Abstract
Background
Infectious complications often occur in acute pancreatitis, related to impaired intestinal barrier function, with prolonged disease course and even mortality as a result. The bile salt nuclear receptor farnesoid X receptor (FXR), which is expressed in the ileum, liver and other organs including the pancreas, exhibits anti-inflammatory effects by inhibiting NF-κB activation and is implicated in maintaining intestinal barrier integrity and preventing bacterial overgrowth and translocation. Here we explore, with the aid of complementary animal and human experiments, the potential role of FXR in acute pancreatitis.
Methods
Experimental acute pancreatitis was induced using the CCK-analogue cerulein in wild-type and Fxr-/- mice. Severity of acute pancreatitis was assessed using histology and a semi-quantitative scoring system. Ileal permeability was analyzed in vitro by Ussing chambers and an in vivo permeability assay. Gene expression of Fxr and Fxr target genes was studied by quantitative RT-PCR. Serum FGF19 levels were determined by ELISA in acute pancreatitis patients and healthy volunteers. A genetic association study in 387 acute pancreatitis patients and 853 controls was performed using 9 tagging single nucleotide polymorphisms (SNPs) covering the complete FXR gene and two additional functional SNPs.
Results
In wild-type mice with acute pancreatitis, ileal transepithelial resistance was reduced and ileal mRNA expression of Fxr target genes Fgf15, SHP, and IBABP was decreased. Nevertheless, Fxr-/- mice did not exhibit a more severe acute pancreatitis than wild-type mice. In patients with acute pancreatitis, FGF19 levels were lower than in controls. However, there were no associations of FXR SNPs or haplotypes with susceptibility to acute pancreatitis, or its course, outcome or etiology.
Conclusion
We found no evidence for a major role of FXR in acute human or murine pancreatitis. The observed altered Fxr activity during the course of disease may be a secondary phenomenon.
Introduction
Acute pancreatitis (AP) is the acute inflammation of the pancreas, and is mostly caused by gallstones or alcohol abuse [1]. In the majority of patients the course of the disease is mild, but in around 20% of patients, AP is severe with organ failure and/or local complications [2]. Mortality from AP is especially caused by infectious complications, such as bacterial infection of pancreatic necrosis [3], [4]. Failure of the intestinal barrier function plays a critical role, as it allows for bacterial translocation, facilitating such infectious complications [5]-[8].
The intracellular bile salt receptor farnesoid X receptor (FXR) is mainly expressed in ileum and liver, and to some extent in other organs, such as the pancreas [9], with little information available on its function in the latter organ. FXR is considered the master regulator of bile acid homeostasis, which regulates various genes encoding for bile acid transport proteins, including apical sodium-dependent bile acid transporter (ASBT) and ileal bile acid binding protein (IBABP) [10], [11]. Also, the enterokine fibroblast growth factor 15 (Fgf15, human orthologue FGF19), whose expression is controlled by FXR, exerts a negative feedback regulation of hepatic bile salt neo-synthesis and, at least in mice, induces gallbladder refilling at the end of the postprandial phase [12].
More recently, FXR has been implicated in the regulation of fat and glucose metabolism, in the maintenance of intestinal barrier integrity and prevention of intestinal bacterial overgrowth, by affecting putative FXR-dependent genes such as angiogenin-1, iNOS, CAR12 and IL18 [13]. In patients with Crohn's colitis, who show impaired antibacterial defense and impaired intestinal barrier function, FXR expression was altered in areas of inflamed mucosa [14]. Furthermore, we recently showed in two murine models for colitis that administering the semi-synthetic FXR agonist INT747 (Obeticholic acid) ameliorates intestinal inflammation, improving colitis symptoms, preserving intestinal barrier function, and reducing goblet cell loss [15]. The underlying mechanism for these anti-inflammatory effects is thought to be inhibition of NF-κB [16]. We also recently detected impaired mRNA expression of FXR target genes in the ileum of patients with clinically quiescent Crohn's colitis [17]. FGF19 signaling has been implicated in regulating inflammation by antagonizing NF-κB signaling in FGF19 target tissues, which may include the pancreas [18], [19].
Because of its role in intestinal barrier function, i.e. prevention of bacterial translocation and modulation of inflammation, we hypothesized that FXR might play an important role in AP. Deficiency of FXR could result in increased severity of the pancreatitis, increased bacterial translocation, and infectious complications. In this study, we therefore explored, with the aid of complementary animal and human experiments, whether FXR could affect AP.
Materials and Methods
Animals
In the first series of experiments, we used adult male wild-type C57BL/6 mice of 10–12 weeks and 20–30 grams of weight (Harlan, Horst, the Netherlands). For the second series of experiments, mice with global Fxr deficiency (Fxr-/-) on a C57BL/6 genetic background [20] were obtained by breeding of heterozygous mice. We used male adult Fxr-/- and wild-type C57BL/6 littermates of 11–16 weeks and 25–35 grams of weight. All mice were kept under constant housing conditions (22°C, 60% relative humidity and a 12-hour light/dark cycle) for at least two weeks prior to the start of the experiment, and had free access to water and food (CRM (E), B.M.I. – Technilab, Someren, the Netherlands) throughout the experiment.
Animal experiments
AP was induced by ten intraperitoneal injections with an hourly interval of cerulein, a CCK analogue (Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands; 50 µg/kg in 0.9% NaCl). Controls received an equal volume of saline. In an initial experiment, pancreatic injury was assessed 24 and 72 hrs after induction of AP. For this purpose, 30 wild-type mice (Harlan) were randomly allocated to a control group (n = 10, sacrificed after 72 hrs) and two experimental groups that were terminated after 24 hrs (early pancreatitis, n = 10) and 72 hrs (late pancreatitis, n = 10). To assess the impact of Fxr deficiency on AP, Fxr+/+ (wild-type) and Fxr-/- mice received control (n = 5) or cerulein (n = 10 per genotype) treatment and were sacrificed after 24 hrs.
Animals were terminated by cervical dislocation or by cardiac puncture under isoflurane anesthesia. For histopathologic evaluation, parts of the ileum and pancreas were fixated in 4% formaldehyde. For RNA isolation, parts of the ileum and liver were immediately snap frozen in liquid nitrogen and stored at −80°C. Plasma samples were stored at −80°C for determination of amylase and bilirubin by standard clinical chemical assays.
The experimental design was approved by the animal experiments committee of Utrecht University, Utrecht, the Netherlands (2007.III.09.117; 2009.III.08.074).
Histopathology
After fixation in 4% formaldehyde, tissues were embedded in paraffin and cut in serial sections of 4 µm for hematoxylin and eosin (H&E) staining. Qualitative assessment of the severity of AP was performed in the initial experiment and, in the second experiment, a slightly modified semi-quantitative scoring system was used [21], [22]. The following items were scored: edema (0-4 points), number of neutrophils in the edema (0–4 points), pancreatic ductal pathology (inflammatory cells; present = 1; absent = 0), intralobular inflammatory infiltrate (0–3 points) and peripheral necrosis of pancreatic tissue (0–4 points). The maximum composite score was 16. To assess the ileal brush border in the second experiment, PAS-diastase staining was performed. Histopathological evaluation was performed by two experienced pathologists (AJJS, MEIS), blinded for experimental study groups.
Measurement of transepithelial electrical resistance
In the initial experiment, a 4 cm segment of the distal ileum was removed for electrical resistance measurements in Ussing chambers, as described elsewhere [22]. Briefly, flat sheets of mucosa were mounted in Ussing chambers with both sides of the epithelium in contact with Krebs-Ringer's solution, stirred and gassed with humidified carbogen at 37°C. Three ileal samples per animal were used. The transepithelial potential difference Vte (mV) was continuously monitored and transepithelial electrical resistance R (Ω.cm2) was calculated [22]. The reported values for the resistance were obtained at the end of the 20 min equilibration period. At the end of the experiment, viability of the tissue segments was confirmed based on carbachol-induced voltage increase [22].
In vivo intestinal permeability assay
In the second experiment, intestinal permeability was assessed with fluorescein isothiocyanate (FITC)-conjugated dextran as previously described [15]. Briefly, two hours before termination, mice were gavaged with 0.6 mg/g body weight of FITC-conjugated dextran (MW 3,000–5,000 Da; Sigma-Aldrich). After termination, FITC fluorescence was measured in plasma with the aid of a fluorometer (BMG Polarstar Galaxy, MTX Lab Systems, Inc., Vienna, Virginia, USA) and compared to a calibration line of standard concentrations of FITC-conjugated dextran.
Analysis of gene expression
Total RNA was isolated from murine ileum and liver (RNeasy Midi Kit, Qiagen, Hilden, Germany). RNA integrity was tested by RNA gel electrophoresis. cDNA was synthesized from total RNA using the iScript cDNA synthesis kit (BioRad, Hercules, CA, USA). Quantitative RT-PCR was performed using SYBR Green Supermix (BioRad) on an iCycler iQ system using diluted cDNA as template (primer sequences are provided in Table S1 in File S1). Expression levels were estimated using the comparative threshold cycle method. Cyclophilin was used as housekeeping gene, with similar expression levels in ileum and liver under all experimental conditions.
Determination of plasma FGF19 levels in patients with acute pancreatitis
FGF19 levels were determined by ELISA in plasma samples of 15 randomly selected patients with predicted severe AP [23]. Patients were participants in an earlier clinical trial (trial registry ISRCTN38327949) and were fed by continuous enteral nutrition [24]. Clinical data were available from the prospectively collected trial database [24]. As a control group, FGF19 levels were also determined in a group of 28 healthy volunteers receiving an oral fat load [25]. In this group, fed FGF19 levels were calculated as the average of postprandrial FGF19 levels at 2, 3, 4, and 6 hrs.
Genetic association study
For the genetic association study, a previously described cohort of 387 patients with a first episode of AP was used [26]. All patients or their legal representatives gave their written informed consent, and the ethics review boards of all participating hospitals approved the study protocol. Genomic DNA was isolated from whole blood using a DNA isolation kit I (Magna Pure LC, Roche Diagnostics, Indianapolis, USA). Clinical data on the severity of disease and outcome of all patients were available from the prospectively collected trial database [24]. The controls consisted of 853 healthy, voluntary, Dutch blood donors [27]. All control genotypes were in Hardy-Weinberg equilibrium (data not shown, p>0.05). Call rates for all SNPs were>95%.
Nine tagging single nucleotide polymorphisms (SNPs) covering the complete FXR gene were selected using Haploview v4.2 [28]. In addition, two functional SNPs affecting FXR expression (-1G/T, rs56163822) and FXR function (518T/C, rs61755050) were analyzed [29]. Details of the SNPs studied are given in Table S2 in File S1. Genotyping was performed using TaqMan assays on a TaqMan 7900 HT (Applied Biosystems, Foster City, California, USA). Haplotype analysis was performed in Haploview [28].
Statistical analysis
Statistical analyses were performed using GraphPad PRISM software (Graphpad Software, La Jolla, CA, USA). Electrical resistance, histology scores, and clinical parameters were compared using one-way ANOVA with Tukey's post-hoc test or the non-parametric Kruskal-Wallis test with Dunn's post-hoc test where appropriate. Differences in gene expression levels were evaluated using the non-parametric Kruskal-Wallis test with Dunn's post-hoc test. Plasma FGF19 levels were compared between AP patients and healthy controls by ANOVA with Tukey's post-hoc test. Statistical analysis of the genetic association study was performed using two-tailed chi-squared for independence tests of case versus control allele and haplotype counts in Haploview v4.2 [28]. Uncorrected P-values, odds ratios (OR) and 95% confidence intervals (95% CI) are given (Table 1 and Table S3 in File S1). The Bonferroni method was used to correct for multiple testing. Data of continuous values are shown as mean ± standard deviation (SD). P-values below 0.05 were considered statistically significant.
Table 1. Association of genetic variants of FXR with acute pancreatitis.
| Acute pancreatitis patients | Controls | P value* | OR | 95% CI | ||||||
| Allele counts | Allele counts | |||||||||
| Major | Minor | MAF | Major | Minor | MAF | |||||
| -1 G>T | C/A# | 732 | 14 | 0.981 | 1588 | 36 | 0.978 | 0.5926 | 1.14 | 0.62–2.10 |
| 518 T>C | A/G | 743 | 5 | 0.993 | 1616 | 6 | 0.996 | 0.3203 | 1.86 | 0.60–5.82 |
| rs11837065 | C/T | 452 | 250 | 0.644 | 1014 | 592 | 0.631 | 0.5663 | 1.05 | 0.88–1.27 |
| rs12313471 | A/G | 699 | 39 | 0.947 | 1548 | 76 | 0.953 | 0.5268 | 1.15 | 0.77–1.71 |
| rs11110390 | C/T | 497 | 247 | 0.668 | 1070 | 544 | 0.663 | 0.8088 | 1.02 | 0.85–1.23 |
| rs4764980 | G/A | 384 | 354 | 0.520 | 832 | 778 | 0.517 | 0.8728 | 1.01 | 0.85–1.21 |
| rs11110395 | G/T | 692 | 44 | 0.940 | 1538 | 84 | 0.948 | 0.4272 | 1.18 | 0.81–1.71 |
| rs17030285 | C/G | 610 | 102 | 0.857 | 1415 | 215 | 0.868 | 0.4599 | 1.11 | 0.86–1.42 |
| rs11610264 | T/C | 536 | 200 | 0.728 | 1160 | 458 | 0.717 | 0.5703 | 1.06 | 0.87–1.28 |
| rs10860603 | G/A | 616 | 122 | 0.835 | 1398 | 214 | 0.867 | 0.0364 | 1.30 | 1.02–1.65 |
| rs35739 | T/C | 398 | 350 | 0.532 | 900 | 712 | 0.558 | 0.2334 | 1.11 | 0.93–1.32 |
OR = odds ratio; 95% CI = 95% confidence interval.
Major allele/minor allele; MAF = major allele frequency.
*Two-tailed p values were calculated by χ2 analysis of allele count.
Results
Acute pancreatitis results in decreased transepithelial resistance and altered expression of Fxr targets in the ileum
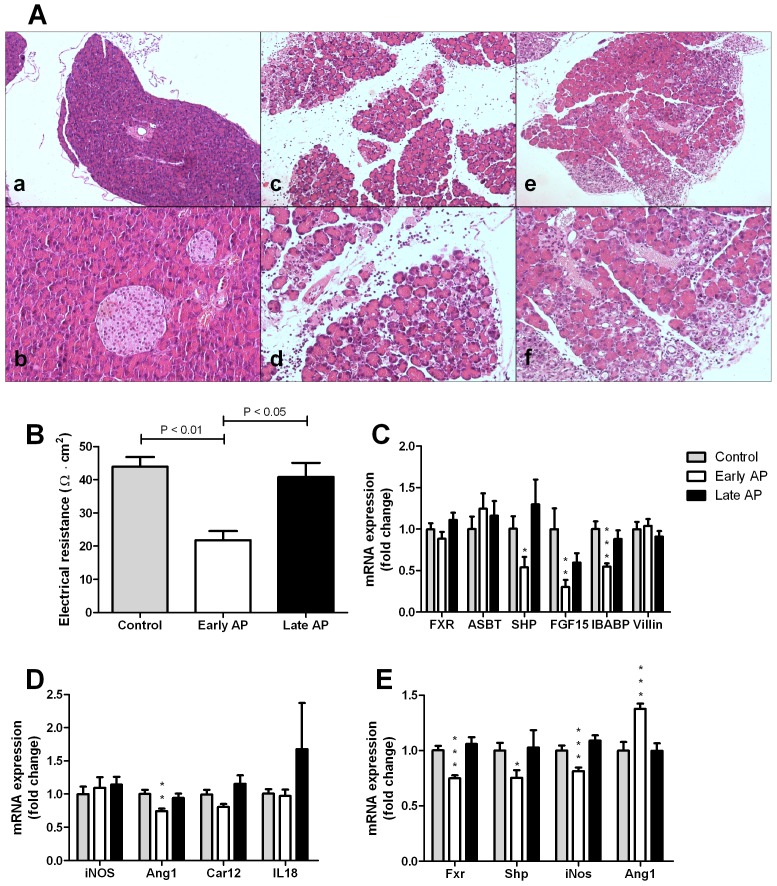
Pancreatic injury was initially assessed in wild-type mice sacrificed at 24 hrs (early AP) or 72 hrs (late AP) after induction of AP. Plasma amylase levels were twice as high in the early and late AP mice compared to the control group (mean ± SEM, 4521±527 U/L vs. 2186±109 U/L, p<0.001). Histopathological examination of the pancreas revealed edema, influx of neutrophils and necrosis in all mice of the early pancreatitis group (Figure 1A). In contrast, the pancreata of all mice in the late pancreatitis group showed no signs of edema or necrosis and displayed infiltration of lymphocytes and fibroblasts rather than neutrophils. There were no histopathological abnormalities in the control group. Histopathological examination of the ileum revealed normal enterocytes without any signs of inflammatory infiltrate in all groups. Nevertheless, the transepithelial electrical resistance of the ileum was approximately half in the early AP group compared to controls and the late AP group (Figure 1B). This indicates that AP induces a transient increase in ileal permeability.
Figure 1.
A – Representative pancreatic histology of wild-type mice from the control group (a, b) and mice with early (c, d) and late (e, f) acute pancreatitis (H&E staining, 20x and 100x magnifications consecutively). Control mice have normal pancreatic morphology, whereas mice of the early pancreatitis group exhibit edema, influx of neutrophils and necrosis. Mice of the late pancreatitis group have no edema or necrosis, but show influx of lymphocytes and fibroblasts. B – Transepithelial electrical resistance of the ileum measured by Ussing chamber experiments. The resistance of the ileum was lower in the early pancreatitis group in comparison to both controls and the late pancreatitis group. C – Ileal mRNA expression of Fxr and FXR target-genes Asbt, Shp, Fgf15, and Ibabp, and Villin in wild-type mice of the control group, and the early and late pancreatitis groups. Expression of Fxr, Asbt and Villin did not differ between experimental groups. Expression of the other Fxr target genes was lower in early acute pancreatitis, but not in late pancreatitis. D – Ileal mRNA expression of genes implicated in intestinal barrier function, iNos, Ang1, Car12, IL18. Expression of Ang1 was lowered in the early pancreatitis group, the other genes remained similar in the different experimental conditions. E – Hepatic expression of Fxr, Shp, iNos and Ang1. Hepatic Ang1 was increased in early pancreatitis, whereas the expression of the other genes was lowered in the early acute pancreatitis group. Expression levels were normalized to cyclophilin expression. Bars indicate means and SEM, * p<0.05, ** p<0.01, *** p<0.001.
Impaired intestinal barrier function in patients with inflammatory bowel disease is accompanied by reduced ileal expression of FXR targets [17]. Ileal gene expression in mice was therefore analyzed to test the consequences of an AP-induced decline of transepithelial electrical resistance. Fxr mRNA expression was comparable between the three experimental groups, as was mRNA expression of the Fxr-target gene Asbt (Figure 1C). In contrast, mRNA expression of Fxr-target genes Shp, Fgf15 and Ibabp was reduced in the early AP group compared to control mice (Figure 1C). In the late pancreatitis group, expression of all Fxr target genes was normalized (Figure 1C). Fxr and its target genes are exclusively expressed in the villous lining of differentiated enterocytes [13]. We therefore also assessed mRNA expression of Villin, which is expressed exclusively in these differentiated enterocytes [17]. mRNA expression of Villin showed no differences between the groups, including the early AP group (Figure 1C), indicating that no intestinal damage was present in this mouse model of AP. Regarding Fxr-dependent genes implicated in intestinal barrier function [13]: Angiogenin-1 (Ang1) mRNA expression in the ileum was reduced in the early pancreatitis group, whereas iNos, Car12 and Il18 were similar in all groups (Figure 1D).
In the liver, mRNA expression of Fxr and its target gene Shp were diminished after 24 hours, but normalized after 72 hours (Figure 1E). Fgf15 could not be detected in the liver. Hepatic iNos expression was also lowered after 24 hours, whereas Ang1 expression increased in the early phase and returned to baseline expression in the late phase (Figure 1E).
Deficiency of Fxr does not lead to more severe acute pancreatitis in mice
The above findings indicate that ileal Fxr activity is disturbed in the early phase of murine AP (i.e. 24 hrs after induction). To test whether Fxr dysfunction contributes to the pathology of AP, mice deficient for Fxr were given ten hourly injections of cerulein to induce AP and sacrificed after 24 hrs. Weight loss due to pancreatitis induction did not differ between wild-type and Fxr-/- mice (mean ± SEM: 6.1±0.37 and 5.2±1.78% of body weight, respectively, p = 0.48). In both wild-type and Fxr-/- pancreatitis groups, plasma amylase levels were significantly higher than in corresponding groups without AP (mean ± SEM; wild-type controls 2160±149 U/L, wild-type AP 8013±923 U/L, p<0.01; Fxr-/- controls without AP 2285±96 U/L, Fxr-/- AP 6801±671 U/L, p<0.01). C-reactive protein (CRP) in serum of mice of all four experimental groups was always very low (<5 mg/L), indicating absence of significant systemic inflammation.
In order to identify whether cholestasis was present in these mice as a sign of post-hepatic bile duct obstruction by the inflamed pancreas, we determined plasma bilirubin levels. In wild-type and Fxr-/- mice, AP did not affect the plasma bilirubin levels (mean ± SEM; wild-type controls 1.8±0.5 µmol/L, wild-type AP 1.5±0.2 µmol/L; Fxr-/- without AP 8.0±2.1 µmol/L, Fxr-/- with AP 5.8±2.6 µmol/L). Fxr deficiency resulted in elevated bilirubin levels (p<0.05). As a potential explanation for this phenomenon, we found elevated hepatic expression of the basolateral bilirubin glucuronide efflux pump Mrp1 in Fxr-/- mice (data not shown) [30].
We subsequently investigated whether Fxr deficiency affects epithelial permeability. Plasma levels of FITC-conjugated dextran were not increased by AP induction in either wild-type or Fxr-/- mice. Nevertheless, Fxr-/- mice had significantly higher plasma levels of FITC-conjugated dextran than wild-type mice (mean ± SEM: wild-type, 3.41±0.61 µg/ml vs. Fxr-/-, 7.45±2.31 µg/ml, p<0.05), indicating that loss of Fxr leads to increased intestinal permeability.
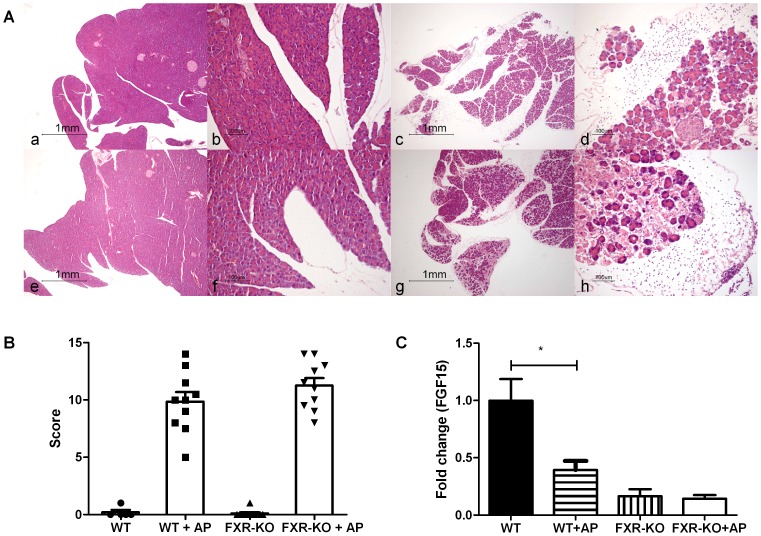
Upon histopathological examination, wild-type and Fxr-/- control mice did not exhibit edema, influx of inflammatory cells, or necrosis of the pancreas. In contrast, all mice in the pancreatitis groups showed clear signs of AP: interlobular and/or interacinar edema, influx of neutrophils, and necrosis (Figure 2A). Pancreatitis severity scores were similar in wild-type and Fxr-/- mice (composite pancreatitis severity score, mean ± SEM: 9.9±0.8 vs. 11.3±0.7, p = 0.27; Figure 2B). When the individual components of the severity score (presence of edema, inflammatory infiltrate, and necrosis), were analyzed, there were also no differences found between wild-type and Fxr-/- mice (data not shown).
Figure 2.
A - Representative pancreatic histology following induction of acute pancreatitis (H&E staining, 20x and 100x consecutive magnifications for each experimental group): wild-type control (a,b); wild-type acute pancreatitis (c,d); Fxr-/- control (e,f); Fxr-/- acute pancreatitis (g,h). B – Semi-quantitative composite pancreatitis severity score of histopathological examination of pancreas samples from wild-type and Fxr-/- mice with and without acute pancreatitis. Absence of Fxr does not result in more severe acute pancreatitis. C – Ileal mRNA expression of Fgf15 in wild-type and Fxr-/- mice with and without early acute pancreatitis. Fgf15 expression was decreased in wild-type mice with acute pancreatitis. Bars indicate mean and SEM.
As expected, AP did not affect the expression of Fxr in the ileum of wild-type mice (results not shown). In contrast to the results depicted in Figure 1C, the effects of AP on expression of Fxr targets Shp and Ibabp in the ileum of wild-type mice did not reach significance in this experiment (results not shown). Nevertheless, a consistent decrease in expression of Fxr target Fgf15 in the ileum was still noted following induction of AP (Figure 2C). Deficiency of Fxr resulted in reduced ileal expression of Fxr targets Shp (data not shown) and Fgf15 (Figure 2C) in mock-treated mice, with AP having no additional suppressive effect. Fxr mRNA could still be detected, albeit at a lower level, in the ileum of Fxr-/- mice. The strategy used for disruption of the Fxr gene in these mice [20] results in a non-functional transcript as is evident from the near absence of ileal Ibabp expression in Fxr-/- mice (results not shown).
Upon histopathological examination, there were no inflammatory infiltrates in the ileum of wild-type or Fxr-/- mice with and without AP and PAS-diastase staining showed intact brush borders (results not shown). There were no differences in mRNA expression of inflammatory genes Car12 and iNos in the enterocyte (results not shown). As an additional marker of pro-inflammatory response, we determined Tnf-α mRNA expression, but found no differences in expression (results not shown). These findings indicate that there were no signs or very limited signs of inflammation on the molecular level in the ileum.
Plasma FGF19 levels are lowered in patients with acute pancreatitis
To obtain an impression of FXR activation in patients with AP, we studied plasma FGF19 levels in patients with predicted severe pancreatitis. Plasma FGF19 levels in AP patients under digestive conditions were significantly lower than in healthy volunteers after ingestion of a single bolus of fat (0.33±0.19 vs. 0.62±0.30 ng/mL, p<0.001; Figure 3). This suggests that in patients with AP, FGF19 may be decreased in a similar way as to that observed in the AP mouse model.
Figure 3. FGF19 plasma levels in patients with predicted severe acute pancreatitis during continuous enteral nutrition.
For comparison, FGF19 plasma levels of healthy controls in the fasting state and after a single bolus of fat are also shown. The postprandial levels represent the average of plasma FGF19 levels at 2, 3, 4 and 6 hours after fat ingestion. There appears to be a blunted FGF19 release in the pancreatitis group. Bars indicate mean and SD.
Genetic polymorphisms in FXR are not associated with acute pancreatitis
To study a potential association between AP and FXR, 387 patients with AP and 853 controls were genotyped for 9 tagging and 2 functional SNPs in the FXR locus. An association with AP was seen for one of the variants (rs10860603, p = 0.0364, OR 1.30, 95% CI 1.02–1.65) (Table 1). This association did not, however, withstand correction for the number of tested SNPs (pcorrected = 0.40). There was no association of haplotypes of FXR with AP (data not shown).
To investigate a potential association between FXR and the course and outcome of AP, we studied the prevalence of the genetic variants in patients with a severe course versus a mild course of AP, patients with infected pancreatic necrosis versus patients without it, and patients who died from the pancreatitis versus those who survived (Table S3 in File S1). One of the tag SNPs seemed to be associated with a severe course of AP (rs10860603, p = 0.0368, OR 1.61, 95% CI 1.00–2.60) and one with infection of pancreatic necrosis (rs11110395, p = 0.0099, OR 2.55, 95% CI 1.28–5.07). Haplotypes containing the same risk allele also seemed to show association (data not shown). Another tag SNP showed a significant difference between patients who died and those who survived (rs11837065, p = 0.0272, OR 2.09, 95% CI 1.07–4.06). After Bonferroni correction for multiple testing, however, there were no associations of SNPs or haplotypes in the FXR gene with course or outcome of AP. Finally, we compared patients with biliary AP to patients with AP of non-biliary origin. None of the SNPs was associated with a biliary cause of AP (Table S3 in File S1).
Discussion
Failure of the intestinal barrier plays an important role in human acute pancreatitis, because it facilitates bacterial translocation which can lead to infectious complications. Such complications strongly increase mortality due to pancreatitis. Which specific molecular events eventually lead to failure of the intestinal barrier in acute pancreatitis is still largely unclear. In mouse [31] and rat [32], [33] experimental acute pancreatitis, however, it has been shown that tight junction failure in the pancreas is a very early event. In rats, more specifically, disruption of the actin cytoskeleton and of tight junctions occurring in experimental pancreatitis leads to increased paracellular permeability [32], [34].
Because of the role of FXR in intestinal barrier function, namely prevention of bacterial translocation and modulation of inflammation, we hypothesized that FXR might play an important role in AP. Deficiency of FXR might lead to increased severity of pancreatitis, increased bacterial translocation and subsequent infectious complications. In this study, we therefore explored, with the aid of complementary animal and human experiments, whether FXR could affect AP. We observed that induction of AP by repeated administration of a supraphysiological dose of the CCK-analogue cerulein was accompanied by decreased expression of Fxr target genes in the ileum. However, the ileal Fxr pathway appears to have no major pathogenic role in this model of AP, as indicated by similar pancreatic histopathology following induction of AP in mice with global deficiency of Fxr and in wild-type controls. Moreover, a case-control association study indicated that genetic variation in the FXR locus is not associated with the risk, etiology or outcome of AP in human subjects. The collective findings of our study indicate that FXR is not a major player in the pathogenesis of AP.
In an initial experiment in wild-type mice, we observed that expression of ileal Fxr target genes Fgf15, Shp and Ibabp (Figure 1C) was disturbed at 24 hrs after induction of AP, while expression levels recovered at the time point that histopathological damage of the pancreas had largely resolved (i.e. 72 hrs after AP induction). Of note, decline of Fxr activity was shown through decreased expression of Fxr target genes, without change of FXR expression. This phenomenon is in line with previous data obtained in patients with Crohn's disease, in animal colitis models, and in vitro and ex vivo models, where FXR expression itself was not significantly changed by pro-inflammatory cytokines. These findings indicate that the inhibition of FXR target gene expression is due to decreased FXR activity [35]. Decline in ileal FXR target gene expression is likely to be due to impaired delivery of its activating bile salt ligands. It is well known that under pro-inflammatory conditions such as AP, small intestinal motility is decreased, both under fasting and fed conditions, with decreased ileal bile salt delivery as a result [36]. Since AP did not affect bilirubin levels in our wild-type and Fxr-/- mice, post-hepatic obstruction by the inflamed pancreas is unlikely. The transient decline in ileal Fgf15 expression likely accounted for de-repression of hepatic Cyp7a1, as higher expression of this bile salt synthetic gene was found after 24 hrs (data not shown). In our Ussing chamber experiments in wild-type mice, impaired ileal Fxr activation was accompanied by decreased transepithelial resistance at the early time point (Figure 1B), indicative for increased intestinal permeability, without ileal inflammation. In contrast, in our second series of experiments, plasma levels of FITC-conjugated dextran were not increased by AP induction in either wild-type or Fxr-/- mice. These findings indicate that transepithelial resistance measurements are a more sensitive marker for disturbed intestinal permeability than FITC-conjugated dextran. Nevertheless, Fxr-/- mice had significantly higher plasma levels of FITC-conjugated dextran than wild-type mice, which was in line with the increased intestinal permeability in Fxr-/- mice previously reported [13].
Although transient Fxr dysfunction was apparent in the early phase of acute murine pancreatitis, this likely did not have a pathogenic contribution, as the severity of AP was similar in mice with genetic disruption of Fxr and controls (Figure 2A and B). The lack of effect upon loss-of-function may relate to decreased intestinal transport of activating bile salt ligands to Fxr in the ileum, as discussed above, and other Fxr expressing tissues of wild-type mice, and result in a phenotype resembling that of the true Fxr-deficient mouse. If this interpretation is correct, gain-of-function studies (e.g. Fxr agonism) may, in theory, be more suitable to address the role of Fxr in AP. Likewise, other models of AP that do not rely on overstimulation of the gallbladder and exocrine pancreas function could, in theory, be employed to further delineate a role of Fxr in AP. Nevertheless, our combined human and murine data strongly argue against a critical role of Fxr in human AP.
In line with our data in wild-type mice, our analysis of non-fasted serum FGF19 levels suggests that ileal FXR dysfunction could also occur in patients with AP. Being a bile salt-regulated enterokine, circulating FGF19 levels increase postprandially in healthy controls (Figure 3). FGF19 levels in enterally fed patients with AP, however, are close to values observed in fasted controls. A possible role of FXR in human AP was further addressed by studying genetic variation at the FXR locus in a cohort of 387 cases and 853 controls. None of the 11 SNPs tested (9 tagging and 2 functional variants), nor the inferred haplotypes, were independently associated with AP. Likewise, none of the variants were independently associated with the risk or course of AP, nor in haplotypes. Thus, genetic variation in the FXR locus does not predispose to, or have a major impact on the course of AP in human subjects.
Of note, genetic variation in the FXR locus did not differ between subjects with a biliary (i.e. gallstone) or non-biliary (mainly alcohol) cause of AP. Fxr-/- mice on a lithogenic diet are highly susceptible to cholesterol gallstone formation due to altered biliary lipid composition [37], although data on the role of FXR in human cholesterol gallstone formation are extremely limited. In female, non-obese gallstone patients, decreased expression of FXR and its target genes ASBT, ileal lipid binding protein (ILBP) and OSTα-OSTβ (all involved in bile acid transport) has been described in the enterocyte [38], [39]. These findings suggest an intestinal defect with decreased absorption and subsequently a diminished bile acid pool [40]. Data on FXR gene polymorphisms in biliary disease show conflicting results. In a Mexican population, the most commonly found FXR haplotype was associated with gallstone prevalence in males, whereas no association was found in German and Chilean populations [41]. Our data yielding an absence of association of FXR polymorphisms or haplotypes contribute to knowledge in the subgroup of patients with gallstone pancreatitis. This subgroup is noteworthy for the presence of small gallstones and biliary sludge [42].
In conclusion, loss-of-function of Fxr did not affect the severity of pancreatitis in the relatively mild model of cerulein-induced AP (fast recovery, no infection, mild histopathological abnormalities). Moreover, our genetic study does not support a major role for variation in the FXR locus as a determinant of human AP.
Supporting Information
Supporting tables. Table S1. Primer sequences. Table S2. SNP information. Table S3. Association analysis of genetic variants in FXR with subgroups of acute pancreatitis patients.
(DOC)
Acknowledgments
We thank Professor A.K. Groen (Department of Pediatrics, Center for Liver, Digestive and Metabolic Diseases, University Medical Center Groningen) for kindly providing Fxr-/- mice. We also thank José Terlinde (Department of Gastroenterology and Hepatology, University Medical Center Utrecht) and Ben de Jong (Department of Medical Microbiology and Immunology, St. Antonius Hospital, Nieuwegein) for technical assistance; Professor K.N. Faber (Department of Gastroenterology and Hepatology, University Medical Center Groningen) for biochemical analyses, and Jackie Senior (Department of Genetics, University Medical Center Groningen) for critically reading the manuscript. We thank the Dutch Pancreatitis Study Group for DNA, plasma and clinical data of acute pancreatitis patients.
Funding Statement
RMN was supported by an Alexandre Suerman stipend from the University Medical Center Utrecht. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Frossard JL, Steer ML, Pastor CM (2008) Acute pancreatitis. Lancet 371:143–152. [DOI] [PubMed] [Google Scholar]
- 2. Banks PA, Freeman ML (2006) Practice guidelines in acute pancreatitis. Am J Gastroenterol 101:2379–2400. [DOI] [PubMed] [Google Scholar]
- 3. Beger HG, Rau B, Mayer J, Pralle U (1997) Natural course of acute pancreatitis. World J Surg 21:130–135. [DOI] [PubMed] [Google Scholar]
- 4. Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, et al. (2009) Timing and impact of infections in acute pancreatitis. Br J Surg 96:267–273. [DOI] [PubMed] [Google Scholar]
- 5. Besselink MG, van Santvoort HC, Renooij W, de Smet MB, Boermeester MA, et al. (2009) Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg 250:712–719. [DOI] [PubMed] [Google Scholar]
- 6. Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, et al. (1999) Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg 3:252–262. [DOI] [PubMed] [Google Scholar]
- 7. Deitch EA (1990) The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg 125:403–404. [DOI] [PubMed] [Google Scholar]
- 8. Van Leeuwen PA, Boermeester MA, Houdijk AP, Ferwerda CC, Cuesta MA, et al. (1994) Clinical significance of translocation. Gut 35:S28–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, et al. (2006) Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rizzo G, Renga B, Mencarelli A, Pellicciari R, Fiorucci S (2005) Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr Drug Targets Immune Endocr Metabol Disord 5:289–303. [DOI] [PubMed] [Google Scholar]
- 11. Wang YD, Chen WD, Moore DD, Huang W (2008) FXR: a metabolic regulator and cell protector. Cell Res 18:1087–1095. [DOI] [PubMed] [Google Scholar]
- 12. Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, et al. (2006) Identification of a hormonal basis for gallbladder filling. Nat Med 12:1253–1255. [DOI] [PubMed] [Google Scholar]
- 13. Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, et al. (2006) Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U. S. A. 103:3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S (2009) The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 183:6251–6261. [DOI] [PubMed] [Google Scholar]
- 15. Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, et al. (2011) Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60:463–472. [DOI] [PubMed] [Google Scholar]
- 16. Wang YD, Chen WD, Wang M, Yu D, Forman BM, et al. (2008) Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 48:1632–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nijmeijer RM, Gadaleta RM, van Mil SW, van Bodegraven AA, Crusius JB, et al. (2011) Farnesoid X receptor (FXR) activation and FXR genetic variation in inflammatory bowel disease. PLOS ONE 6:e23745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drafahl KA, McAndrew CW, Meyer AN, Haas M, Donoghue DJ (2010) The receptor tyrosine kinase FGFR4 negatively regulates NF-kappaB signaling. PLOS ONE 5:e14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zweers SJ, Booij KA, Komuta M, Roskams T, Gouma DJ, et al. (2012) The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology 55:575–583. [DOI] [PubMed] [Google Scholar]
- 20. Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, et al. (2003) Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem 278:41930–41937. [DOI] [PubMed] [Google Scholar]
- 21. Demols A, Le Moine O, Desalle F, Quertinmont E, van Laethem JL, et al. (2000) CD4(+)T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology 118:582–590. [DOI] [PubMed] [Google Scholar]
- 22. Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, et al. (2009) Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery 145:157–167. [DOI] [PubMed] [Google Scholar]
- 23. Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL (2009) High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 49:1228–1235. [DOI] [PubMed] [Google Scholar]
- 24. Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, et al. (2008) Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 371:651–659. [DOI] [PubMed] [Google Scholar]
- 25. Nierman MC, Rip J, Kuivenhoven JA, van Raalte DH, Hutten BA, et al. (2005) Carriers of the frequent lipoprotein lipase S447X variant exhibit enhanced postprandial apoprotein B-48 clearance. Metabolism 54:1499–1503. [DOI] [PubMed] [Google Scholar]
- 26. Nijmeijer RM, van Santvoort HC, Zhernakova A, Teller S, Scheiber JA, et al. (2013) Association analysis of genetic variants in the Myosin IXB gene in acute pancreatitis. PLOS ONE 8:e85870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wapenaar MC, Monsuur AJ, van Bodegraven AA, Weersma RK, Bevova MR, et al. (2008) Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut 57:463–467. [DOI] [PubMed] [Google Scholar]
- 28. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265. [DOI] [PubMed] [Google Scholar]
- 29. Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, et al. (2007) Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology 133:507–516. [DOI] [PubMed] [Google Scholar]
- 30. Jedlitschky G, Leier I, Buchholz U, Hummel-Eisenbeiss J, Burchell B, et al. (1997) ATP-dependent transport of bilirubin glucuronides by the multidrug resistance protein MRP1 and its hepatocyte canalicular isoform MRP2. Biochem J 327 (Pt 1):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitt M, Klonowski-Stumpe H, Eckert M, Luthen R, Haussinger D (2004) Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas 28:181–190. [DOI] [PubMed] [Google Scholar]
- 32. Fallon MB, Gorelick FS, Anderson JM, Mennone A, Saluja A, et al. (1995) Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology 108:1863–1872. [DOI] [PubMed] [Google Scholar]
- 33. Lutgendorff F, Nijmeijer RM, Sandstrom PA, Trulsson LM, Magnusson KE, et al. (2009) Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLoS ONE 4:e4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schnekenburger J, Mayerle J, Kruger B, Buchwalow I, Weiss FU, et al. (2005) Protein tyrosine phosphatase kappa and SHP-1 are involved in the regulation of cell-cell contacts at adherens junctions in the exocrine pancreas. Gut 54:1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gadaleta RM, Oldenburg B, Willemsen EC, Spit M, Murzilli S, et al. (2011) Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-kappaB signaling in the intestine. Biochim Biophys Acta 1812:851–858. [DOI] [PubMed] [Google Scholar]
- 36. Van Felius I, Akkermans LM, Bosscha K, Verheem A, Harmsen W, et al. (2003) Interdigestive small bowel motility and duodenal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol Motil 15:267–276. [DOI] [PubMed] [Google Scholar]
- 37. Moschetta A, Bookout AL, Mangelsdorf DJ (2004) Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 10:1352–1358. [DOI] [PubMed] [Google Scholar]
- 38. Bergheim I, Harsch S, Mueller O, Schimmel S, Fritz P, et al. (2006) Apical sodium bile acid transporter and ileal lipid binding protein in gallstone carriers. J Lipid Res 47:42–50. [DOI] [PubMed] [Google Scholar]
- 39. Renner O, Harsch S, Strohmeyer A, Schimmel S, Stange EF (2008) Reduced ileal expression of OSTalpha-OSTbeta in non-obese gallstone disease. J Lipid Res 49:2045–2054. [DOI] [PubMed] [Google Scholar]
- 40. Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, et al. (2010) Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta 1801:683–692. [DOI] [PubMed] [Google Scholar]
- 41. Kovacs P, Kress R, Rocha J, Kurtz U, Miquel JF, et al. (2008) Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol 48:116–124. [DOI] [PubMed] [Google Scholar]
- 42. Venneman NG, Renooij W, Rehfeld JF, VanBerge-Henegouwen GP, Go PM, et al. (2005) Small gallstones, preserved gallbladder motility, and fast crystallization are associated with pancreatitis. Hepatology 41:738–746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting tables. Table S1. Primer sequences. Table S2. SNP information. Table S3. Association analysis of genetic variants in FXR with subgroups of acute pancreatitis patients.
(DOC)