Abstract
Aortocaval fistula (ACF)-induced volume overload (VO) heart failure (HF) results in progressive left ventricular (LV) dysfunction. Hemodynamic load reversal during pre-HF (4 wk post-ACF; REV) results in rapid structural but delayed functional recovery. This study investigated myocyte and myofilament function in ACF and REV and tested the hypothesis that a myofilament Ca2+ sensitizer would improve VO-induced myofilament dysfunction in ACF and REV. Following the initial sham or ACF surgery in male Sprague-Dawley rats (200–240 g) at week 0, REV surgery and experiments were performed at weeks 4 and 8, respectively. In ACF, decreased LV function is accompanied by impaired sarcomeric shortening and force generation and decreased Ca2+ sensitivity, whereas, in REV, impaired LV function is accompanied by decreased Ca2+ sensitivity. Intravenous levosimendan (Levo) elicited the best inotropic and lusitropic responses and was selected for chronic oral studies. Subsets of ACF and REV rats were given vehicle (water) or Levo (1 mg/kg) in drinking water from weeks 4–8. Levo improved systolic (% fractional shortening, end-systolic elastance, and preload-recruitable stroke work) and diastolic (τ, dP/dtmin) function in ACF and REV. Levo improved Ca2+ sensitivity without altering the amplitude and kinetics of the intracellular Ca2+ transient. In ACF-Levo, increased cMyBP-C Ser-273 and Ser-302 and cardiac troponin I Ser-23/24 phosphorylation correlated with improved diastolic relaxation, whereas, in REV-Levo, increased cMyBP-C Ser-273 phosphorylation and increased α-to-β-myosin heavy chain correlated with improved diastolic relaxation. We concluded that Levo improves LV function, and myofilament composition and regulatory protein phosphorylation likely play a key role in improving function.
Keywords: myofilament dysfunction, myofilament Ca2+ sensitization, levosimendan, myosin-binding protein-C, troponin I
mitral regurgitation (MR) is the most and second most common valve lesion in the United States and Europe, respectively, affecting >2 million Americans (10, 16). The pathophysiological consequences of MR include chronic left ventricular (LV) hemodynamic volume overload (VO) followed by LV chamber dilation, progressive LV contractile dysfunction, and heart failure (HF). Despite the clinical importance of VO, few models mimic the pathophysiological progression of chronic VO with and without hemodynamic load reduction. The aortocaval fistula (ACF) model of VO HF in the rodent mimics increased hemodynamic preload observed in human disease, irrespective of etiology. In this model, chronically increased LV preload leads to progressive LV pump failure, which is classified into three clinically relevant stages: 1) pre-HF [4-wk ACF; marked LV dilation, increased LV wall stress, mild LV dysfunction (∼10% decrease in % fractional shortening, %FS); no pulmonary congestion/edema], 2) established HF [8-wk ACF; severe LV dilation, significant LV systolic (25% decrease in %FS, ∼50% decrease in end-systolic elastance, Ees, and preload-recruitable stroke work, PRSW) and diastolic dysfunction (10–27% increase in τ and dP/dtmin, respectively); no pulmonary congestion or edema], and 3) end-stage HF (15–21-wk ACF; LV pump failure with pulmonary congestion/edema) (17, 19, 34). This model is commonly used to identify molecular and cellular mechanisms driving disease progression and to test novel therapeutic approaches for improving LV structure and function (13, 21, 40).
We have previously characterized a technique to reverse (REV, close) the ACF (19). In that report, REV during pre-HF (i.e., 4 wk post-ACF) results in rapid structural recovery but delayed functional recovery (19). Although this mirrors human data where structure, but not function, returns postsurgically in 17% of patients with MR (27), the mechanism underlying the delayed functional recovery remains elusive. Although the cellular mechanisms that control excitation-contraction coupling have been studied at end-stage HF (17), less is known about cellular dysfunction at earlier stages of HF. Specifically, myocyte and myofilament function has not previously been evaluated in ACF or REV, and our model provides an attractive system for studying the physical and biochemical mechanics of VO before and after surgical intervention.
Additionally, this model is attractive because pharmacological agents can be added in the presence or absence of hemodynamic load reversal. VO HF therapeutic options generally target neurohormonal pathways by disrupting receptor-ligand interactions or modulating downstream signaling pathways (e.g., Ca2+-cAMP). Although these therapies successfully manage LV hypertrophy, their inotropic actions do not directly target impaired LV contractility, the central feature of systolic HF (14). This can result in increased myocardial oxygen consumption and myocardial Ca2+ overload (30). Newer therapeutics target myofilament activation without altering the Ca2+ transient, and these myofilament Ca2+ sensitizers (e.g., levosimendan, Levo) and myosin activators (e.g., omecamtiv mecarbil, OM) are used to treat acute and chronic HF (5, 20, 37).
The goal of the present study was to investigate the effects of VO and VO reversal on myocyte and myofilament function. On the basis of these results, we then tested the hypothesis that a myofilament Ca2+ sensitizer would improve VO-induced myofilament dysfunction in ACF and REV.
MATERIALS AND METHODS
ACF and REV surgical model.
Male Sprague-Dawley rats (200–240 g, Harlan; Charles River Laboratories, Wilmington, MA) were housed in a temperature- and humidity-controlled room using a 12-h:12-h light/dark cycle with free access to standard rat chow and water. Studies, approved by the Institutional Animal Care and Use Committee, conformed to the NIH Guide for the Care and Use of Laboratory Animals.
At week 0, VO was induced under isoflurane anesthesia (2–2.5%) as described (17, 19). Following abdominal incision, the abdominal aorta and caudal vena cava were exposed. Cranial and caudal to the fistula site, the adventitia was bluntly separated, and 5–0 Ethilon suture (Ethicon, Cincinnati, OH) was preplaced for reversal. An 18-g needle was used to create the ACF. The aortic puncture was sealed with cyanoacrylate glue, and the preplaced suture was loosely tied, with care taken to not occlude blood flow. In a subset of rats, the fistula site was exposed, and the preplaced suture was ligated 4 wk post-ACF (REV). Shunt patency and subsequent closure were confirmed by the presence or absence, respectively, of bright red arterial blood in the vena cava. The abdominal wall and skin were closed. Sham animals underwent a similar procedure except for suture preplacement and aortic puncture. Buprenorphine (0.03 mg/kg sc) was given immediately postoperatively and every 12 h for 72 h postoperatively as needed for pain.
Echocardiography.
Transthoracic echocardiograms were performed biweekly (8.5-MHz, Xario; Toshiba Medical Systems, Tustin, CA) under isoflurane anesthesia (1.5–2.0%) (17, 19). Midwall parasternal short-axis M-mode images were obtained to assess chamber diameters in end-systole (LVESD) and end-diastole (LVEDD) and LV posterior wall thickness in systole (PWTs) and diastole (PWTd). These indices were calculated as follows: %FS = (LVEDD − LVESD)/LVEDD × 100; dilation index = (2 × PWTd)/LVEDD.
Measurement of sarcomere shortening and Ca2± transients in isolated LV myocytes.
Hearts were removed from anesthetized rats for LV myocyte isolation (17). The heart, mounted on a Langendorff apparatus, was perfused with Tyrode's solution supplemented with 10 mM 2,3-butanedione monoxime, followed by perfusion buffer and then digestion with trypsin/Liberase-TH. LV myocytes were mechanically dispersed, filtered, and resuspended in increasing [CaCl2] to achieve [CaCl2]final = 1 mM. Isolated myocytes were incubated on laminin-coated chambers in plating media and then culture media for 1 h each.
Single myocyte sarcomere shortening (1-Hz field stimulation) was measured with a Myocam (IonOptix, Milton, MA) within 2–3 h of initial plating. The following parameters were analyzed: sarcomere peak shortening normalized to resting sarcomere length (%PS), maximal cell shortening (−dl/dt) and relengthening (+dl/dt) velocities, and time to 90% peak contraction and relaxation. To evaluate Ca2+, myocytes were loaded with fura 2-AM (17); they were excited during field stimulation with light in an interleaved pattern at 340/12 nm and 380/12 nm, and emission was collected at 510/40 nm. Data were expressed as peak Ca2+ amplitudes normalized to resting diastolic Ca2+ (peak[Ca2+]i), maximum rates in rise of systolic Ca2+ and Ca2+ decay, and times to 90% peak Ca2+ release and Ca2+ reuptake. All measurements were analyzed using IonWizard data acquisition system (IonOptix).
Myofilament pCa force.
Myocyte fragments were isolated from frozen LV tissue by mechanical dissociation and chemically permeabilized with 0.3% Triton X-100 and used as described (2, 4, 22). Active force development was fit to a modified Hill equation that yields maximum force development, Hill coefficient (a measure of cooperativity), and pCa50 (−log[Ca2+], where 50% of maximum force is developed, a measure of myofilament Ca2+ sensitivity).
Acute intravenous Levo, milrinone, and OM treatment.
To investigate the effects of three positive inotropes with varying mechanisms of action, 8-wk ACF rats were infused with Levo (IV Levo, 2.8 μg/kg per min; LKT Laboratories, St. Paul, MN), milrinone (9.9 μg/kg per min; LKT Laboratories), or OM (11.7 μg/kg per min; Selleck Chemicals, Houston, TX) for 30 min. Additional sham and ACF rats were infused with vehicle (Veh, 1% DMSO in 5% dextrose in water). %FS and mean arterial pressure (MAP) were measured before and after infusion, and τ and dP/dtmin (see below) were measured postinfusion.
Chronic oral Levo treatment.
From weeks 4–8 (Fig. 1), ACF and REV rats were given Veh (water) or Levo [oral Levo; L-enantiomer of ([4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl]-hydrazono)-propanedinitrile; LKT Laboratories]. Levo (0.0133 mg/ml), prepared twice weekly in drinking water, was delivered at a dose of ∼1 mg/kg per day, which has improved LV function in previous rodent studies (6, 24, 25).
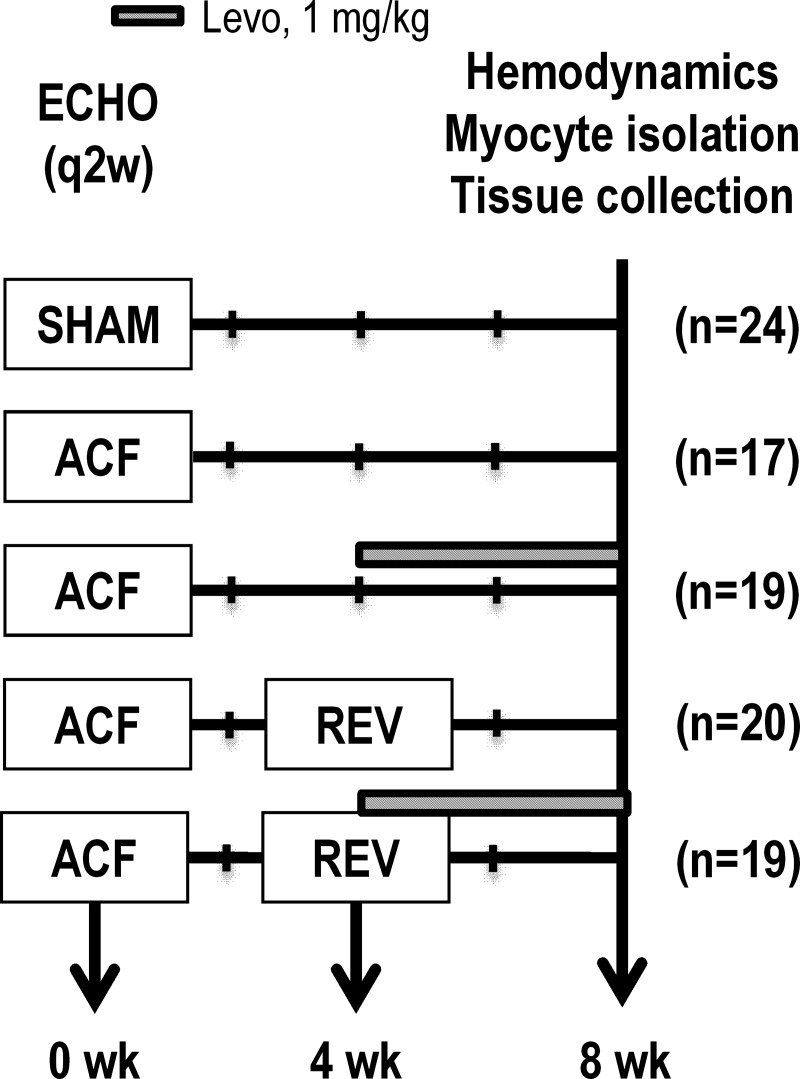
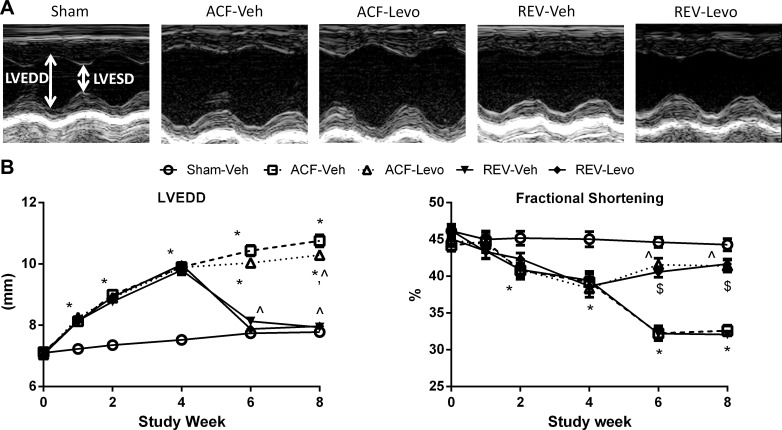
Fig. 1.
Experimental time course for chronic, oral levosimendan (Levo) treatment. Aortocaval fistula (ACF) was induced at week 0. In a subset of rats, the ACF was reversed (REV) at week 4. Subsets of ACF and REV rats were given Levo (1 mg/kg per day in drinking water) from weeks 4–8. Echo was performed biweekly, and hemodynamics, myocyte isolation, and tissue collection were performed at week 8.
Radiotelemetry.
Radiotelemetry catheters (PhysioTel PA-C40; Data Sciences International, St. Paul, MN) were implanted immediately following sham or ACF surgery. Following ventral neck incision, the catheter was inserted into the right carotid artery to the aortic arch. The radiotransmitter was fed into a subcutaneous pocket positioned dorsally over the right shoulder, and the neck incision was closed. Blood pressure was recorded using Dataquest A.R.T (Data Sciences International) acquisition software. Final analysis averaged data from 24-h periods.
Dobutamine stress echocardiography.
Following baseline echo at week 8, dobutamine (0.5 mg/kg ip) was given to a subset of ACF and REV rats given Veh or oral Levo. M-mode images were obtained 2.5–5.0 min postinjection; %FS at the peak increase was analyzed.
Invasive LV hemodynamics.
LV hemodynamics were assessed during week 8 with a pressure-volume catheter (1.9F; SciSense, London, ON, Canada) as described (17, 19). Rats were anesthetized with 3% isoflurane, intubated by tracheostomy, ventilated (SAR-830; CWE, Ardmore, PA), and maintained under 1.75% isoflurane anesthesia. Baseline LV hemodynamic parameters were acquired; preload was varied by brief occlusion of the vena cava to obtain PRSW and Ees, the slope of the end-systolic pressure-volume relationship. Data were acquired and analyzed using Labscribe2 software (iWORX, Dover, NH). Measures of LV function include stroke volume (SV), heart rate (HR), maximum and minimum dP/dt, end-systolic and end-diastolic volume (ESV and EDV), end-systolic and end-diastolic pressure, Ees, PRSW, and τ (Weiss correction). Analysis of covariance (ANCOVA)-adjusted marginal means of Ees and PRSW are presented and account for changes in the volume-axis intercept (8).
Immunoblot analysis.
Immunoblots of LV tissue lysates were performed and analyzed as described (17, 19) with antibodies against SERCA-2a (1:2,000; Thermo Fisher, Waltham, MA), phospholamban (PLB, 1:2,000; Millipore, Billerica, MA), phospho-PLB Ser16 (p-PLB, 1:2,000, Millipore), cardiac troponin I (cTnI) (1:2,000; Cell Signaling, Beverly, MA), phospho-TnI Ser23/24 (1:1,000, Cell Signaling), cMyBP-C (1:5,000) (15), and phospho-cMyBP-C Ser-273, Ser-282, and Ser-302 (1:5,000) (15).
Quantitative real-time PCR analysis.
RNA was isolated from LV tissue and amplified for α- and β-myosin heavy chain (MHC) as described (17, 19). Data were analyzed for relative expression using the 2-ΔΔCt method, with ribosomal protein Rpl13a as the internal control and the average sham value as a second normalizer.
Statistical analysis.
Data are expressed as means ± SE. Statistical analyses were performed using GraphPad Prism v.6.0 or SPSS v.19. One-way or two-way ANOVA or ANCOVA, followed by Bonferroni's post hoc test, was used to measure differences between groups. P < 0.05 was considered significant.
RESULTS
REV improves impaired myocyte contraction kinetics.
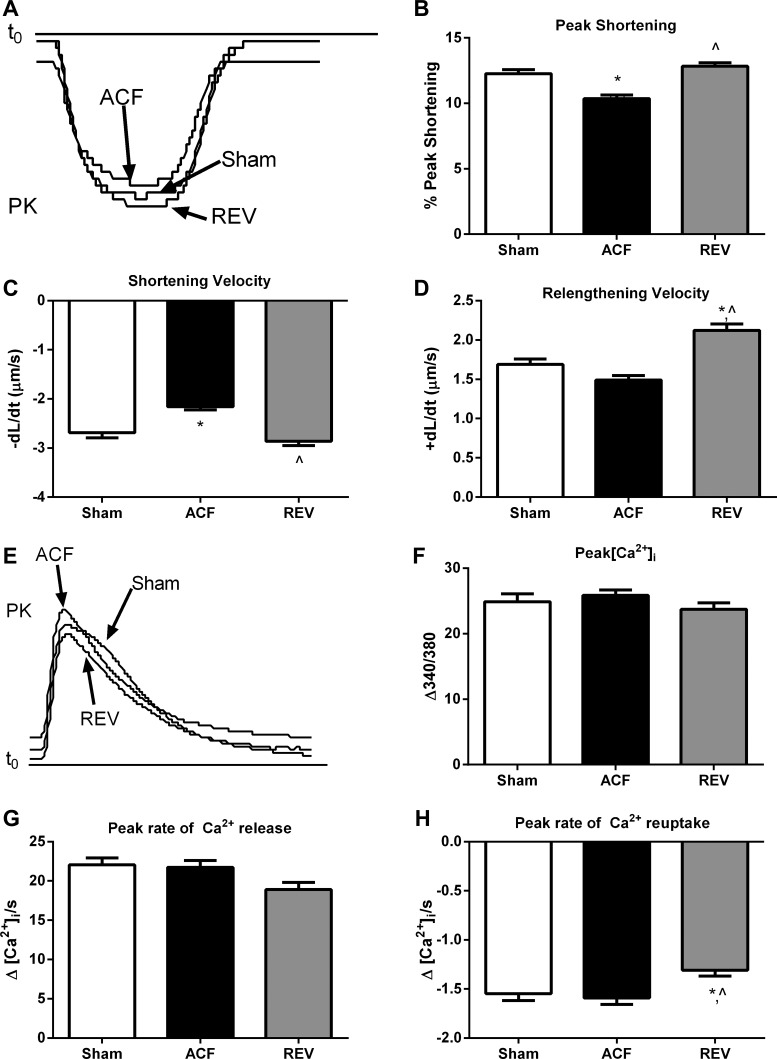
To determine whether the observed in vivo LV systolic dysfunction was attributable to impaired excitation-contraction coupling in cardiomyocytes, we isolated LV myocytes from sham, ACF, and REV rats. Representative sarcomere shortening recordings are shown in Fig. 2A. Compared with sham, there was a 15% decrease in %PS in freshly isolated ACF myocytes that was normalized in REV myocytes (Fig. 2B). Sarcomere shortening velocity (−dl/dt) was also reduced in ACF and REV myocytes by 20% and 6% (Fig. 2C), respectively, whereas relaxation velocity (+dl/dt) was reduced in ACF myocytes by 11% but increased in REV myocytes by 26% (Fig. 2D). In sham, ACF, and REV myocytes, the time to 90% of peak contraction (0.14 ± 0.00 ms, 0.15 ± 0.00 ms, 0.14 ± 0.00 ms, respectively; P > 0.05) and the time to 90% of peak relaxation (0.51 ± 0.01 ms, 0.51 ± 0.01 ms, 0.49 ± 0.01 ms, respectively; P > 0.05) were not different.
Fig. 2.
REV improves impaired myocyte contraction kinetics without altering Ca2+-transient kinetics. A: representative sarcomere shortening. PK, peak. B: peak shortening. C: peak shortening velocity (−dl/dt). D: peak relengthening velocity (+dl/dt). E: representative Ca2+ transients. F: intracellular calcium amplitude (peak[Ca2+]i). G: peak rate of Ca2+ release. H: peak rate of Ca2+ reuptake. *P < 0.05 vs. sham; ^P < 0.05 vs. ACF. Data represent means ± SE from 100–125 cells from 7 rats/group.
ACF alters myocyte force generation and myofilament Ca2± sensitivity without altering Ca2±-transient kinetics.
To further investigate changes in excitation-contraction coupling, we next measured the amplitude and kinetics of the Ca2+ transient. Representative Ca2+ transients are depicted in Fig. 2E. The mean peak amplitude of Ca2+ fluorescence was not different in sham, ACF, and REV myocytes (Fig. 2F). Additionally, there were no differences in the peak rate of Ca2+ release (Fig. 2G), the time to 90% of peak [Ca2+]i (0.03 ± 0.00 ms, 0.03 ± 0.00 ms, 0.03 ± 0.00 ms, respectively; P > 0.05), or the time to remove 90% of [Ca2+]i toward baseline (0.54 ± 0.01 ms, 0.54 ± 0.01 ms, 0.55 ± 0.01 ms, respectively; P > 0.05). Compared with sham, the peak rate of Ca2+ reuptake was decreased (less negative) in REV but unchanged in ACF (Fig. 2H).
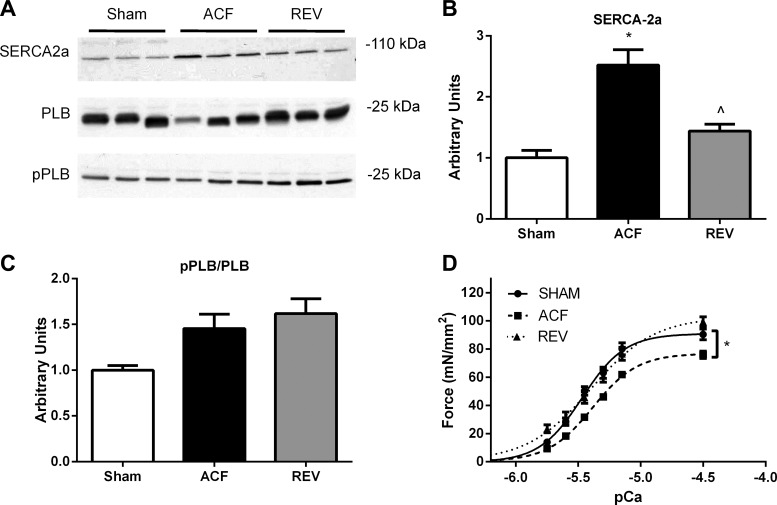
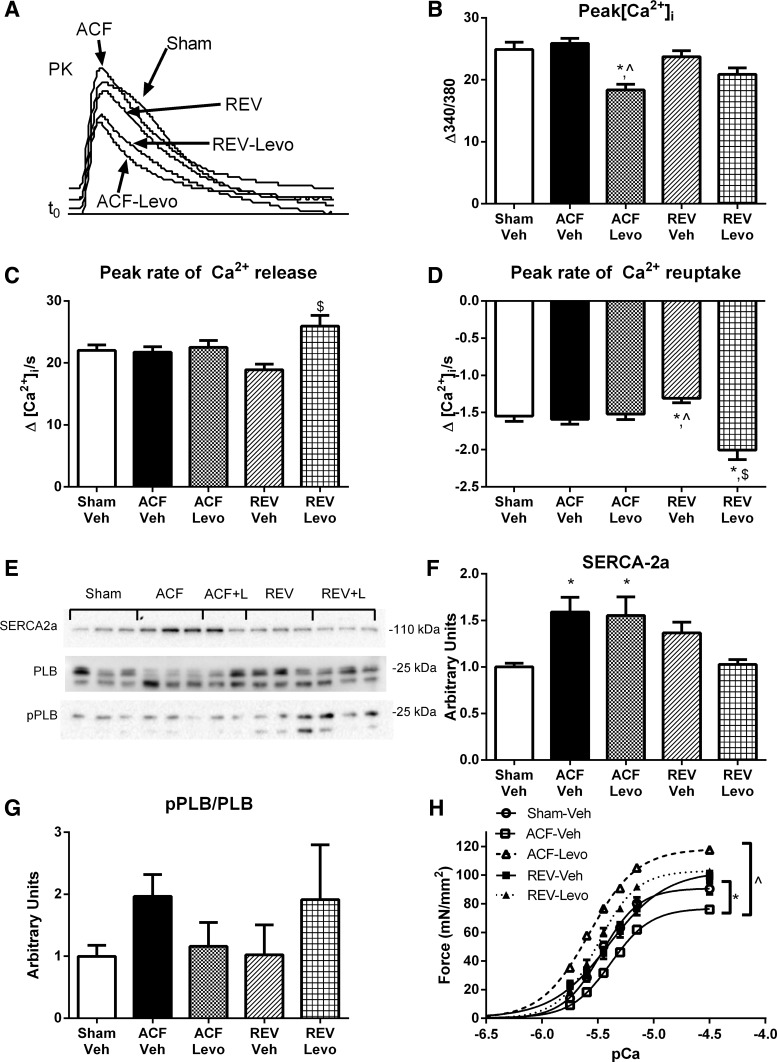
Finally, we evaluated alterations in the expression of SERCA-2a and total and phosphorylated PLB in LV tissue. Representative immunoblots are shown in Fig. 3A, and summary data are shown in Fig. 3, B and C. SERCA-2a was increased 2.5-fold in ACF and normalized in REV. Although these changes in SERCA-2a may be compensatory, there is a poor correlation with the peak rate of Ca2+ reuptake or the peak rate of myocyte relengthening. This suggests that the changes in SERCA-2a may be unrelated to the pathophysiology of myocyte and myocardial dysfunction in ACF and REV. Total and phospho-PLB/PLB were not statistically different among sham, ACF, and REV.
Fig. 3.
REV improves increased SERCA-2a, whereas ACF and REV decrease myofilament Ca2+ sensitivity. A: representative immunoblots for SERCA-2a, phospho-phospholamban (pPLB), phospholamban (PLB). Cumulative data for SERCA-2a (B) and pPLB/PLB (C). D: force-pCa for left ventricular (LV) myofilaments. Data are means ± SE for 5–6 rats/group. *P < 0.05 vs. sham; ^P < 0.05 vs. ACF.
With the limited alterations in ACF or REV myocyte calcium transient or kinetics, we next measured myofilament Ca2+ sensitivity and force generation. Myofilament force generation at varying [Ca2+] was measured in skinned myocytes from frozen LV tissue. Compared with sham, there was a rightward shift in the force-pCa relationship for ACF and REV (5.37 ± 0.01 and 5.40 ± 0.02 vs. 5.47 ± 0.02, respectively), indicating decreased Ca2+ sensitivity, and 16% decrease in maximal force generation for ACF (Fig. 3D). Maximal force generation was comparable in sham and REV.
Acute IV Levo and milrinone improve LV systolic and diastolic function.
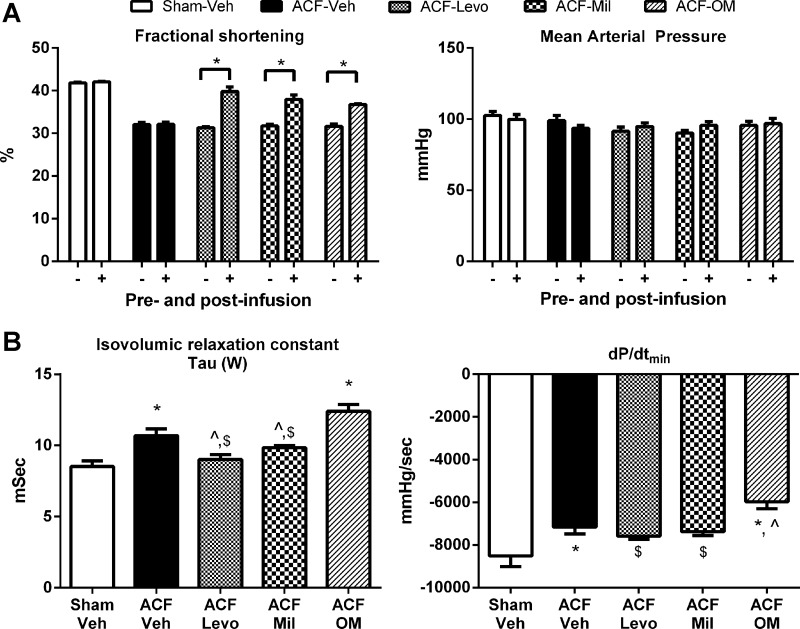
The findings from the myocyte and myofilament studies demonstrate decreased myofilament Ca2+ sensitivity in ACF and REV without alterations in Ca2+ handling. On the basis of these findings, we next treated ACF rats with pharmacological agents that improve myofilament function (i.e., milrinone, Levo, or OM) intravenously to determine their effects on systolic and diastolic function. Following 30-min infusion, %FS in ACF (32%) was increased by IV Levo (40%), milrinone (38%), and OM (37%); MAP remained unchanged in all groups (Fig. 4A). Diastolic relaxation differed among groups (Fig. 4B). Diastolic relaxation was normal in sham and impaired in ACF-Veh. Diastolic relaxation as measured by τ and dP/dtmin was improved in ACF-Levo and ACF-milrinone but impaired in ACF-OM. On the basis of these results, Levo was selected for chronic oral treatment to determine whether IV Levo preserves/improves LV function in ACF and enhances functional recovery in REV (Fig. 1).
Fig. 4.
Acute treatment with phosphodiesterase 3 inhibitor improves lusitropy in 8-wk ACF rats. A: Levo (ACF-Levo), milrinone (ACF-Mil), and omecamtiv mecarbil (ACF-OM) improved percent of fractional shortening (%FS) without altering mean arterial pressure. B: Levo and milrinone improved, whereas OM worsened τ and dP/dtmin. Data are means ± SE. N = 5/group. *P < 0.05 vs. vehicle sham (Sham-Veh); ^P < 0.05 vs. ACF-Veh; $P < 0.05 vs. ACF-OM.
Chronic oral Levo does not significantly alter LV chamber morphology.
Compared with sham at weeks 6 and 8, LVEDD was increased in ACF-Veh and ACF-Levo and normalized in REV-Veh and REV-Levo (Fig. 5, Table 1). At these time points, LVESD was increased to a greater degree in ACF-Veh and REV-Veh compared with ACF-Levo and REV-Levo. Because PWTd remained largely unchanged, the dilation index was significantly lower in ACF but normalized in REV, indicating eccentric hypertrophy and reverse remodeling, respectively (Table 1). EDV and ESV were significantly increased in ACF but reduced in REV (Table 1). In ACF-Veh and ACF-Levo, these increases were accompanied by increased heart and wet lung weights (Table 1), consistent with pathological LV hypertrophy and pulmonary congestion/edema. By contrast, heart and lung weights normalized to sham levels in REV-Veh and REV-Levo.
Fig. 5.
Chronic Levo improves %FS but not LV end-diastolic diameter (LVEDD). LVESD, LV end-systolic diameter A: representative M-mode images at week 8. B: LVEDD and %FS. Data are means ± SE (n = 15–24/group). *P < 0.05 vs. Sham-Veh; ^P < 0.05 vs. ACF-Veh; $P < 0.05 vs. REV-Veh.
Table 1.
LV morphological, echocardiographic, and hemodynamic parameters at week 8 in rats given Levo chronically
| Sham | ACF-Veh | ACF-Levo | REV-Veh | REV-Levo | |
|---|---|---|---|---|---|
| N | 10–24 | 7–17 | 10–19 | 10–20 | 10–19 |
| Body weight, g | 392.2 ± 7.7 | 415.0 ± 7.0 | 412.4 ± 6.7 | 352.0 ± 6.6 | 383.8 ± 5.3 |
| Heart, g | 1.00 ± 0.02 | 1.91 ± 0.06* | 1.85 ± 0.07* | 1.13 ± 0.02† | 1.18 ± 0.03† |
| Lung, g | 1.39 ± 0.03 | 2.24 ± 0.14* | 2.12 ± 0.12* | 1.46 ± 0.04† | 1.59 ± 0.05† |
| LVESD, mm | 4.3 ± 0.08 | 7.4 ± 0.22* | 6.1 ± 0.14*† | 5.2 ± 0.25*† | 4.7 ± 0.05† |
| PWTd, mm | 1.7 ± 0.02 | 1.8 ± 0.04* | 1.9 ± 0.05* | 1.8 ± 0.03 | 1.8 ± 0.04* |
| PWTs, mm | 2.8 ± 0.05 | 2.6 ± 0.07 | 3.1 ± 0.05*† | 2.5 ± 0.04* | 2.8 ± 0.05‡ |
| Dilation index | 0.44 ± 0.01 | 0.34 ± 0.01* | 0.38 ± 0.01* | 0.45 ± 0.01† | 0.45 ± 0.01† |
| HR, beats/min | 358 ± 12 | 315 ± 16 | 331 ± 7 | 345 ± 6 | 362 ± 21 |
| SV, μl | 152 ± 7 | 220 ± 9* | 209 ± 11* | 140 ± 9† | 146 ± 9† |
| EDV, μl | 312 ± 15 | 396 ± 13* | 381 ± 5* | 341 ± 15 | 323 ± 15† |
| dP/dtmax, mmHg/s | 7522 ± 296 | 7456 ± 414 | 9788 ± 459*† | 6907 ± 230 | 8327 ± 645 |
Data are expressed as means ± SE. Dilation index [2× posterior wall thickness in diastole/left ventricular end-diastolic diameter (PWTd/LVEDD)].
P < 0.05 vs. vehicle sham (Sham-Veh);
P < 0.05 vs. vehicle aortocaval fistula (ACF-Veh);
P < 0.05 vs. vehicle reverse (REV-Veh).
Levo, levosimendan; LVESD, LV end-systolic diameter; PWTs, posterior wall thickness in systole; HR, heart rate; SV, stroke volume; EDV, end-diastolic volume.
Chronic oral Levo improves LV systolic and diastolic function without altering β-adrenergic responsiveness.
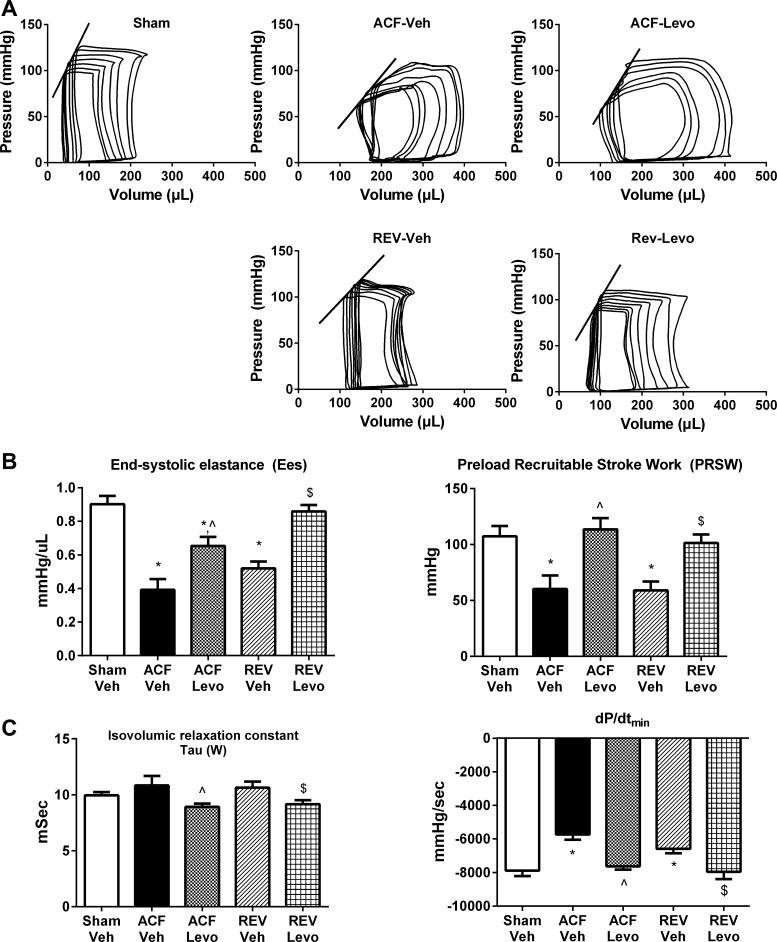
%FS was measured by serial echocardiography (Fig. 5). At 2–4 wk post-ACF, %FS was significantly lower in ACF compared with sham. Oral Levo significantly improved %FS (8% increase compared with week 4, P < 0.05), whereas %FS continued to decline in ACF-Veh and REV-Veh. During week 8, load-independent measures of systolic and diastolic function were obtained through pressure-volume analysis (Fig. 6, Table 1). As previously reported (19), Ees and PRSW were decreased in ACF-Veh and REV-Veh vs. sham. Oral Levo significantly increased Ees and PRSW over ACF-Veh (67% and 89%, respectively) and REV-Veh (65% and 71%, respectively). In ACF-Veh and REV-Veh, there was significantly impaired diastolic relaxation (increased τ, a less negative dP/dtmin; Fig. 6C, Table 1), which was improved by oral Levo.
Fig. 6.
Chronic Levo improves LV systolic and diastolic function. A: representative pressure-volume loops. LV systolic (B) and diastolic (C) functional measurements. Data are means ± SE (n = 7–10/group). *P < 0.05 vs. Sham-Veh; ^P < 0.05 vs. ACF-Veh; $ P < 0.05 vs. REV-Veh.
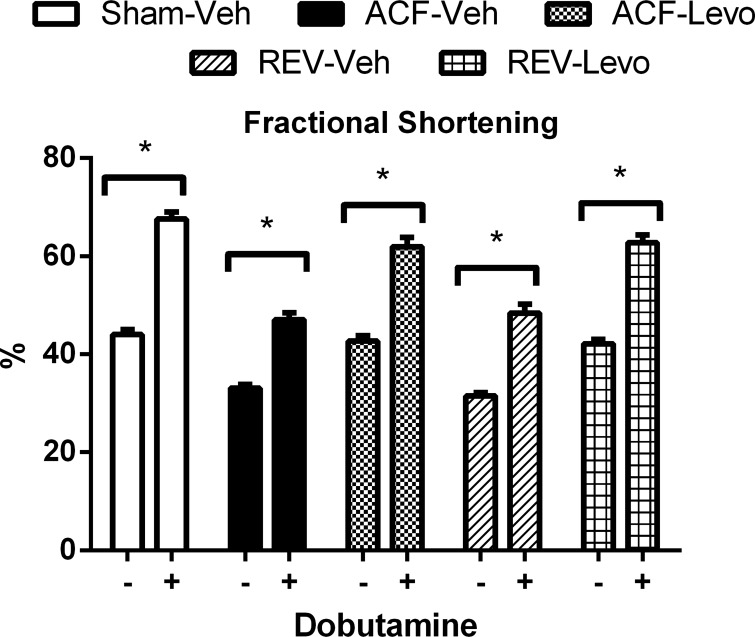
Decreased β-adrenergic responsiveness occurs in end-stage VO (17). To determine whether Levo alters β-adrenergic responsiveness, we measured %FS following dobutamine injection at week 8. Sham, REV-Veh, and REV-Levo had comparable responses to dobutamine (54 ± 3%, 54 ± 6%, and 49 ± 5% increase, respectively; Fig. 7), which was mildly blunted but not significantly different in ACF-Veh and ACF-Levo (42 ± 4% and 46 ± 4% increase, respectively). This suggests that β-adrenergic responsiveness is preserved in ACF and REV at this time point.
Fig. 7.
β-Adrenergic responsiveness is preserved in ACF and REV. Dobutamine (0.5 mg/kg ip) was given during week 8, and the increase in %FS was measured. Data are means ± SE (n = 11–12/group). *P < 0.05.
Chronic oral Levo does not significantly alter MAP.
Short-term IV administration of Levo in patients with acutely decompensated HF reportedly causes vasodilation and hypotension (29). To determine whether these effects occurred with our oral Levo dose, we measured blood pressure in conscious rats by radiotelemetry. At week 8, MAP was comparable (P > 0.05) among all five groups: sham (110 ± 3 mmHg), ACF-Veh (103 ± 2 mmHg), ACF-Levo (111 ± 4 mmHg), REV-Veh (120 ± 2 mmHg), and REV-Levo (116 ± 3 mmHg).
Chronic oral Levo improves LV myocyte sarcomere shortening kinetics without significantly altering PS.
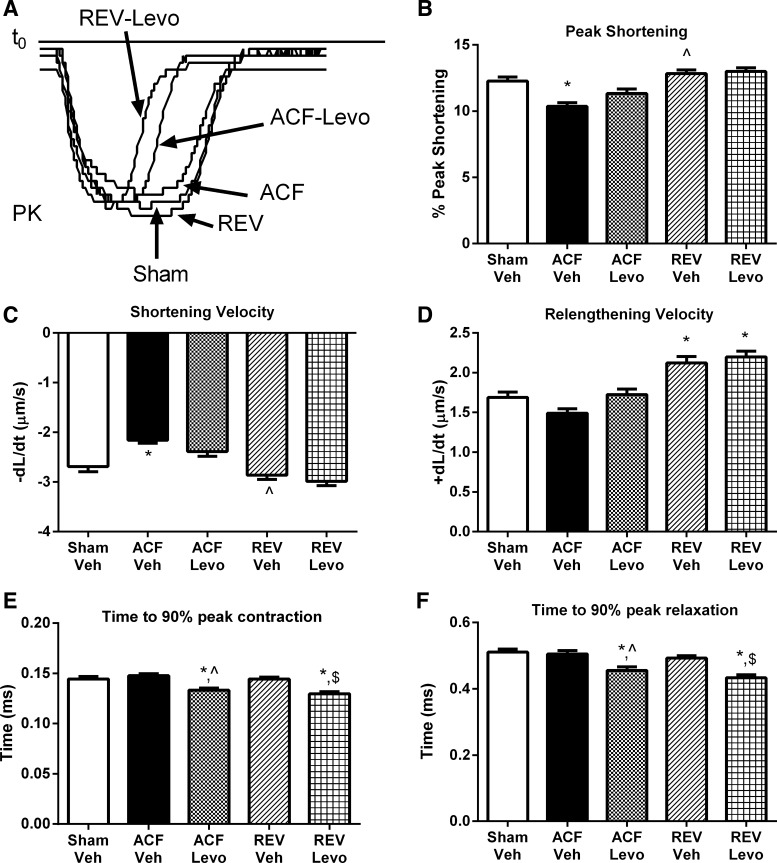
To evaluate the effects of oral Levo on PS, LV myocytes from Levo-treated rats were isolated at week 8. Representative sarcomere shortening recordings are shown in Fig. 8A. PS and maximal sarcomere shortening velocity (−dl/dt) in ACF-Levo and REV-Levo were not different from ACF-Veh or REV-Veh, respectively, or sham (Fig. 8, B and C). Maximal sarcomere relengthening velocity (+dl/dt) in ACF-Levo was not different from ACF-Veh or sham but increased in REV-Levo compared with sham and was no different from REV-Veh (Fig. 8D). By contrast, compared with sham, ACF-Veh, and REV-Veh, the time to 90% of peak contraction and relaxation was decreased in ACF-Levo and REV-Levo myocytes (Fig. 8, E and F).
Fig. 8.
Chronic Levo improves LV myocyte sarcomere shortening kinetics without significantly altering peak shortening. A: representative sarcomere shortening. B: peak shortening. C: peak shortening velocity (−dl/dt). D: peak relengthening velocity (+dl/dt). E: time to 90% peak contraction. F: time to 90% of peak relaxation. *P < 0.05 vs. sham; ^P < 0.05 vs. ACF-Veh; $P < 0.05 vs. REV-Veh. Data represent means ± SE from 60–125 cells from 4–7 rats/group. Please note that sham, ACF, and REV data are redrawn from Fig. 2.
Chronic oral Levo improves myofilament Ca2± sensitivity and maximal force generation without altering myocyte Ca2±-transient kinetics.
To further investigate changes in excitation-contraction coupling in Levo-treated rats, we next measured the amplitude and kinetics of the Ca2+ transient. Representative Ca2+ transients are depicted in Fig. 9A. The mean peak amplitude of Ca2+ fluorescence was 30% decreased in ACF-Levo compared with ACF-Veh (P > 0.0001) and 11% decreased in REV-Levo compared with REV-Veh (P > 0.05; Fig. 9B). In ACF-Levo, the peak rates of Ca2+ release and reuptake were not different from sham or ACF-Veh (Fig. 9, C and D); however, in REV-Levo, the peak rate of Ca2+ release was 37% greater than REV-Veh, and the peak rate of Ca2+ reuptake was 29% and 53% greater than sham and Rev-Veh, respectively (Fig. 9, C and D). Finally, in ACF-Levo and REV-Levo, the time to 90% of peak [Ca2+]i and the time to remove 90% of [Ca2+]i toward baseline were not different from sham, ACF-Veh, or REV-Veh.
Fig. 9.
Chronic Levo improves myofilament Ca2+ sensitivity and maximal force generation without altering myocyte Ca2+-transient kinetics. A: representative Ca2+ transients. B: intracellular calcium amplitude (peak[Ca2+]i). C: peak rate of Ca2+ release. D: peak rate of Ca2+ reuptake. E: representative immunoblots for SERCA-2a, pPLB, and PLB. Cumulative data for SERCA-2a (F) and pPLB/PLB (G). H: force-pCa for LV myofilaments. Myocyte data are means ± SE for 60–125 cells from 4–7 rats/group. (Note: sham, ACF, and REV data are redrawn from Fig. 2.) Immunoblot and force-pCa data are means ± SE for 5–6 rats/group. *P < 0.05 vs. sham; ^P < 0.05 vs. ACF-Veh; $P < 0.05 vs. REV-Veh.
Finally, we evaluated alterations in the expression of SERCA-2a and total and phosphorylated PLB in LV tissue. Representative immunoblots are shown in Fig. 9E, and summary data are shown in Fig. 9, F and G. SERCA-2a was increased 1.6-fold in ACF-Levo but comparable with ACF-Veh and normalized in REV-Levo. Total and phospho-PLB/PLB were not statistically different among the groups.
We next measured myofilament force generation at varying [Ca2+] in skinned myocytes from frozen LV tissue (Fig. 9H). Oral Levo caused the leftward shift in the force-pCa relationship expected for a myofilament Ca2+ sensitizer, and, in ACF-Levo, there was an ∼50% increase in maximal force generation compared with ACF-Veh.
The positive lusitropy of chronic oral Levo is associated with increased myofilament regulatory protein phosphorylation and increased α-to-β-MHC ratio.
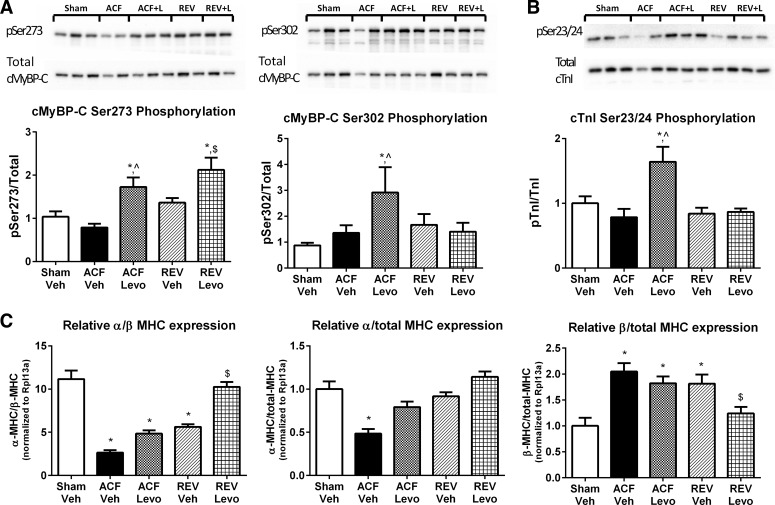
Diastolic relaxation is controlled by changes in Ca2+ cycling, myofilament composition, Ca2+ desensitization, and cross-bridge cycling kinetics (1, 7). Coordinated phosphorylation of cMyBP-C and cTnI are central to these processes; therefore, we measured cMyBP-C and cTnI phosphorylation at key serine residues (cMyBP-C Ser-273, Ser-282 and Ser-302, and cTnI Ser-23/24). Total protein levels, although variable, were not different among groups (n = 5–6; Fig. 10). In ACF-Levo, cMyBP-C phosphorylation at Ser-273 (∼2-fold) and Ser-302 (∼3-fold), but not Ser-282 (not shown), were significantly increased compared with ACF-Veh (Fig. 6A). Additionally, in ACF-Levo, cTnI Ser-23/24 phosphorylation (∼2-fold) was significantly increased (Fig. 10B). By comparison, in REV-Levo, only cMyBP-C phosphorylation at Ser-273 was significantly increased (∼2-fold).
Fig. 10.
The positive lusitropy of chronic Levo is associated with increased myofilament regulatory protein phosphorylation and increased α-to-β-myosin heavy chain (MHC). A: total cMyBP-C and Ser-273 and Ser-302 phosphorylation. B: total cardiac troponin I (cTnI) and Ser-23/24 phosphorylation. Representative immunoblots and cumulative data. C: MHC mRNA. Immunoblot data are means ± SE from n = 5–6/group, and mRNA data are means ± SE from n = 7–8/group. *P < 0.05 vs. Sham-Veh; ^P < 0.05 vs. ACF-Veh; $P < 0.05 vs. REV-Veh.
Because of the role of myofilament composition in cross-bridge cycling kinetics, we measured α- and β-MHC mRNA in LV tissue. As previously demonstrated in ACF-Veh and REV-Veh (19), there is a relative decrease and increase in α- and β-MHC mRNA, respectively (Fig. 10C). Whereas the α-to-β-MHC mRNA ratio remains depressed in ACF-Levo compared with sham (4.9 vs. 11.2, respectively), the α-to-β-MHC mRNA ratio is normalized in REV-Levo (10.2) and significantly improved relative to REV-Veh (5.6, P < 0.0001). This increase in ratio is driven primarily by decreased β-MHC mRNA (Fig. 10C).
DISCUSSION
In this study, we first investigated the effects of ACF and REV on myocyte function and intracellular calcium transients as well as myofilament calcium sensitivity and force generation to begin to characterize the mechanisms of LV dysfunction. We next investigated the effects of acute IV infusion of two positive inotropes targeting the myofilament (i.e., Levo and OM) and one positive inotrope targeting phosphodiesterase 3 (PDE3) (i.e., milrinone) on LV systolic and diastolic function. Finally, we determined whether oral Levo would improve LV function in ACF rats with pre-HF or reversed pre-HF. Together, our results demonstrate that ACF-induced VO results in myofilament dysfunction (Fig. 3) that can be improved through myofilament Ca2+ sensitization (Fig. 9). We show for the first time that chronic oral administration of Levo to VO HF rats improves systolic (%FS, Ees, and PRSW; Figs. 5–6) and diastolic (τ and dP/dtmin; Fig. 6) function, without significantly altering chamber dilation (Fig. 5). These functional changes are associated with improved myofilament Ca2+ sensitivity with decreased intracellular Ca2+ transient amplitude (Fig. 9) and preserved in vivo β-adrenergic responsiveness (Fig. 7). In ACF, oral Levo increased cMyBP-C and cTnI phosphorylation, whereas, in REV, oral Levo increased cMyBP-C phosphorylation and normalized the α-to-β-MHC ratio (Fig. 10).
ACF is widely used to induce VO in rodents (12, 18, 35). This model replicates the hemodynamics and pathophysiology of VO HF progression, irrespective of the etiology, and is the only rodent model that can be used to model the hemodynamics of VO reversal (17–19, 34). We previously demonstrated that reversal during pre-HF (i.e., 4 wk post-ACF) results in rapid structural recovery with delayed functional recovery, which mirrors human data where structure, but not function, returns postsurgically in 17% of patients with MR (27). The results from the single-myocyte experiments demonstrate that impaired LV function in REV is not explained by impaired sarcomere shortening or Ca2+ handling. The decreased Ca2+ sensitivity and decreased α-to-β-MHC in ACF persists in REV (19) (Fig. 10), suggesting persistent myofilament dysfunction. This is supported by the fact that many dilated cardiomyopathy (DCM)-causing mutations result in a calcium-desensitizing effect on myofilament force generation and ATPase activity, suggesting that the primary mechanism for DCM is Ca2+ desensitization (26).
Because myofilament dysfunction is emerging as the mechanism of dysfunction in VO, we next determined whether compounds targeting myofilaments, including the myofilament Ca2+ sensitizer Levo and the myosin activator OM, could improve LV function acutely via administration of these drugs in 8-wk ACF rats. Both compounds increased LV systolic function as expected, whereas only Levo improved diastolic relaxation. Accordingly, Levo was selected for experiments in which ACF and REV were given Levo orally.
Although Levo is untested in VO HF models, our in vivo findings in rats given Levo orally or intravenously mirror improved systolic function in Goto-Kakizaki rats postinfarct (24), in dogs with pacing-induced HF (28), and in patients with acute decompensated HF (29). To our knowledge, single myocyte mechanics have not been studied in animals treated with Levo. Overall, our results demonstrate that Levo did not significantly alter PS or contraction/relengthening velocity. Instead, Levo shortened the time to 90% peak sarcomere contraction and time to 90% peak sarcomere relaxation without significant changes in the shortening or relengthening velocities. The positive inotropic effects of Levo are likely due to the well-characterized mechanism by which Levo stabilizes the Ca2+-saturated cTnC, prolonging its interaction with cTnI (29), accelerating actin-myosin cross-bridge cycling (1), and increasing contractility without altering intracellular Ca2+ transient or myocardial O2 demand (29). Consistent with these observations, Levo did not significantly increase the amount of intracellular Ca2+ available for contraction and did not alter expression of SERCA-2a, pPLB/PLB (Fig. 9), or RyR2 (data not shown). The decrease in the Ca2+-transient peak amplitude may suggest that more Ca2+ has bound to the sensitized myofilaments, leaving less in the cytosol. Taken together, our data suggest that Levo improves myofilament dysfunction in ACF and REV.
In ACF rats at end-stage HF, β-adrenergic responsiveness decreases (17). In our rats, β-adrenergic responsiveness was preserved in all groups (Fig. 7), and β1-mRNA expression was not significantly different among sham, ACF, and REV (data not shown), suggesting that β-receptor desensitization is not fully manifested in ACF at week 8. Interestingly, treating ACF rats with metoprolol 4–15 wk post-ACF increased mortality and decreased systolic function (unpublished data, K. Wilson and P. Lucchesi). Collectively, these data support the notion that β-adrenergic responsiveness is preserved during pre-HF and established HF. It is possible that PDE3 inhibition contributes to the positive inotropy of Levo (28); however, some studies suggest that PDE3 inhibition occurs at supratherapeutic concentrations and does not contribute to positive inotropy (30).
We also demonstrate positive lusitropy with Levo (Fig. 6C), which has been shown in Dahl/Rapp rats (6), in dogs with pacing-induced HF (28), and in human clinical trials (29). The mechanism of the positive lusitropy of Levo is poorly understood; proposed mechanisms include relatively mild Ca2+ sensitization and weaker cTnC-Levo interactions at diastolic Ca2+ levels (29, 30). Our results suggest that cMyBP-C phosphorylation, cTnI phosphorylation, and/or MHC isoform contribute to improved relaxation. Levo treatment in the absence of reversal increased phosphorylation of the myofilament regulatory proteins cMyBP-C (Ser-273 and Ser-302) and cTnI (Ser-23/24) with no alteration in MHC isoform distribution. Conversely, the predominant effects of hemodynamic load reversal alone were on the ratio of α-to-β-MHC, which was partially normalized to sham levels. Interestingly, a different pattern was noted when we combined hemodynamic load reversal and Levo treatment (i.e., REV-Levo). In these samples, both the α-to-β-MHC ratio was normalized to sham levels and phosphorylation of cMyBP-C Ser-273 persisted. The lack of increased phosphorylation of cMyBP-C Ser-302 and cTnT Ser23/24 may be due to differences in hemodynamic load.
According to Fitzsimmons et al. (11), tension relaxation is governed by several interrelated factors, including the rate of cross-bridge detachment, reattachment of previously detached cross-bridges, and calcium dissociation from TnC. Historically, the rate of cross-bridge detachment was thought to be rate limiting because it was significantly slower than the rate of Ca2+ dissociation from TnC (11, 23). However, newer data suggest that the rate of cross-bridge detachment is temperature sensitive, and, at near physiological temperatures, the rate of cross-bridge detachment is faster than the rate of Ca2+ dissociation from TnC, suggesting that Ca2+ is rate limiting (23). Several thick and thin myofilament proteins have been shown to alter cross-bridge cycling kinetics as well as myofilament Ca2+ sensitivity, and, as mentioned, we have demonstrated changes in these proteins in our model. cMyBP-C regulates thick-filament function, and phosphorylation at Ser-273, Ser-282, and Ser-302 appears to accelerate cross-bridge cycling, increasing force development as well as relaxation (9, 33). cTnI regulates thin-filament function, and Ser-23/24 phosphorylation by PKA decreases Ca2+ sensitivity and myofilament cross-bridge cycling kinetics (31, 36) and increases the rate of cTnC-Ca2+ dissociation (23). Finally, chemically skinned myocardium containing primarily α-MHC has a higher ATPase rate, faster sarcomeric shortening velocity, faster rate of tension relaxation, higher power production, and greater rate of force development compared with β-MHC (11, 39). Taken together, we suggest that there is a complex interrelationship among these three proteins, and the relative importance of each in our model depends on hemodynamic load as well as presence or absence of the myofilament Ca2+ sensitizer. Further studies are needed to elucidate the relative contribution of each of these changes.
The mechanism underlying the positive lusitropy of Levo may involve the phosphorylation of cMyPB-C and cTnI secondary to PDE3a inhibition. Data from the short-term IV infusion study allowed us to, not only select an agent for oral treatment, but also further characterize the role of PDE3a inhibition in lusitropy. In addition to evaluating OM and Levo, we evaluated diastolic relaxation in rats given the PDE3a inhibitor milrinone. Lusitropy was improved with Levo and milrinone but not with OM (Fig. 4B). This suggests that the positive inotropy and lusitropy of Levo are mediated by improved myofilament Ca2+ sensitivity and PDE3a inhibition, respectively, whereas the positive inotropy and lusitropy of milrinone are mediated by PDE3a inhibition and increased phosphorylation of Ca2+ regulatory proteins (3). Our data suggest that the lusitropic actions of Levo are associated with increased cTnI and cMyBP-C phosphorylation. The reason for the difference in phosphorylation patterns that result from these two distinct PDE3a inhibitors is unclear. However, one possibility is that the drugs interact with PDE3a localized to different subcellular compartments (32, 38).
One potential clinical limitation of our study is that high-dose, short-term infusions are associated with arrhythmias and hypotension in patients with acute decompensated HF (29). We noted no significant increase in arrhythmias in Levo-treated rats (not shown); however, heart rhythm was not chronically measured, and arrhythmias are difficult to characterize in rodents because of their high heart rates. The oral Levo dose used (1 mg/kg per day) did not significantly alter MAP, suggesting that the inotropic and lusitropic effects of Levo are not secondary to changes in afterload.
In summary, we demonstrate that 1) Levo improves systolic and diastolic function in rats with pre-HF and reversed pre-HF, 2) targeting the myofilament improved function during early HF even with continued VO, and 3) improved diastolic function correlates with myofilament regulatory protein phosphorylation and normalization of the α-to-β-MHC ratio. Myofilament-targeting compounds may offer alternate pharmacological treatment options for patients with VO HF.
GRANTS
This work was supported by the National Institutes of Health [R01 HL-056046 (to P. Lucchesi), R01 HL-62426 (to P. de Tombe), R01 HL105826 and K02 HL 114749 (to S. Sadayappan), and T32 HL-098039 (to A. Trask)], the American Heart Association (13SDG16840035 to A. Trask), funds provided by The Heart Center and Research Institute at Nationwide Children's Hospital, and by a fellowship from Genentech through the American College of Veterinary Pathologists and Society of Toxicologic Pathology Coalition for Veterinary Pathology Fellows (to K. Wilson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.W., A.G., A.J.T., and P.A.L. conception and design of research; K.W., A.G., T.A.W., X.Z., A.J.T., M.J.C., and P.d.T. performed experiments; K.W., A.G., T.A.W., M.J.C., and P.d.T. analyzed data; K.W., A.G., T.A.W., P.d.T., S.S., and P.A.L. interpreted results of experiments; K.W. and M.J.C. prepared figures; K.W. and P.A.L. drafted manuscript; K.W., A.G., T.A.W., X.Z., A.J.T., M.J.C., P.d.T., S.S., and P.A.L. edited and revised manuscript; K.W., A.G., T.A.W., X.Z., A.J.T., M.J.C., P.d.T., S.S., and P.A.L. approved final version of manuscript.
REFERENCES
- 1.Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, Denney T, Jr, Powell P, McGiffin DC, Dell'Italia LJ. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60%. J Am Coll Cardiol 55: 671–679, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ait Mou Y, Toth A, Cassan C, Czuriga D, de Tombe PP, Papp Z, Lacampagne A, Cazorla O. Beneficial effects of SR33805 in failing myocardium. Cardiovasc Res 91: 412–419, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beca S, Aschars-Sobbi R, Panama BK, Backx PH. Regulation of murine cardiac function by phosphodiesterases type 3 and 4. Curr Opin Pharmacol 11: 714–719, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Belin RJ, Sumandea MP, Sievert GA, Harvey LA, Geenen DL, Solaro RJ, de Tombe PP. Interventricular differences in myofilament function in experimental congestive heart failure. Pflügers Arch 462: 795–809, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergh CH, Andersson B, Dahlstrom U, Forfang K, Kivikko M, Sarapohja T, Ullman B, Wikstrom G. Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur J Heart Fail 12: 404–410, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biala A, Finckenberg P, Korpi A, Loytainen M, Martonen E, Levijoki J, Mervaala E. Cardiovascular effects of the combination of levosimendan and valsartan in hypertensive Dahl/Rapp rats. J Physiol Pharmacol 62: 275–285, 2011. [PubMed] [Google Scholar]
- 7.Borlaug BA, Kass DA. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med 16: 273–279, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Coulton AT, Stelzer JE. Cardiac myosin binding protein C and its phosphorylation regulate multiple steps in the cross-bridge cycle of muscle contraction. Biochemistry 51: 3292–3301, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 373: 1382–1394, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J Physiol 513: 171–183, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaim SF, Minteer WJ, Nellis SH, Clark DP. Chronic arteriovenous shunt: evaluation of a model for heart failure in rat. Am J Physiol 236: H698–H704, 1979. [DOI] [PubMed] [Google Scholar]
- 13.Gardner JD, Murray DB, Voloshenyuk TG, Brower GL, Bradley JM, Janicki JS. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol 298: H497–H504, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg V, Frishman WH. A new approach to inotropic therapy in the treatment of heart failure: cardiac myosin activators in treatment of HF. Cardiol Rev 21: 155–159, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, Gongora E, Greis KD, Luther PK, Winegrad S, Henderson KK, Sadayappan S. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol 52: 154–164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grayburn PA, Weissman NJ, Zamorano JL. Quantitation of mitral regurgitation. Circulation 126: 2005–2017, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Guggilam A, Hutchinson KR, West TA, Kelly AP, Galantowicz ML, Davidoff AJ, Sadayappan S, Lucchesi PA. In vivo and in vitro cardiac responses to beta-adrenergic stimulation in volume-overload heart failure. J Mol Cell Cardiol 57: 47–58, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ, American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111: 131–150, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson KR, Guggilam A, Cismowski MJ, Galantowicz ML, West TA, Stewart JA, Jr, Zhang X, Lord KC, Lucchesi PA. Temporal pattern of left ventricular structural and functional remodeling following reversal of volume overload heart failure. J Appl Physiol 111: 1778–1788, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalanko M, Kivikko M, Harjola VP, Nieminen MS, Laine M. Oral levosimendan improves filling pressure and systolic function during long-term treatment. Scand Cardiovasc J 45: 91–97, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Jobe LJ, Melendez GC, Levick SP, Du Y, Brower GL, Janicki JS. TNF-α inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. Am J Physiol Heart Circ Physiol 297: H1462–H1468, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koshman YE, Chu M, Kim T, Kalmanson O, Farjah M, Kumar M, Lewis W, Geenen DL, de Tombe P, Goldspink PH, Solaro RJ, Samarel AM. Cardiomyocyte-specific expression of CRNK, the C-terminal domain of PYK2, maintains ventricular function and slows ventricular remodeling in a mouse model of dilated cardiomyopathy. J Mol Cell Cardiol 72: 281–291, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little SC, Biesiadecki BJ, Kilic A, Higgins RS, Janssen PM, Davis JP. The rates of Ca2+ dissociation and cross-bridge detachment from ventricular myofibrils as reported by a fluorescent cardiac troponin C. J Biol Chem 287: 27930–27940, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louhelainen M, Vahtola E, Forsten H, Merasto S, Kyto V, Finckenberg P, Leskinen H, Kaheinen P, Tikkanen I, Levijoki J, Mervaala E. Oral levosimendan prevents postinfarct heart failure and cardiac remodeling in diabetic Goto-Kakizaki rats. J Hypertens 27: 2094–2107, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Louhelainen M, Vahtola E, Kaheinen P, Leskinen H, Merasto S, Kyto V, Finckenberg P, Colucci WS, Levijoki J, Pollesello P, Haikala H, Mervaala EM. Effects of levosimendan on cardiac remodeling and cardiomyocyte apoptosis in hypertensive Dahl/Rapp rats. Br J Pharmacol 150: 851–861, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu QW, Wu XY, Morimoto S. Inherited cardiomyopathies caused by troponin mutations. J Geriatr Cardiol 10: 91–101, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascle S, Schnell F, Thebault C, Corbineau H, Laurent M, Hamonic S, Veillard D, Mabo P, Leguerrier A, Donal E. Predictive value of global longitudinal strain in a surgical population of organic mitral regurgitation. J Am Soc Echocardiogr 25: 766–772, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Masutani S, Cheng HJ, Hyttila-Hopponen M, Levijoki J, Heikkila A, Vuorela A, Little WC, Cheng CP. Orally available levosimendan dose-related positive inotropic and lusitropic effect in conscious chronically instrumented normal and heart failure dogs. J Pharmacol Exp Ther 325: 236–247, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Papp Z, Edes I, Fruhwald S, De Hert SG, Salmenpera M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikstrom BG, Jorgensen K, Filippatos G, Parissis JT, Gonzalez MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 159: 82–87, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Pathak A, Lebrin M, Vaccaro A, Senard JM, Despas F. Pharmacology of levosimendan: inotropic, vasodilatory and cardioprotective effects. J Clin Pharm Ther 38: 341–349, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Pena JR, Wolska BM. Troponin I phosphorylation plays an important role in the relaxant effect of beta-adrenergic stimulation in mouse hearts. Cardiovasc Res 61: 756–763, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Pidoux G, Tasken K. Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins. J Mol Endocrinol 44: 271–284, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Previs MJ, Beck Previs S, Gulick J, Robbins J, Warshaw DM. Molecular mechanics of cardiac myosin-binding protein C in native thick filaments. Science 337: 1215–1218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan TD, Rothstein EC, Aban I, Tallaj JA, Husain A, Lucchesi PA, Dell'Italia LJ. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J Am Coll Cardiol 49: 811–821, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Scheuermann-Freestone M, Freestone NS, Langenickel T, Hohnel K, Dietz R, Willenbrock R. A new model of congestive heart failure in the mouse due to chronic volume overload. Eur J Heart Fail 3: 535–543, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Solaro RJ, Henze M, Kobayashi T. Integration of troponin I phosphorylation with cardiac regulatory networks. Circ Res 112: 355–366, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sponga S, Ivanitskaia E, Potapov E, Krabatsch T, Hetzer R, Lehmkuhl H. Preoperative treatment with levosimendan in candidates for mechanical circulatory support. ASAIO J 58: 6–11, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Sumandea CA, Garcia-Cazarin ML, Bozio CH, Sievert GA, Balke CW, Sumandea MP. Cardiac troponin T, a sarcomeric AKAP, tethers protein kinase A at the myofilaments. J Biol Chem 286: 530–541, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Tanner BC, Lombardo AT, Tremble SM, Maughan DW, Vanburen P, Lewinter MM, Robbins J, Palmer BM. Cardiac myosin isoforms exhibit differential rates of MgADP release and MgATP binding detected by myocardial viscoelasticity. J Mol Cell Cardiol 54: 1–8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei CC, Chen Y, Powell LC, Zheng J, Shi K, Bradley WE, Powell PC, Ahmad S, Ferrario CM, Dell'Italia LJ. Cardiac kallikrein-kinin system is upregulated in chronic volume overload and mediates an inflammatory induced collagen loss. PloS One 7: e40110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]