PIP2 in myocytes isolated from adult mice treated with ANG II (40). In addition, the PIP2 level could be converted at plasma membrane to PIP3 by PTEN, which is one of the regulators of PIP2 located there. In our results, the PTEN was activated by ANG II in NRVMs (Fig. 4), and PIP2 and PIP3 levels induced by ANG II were conversely changed by PTEN inhibition, suggesting that PTEN plays a role in PIP2 production by ANG II.
There are two main explanations of how PTEN binds to the plasma membrane. The binding of PTEN to PIP2 might be critical for membrane localization (9). In contrast, PTEN might interact with the nonspecific electrostatic charges at the plasma membrane arising from lipids, such as phosphatidylserine (25). In our time course results, PTEN significantly increased at plasma membrane after only 10 min of ANG II stimulation (Fig. 6, B and E), whereas increased PIP2/decreased PIP3 did not change significantly until 30 min (Fig. 5). This time sequence suggests that PTEN interacted with the plasma membrane first and then converted PIP3 to PIP2 at plasma membrane.
Since the majority of cellular PTEN is found in the cytosol (30), how is PTEN able to execute different cellular functions that require its membranous lipid phosphatase activity? The lipid phosphatase activity of PTEN is at the plasma membrane. The plasma membrane PTEN increased in a time-dependent manner with ANG II treatment (Fig. 6, B and E). A decrease of cytosolic PTEN was expected but not found (Fig. 6, C and F) perhaps because most of the PTEN remains in this compartment. The level of PTEN in unstimulated myocytes was over 50-fold higher in the cytosol than in the membrane detected by Western blotting (data not shown).
The phosphatase activity of PTEN was significantly increased in ANG II-treated cardiomyocytes, whereas it was blunted by PTEN inhibitors bpV or SF1670 (Fig. 4). The subcellular localization of PTEN and its enzymatic activity are regulated by its various posttranslational modifications. PTEN contains multiple domains, including an NH2-terminal phosphatase domain, a central C2 domain, and a COOH terminal tail. The phosphorylation sites mapped on PTEN could be serine and threonine residues (39), Ser370 and Ser385 (38) and Ser362 and Thr366 (1) in its COOH-terminal tail. Furthermore, PTEN was phosphorylated by RhoA-associated kinase at Ser229, Thr232, Thr319, and Thr321 in the C2 domain (16). In contrast, Thr366 phosphorylation promoted PTEN degradation (21). In this study, the focus was on the membrane translocation of PTEN that may contribute to its lipid phosphatase activity, since there are so many phosphorylation sites in inactive PTEN in cytosol. Our results were consistent with changes seen in cultured cardiomyocytes whereby ANG II-induced increased actin dynamics was blocked by the PTEN inhibitor bpV or SF1670 (Fig. 3C).
In conclusion, PIP2 plays a major role in regulating actin cytoskeleton dynamics. Furthermore, our results provide a crucial link between PTEN and the cellular responses to ANG II in which the translocation of PTEN was associated. We speculate that similar mechanisms may be responsible for ventricular remodeling and progression to heart failure.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-62426.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L. conception and design of research; J.L. and E.J.T. performed experiments; J.L. analyzed data; J.L. and B.R. interpreted results of experiments; J.L. prepared figures; J.L. drafted manuscript; B.R. edited and revised manuscript; B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Yunbo Ke for the gift of the hypertrophic heart tissues treated by ANG II. We thank Dr. Ke Ma for expert assistance with the FRAP experiments.
REFERENCES
- 1.Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem 280: 35195–31202, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, de Tombe PP, Russell B. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am J Physiol Heart Circ Physiol 292: H259–H269, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. Am J Physiol Cell Physiol 285: C171–C182, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 110: 737–749, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Das S, Dixon JE, Cho Dixon W, Cho W. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci USA 100: 7491–7496, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFea KA. Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol 69: 535–560, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Ganguly S, Saxena R, Chattopadhyay A. Reorganization of the actin cytoskeleton upon G-protein coupled receptor signaling. Biochim Biophys Acta 1808: 1921–1929, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Hartman TJ, Martin JL, Solaro RJ, Samarel AM, Russell B. CapZ dynamics are altered by endothelin-1 and phenylephrine via PIP2- and PKC-dependent mechanisms. Am J Physiol Cell Physiol 296: C1034–C1039, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem 279: 16606–16613, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Janmey PA, Stossel TP. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature 325: 362–364, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto H, Hamada K, Saunders M, Backman S, Sasaki T, Nakano T, Mak TW, Suzuki A. Physiological functions of Pten in mouse tissues. Cell Struct Funct 28: 11–21, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Lassing I, Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature 314: 472–474, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Leslie NR, Downes CP. PTEN: the down side of PI 3-kinase signalling. Cell Signal 14: 285–295, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Russell B. Phosphatidylinositol 4,5-bisphosphate regulates CapZbeta1 and actin dynamics in response to mechanical strain. Am J Physiol Heart Circ Physiol 305: H1614–H1623, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Prasad A, Jia Y, Roy SG, Loison F, Mondal S, Kocjan P, Silberstein LE, Ding S, Luo HR. Pretreatment with phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibitor SF1670 augments the efficacy of granulocyte transfusion in a clinically relevant mouse model. Blood 117: 6702–6713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol 7: 399–404, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Lin YH, Li J, Swanson ER, Russell B. CapZ and actin capping dynamics increase in myocytes after a bout of exercise and abates in hours after stimulation ends. J Appl Physiol (1985) 114: 1603–1609, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin. J Cell Biol 176: 343–353, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420: 89–93, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, Prehar S, Gui J, Tsui H, Xiao RP, Neyses L, Solaro RJ, Ke Y, Cartwright EJ, Lei M, Wang X. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation 124: 2702–2715, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccario H, Perera NM, Davidson L, Downes CP, Leslie NR. PTEN is destabilized by phosphorylation on Thr366. Biochem J 405: 439–444, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem 70: 247–279, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Mellman DL, Gonzales ML, Song C, Barlow CA, Wang P, Kendziorski C, Anderson RA. A PtdIns4,5P2-regulated nuclear polyA polymerase controls expression of select mRNAs. Nature 451: 1013–1017, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Mulgrew-Nesbitt A, Diraviyam K, Wang J, Singh S, Murray P, Li Z, Rogers L, Mirkovic N, Murray D. The role of electrostatics in protein-membrane interactions. Biochim Biophys Acta 1761: 812–826, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Ohanian V, Gatfield K, Ohanian J. Role of the actin cytoskeleton in G-protein-coupled receptor activation of PYK2 and paxillin in vascular smooth muscle. Hypertension 46: 93–99, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Oudit GY, Kassiri Z, Zhou J, Liu QC, Liu PP, Backx PH, Dawood F, Crackower MA, Scholey JW, Penninger JM. Loss of PTEN attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc Res 78: 505–514, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem 278: 51334–51339, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Pyle WG, Hart MC, Cooper JA, Sumandea MP, de Tombe PP, Solaro RJ. Actin capping protein: an essential element in protein kinase signaling to the myofilaments. Circ Res 90: 1299–1306, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Ross AH, Gericke A. Phosphorylation keeps PTEN phosphatase closed for business. Proc Natl Acad Sci USA 106: 1297–1298, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy P, Rajfur Z, Pomorski P, Jacobson K. Microscope-based techniques to study cell adhesion and migration. Nat Cell Biol 4: E91–E96, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Sanger JM, Sanger JW. The dynamic Z bands of striated muscle cells. Sci Signal 1: pe37, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Schafer DA, Jennings PB, Cooper JA. Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J Cell Biol 135: 169–179, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartzbauer G, Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J Biol Chem 276: 35786–35793, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Tang DD, Turner CE, Gunst SJ. Expression of non-phosphorylatable paxillin mutants in canine tracheal smooth muscle inhibits tension development. J Physiol 553: 21–35, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thapa N, Sun Y, Schramp M, Choi S, Ling K, Anderson RA. Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells. Dev Cell 22: 116–130, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem 277: 9429–9436, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem 276: 993–998, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol 20: 5010–5018, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu JX, Si M, Zhang HR, Chen XJ, Zhang XD, Wang C, Du XN, Zhang HL. Phosphoinositide kinases play key roles in norepinephrine- and angiotensin II-induced increase in phosphatidylinositol-4,5-bisphosphate and modulation of cardiac function. J Biol Chem 289: 6941–6948, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]