Abstract
A large number of foreign compounds, including many drugs, industrial pollutants, and environmental chemicals, can be oxidized under appropriate conditions to potentially toxic free radical intermediates. We evaluated the ability of the oxidants produced by the neutrophil myeloperoxidase system to generate free radical intermediates from several such compounds. Sodium hypochlorite or hypochlorous acid produced by human peripheral blood neutrophils and trapped in the form of taurine chloramine were both found to be capable of producing free radicals from chlorpromazine, aminopyrine, and phenylhydrazine. These radical intermediates were demonstrated by visible light spectroscopy and by direct electron spin resonance (for the chlorpromazine and aminopyrine radicals) or by spin-trapping (for the phenyl radical generated from phenylhydrazine). Stable oxidants produced by the neutrophils (i.e., those present in the supernatants of stimulated neutrophils in the absence of added taurine) also were found to be capable of generating free radical intermediates. The production of the oxidants and the ability of neutrophil supernatants to generate these radicals were almost completely eliminated by sodium azide, a myeloperoxidase inhibitor. We suggest that the oxidation by neutrophils of certain chemical compounds to potentially damaging electrophilic free radical forms may represent a new metabolic pathway for these substances and could be important in the processes of drug toxicity and chemical carcinogenesis.
Full text
PDF
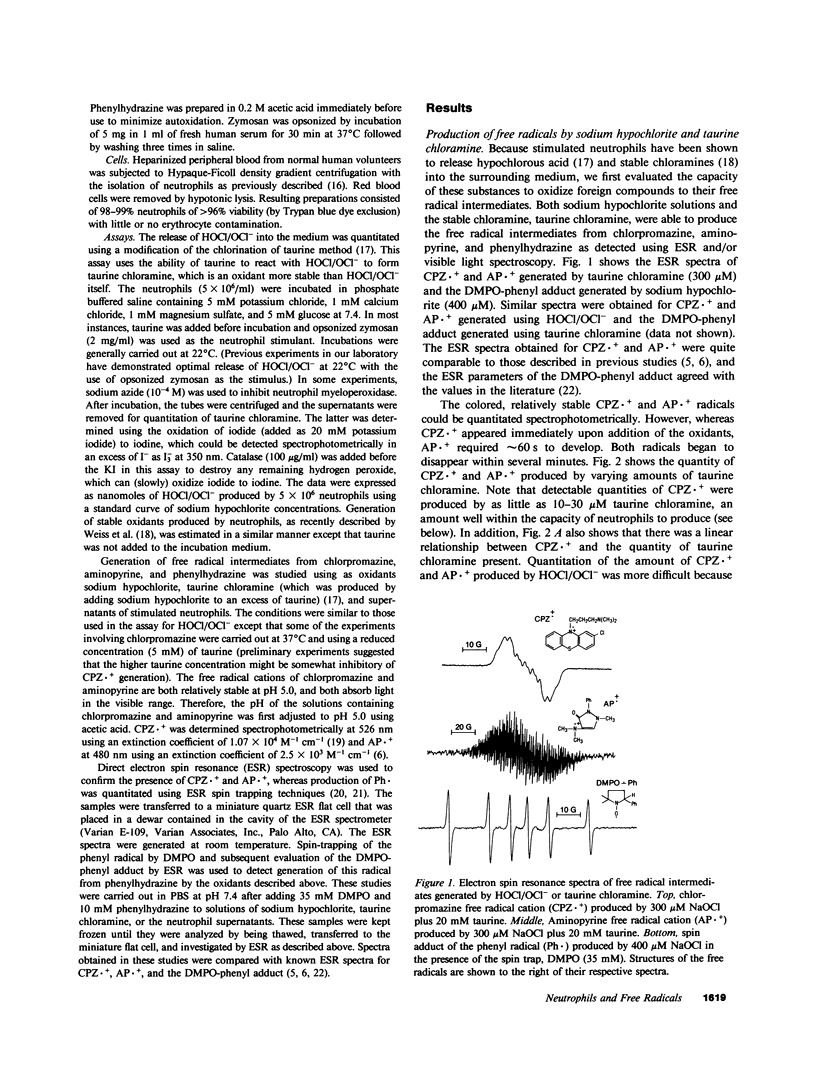
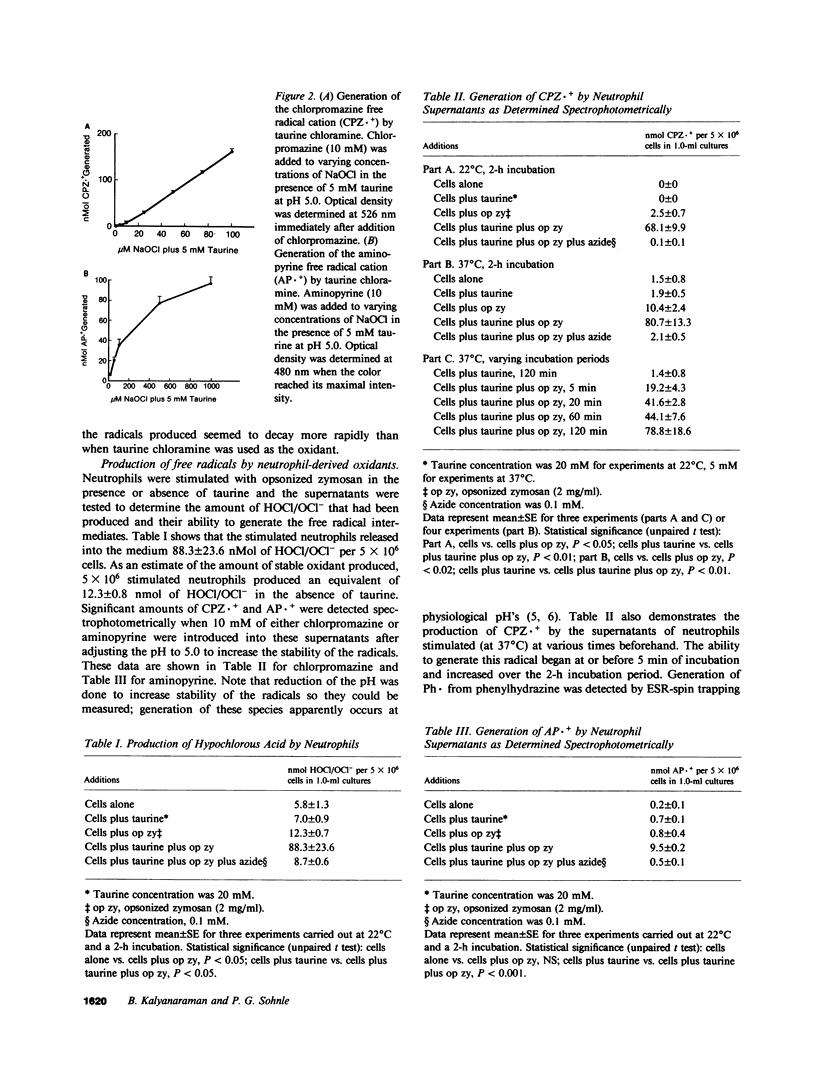


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Augusto O., Kunze K. L., Ortiz de Montellano P. R. N-Phenylprotoporphyrin IX formation in the hemoglobin-phenylhydrazine reaction. Evidence for a protein-stabilized iron-phenyl intermediate. J Biol Chem. 1982 Jun 10;257(11):6231–6241. [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R., Moreno S. N. Free radical metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev Infect Dis. 1984 Mar-Apr;6(2):223–238. doi: 10.1093/clinids/6.2.223. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Kuntz R. E., Cheever A. W., Myers B. J. Proliferative epithelial lesions of the urinary bladder of nonhuman primates infected with Schistosoma haematobium. J Natl Cancer Inst. 1972 Jan;48(1):223–235. [PubMed] [Google Scholar]
- Lasker J. M., Sivarajah K., Mason R. P., Kalyanaraman B., Abou-Donia M. B., Eling T. E. A free radical mechanism of prostaglandin synthase-dependent aminopyrine demethylation. J Biol Chem. 1981 Aug 10;256(15):7764–7767. [PubMed] [Google Scholar]
- Leibovitz B. E., Siegel B. V. Aspects of free radical reactions in biological systems: aging. J Gerontol. 1980 Jan;35(1):45–56. doi: 10.1093/geronj/35.1.45. [DOI] [PubMed] [Google Scholar]
- MASON H. S. Comparative biochemistry of the phenolase complex. Adv Enzymol Relat Subj Biochem. 1955;16:105–184. doi: 10.1002/9780470122617.ch3. [DOI] [PubMed] [Google Scholar]
- Manale B. L., Brower T. D. The significance of bacterial flora in carcinoma in chronic osteomyelitis. Surg Gynecol Obstet. 1973 Jan;136(1):63–64. [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981 May 15;47(10):2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- PIETTE L. H., BULOW G., YAMAZAKI I. ELECTRON-PARAMAGNETIC-RESONANCE STUDIES OF THE CHLORPROMAZINE FREE RADICAL FORMED DURING ENZYMIC OXIDATION BY PEROXIDASE-HYDROGEN PEROXIDE. Biochim Biophys Acta. 1964 Jul 29;88:120–129. doi: 10.1016/0926-6577(64)90160-3. [DOI] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Saeed W., Kim S., Burch B. H. Development of carcinoma in regional enteritis. Arch Surg. 1974 Mar;108(3):376–379. doi: 10.1001/archsurg.1974.01350270106021. [DOI] [PubMed] [Google Scholar]
- Sohnle P. G., Collins-Lech C. Cell-mediated immunity to Pityrosporum orbiculare in tinea versicolor. J Clin Invest. 1978 Jul;62(1):45–53. doi: 10.1172/JCI109112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979 Feb;23(2):522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth B. Synthetic and naturally occurring hydrazines as possible cancer causative agents. Cancer Res. 1975 Dec;35(12):3693–3697. [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Lampert M. B., Test S. T. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science. 1983 Nov 11;222(4624):625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]
- Weitberg A. B., Weitzman S. A., Destrempes M., Latt S. A., Stossel T. P. Stimulated human phagocytes produce cytogenetic changes in cultured mammalian cells. N Engl J Med. 1983 Jan 6;308(1):26–30. doi: 10.1056/NEJM198301063080107. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Mutation caused by human phagocytes. Science. 1981 May 1;212(4494):546–547. doi: 10.1126/science.6259738. [DOI] [PubMed] [Google Scholar]



