Abstract
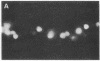
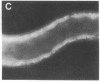
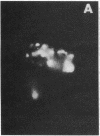
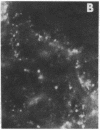
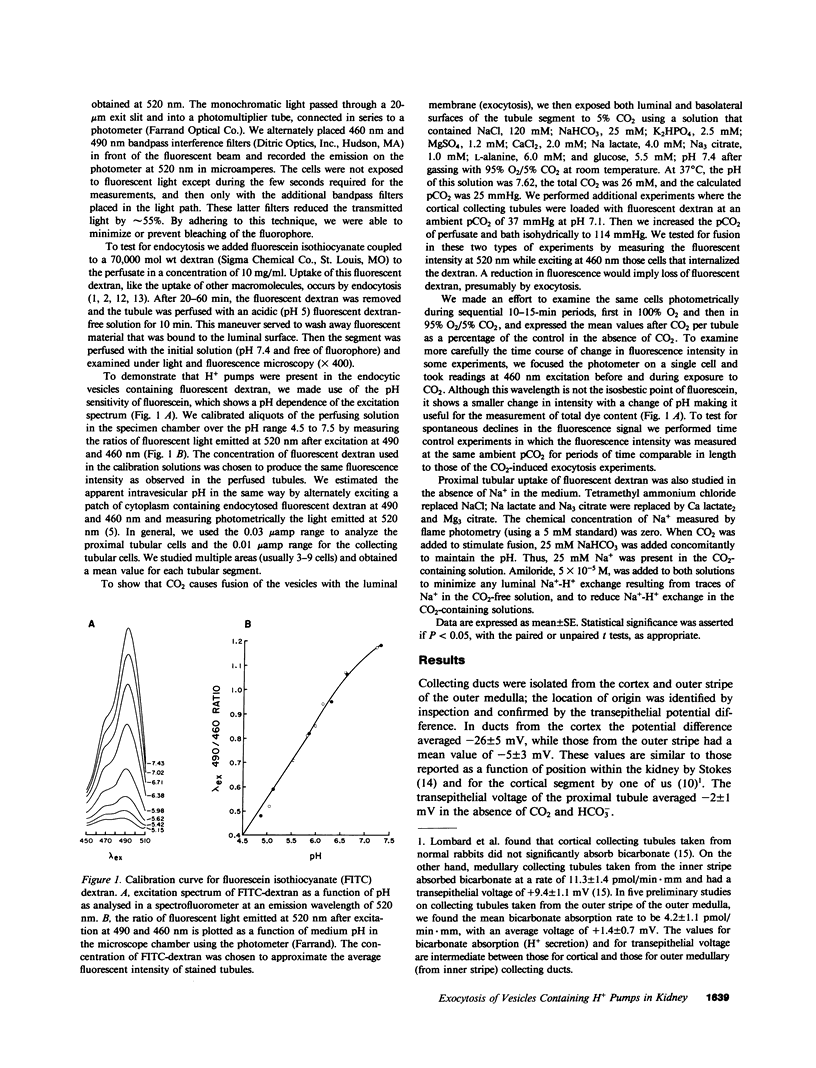
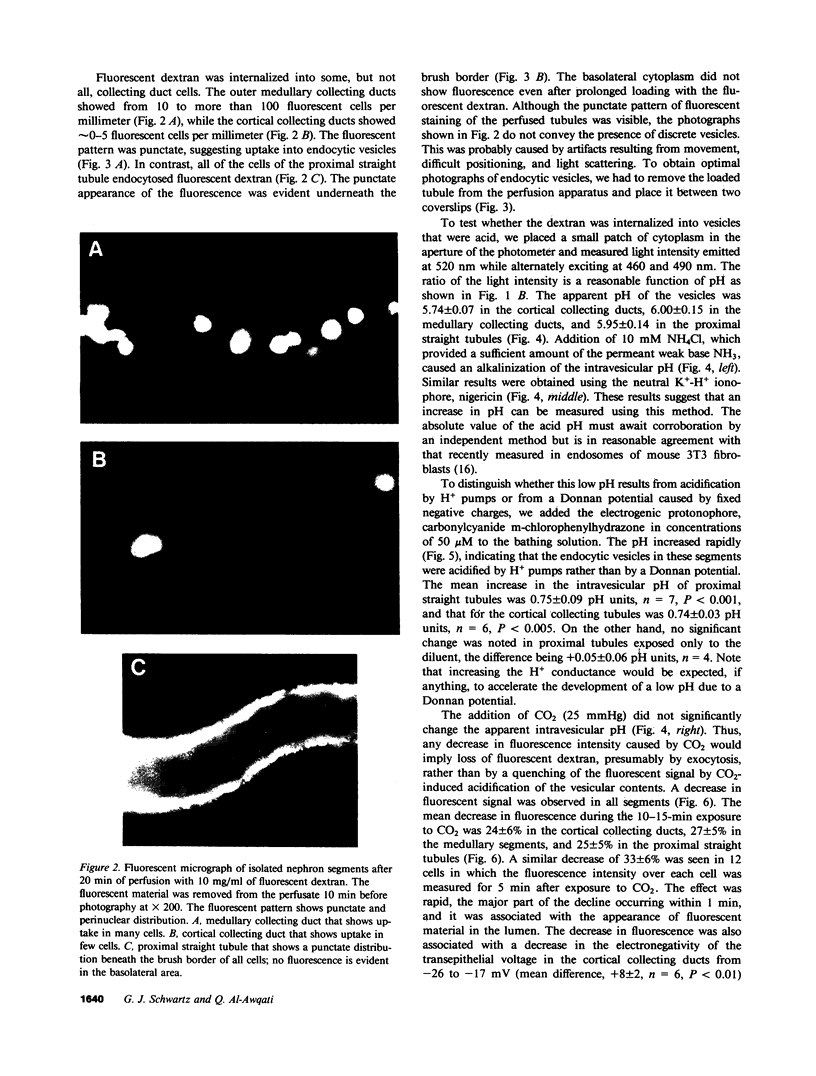
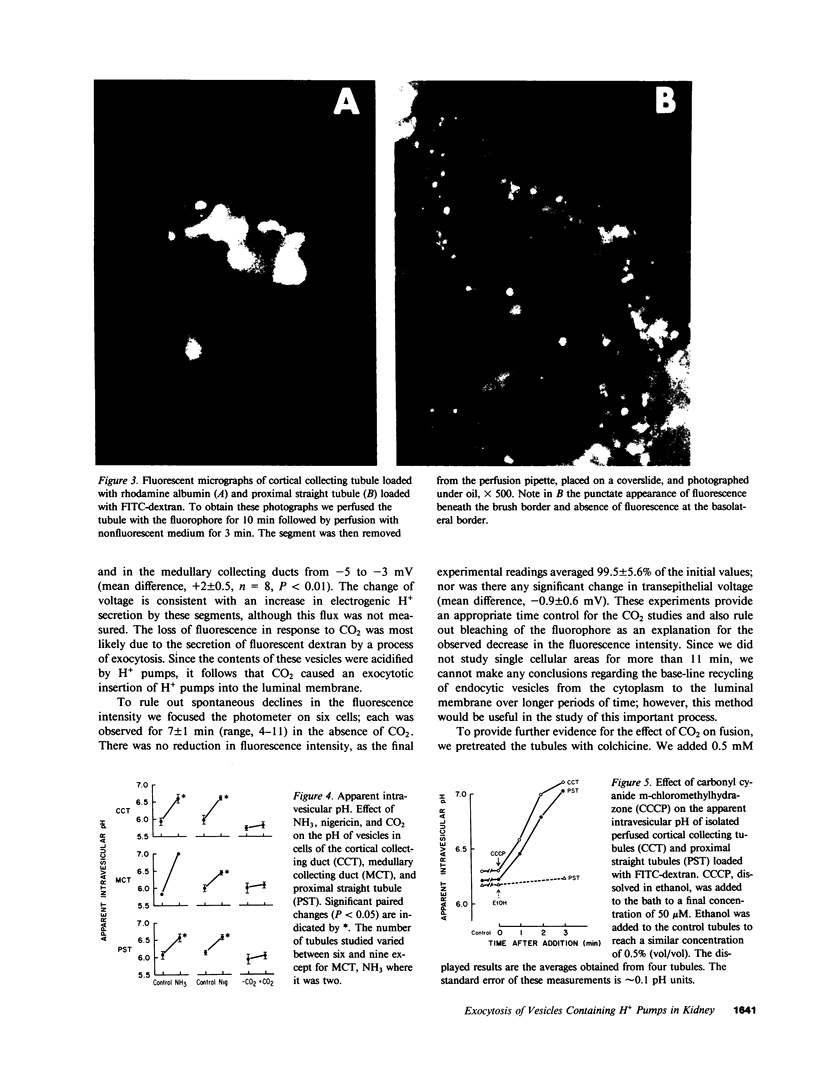
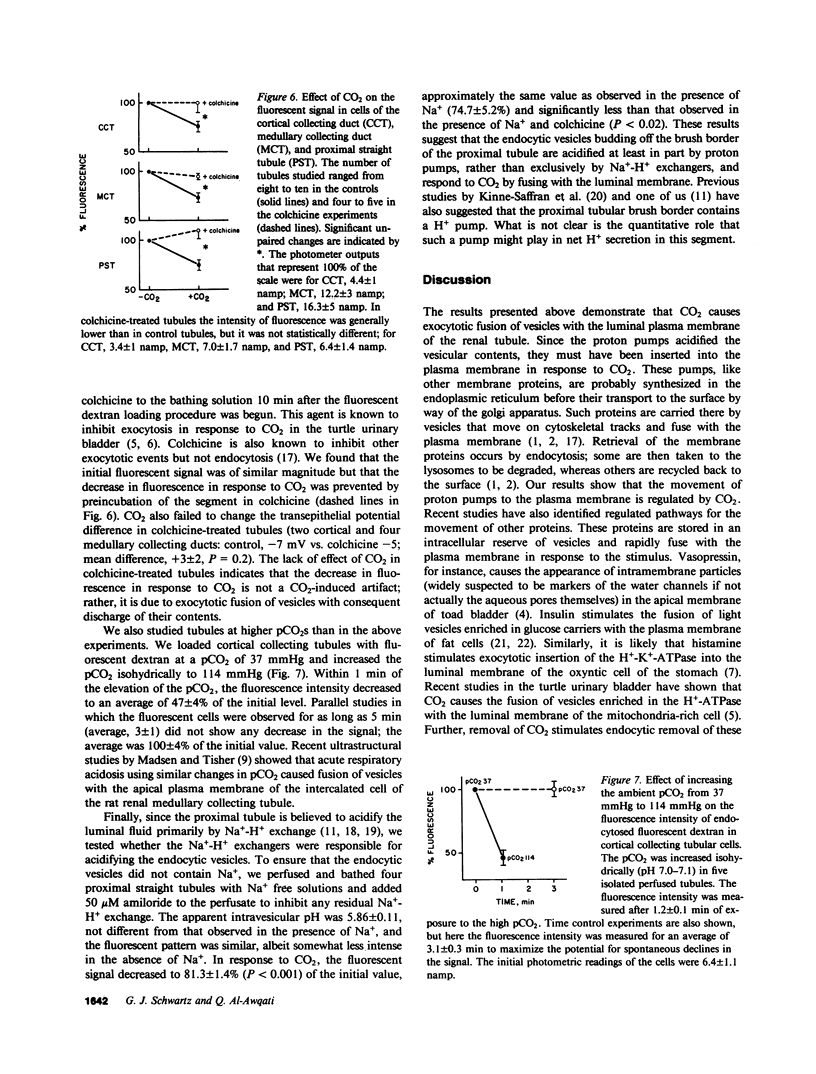
In the turtle bladder it has recently been shown that CO2 stimulates H+ secretion, at least in part, by causing fusion of vesicles enriched in H+ pumps with the luminal plasma membrane. To test for the presence of this mechanism in the kidney we perfused collecting ducts and proximal straight tubules on the stage of an inverted epifluorescence microscope with fluorescein isothiocyanate dextran (70,000 mol wt) in CO2-free medium. After washout we noted punctate fluorescence in endocytic vesicles in some collecting ducts and in all proximal straight tubule cells. More cells took up fluorescent dextran in outer medullary than in cortical collecting ducts. Using the pH dependence of the excitation spectrum of fluorescein we found the pH of the vesicles to be acid (approximately pH 6). Addition of proton ionophores increased vesicular pH by 0.6 +/- 0.1 U, suggesting that the acidity of the vesicles was caused by H+ pumps. CO2 added to the medium (25 mmHg, pH 7.6 at 37 degrees C) reduced fluorescence intensity by 24 +/- 5% in cortical collecting ducts, 27 +/- 5% in medullary collecting ducts, and 25 +/- 5% in proximal straight tubules. Since this effect was prevented by the prior addition of colchicine to the bath, we believe that CO2 caused a decrease in cytoplasmic fluorescence by stimulating exocytotic fusion of the vesicles and thereby secretion of fluorescent dextran. This exocytotic fusion also occurred when tubules that were loaded with fluorescent dextran at a pCO2 of 37 mmHg were exposed isohydrically to a pCO2 of 114 mmHg; the mean decrease was 53 +/- 4%. We conclude that some cells in the collecting ducts and all cells in the proximal straight tubule incorporate fluorescent dextran into the apical cytoplasmic vesicles and acidify them with H+ pumps. CO2 causes fusion of these vesicles with the luminal membrane, but whether CO2 stimulates H+ secretion by increasing the number of functioning H+ pumps remains to be determined.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Honerjäger P. Influence of carbon dioxide on level of ionised calcium in squid axons. Nature. 1978 May 11;273(5658):160–161. doi: 10.1038/273160a0. [DOI] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte T. M., Machen T. E., Forte J. G. Ultrastructural and physiological changes in piglet oxyntic cells during histamine stimulation and metabolic inhibition. Gastroenterology. 1975 Dec;69(6):1208–1222. [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J., Croen K., Kelly S., Al-Awqati Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance. J Cell Biol. 1983 Oct;97(4):1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Al-Awqati Q. An electrogenic proton-translocating adenosine triphosphatase from bovine kidney medulla. J Clin Invest. 1984 Jun;73(6):1704–1710. doi: 10.1172/JCI111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Cannon C., Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V., Farquhar M. G. Luminal membrane retrieved after exocytosis reaches most golgi cisternae in secretory cells. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5073–5077. doi: 10.1073/pnas.74.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson H. R. Effects of CO2 and acetazolamide on bicarbonate and fluid transport in rabbit proximal tubules. Am J Physiol. 1981 Jan;240(1):F54–F62. doi: 10.1152/ajprenal.1981.240.1.F54. [DOI] [PubMed] [Google Scholar]
- Kaissling B., Kriz W. Structural analysis of the rabbit kidney. Adv Anat Embryol Cell Biol. 1979;56:1–123. doi: 10.1007/978-3-642-67147-0. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kinne-Saffran E., Beauwens R., Kinne R. An ATP-driven proton pump in brush-border membranes from rat renal cortex. J Membr Biol. 1982;64(1-2):67–76. doi: 10.1007/BF01870769. [DOI] [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Blevins T. L., Ezaki O. Evidence that translocation of the glucose transport activity is the major mechanism of insulin action on glucose transport in fat cells. J Biol Chem. 1982 Sep 25;257(18):10942–10947. [PubMed] [Google Scholar]
- Lea T. J., Ashley C. C. Increase in free Ca2+ in muscle after exposure to CO2. Nature. 1978 Sep 21;275(5677):236–238. doi: 10.1038/275236a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., de Moura J. L. Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature. 1982 Jun 24;297(5868):685–688. doi: 10.1038/297685a0. [DOI] [PubMed] [Google Scholar]
- Lombard W. E., Kokko J. P., Jacobson H. R. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol. 1983 Mar;244(3):F289–F296. doi: 10.1152/ajprenal.1983.244.3.F289. [DOI] [PubMed] [Google Scholar]
- Lorenzen M., Taylor A., Windhager E. E. pH effect on osmotic response of collecting tubules to vasopressin and 8-CPT-cAMP. Am J Physiol. 1983 Aug;245(2):F188–F197. doi: 10.1152/ajprenal.1983.245.2.F188. [DOI] [PubMed] [Google Scholar]
- Madsen K. M., Tisher C. C. Cellular response to acute respiratory acidosis in rat medullary collecting duct. Am J Physiol. 1983 Dec;245(6):F670–F679. doi: 10.1152/ajprenal.1983.245.6.F670. [DOI] [PubMed] [Google Scholar]
- Masur S. K., Holtzman E., Walter R. Hormone-stimulated exocytosis in the toad urinary bladder. Some possible implications for turnover of surface membranes. J Cell Biol. 1972 Jan;52(1):211–219. doi: 10.1083/jcb.52.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. F., Powers S., Cantor C. R. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J Cell Biol. 1984 May;98(5):1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees-Jones R., Al-Awqati Q. Proton-translocating adenosinetriphosphatase in rough and smooth microsomes from rat liver. Biochemistry. 1984 May 8;23(10):2236–2240. doi: 10.1021/bi00305a022. [DOI] [PubMed] [Google Scholar]
- Rodman J. S., Kerjaschki D., Merisko E., Farquhar M. G. Presence of an extensive clathrin coat on the apical plasmalemma of the rat kidney proximal tubule cell. J Cell Biol. 1984 May;98(5):1630–1636. doi: 10.1083/jcb.98.5.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J. Na+-dependent H+ efflux from proximal tubule: evidence for reversible Na+-H+ exchange. Am J Physiol. 1981 Oct;241(4):F380–F385. doi: 10.1152/ajprenal.1981.241.4.F380. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D. L., Steinmetz P. R. Role of membrane fusion in CO2 stimulation of proton secretion by turtle bladder. Am J Physiol. 1983 Jul;245(1):C113–C120. doi: 10.1152/ajpcell.1983.245.1.C113. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Ion transport by the cortical and outer medullary collecting tubule. Kidney Int. 1982 Nov;22(5):473–484. doi: 10.1038/ki.1982.200. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. An ATP-driven proton pump in clathrin-coated vesicles. J Biol Chem. 1983 Apr 10;258(7):4059–4062. [PubMed] [Google Scholar]