Abstract
Background
Bariatric surgery is associated with cognitive benefits, but the nature of such gains may be variable across demographically and clinically diverse persons. Older adults achieve less weight loss and resolution of fewer medical comorbidities after surgery compared to younger patients, and are also at heightened risk for nutritional deficiencies. However, no study has examined the influence of age on cognitive improvements after bariatric surgery.
Objective
To determine the effects of age on cognitive function post-bariatric surgery.
Setting
Hospital.
Methods
95 participants enrolled in the Longitudinal Assessment for Bariatric Surgery completed a computerized cognitive test battery prior to bariatric surgery and at 12-weeks, and 12-months post-operatively.
Results
Baseline cognitive impairments were common. Significant improvements were found in attention/executive function and memory abilities 12-weeks and 12-months after surgery. Age was not associated with baseline cognitive test performance. Separate multivariable regression analyses controlling for baseline attention/executive function and memory also showed that age was not a significant predictor of 12-week or 12-month performances in these domains (p > 0.05 for all).
Conclusions
The current study provides preliminary evidence suggesting that older age does not preclude post-bariatric surgery cognitive benefits. Prospective studies in more age diverse samples (i.e., up to 70 years) are needed to determine whether bariatric surgery can reduce risk of age-related neurological conditions like Alzheimer’s disease and stroke.
Keywords: Obesity, bariatric surgery, cognitive function, age, older adults
Introduction
Obese persons are at elevated risk for dementia (e.g., Alzheimer’s disease, vascular dementia).1 More subtle deficits in cognitive function are also evident in obese individuals before the onset of these severe neurological conditions.2 Fortunately, extant evidence suggests weight loss may help to at least partially reverse poor cognitive outcomes in severely obese persons. Most notably, bariatric surgery has been shown to yield post-operative cognitive benefits relative to obese controls at 12-weeks and 12-month follow-ups3,4 and such gains may even be sustained up to three years later.5
The nature of post-operative cognitive benefits is likely complicated by the influence of interindividual differences in demographic and medical characteristics on post-surgery outcomes (e.g., weight loss) and cognition. Although not previously examined, age is likely an important modifier of cognitive changes after bariatric surgery. Bariatric surgery has been shown to be safe and effective in older adults6 and is becoming increasingly popular among older individuals.7 However, recent work shows that advancing age is associated with differential outcomes in some studies, including reduced weight loss, comorbid medical condition resolution, and risk for nutritional deficiencies.8–10
It is possible that increasing age may also attenuate post-operative cognitive benefits. Increasing age is the number one risk factor for neurodegenerative conditions such as Alzheimer’s disease11 and older adults are at risk for adverse brain changes (e.g., atrophy).12 Indeed, these neurocognitive impairments are exacerbated in older adults with obesity.13 Older adults also exhibit higher rates of post-operative nutritional deficiencies than younger patients, placing them at elevated risk for neurological complications.14,15 In addition, increasing age may be accompanied by greater obesity duration and associated comorbid medical conditions (e.g., hypertension) and physiological disturbances (e.g., inflammation) that further increase risk for neurocognitive impairment.
No study to date has examined the effects of age on cognitive changes following bariatric surgery. The purpose of the current study was to examine the influence of age on post-operative cognitive changes 12-weeks and 12-months after bariatric surgery. We hypothesized that increasing age would attenuate acute and long-term post-operative cognitive improvements.
Materials and Methods
Participants
A total of 95 participants were recruited into a multi-site prospective study examining the neurocognitive effects of bariatric surgery. All bariatric surgery patients were part of the Longitudinal Assessment of Bariatric Surgery (LABS) parent project and were recruited from existing LABS sites (Columbia, Cornell, and Neuropsychiatric Research Institute).16 For study inclusion, Bariatric Surgery Patients were required to be enrolled in LABS, between 20–70 years of age, and English-speaking. Exclusion criteria included history of neurological disorder or injury (e.g. dementia, stroke, seizures), moderate or severe head injury (defined as >10 minutes loss of consciousness), past or current history of severe psychiatric illness (e.g. schizophrenia, bipolar disorder), past or current history of alcohol or drug abuse (defined by DSM-IV criteria), history of a learning disorder or developmental disability (defined by DSM-IV criteria), or impaired sensory function.
Within the sample, almost all patients underwent Roux-en-Y gastric bypass surgery (RYGB). Only 1 bariatric surgery patient underwent a gastric banding procedure and thus no comparisons for type of surgery were conducted. The present sample represents individuals that have completed 12-weeks and 12 months of follow-up cognitive data.
Predictors and Outcomes
Cognitive Function
The IntegNeuro is a computerized cognitive test battery that assesses cognitive function in multiple domains. It can be completed in 45–60 minutes and individual scores obtained are compared to a normative sample to automatically derive standardized scores (Brain Resource Company, Ltd). This instrument demonstrates excellent psychometric properties, including convergent validity with standardized neuropsychological measures (r = 0.53 to r = 0.77) and test-retest reliability across the tasks ranging from 0.52 to 0.89.17,18 Alternate forms of the IntegNeuro also help limit practice effects. The cognitive domain and specific tests included:
Attention/Executive Function
Switching of Attention
This task is a computerized adaptation of the Trail Making Test A and B.19 Participants are first asked to touch a series of 25 numbers in ascending order as quickly as possible. This is followed by the presentation of 13 numbers (1–13) and 12 letters (A-L) that participants alternately touch in ascending order. These tests assess attention and psychomotor speed as well as executive function. Time to completion served as the outcome measure in the current study.
Maze Task
This task is a computerized adaptation of the Austin Maze20 and assesses executive function. Participants are presented with a grid (8×8 matrix) of circles and asked to identify the hidden path through the grid. Distinct auditory and visual cues are presented for correct and incorrect responses. The trial ends when the subject completed the maze twice without error or after 10 minutes has elapsed.
Memory
Verbal List-learning
Participants are read a list of 12 words a total of 4 times and asked to recall as many words as possible after each trial. Following presentation and recall of a distraction list, participants are then asked to recall words from the original list. After a 20- minute filled delay, participants are asked to freely recall the learned list and perform a recognition trial comprised of target words and non-target words. Total long delayed free recall and recognition of these verbal list items assessed memory in the current study.
Language
Letter Fluency
Participants are asked to generate words beginning with a given letter of the alphabet for 60 seconds. A different letter is used for each of the three trials. Total number of correct words generated across the three trials served as the dependent variable.
Animal Fluency
In this task, participants generate as many animal names as possible in 60 seconds. Total correct served as the dependent variable.
Demographic and Medical Characteristics
Demographic and medical characteristics were ascertained via participant self-report. Train research staff also performed a chart review on all participants in order to corroborate participant self-reported medical information. Through these procedures, demographic variables (i.e., age, sex, race), and medical factors such as diagnostic history of hypertension, type 2 diabetes mellitus (T2DM), and sleep apnea were collected.
Procedures
All procedures were approved by the Institutional Review Boards and all participants provided written informed consent prior to study involvement. Research was completed in accordance with the Helsinki Declaration. The bariatric surgery participants completed a computerized cognitive test battery at baseline (within 30 days prior to surgery), 12-weeks (± 7 days), and 12-months (± 14 days) following surgery. At each time point, participants completed demographic and medical history questionnaires and a medical chart review was performed. Participants’ height and weight were also measured at each time point and BMI was calculated using the standard formula.
Statistical Analysis
All raw scores of neuropsychological measures assessing cognitive function were transformed to T-scores (a distribution with a mean of 50, and a standard deviation of 10) using existing IntegNeuro normative data correcting for age, gender, and premorbid intelligence. Consistent with clinical interpretation, a T-score < 35 was used to detect significant cognitive impairment (i.e., 1.5 SD from the normative mean).21 A composite score was computed at each time point for attention/executive function, memory, and language that consisted of the mean of T-scores of the respective neuropsychological measures that comprise each domain.
Descriptive statistics (i.e., mean and standard deviations) were performed to characterize the demographic, medical, and clinical status of the sample. Hypertension and T2DM variables each had one case with missing baseline data and the sample size for analyses that included these medical factors was 94. Similarly, due to missing weight data at follow-up time points the sample size for analyses that included 12-week and 12-month BMI was reduced to 91 and 87, respectively. Independent samples t-tests examined the associations between age and medical and demographic variables.
Repeated measures analyses of variance (ANOVA) were performed to examine changes in attention/executive function, memory, and language across each time point (i.e., baseline, 12-weeks, and 12-months) among the sample of bariatric surgery patients (N = 95). For those domains with significant time effects, follow-up repeated measure ANOVA examined cognitive changes between each time point.
Regression analyses first examined the effects of age on baseline cognitive function for each of the domains. Multivariable linear regression analyses were then performed to examine the acute and long-term effects of age on post-operative cognitive changes. Separate regression models were performed for each cognitive domain that demonstrated significant changes over time. Specifically, in separate models, the dependent variable included the 12-week and 12-month cognitive domain. For all analyses, block 1 of the regression model included the baseline cognitive domain and age was entered in block 2. These same analyses were also performed to examine the effects of age on 12-week and 12-month post-operative changes in BMI.
Results
Age and Pre- and Post-Operative Medical and Clinical Characteristics
The average baseline BMI of the sample was 46.2 (SD = 5.9) kg/m2, placing them in the severely obese classification. Bariatric surgery participants demonstrated significant weight loss after surgery with an average 12-month BMI of 30.7 (SD = 5.2) kg/m2 (i.e., obese range). Pre-operative status of hypertension (46.2%), T2DM (26.9%), and sleep apnea (38.7%) were also prevalent in the sample of bariatric surgery patients.
Of the sample, 89.5% were women and the mean age was 43.2 (SD = 10.8), with 18.9% of persons aged 55 years or older. Bivariate correlations showed no significant association between age and pre-operative BMI (p > 0.05). However, at baseline, independent samples t-tests showed that patients with hypertension, T2DM, and/or sleep apnea were more likely to be older those patients without these conditions (p < 0.05 for all). Regression analyses controlling for baseline BMI revealed age was not a significant predictor of 12-week (N = 91) or 12-month (N = 87) post-operative changes in BMI (p > 0.05 for all).
Pre- and Post-Operative Changes in Cognitive Function
Table 1 presents cognitive test performance at each time point. Clinically meaningful baseline impairments (i.e., T-score < 35) were most prevalent in memory and language abilities among the overall sample of bariatric surgery patients. Impairments on tasks of attention/executive function were prevalent, but not as common. At 12-months after surgery, impairments on almost all neuropsychological measures became less prevalent, particularly on tests of memory.
Table 1.
Baseline, 12-Week, and 12-Month Neuropsychological Test Performance in the Full Sample (N = 95)
| Neuropsychological Domains/Tests |
Baseline M (SD) |
% T-score < 35 |
12-week M (SD) |
% T-score < 35 |
12-month M (SD) |
% T-score < 35 |
|---|---|---|---|---|---|---|
| Attention/Executive Function | ||||||
| SOA-A | 54.6(14.5) | 10.5 | 58.5(13.7) | 4.2 | 61.4(15.0) | 5.3 |
| SOA-B | 52.3(15.2) | 9.5 | 57.8(12.7) | 6.3 | 58.0(13.4) | 8.4 |
| Maze Errors | 49.8(12.9) | 12.6 | 55.7(11.8) | 6.3 | 53.8(11.3) | 7.4 |
| Memory | ||||||
| LDFR | 45.9(10.9) | 15.8 | 48.6(13.1) | 18.9 | 53.0(9.8) | 5.3 |
| Recognition | 41.3(10.4) | 22.1 | 51.4(10.5) | 8.4 | 49.1(9.4) | 5.3 |
| Language | ||||||
| Verbal Fluency | 47.0(11.5) | 15.8 | 46.9(10.1) | 11.6 | 47.5(11.3) | 12.6 |
| Animals | 50.7(10.6) | 4.2 | 50.2(10.1) | 5.3 | 50.4(11.1) | 7.4 |
Note. Test scores are reported as T-Scores. SOA = Switching of Attention; LDFR = Long Delay Free Recall.
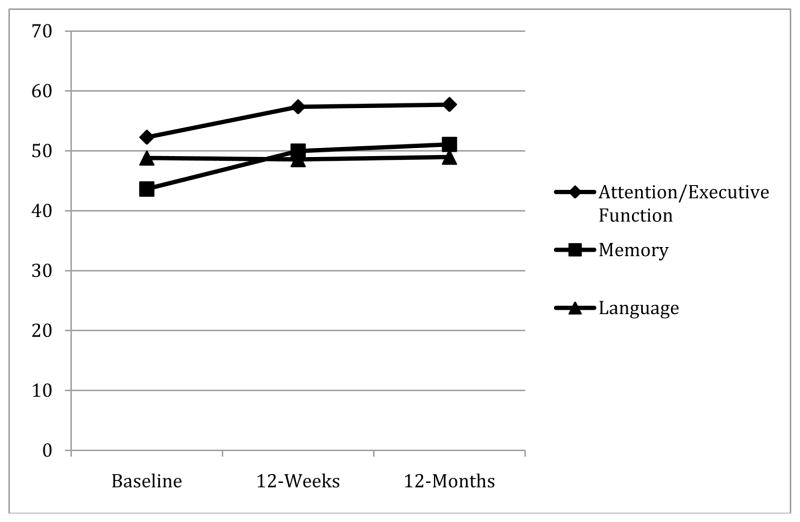
See Figure 1 for changes in cognitive function across the time points in the overall sample. Repeated measures ANOVA showed a significant time effect across the three time points (i.e., baseline, and 12-weeks, and 12-months after surgery) for attention/executive function (F(2, 93) = 30.1, p < 0.001) and memory (F(2, 93) = 39.1, p < 0.001). For both domains, follow up analyses showed that cognitive function improved from baseline to 12-weeks [attention/executive function: F(1, 94) = 47.2, p < 0.001); memory: F(1, 94) = 55.6, p < 0.001] and 12-months post-operatively [attention/executive function: F(1,94) = 46.4, p < 0.001; memory: F(1, 94) = 67.7, p < 0.001]. In contrast, attention/executive function and memory abilities remained largely unchanged from 12-weeks to 12-months post-surgery (p > 0.10). There was no time effect for language abilities across the three time points: F(1, 94) = 0.20, p = 0.8.
Figure 1.
Pre- to Post-Bariatric Surgery Cognitive Changes (N = 95)
Y-axis represents T-scores with higher values reflective of better performance. There is a significant time effect across the three time points for attention/executive function and memory (p < 0.05), but not language.
The Impact of Age on Pre- and Post-Operative Cognitive Changes
Regression analyses showed no association between age and baseline cognitive test performance in any of the domains (p > 0.05 for all). Regression analyses controlling for baseline of the dependent variable were then performed to examine the predictive validity of age on post-operative changes in attention/executive function and memory. Age did not emerge as a predictor of 12-week (ΔF(1, 92) = 0.07, p = 0.25, β = 0.07) or 12-month (ΔF(1, 92) = 0.80, p = 0.37, β = 0.06) attention/executive function. Age also did not predict 12-week (ΔF(1, 92) = 0.39, p = 0.54, β = -0.05) or 12-month (ΔF(1, 92) = 1.24, p = 0.27, β = -0.10) memory performance.
Discussion
Bariatric surgery patients exhibited acute and long-term post-operative improvements in cognitive function, suggesting obesity related cognitive impairment may be partially reversible through weight loss surgery. The mechanisms for this phenomenon are not entirely clear, but may involve substantial weight loss, comorbid medical condition resolution, or more novel mechanisms such as in improvements in appetitive neurohormones (i.e., leptin, ghrelin)22,23 and decreased inflammation.24–26 Regardless of the exact etiology, the nature of cognitive gains following bariatric surgery appears variable across persons and dependent on demographic and clinical factors. No previous study has examined the modifying role of age and we hypothesized older age would attenuate improvements in cognitive function following surgery. Counter to expectations, our findings provide preliminary evidence that age may not influence patterns of cognitive improvements after bariatric surgery.
Although bariatric surgery is effective in all age groups,6,8 older adults have been shown to benefit less from surgery (e.g., weight loss, medical comorbidity resolution) relative to younger adults in some studies.8–10 It is thus interesting that older age was not associated with decreased cognitive gains post-operatively. Mid-life obesity is a significant risk factor for cognitive decline, and Alzheimer’s disease.27 It is possible that bariatric surgery in older obese individuals may attenuate the pathogenesis of obesity accelerated cognitive decline and/or dementia related processes. Supporting this notion is a recent study that shows bariatric surgery is associated with reduced expression of Alzheimer’s disease markers (e.g., amyloid precursor protein)18 as well as evidence for the effectiveness of weight loss surgery in individuals >65 years.6 Future studies with larger and more age diverse samples are needed to fully elucidate the effects of age on post-operative cognitive function.
Age was also not associated with baseline cognitive test performance and there are multiple possible explanations for the overall lack of age effects in this study. Interestingly, older age was also not related to pre- or post-operative BMI and this might be one potential explanation for the lack of association between age and baseline cognition. Alternatively, age may impact cognitive function in this sample through indirect mechanisms. For example, older age was indeed linked with a baseline history of comorbid medical conditions that negatively influence cognitive outcomes such as hypertension, T2DM, and sleep apnea. Another possibility is that that effects of severe obesity are significant enough to obscure age effects in this otherwise neurologically-healthy sample. However, a more likely explanation for the lack of age effects in this study likely involves range restriction. Greater neuropathology is correlated with higher BMI in older adults 28 and adverse brain changes in this rather younger sample (i.e., mean age = 43) perhaps did not reach threshold to negatively impact baseline cognition or attenuate cognitive improvements. Likewise, the short 12-month follow-up period may have precluded age-related brain changes that possibly interfere with post-surgery cognitive outcomes. A longer follow-up period (e.g., 5–10 years) among older aged obese persons may be warranted to fully capture the negative effects of age on post-surgery cognition and future studies should explore this possibility.
There are several limitations of the current study that may provide clearer insight into the impact of age on cognitive function after bariatric surgery. Most notably, the relatively modest sample size of the current sample may have precluded detection of statistical significance. Thus, future studies with larger samples of both younger and older obese adults would help better understand the clinical implications for the effects of age on cognitive outcomes after bariatric surgery. Variability in obesity duration across the current sample may also help to explain the current findings and future studies should account for this possible confound. It is also possible that advanced age in bariatric surgery patients is associated with longer disease duration and greater medical comorbidities. Although we hypothesized that worse disease status would preclude post-surgery cognitive benefits, older adult bariatric surgery patients may also experience cognitive gains due to greater opportunity for remission of comorbid conditions that negatively affect cognitive function, particularly in younger individuals (e.g., < 60 years) in which adverse brain pathology may still be reversible or less severe. Indeed, older aged bariatric samples of (e.g., 65–80) would be more likely to include individuals with significant irreversible neurological impairments, particularly in a severely obese population.27,28 Future studies with extended follow-ups among bariatric surgery patients across the adult lifespan (i.e., 40–80 years) would better clarify whether weight loss surgery can reduce the heightened risk of age associated cognitive worsening and/or dementia. Finally, alternate form of the IntegNeuro was utilized and past work shows practice effects on computerized cognitive test batteries are minimal29 and bariatric surgery has also been shown to yield cognitive improvements relative to obese controls that do not undergo surgery.3 However, case-controlled studies are needed to better understand the role of aging on cognitive changes after surgery.
Conclusions
This study provides initial evidence suggesting cognitive benefits following bariatric surgery may not be influenced by age. Prospective studies with extended follow-ups among older obese individuals (e.g., between 60–70 years) are needed to determine whether bariatric surgery continues to produce cognitive benefits and possibly attenuates age associated cognitive decline.
Acknowledgments
Data collection supported by National Institutes of Health (grant number DK075119). Manuscript supported in part by HL089311.
Research reported in this publication was supported by National Institutes of Health under award numbers number R01 DK075119 andR01HL089311.
Footnotes
The authors disclose no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanek KM, Strain G, Devlin M, et al. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology. 2013;27:141–151. doi: 10.1037/a0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller LA, Crosby RD, Galioto R, et al. Bariatric surgery patients exhibit improved memory function 12 months post-operatively. Obes Surg. 2013;23:1527–1535. doi: 10.1007/s11695-013-0970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunstad J, Strain G, Devlin MJ, et al. Improved memory function 12 weeks after bariatric surgery. Surg Obes Relat Dis. 2011;7:465–472. doi: 10.1016/j.soard.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alosco ML, Galioto R, Spitznagel MB, et al. Cognitive Function Following Bariatric Surgery: Evidence for Improvement 3 Years Post- Surgery. Am J Surgery. doi: 10.1016/j.amjsurg.2013.05.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patents aged 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obes Surg. 2010;20:1199–1205. doi: 10.1007/s11695-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 7.Picket-Blakely OE, Huizinga MM, Clark JM. Sociodemographic trends in bariatric surgery utilization in the USA. Obes Surg. 2012;22:838–842. doi: 10.1007/s11695-012-0629-9. [DOI] [PubMed] [Google Scholar]
- 8.Contreras JE, Santander C, Court I, Braco J. Correlation between age and weight loss after bariatric surgery. Obes Surg. 2013;23:1286–1289. doi: 10.1007/s11695-013-0905-3. [DOI] [PubMed] [Google Scholar]
- 9.Scozzari G, Passera R, Benvenga R, Toppino M, Morino M. Age as a long-term prognostic factor in bariatric surgery. Ann Surg. 2012;256:724–728. doi: 10.1097/SLA.0b013e3182734113. [DOI] [PubMed] [Google Scholar]
- 10.Lynch J, Belgaumkar A. Bariatric surgery is effective and safe in patients over 55: a systematic review and meta-analysis. Obes Surg. 2012;22:1507–1516. doi: 10.1007/s11695-012-0693-1. [DOI] [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention. Alzheimer’s disease. Available at: http://www.cdc.gov/aging/aginginfo/alzheimers.htm.
- 12.Takeda S, Matsuzawa T. Age-related brain atrophy: A study with computed tomography. J Gerontol. 1985;40:159–163. doi: 10.1093/geronj/40.2.159. [DOI] [PubMed] [Google Scholar]
- 13.Alosco ML, Spitznagel MB, Gunstad J. Obesity as a risk factor for poor neurocognitive outcomes in older adults with heart failure. Heart Fail Rev. 2013 doi: 10.1007/s10741-013-9399-2. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John S, Hoegerl C. Nutritional deficiencies after gastric bypass surgery. The J Am Osteopath Assoc. 2009;109:601–604. [PubMed] [Google Scholar]
- 15.Becker D, Balcer L, Galetta S. The neurological complications of nutritional deficiency following bariatric surgery. J Obes. 2012;2012:608534. doi: 10.1155/2012/608534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of “integneuro”“ a new computerized battery of neurocognitive tests. Int J Neuroscie. 2005;115:1549–1567. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- 18.Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gorodn E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. Int J Neurosci. 2005;115:1605–1630. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- 19.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills. 1958;8:271–276. [Google Scholar]
- 20.Walsh K. Understanding Brain Damage – A Primer of Neuropsychological Evaluation. Melbourne: Churchill Livingstone; 1985. [Google Scholar]
- 21.Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: a challenge to current thinking. The British Journal of Psychiatry. 2006;189:399–404. doi: 10.1192/bjp.bp.105.014779. [DOI] [PubMed] [Google Scholar]
- 22.Terra X, Auguet T, Guiu-Jurado E, et al. Long-term changes in leptin, chemerin, and Ghrelin levels following different bariatric surgery procedures: Roux-en-Y Gastric bypass and sleeve gastrectomy. Obes Surg. 2013 doi: 10.1007/s11695-013-1033-9. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Dimitriadis E, Daskalakis M, Kampa M, Peppe A, Papadakis JA, Melissas J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257:647–654. doi: 10.1097/SLA.0b013e31826e1846. (2013) [DOI] [PubMed] [Google Scholar]
- 24.Ghanim H, Monte SV, Sia CL, et al. Reduction in inflammation and the expression of amyloid precursor protein and other proteins related to alzheimer’s disease following gastric bypass surgery. J Clin Endocrinol. 2012;97:E1197–E1201. doi: 10.1210/jc.2011-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeki AL, Hazzouri A, Haan MN, Whitmer RA, Yaffe K, Neuhaus J. Central obesity, leptin and cognitive decline: the sacramenta Area Latino Study on Aging. Dement Geriatr Cogn. 2012;33:400–409. doi: 10.1159/000339957. (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dos Santos VV, Rodrigues AL, De Lima TC, et al. Ghrelin as a neuroprtoective and palliative agent in Alzheimer’s and Parkinsons disease. Current Pharmaceutical Design. 2013 doi: 10.2174/13816128113199990411. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Loef M, Walach H. Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity. 2013;21:E51–E55. doi: 10.1002/oby.20037. [DOI] [PubMed] [Google Scholar]
- 28.Gustafson D, Steen B, Skoog I. Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. Int Psychogeriatr. 2004;16:327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- 29.Falleti MG, Maruff P, Collie A, et al. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J Clin Exp Neuropsychol. 2006;28:1095–1112. doi: 10.1080/13803390500205718. [DOI] [PubMed] [Google Scholar]