Summary
Background
Observational evidence suggests that community-based services for people with schizophrenia can be successfully provided by community health workers, when supervised by specialists, in low-income and middle-income countries. We did the COmmunity care for People with Schizophrenia in India (COPSI) trial to compare the effectiveness of a collaborative community-based care intervention with standard facility-based care.
Methods
We did a multicentre, parallel-group, randomised controlled trial at three sites in India between Jan 1, 2009 and Dec 31, 2010. Patients aged 16–60 years with a primary diagnosis of schizophrenia according to the tenth edition of the International Classification of Diseases, Diagnostic Criteria for Research (ICD-10-DCR) were randomly assigned (2:1), via a computer-generated randomisation list with block sizes of three, six, or nine, to receive either collaborative community-based care plus facility-based care or facility-based care alone. Randomisation was stratified by study site. Outcome assessors were masked to group allocation. The primary outcome was a change in symptoms and disabilities over 12 months, as measured by the positive and negative syndrome scale (PANSS) and the Indian disability evaluation and assessment scale (IDEAS). Analysis was by modified intention to treat. This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN 56877013.
Findings
187 participants were randomised to the collaborative community-based care plus facility-based care group and 95 were randomised to the facility-based care alone group; 253 (90%) participants completed follow-up to month 12. At 12 months, total PANSS and IDEAS scores were lower in patients in the intervention group than in those in the control group (PANSS adjusted mean difference −3·75, 95% CI −7·92 to 0·42; p=0·08; IDEAS −0·95, −1·68 to −0·23; p=0·01). However, no difference was shown in the proportion of participants who had a reduction of more than 20% in overall symptoms (PANSS 85 [51%] in the intervention group vs 44 [51%] in the control group; p=0·89; IDEAS 75 [48%] vs 28 [35%]). We noted a significant reduction in symptom and disability outcomes at the rural Tamil Nadu site (−9·29, −15·41 to −3·17; p=0·003). Two patients (one in each group) died by suicide during the study, and two patients died because of complications of a road traffic accident and pre-existing cardiac disease. 18 (73%) patients (17 in the intervention group) were admitted to hospital during the course of the trial, of whom seven were admitted because of physical health problems, such as acute gastritis and vomiting, road accident, high fever, or cardiovascular disease.
Interpretation
The collaborative community-based care plus facility-based care intervention is modestly more effective than facility-based care, especially for reducing disability and symptoms of psychosis. Our results show that the study intervention is best implemented as an initial service in settings where services are scarce, for example in rural areas.
Funding
Wellcome Trust.
Introduction
The absence of accessible services for people with schizophrenia in low-income and middle-income countries contributes to the substantial public health burden of schizophrenia; poor health and social outcomes, including poverty;1 social exclusion attributable to stigma and discrimination; and human rights violations in these settings.2 In many high-income countries, clinical and social services for people with schizophrenia are coordinated by specialist community-based multidisciplinary teams. However, such specialist services are not presently feasible in low-income and middle-income countries because of serious human and financial resource constraints. Hence, development of alternative methods for provision of accessible, community-based services for people with schizophrenia within these countries is a global public health priority.3
Increasing observational evidence from low-income and middle-income countries shows that the community-based rehabilitation method of services, delivered through community health workers working with mental health specialists, might be a feasible way to deliver services that improve clinical and social outcomes of people with schizophrenia.4 However, such evidence from randomised controlled trials in these settings is scarce.
We did the COmmunity care for People with Schizophrenia in India (COPSI) study to compare the clinical effectiveness of two service delivery methods for people with schizophrenia and their caregivers. We postulated that a combination of facility-based care and collaborative community-based care would be more effective than facility-based care alone for people with moderate to severe schizophrenia for changes in symptoms and disabilities.
Methods
Study design and patients
A description of the study design, methods, and analysis plan has been published elsewhere.5 We undertook this multicentre, parallel-group, randomised controlled trial between Jan 1, 2009 and Dec 31, 2010, at three sites in India (four subdistricts of Kancheepuram district, Tamil Nadu; Goa; and Satara District in Maharashtra). The Tamil Nadu site was rural with no locally accessible mental health services; Satara and Goa had a mixed urban and rural population with easier access to specialist care in public and private facilities.
Because COPSI was designed as a pragmatic randomised trial, we used broad inclusion criteria. Eligible patients aged 16–60 years had a primary diagnosis of schizophrenia according to the tenth edition of the International Classification of Diseases, Diagnostic Criteria for Research (ICD-10-DCR); an illness duration of at least 12 months of at least moderate severity overall, as rated by the clinical global impression-schizophrenia scale;6 and intended to reside in the study regions for 12 months. In Tamil Nadu, a two-staged, key-informant community survey was done to identify participants who met inclusion criteria, whereas in Goa and Satara, participants were recruited from the clinical practices of the collaborating psychiatrists. Thus, we used two pathways for recruitment: (1) people with schizophrenia from within the existing caseloads, and (2) people with schizophrenia who presented for care at treatment facilities for the first time. Across the study sites, collaborating psychiatrists screened people with a primary ICD-10-DCR diagnosis of schizophrenia for eligibility. For each participant, one primary caregiver was identified and asked for consent to participate. All eligible participants were provided with an overview of the study in the relevant local language before seeking informed consent (appendix).
The COPSI-informed consent procedure was undertaken by a team of trained interviewers using a flipchart to enable people who were not literate to understand the implications and risks of participation. We enrolled participants and caregivers if both provided written or independently witnessed (for people who were not literate) informed consent. Ethics approval was obtained from the institutional review boards of Sangath and SCARF, and from King's College London and the London School of Hygiene & Tropical Medicine. The trial monitoring committee provided additional oversight of the trial.
Randomisation and masking
Patients were randomly assigned in a 2:1 ratio, via computer-generated randomisation list with block sizes of three, six, or nine, to receive either collaborative community-based care plus facility-based care or facility-based care alone. Randomisation was stratified by study site. For each site, the randomisation list was generated independently by the trial statistician and transferred to the site data manager before recruitment. The data manager had no role in the recruiting of participants and held the passwords for the randomisation lists; individuals recruiting participants did not have access to the randomisation lists or the passwords.
Outcome assessors were masked to group allocation. Because neither participants nor the family caregivers were masked to their allocation status at the 6 month and 12 month interviews, unmasking was possible during the outcome assessments. To minimise this possibility, we kept the research and intervention teams physically separate during the trial, asked participants and caregivers first at the time of assessments to not disclose whether they had received home visits from the community health worker, and the primary outcome measures (positive and negative syndrome scale [PANSS] and the Indian disability evaluation assessment scale [IDEAS]) were completed first. Instances of unmasking were recorded by the researchers. If unmasking happened at the time of the 6 month assessment, a separate researcher undertook the 12 month assessments. Facility-based care was available to participants in both study groups; thus, treating psychiatrists were aware of the allocation status of participants.
Procedures
The collaborative community-based care intervention was motivated by our experience with development of a similar intervention in a rural resource-poor setting in India.4 This intervention was further refined in iterative formative phases as recommended by guidelines from the Medical Research Council for development and assessment of complex interventions. Initially, we selected components of the intervention from a systematic review of the scientific literature. To help match the concerns of people with schizophrenia and their caregivers with the intervention components, we additionally identified outcomes that mattered to these individuals. We undertook further pilot work to address operational challenges in delivery of the interventions. This process identified challenges in delivery of the home-based sessions, organisation of the supervision for community health workers as planned, and the need for a simpler set of information materials. This identification led to adaptations such as specific strategies to address planning for the home-based sessions, the risk of attrition, and the development of material such as the flipchart element of the manual to help community health workers communicate the contents of the psychoeducation sessions.
The collaborative community-based care intervention was designed to promote collaboration between the person with schizophrenia, their caregivers, and the treatment team to deliver a flexible, individualised, and needs-based intervention.7 The intervention was delivered by community health workers who had at least 10 years of schooling and good interpersonal skills. These workers were systematically trained over 6 weeks and assessed for competence with the intervention manual.
To meet the prespecified quality assurance and fidelity standards, community health workers at each site were supervised by intervention coordinators. Coordinators were psychiatric social workers trained in the necessary supervision and monitoring skills. The treating psychiatrists supervised the intervention delivery through quarterly team reviews and ongoing supervision of community health workers. Continued education and training of the health workers was an essential part of the intervention. Panel 1 describes components of the collaborative community-based care intervention. To standardise the intervention across sites, the COPSI study team developed a manual to be used flexibly according to the individual needs of participants. The intervention was delivered in three phases: (1) the intensive engagement phase (0–3 months), including six to eight home visits made by community health workers; (2) the stabilisation phase (4–7 months), with sessions delivered once every 15 days; and (3) the maintenance phase (8–12 months), with sessions delivered once a month.
Panel 1. Components of the collaborative community-based care intervention.
-
•
Structured needs assessments at enrolment and every 3 months thereafter to develop matched individualised treatment plans
-
•
Structured clinical reviews by treating team and supervision for community health workers
-
•
Psychoeducational information for participants and caregivers
-
•
Adherence management strategies
-
•
Strategies of health promotion to address physical health problems in participants
-
•
Individualised rehabilitation strategies to improve the personal, social, and work functioning of participants
-
•
Specific efforts with participants and caregivers to deal with experiences of stigma and discrimination
-
•
Linkage to self-help groups and other methods of user-led support
-
•
Networks with community agencies to address social issues, to help with social inclusion, access to legal benefits, and employment opportunities
The control group received the facility-based care indication to show the crucial differences compared with collaborative community-based and facility-based care—ie, the location of services and the use of community health workers. Facility-based care represents the usual care provided by specialist mental health practitioners for people with schizophrenia and their families in India. The operational aspects of the facilities differed across trial sites. In Goa there were four private clinics, supported by inpatient facilities. In Satara, the four private psychiatrists operated as a group practice from one facility and shared an inpatient service. In Tamil Nadu, care was from a clinical team consisting of psychiatrists and assistants at a designated facility some distance away from most patients; this service is similar to those in many rural areas in India.
Although the sites varied somewhat, the content of the control-based care provided was similar. Briefly, the intervention was delivered by the psychiatrists through consultations, lasted 10–15 min, and all people with schizophrenia were prescribed antipsychotic drugs. Psychiatrists also provided information about the illness, encouraged adherence to drugs, and discussed specific concerns raised.
Outcomes
The primary outcome was a change in symptoms and disabilities in 12 months. Secondary outcomes were adherence to antipsychotic treatment and experiences of stigma and discrimination. For caregivers of people with schizophrenia, the secondary outcomes were changes in knowledge and attitudes about the illness, changes in felt burden of caring, and experiences of stigma and discrimination. Additionally, we included an analysis of health economics with an assessment of the costs and cost-effectiveness of the collaborative community-based care and facility-based care intervention compared with facility-based care alone.
Symptoms were measured at baseline and 12 months with the PANSS,8 which has been previously used in India. PANSS comprises 30 items measuring positive and negative symptoms and general psychopathology. To assess disability, we used the IDEAS at baseline, 6, and 12 months. IDEAS has been validated in India9 and measures self-care, interpersonal activities, communication and understanding, and work.10
The trial protocol has full details of the measures used for the secondary outcomes.5 In brief, we used the following scales: a 5-point ordinal scale developed for the study (adherence); discrimination and stigma scale,11 the alienation subscale of the internalised stigma of mental illness scale,12 and an item on willingness to disclose the illness (stigma and discrimination of participants); the knowledge about schizophrenia interview scale13 (caregiver knowledge and attitudes); the burden assessment schedule (family burden of caring);14 the relevant section of the family interview schedule;15 and a modified version of the item on willingness to disclose the illness (caregiver experiences of stigma and discrimination); and cost of illness schedule (health economic outcomes).16
Statistical analysis
On the basis of data from the only relevant study in India,17 we assumed that an absolute difference of a 20% reduction in mean PANSS total score, from 65 to 52 (SD 10), would be highly clinically significant. The intraclass correlation between sites in the facility-based care group was estimated as 0·05; for the intervention group (which also includes between-community health worker clustering), this correlation was assumed to be 0·1. These estimates were conservative on the basis of a review of primary care variables.18 An allocation ratio of 2:1 was used to allow for the between-health worker clustering effect in the intervention group.19 The estimated required total sample size of 241 was increased to 282 to allow for 15% attrition during the study. With α=0·05, this sample size had 80% power to detect an effect size of 0·8 (difference in PANSS score of 7·2 units [SD 9]), and 90% power to detect an effect size of 1 (difference in PANSS score of 9 units [9]). We obtained data for IDEAS and adherence with palmtop computers, whereas PANSS data were obtained with pen and paper. Analyses followed the prespecified data analysis plan and no interim analyses were done. All analyses used Stata version 11.2.
We analysed descriptive summaries of sociodemographic and clinical data for all participants at baseline, and for outcome measures at baseline, and at 6 month and 12 month follow-up points, and compared them by group. PANSS and IDEAS scores used were total score, domain sub-scores, and proportion of patients improving by more than 20% from baseline. For PANSS, a value of 30 was first subtracted from the proportion of patients improving by more than 20% from baseline because this is the minimum total score. We used histograms of data for the control group to assess normality of the distribution of the data and to identify outliers.
For inferential analysis, participants were analysed by original randomised group (a modified intention-to-treat approach). We used linear mixed-effect regression to analyse the intervention effect on continuous outcomes (the 12 month PANSS and IDEAS total scores), adjusted for baseline scores and site as fixed terms, and a random effect to allow for community health workers clustering in the intervention group. We used mixed-effects logistic regression to analyse the intervention effect on binary outcomes, and mixed-effects ordinal logistic regression for ordered categorical outcomes. For the IDEAS score, we did a longitudinal analysis, including both 6 month and 12 month outcomes, using a random-effects model including a time×treatment term allowing for intervention effect changes over time. The effect of adherence was assessed by categorisation of self-reported adherence at 12 months as complete or most of the time, and including an interaction term between adherence and intervention group. No imputation was made because missing data were fewer than the predefined threshold specified in the trial protocol.
Costs were reported in Indian Rupees (INR) and also International Dollars (I$) with an exchange rate of INR 19·13 to I$1. Total costs were compared and bootstrapped CIs computed. We calculated incremental cost-effectiveness ratios to show the extra cost for collaborative community-based care and facility-based care to achieve an extra unit improvement in the primary outcomes.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
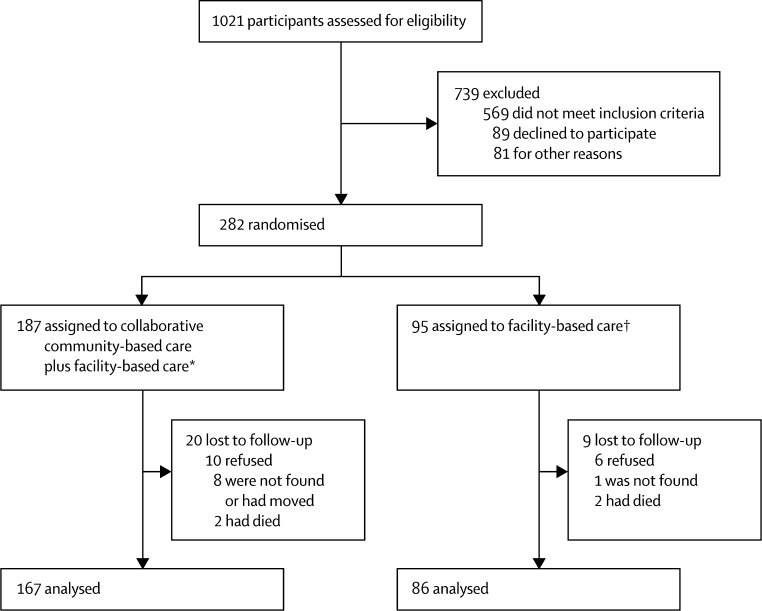
The figure shows the trial profile. 187 eligible patients were randomised to the collaborative community-based care and facility-based care group (70 in Tamil Nadu, 60 in Goa, and 57 at Satara) and 95 were randomised to the facility-based care group (35 in Tamil Nadu, 32 in Goa, and 28 at Satara). Recruitment and follow-up took place between Oct 1, 2009, and Dec 31, 2011. Most participants in both groups (167 [89%] in the intervention group and 86 [91%] in the control group) were available at 12 month assessment. 23 interviews (19 [83%] in the intervention group and four [17%] in the control group) were unmasked during the 6 months of data collection, whereas 22 interviewers (20 [91%] vs two [9%]) were unmasked at the 12 month point. The sociodemographic and clinical characteristics of participants were similar between the treatment groups (table 1).
Figure.
Trial profile
Table 1.
Baseline characteristics
| Collaborative community-based care plus facility-based care(n=187) | Facility-based care (n=95) | ||
|---|---|---|---|
| Age (years) | 36·2 (10·2) | 35·6 (10·4) | |
| Sex | |||
| Women | 86 (46%) | 47 (49%) | |
| Education | |||
| None | 11 (6%) | 6 (6%) | |
| Primary school (up to 5th standard) | 26 (14%) | 14 (16%) | |
| Middle school (6th to 8th standard) | 30 (16%) | 14 (16%) | |
| High school (9th to 12th standard) | 81 (43%) | 39 (43%) | |
| College or above | 34 (18%) | 17 (19%) | |
| Missing | 5 (3%) | 5 (5%) | |
| Occupation | |||
| Not income generating | 138 (75%) | 66 (69%) | |
| Income generating | 38 (21%) | 25 (26%) | |
| Other* | 8 (4%) | 4 (4%) | |
| Marital status | |||
| Married | 87 (47%) | 34 (36%) | |
| Single | 77 (42%) | 44 (46%) | |
| Separated or divorced | 15 (8%) | 8 (8%) | |
| Widow | 5 (3%) | 9 (9%) | |
| Residence | |||
| Rural | 132 (71%) | 63 (66%) | |
| Urban | 55 (29%) | 32 (34%) | |
| Caste | |||
| Schedule caste | 46 (25%) | 20 (21%) | |
| Schedule tribe | 4 (2%) | 2 (2%) | |
| Other backward caste | 45 (24%) | 28 (29%) | |
| None of the above | 74 (40%) | 30 (32%) | |
| Unknown | 18 (10%) | 15 (16%) | |
| Duration of illness (years) | 7·0 (3·0–11·0) | 6·25 (3·1–10·8) | |
| Treatment status at entry | |||
| Ongoing treatment at facility | 96 (57%) | 49 (56%) | |
| First contact with facility | 71 (43%) | 39 (44%) | |
Data are mean (SD), n (%), or median (IQR).
Student or retired.
We monitored the intervention for fidelity and quality assurance purposes by collecting key process indicators for both groups. The mean number of sessions with community health workers that were received by participants in the community-based care and facility-based care group was 17·97 (SD 7·12, 95% CI 16·94–19·00), and 169 (90%) received the predefined minimally effective 12 sessions. The mean number of contacts with a treating psychiatrist was ten (95% CI 9·53–10·89) in the intervention group and eight (6·98–9·11) in the control group. At 12 months, we noted a reduction in overall symptoms in both groups (table 2). Total PANSS score was lower at 12 months in patients in the intervention group than in those in the control group, after adjustment for baseline PANSS scores and site (table 2). However, no difference was shown in the proportion of participants who had a reduction of more than 20% in overall symptoms (85 [51%] vs 44 [51%]; p=0·89).
Table 2.
Adjusted PANSS and IDEAS total scores over 12 months
| Collaborative community-based care | Facility-based care | Collaborative community-based care vs facility-based care* | p value | ||
|---|---|---|---|---|---|
| All sites | |||||
| PANSS score | |||||
| Baseline | 76·28 (20·19) | 74·65 (19·42) | .. | .. | |
| 12 months | 66·62 (17·25) | 70·53 (17·94) | −3·75 (−7·92 to 0·42) | 0·08 | |
| IDEAS score | |||||
| Baseline | 6·79 (3·90) | 6·13 (3·60) | .. | .. | |
| 12 months | 5·68 (3·54) | 6·40 (3·82) | −0·95 (−1·68 to −0·23) | .. | |
| Individual sites | |||||
| PANSS score | |||||
| Tamil Nadu | 63·5 (13·6) | 73·0 (17·8) | −9·29 (−15·41 to −3·17) | 0·003 | |
| Goa | 73·0 (18·0) | 75·9 (17·0) | −2·59 (−10·20 to 5·01) | 0·50 | |
| Satara | 63·8 (19·1) | 61·6 (16·4) | 1·72 (−5·86 to 9·30) | 0·66 | |
| IDEAS score | |||||
| Chennai | 7·78 (2·57) | 9·23 (2·76) | −1·76 (−2·80 to −0·72) | 0·001 | |
| Goa | 5·78 (3·30) | 5·64 (3·31) | 0·01 (−1·43 to 1·45) | 0·98 | |
| Satara | 2·80 (2·86) | 3·85 (3·27) | −0·96 (−2·33 to 0·40) | 0·17 | |
Data are mean (SD) or adjusted mean difference (95% CI), unless otherwise indicated. PANSS=positive and negative syndrome scale. IDEAS=Indian disability evaluation assessment scale.
Adjusted for baseline score and site.
The effect was stronger for overall disability (table 2). A post-hoc analysis of the odds of patients improving by 20% or more on the IDEAS total score showed that more people improved by this extent in the intervention group than in the control group (75 [48%] vs 28 [35%], adjusted odds ratio [OR] 1·84, 95% CI 0·97–3·46; p=0·06). We noted clear site effects in relation to the primary outcomes (table 2). For the PANSS primary symptom outcome, a significant symptom reduction was shown for Tamil Nadu (p=0·003), but no significant change was reported for the Goa and Satara sites (table 2). The same pattern was shown for the IDEAS disability outcome, with a clear disability reduction in Tamil Nadu (p=0·01), but no change was identified at the other two sites (table 2).
Participants in the intervention group had lower scores across all subdomains of symptoms and disabilities measured at 12 months than did those in the intervention group (table 3). Scores reached statistical significance only for the general psychopathology domain in the PANSS and the self-care domain in the IDEAS (table 3). The community-based care and facility-based care intervention was more effective in reducing total disability scores at 12 months than at 6 months, after adjustment for baseline score and site (p=0·01; appendix).
Table 3.
Changes in PANSS subscale and IDEAS domain scores over 12 months
| Collaborative community-based care | Facility-based care | Collaborative community-based care vs facility-based care (adjusted mean difference [SE]) | 95% CI | ||
|---|---|---|---|---|---|
| PANSS* | |||||
| Positive subscale | |||||
| Baseline | 17·84 (7·07) | 16·68 (5·91) | .. | .. | |
| 12 months | 13·98 (5·68) | 15·03 (6·91) | −1·45 (0·93) | −3·27 to 0·38 | |
| Negative subscale | |||||
| Baseline | 21·47 (7·55) | 21·24 (7·54) | .. | .. | |
| 12 months | 19·59 (6·95) | 20·13 (6·11) | −0·50 (0·77) | −2·00 to 1·00 | |
| General subscale | |||||
| Baseline | 36·97 (10·29) | 36·73 (9·89) | .. | .. | |
| 12 months | 32·88 (8·76) | 35·36 (9·81) | −2·16 (1·05) | −4·23 to −0·09† | |
| IDEAS‡ | |||||
| Self-care item | |||||
| Baseline | 1 (0–2) | 1 (0–2) | .. | .. | |
| 6 months | 1 (0–2) | 1 (0–2) | −0·001 (0·26) | −0·50 to 0·50 | |
| 12 months | 1 (0–2) | 1 (0–2) | −0·86 (0·26) | −1·36 to −0·35§ | |
| Interpersonal activities item | |||||
| Baseline | 2 (1–2) | 1 (1–2) | .. | .. | |
| 6 months | 1 (1–2) | 1 (1–2) | 0·05 (0·25) | −0·45 to 0·54 | |
| 12 months | 2 (1–2) | 2 (1–2) | −0·37 (0·34) | −0·89 to 0·16 | |
| Communication and understanding item | |||||
| Baseline | 1 (1–2) | 1 (1–2) | .. | .. | |
| 6 months | 1 (1–2) | 1 (0·5–2) | 0·39 (0·26) | −0·11 to 0·90 | |
| 12 months | 2 (1–2) | 2 (1–2) | −0·47 (0·25) | −0·97 to 0·26 | |
| Work item | |||||
| Baseline | 2 (1–4) | 2 (1–4) | .. | .. | |
| 6 months | 2 (0–3) | 2 (1–3) | 0·39 (0·25) | −0·09 to 0·87 | |
| 12 months | 2 (0–3) | 2 (1–3) | −0·30 (0·25) | −0·79 to 0·18 | |
Data are mean (SD) or median (IQR), unless otherwise indicated. PANSS=positive and negative syndrome scale. IDEAS=Indian disability evaluation assessment scale.
Linear regression including a random effect for community health-care worker and covariance for baseline score and site.
p=0·04.
Ordinal logistic regression fitted with Generalized Linear Latent and Mixed Models (GLLAMM). Model includes a random effect for community health-care worker and covariance for baseline score and site.
p=0·001.
The appendix shows the association of complete or almost complete adherence between the treatment groups. At 12 months, 153 (61%) participants reported complete adherence to drugs, and a further 57 (23%) reported adherence most of the time. At both 6 months and 12 months, participants in the intervention group were significantly more likely to report adherence all or most of the time to prescribed drugs compared with those in the control group (appendix). We noted a similar effect on adherence reported by caregivers at 12 months (appendix).
The study had low power to detect intervention effects by level of adherence, but there was a trend towards reduced symptoms in patients who had good adherence to drugs (PANSS adjusted mean difference −3·85 [SE 6·94] in patients with good adherence vs −0·52 [5·13] in those with poor adherence; p value for effect modification between adherence and treatment group=0·56). For IDEAS, we noted a similar, but smaller, trend of greater improvement in patients with good adherence compared with those with poor adherence (–1·14 [SE 1·33] vs 0·09 [0·90]; p value for effect modification=0·21).
Participants in the intervention group did not report lower stigma than did those in the control group (adjusted OR for any reported alienation 1·15, 95% CI 0·66–2·02; table 4). Moreover, participants in the intervention group were more likely to be unwilling to disclose their illness at 12 months than were those in the control group (table 4). However, we noted clinically and statistically significant reductions in reported experiences of stigma and discrimination from baseline to 12 months in both groups for three of the four outcome measures—eg, the proportion of participants reporting any negative discrimination (p=0·004; table 4).
Table 4.
Stigma and discrimination outcomes for people with schizophrenia
| Overall (n=246) | Collaborative community-based care (n=162) | Facility-based care (n=84) | Effect measure (OR [95% CI]) | |||
|---|---|---|---|---|---|---|
| Negative discrimination | ||||||
| Proportion with any negative discrimination* | .. | .. | .. | 1·02 (0·54–1·92) | ||
| Baseline | 105 (43%) | 67 (42%) | 38 (45%) | .. | ||
| 12 months | 73 (30%) | 48 (30%) | 25 (30%) | .. | ||
| Anticipated discrimination† | ||||||
| Proportion with any anticipated discrimination | .. | .. | .. | 1·31 (0·66–2·60) | ||
| Baseline | 131 (53%) | 92 (57%) | 39 (46%) | .. | ||
| 12 months | 92 (37%) | 64 (40%) | 28 (33%) | .. | ||
| Alienation‡ | ||||||
| Proportion with high alienation§ | .. | .. | .. | 1·60 (0·82–3·12) | ||
| Baseline | 133 (48%) | 88 (48%) | 45 (48%) | .. | ||
| 12 months | 77 (32%) | 55 (34%) | 22 (27%) | .. | ||
| Proportion unwilling to disclose their illness | .. | .. | .. | 2·77 (1·65–4·67) | ||
| Baseline | 119 (48%) | 80 (49%) | 39 (45%) | .. | ||
| 12 months | 118 (48%) | 82 (51%) | 36 (43%) | .. | ||
Data are n (%), unless otherwise indicated. OR=odds ratio.
Excludes seven participants who had missing items for all 20 items on negative discrimination.
Excludes seven participants who had missing items for all four items on anticipated discrimination.
Excludes five participants who had missing data for all six items on alienation.
Alienation mean score less than 2·5.
We noted no evidence of an intervention effect on mean total score on the knowledge about schizophrenia interview scale (adjusted mean difference 0·34, 95% CI −0·28 to 0·96), or on burden (−0·04, −0·18 to 0·11), reported stigma (1·35, 0·72–2·53), or willingness to disclose their family members' illness (1·43, 0·80–2·53).
The mean number of sessions in the intervention group was 17·1 (6·9) with slight variation between sites (appendix). Travel times were highest in Tamil Nadu (appendix). Costs of input from the community health workers were exceeded by the costs of supervision (appendix). Total intervention costs were highest in Goa and lowest in Satara (appendix). Other service costs varied between sites with the highest for participants in in the intervention group in Goa (appendix). Total costs in the 12 months of follow-up were highest for participants in the intervention group, mainly because of staff supervision costs (appendix). The mean difference between the two groups adjusted for baseline was INR 9427 (95% CI 6584–12 224), which is equivalent to I$493 (344–639). The incremental cost-effectiveness ratio (ICER), based on the PANSS, is defined as the incremental cost of community-based care and facility-based care group compared with facility-based care alone (INR 9427) divided by the incremental reduction in symptoms (3·75). This ratio shows that a cost of INR 2514 (I$131) is needed to achieve a 1 point reduction on the PANSS. The ICER based on the IDEAS is INR 9365 divided by 0·95—ie, INR9923 (I$519) to achieve a 1 point reduction on the IDEAS (appendix).
Four patients died during the study. Two (50%) of these deaths were because of suicide (one in each treatment group), whereas the other two (50%) were due to complications of a road traffic accident and pre-existing cardiac disease. 18 (73%) patients were admitted to hospital during the course of the trial; of these, 17 were in the intervention group. Seven (39%) of these admissions were related to physical health problems, such as acute gastritis and vomiting, road accident, high fever, or cardiovascular disease; only one admission was at the Tamil Nadu site.
Discussion
Our findings show that the collaborative community-based intervention including supervised community health workers was more effective than were facility-based services for people with moderate to severe schizophrenia. The overall benefits of community-based plus facility-based care were modest at 12 months and were most evident in the reducing of disabilities associated with schizophrenia and in promotion of adherence with prescribed drugs. Additionally, we noted an overall trend for improvement in the severity of symptoms with the intervention; however, across the whole study, this trend was not statistically or clinically significantly different in comparison to facility-based care at 12 months.
We have described the association of PANSS score with the intervention (p=0·08) as modestly more effective, which is in line with recommendations about this issue.21 In view of this finding, we focused on the effect size and 95% CI whilst showing p values, which is in line with CONSORT 2010 guidelines for reporting of findings from clinical trials.22 This symptom-related finding is better than those from previous research about community mental health interventions in high-income countries, for which most studies tend to report improvements in service satisfaction, quality of life, and aspects of disability, but do not show symptom changes.23 Because the study intervention included components that were designed to improve self-care, structuring activities, social interactions, and work ability, the greater effectiveness of the intervention in reducing disability, compared with facility-based care alone, is understandable.
The stigma-related findings are consistent with the explanation that facility-based care alone is effective in reducing stigma and discrimination, and that there is little additional contribution from community-based care. The community-based plus facility-based care intervention did not produce any additional changes in caregivers understanding and knowledge of schizophrenia. This finding is surprising and needs further exploration, especially from the available qualitative data available from a subset of participants. The absence of an association between caregivers' burden and the greater reduction of disability levels in participants in the intervention group than in those in the control group is also unexpected. This finding could show that an increased quantum of improvement in disability scores is needed to translate this improvement into matching improvement in caregiver burden.
The health economic findings showed that costs in the intervention group were on average greater than those in the control group, and that about a third of these additional costs were attributable to supervision. Effective staff supervision is increasingly seen as a cornerstone for interventions including community health workers that are sustainable in the long term, and is fundamental to the delivery of WHO's mhGAP Intervention Guide. The average greater cost for participants in the intervention group over the study period was almost INR 9500 (roughly I$500); therefore, a judgment should be taken as to the value in terms of the clinical and social improvements identified for a group of highly vulnerable people.
Recent randomised trials from low-income and middle-income countries have reported the benefits of family psychoeducation and adherence management in improvement of outcomes in people with schizophrenia.24, 25, 26, 27 In all these trials, participants were recruited from hospital settings and the interventions were provided by specialists. By contrast, in our COPSI trial, supervised community health workers delivered the psychosocial intervention in the community. The findings of improved disability and adherence with additional psychosocial interventions are common across COPSI and these other studies.
Although the overall effects of community-based plus facility-based care are moderate in scale, the primary outcomes differ greatly when disaggregated by site. This difference is probably because of the methods of recruitment and the consequent characteristics of the participants. At both Satara and Goa, facility-based care was provided by psychiatrists in well-established clinics and participants were therefore recruited from an existing pool of people with schizophrenia who were already receiving ongoing high-quality treatment. This ongoing usual care included many elements of community-based and facility-based care, albeit unstructured. In this context, community-based and facility-based care might have added little to the cumulative effects of the facility-based care and did not provide substantial additional benefits. However, in Tamil Nadu, participants were recruited from a population-based sample and the additional community-based care intervention did provide substantial benefits.
Were specific, evidence-based interventions actually delivered to the intervention group? We ensured this delivery by first, following an independent assessment procedure for individualised clinical and social needs (using Camberwell assessment of needs). The team also adapted the manual to deliver the intervention according to the needs identified by each participant. Because these interventions were intensively supervised, we are reasonably confident that community-based and facility-based care delivered more than generic support from the community support worker and did in fact deliver interventions matched to individual needs.
Were all participants at least moderately severely disabled as specified in the study protocol? To ensure that first-contact patients did meet this criterion, details were collected by an experienced senior member of the clinical team. The psychiatrist then reviewed the information and did a detailed clinical assessment to decide if each participant fulfilled the criteria for ICD-10 schizophrenia, and if they met the severity threshold by using criteria from the clinical global impression-schizophrenia scale for ICD-10 schizophrenia. Indeed, our trial was designed to include people with schizophrenia with a high overall severity for whom additional, community-based care would be justifiable in the context of scarce resources. Thus, participants in the trial had a median duration of about 7 years of the illness, at least a moderate severity of symptoms and disabilities, and high levels of social adversity. For this group of people with long-term and severely disabling symptoms and various social problems, these modest improvements in disability and symptoms could be viewed as important outcomes. Indeed, it was striking that the outcome that improved the most disability matched the outcome that mattered the most to participants.
Clinical improvement in people with severe schizophrenia is often a gradual process and the fairly short duration of follow-up is a limitation of our trial. The longitudinal analysis of the disability data shows that significant improvements from the intervention happened between the 6 month and 12 month period, suggesting that a longer duration of follow-up is needed to show the additional benefits of the psychosocial intervention.4
These findings are important because they suggest the potential use and limitations of the community-based and facility-based care intervention. With extrapolation from the findings in Tamil Nadu, and in view of the modest overall effectiveness and the additional costs involved, community-based and facility-based care might be best deployed in a defined catchment-area-based population as an initial strategy of making care more accessible and equitable (by circumvention of barriers to treatment). Furthermore, the community-based care intervention is clearly of restricted use in situations when the high-quality facility-based care is provided to a stable group of people with schizophrenia who are well engaged with their psychiatrists. The results also suggest that for people with moderate to severe schizophrenia, the interventions should focus on social and economic recovery and on changing the environmental barriers to care.
In the global mental health context, the results strengthen the case for consideration of the adoption of community-based plus facility-based care as an initial step in the provision of services to where they are needed and scarce (as is the case in many low-income and middle-income countries); this provision might need to be progressively supplemented by intensive or specialist services for patients who are difficult to treat, as is common in high-income countries.28 In our trial, community health workers were trained for 6 weeks because the requirements of working in the context of a highly structured randomised trial are somewhat more complicated than in real-life health programmes, whereas a briefer initial training programme of 3 weeks is likely to be more sustainable.
These findings have both strong internal validity and are potentially generalisable. The large sample size, the successful masking of allocation, the endpoint outcome data collected with fairly low rates of unmasking, the delivery of community-based plus facility-based care according to protocol across the sites, and the low attrition rates, add to the internal validity of the findings. The broad inclusion criteria and the fact that participants with a need for additional community care were recruited from real-world clinical settings across three diverse sites and practice arrangements in India all suggest that the findings of the trial are generalisable to similar settings in other low-income and middle-income countries.
These results add to the evidence base for the use of community health workers in the enhancement of access to services for people with schizophrenia in low-income and middle-income countries where specialist resources are few (panel 2). WHO and the Expert Policy Group of the Ministry of health in India have recommended use of non-specialist health workers to deliver community-based psychosocial interventions to scale up services in low-income and middle-income countries.29. Within this global and national context, our findings are an important addition to the ongoing policy and advocacy efforts to make evidence-based services available for many more people with schizophrenia in low-income and middle income countries in the near future.
Panel 2. Research in context.
Systematic review
Several systematic reviews have been done to assess the evidence base for community services for people with schizophrenia in low-income and middle-income countries.30 Except for some randomised trials of psychoeducational interventions from China, few trials from other low-income and middle-income countries have assessed community services rigorously. However, some recent randomised trials have tested the effectiveness of psycho-educational interventions and adherence management strategies for people with schizophrenia and their caregivers from other low-income and middle-income countries.24, 25, 26, 27 The results show that both psychoeducation and adherence management are effective across a range of outcomes. However, there is an over-representation of studies done in specialist hospital settings and in settings where the interventions were delivered by specialist mental health workers, making generalisation of the results to community settings uncertain. As yet, no randomised trials are available of a community-based, multicomponent intervention delivered by community health workers from low-income and middle-income settings.
Interpretation
The community-based care intervention provided collaboratively by a team consisting of community health workers was more effective for people with moderate to severe schizophrenia for reducing disability and improving adherence compared with usual, specialist care delivered from treatment facilities. Our findings provide rigorous evidence about the effectiveness of use of supervised community health workers in provision of accessible, community-based services to people with schizophrenia—a strategy that has been advocated as a pragmatic method to scale up service coverage when specialist resources are scarce in low-income and middle-income countries.
Acknowledgments
Acknowledgments
We thank the Wellcome Trust for providing the academic grant (number 084355/Z/07/Z) for undertaking the COPSI trial; the generous participation of people with schizophrenia and their families, without whom this study would not have been possible; all COPSI staff members who delivered the intervention across the sites, in particular, Dakshin Lilly and Pratheesh Kumar KK for their wonderful coordination across the three study sites; members of the intervention teams who delivered the collaborative community-based care plus facility-based care intervention superbly across the sites (Binu Gangadharan, Pradnya Umarye, Rachel Andrade, the late Chandrakant Mhambrey, Esmaralda Rego, Dinesh Velip, Dilip Gaonkar, and Mabel Pereira at Goa; Pratiksha Mane, Reshma Momin, Tejas Chowkwale, Nitin Barawade, Jayashree Chowkwale and Yogini Magar at Satara; and S Selvi, A Geetha, Shanthi, S Valarmathi, V Prema, R Kalaiyarasi, and D Kalaivani at Chennai); the research staff who made data collection possible according to highest standards of competence at all three sites (Fatima Gomes, Lisa Cordiero, Aarti Rajan, Swamini Kakodkar, Basvaraj Katti, and Anthony Lobo at Goa; Vaishali Chavan, Prashant Potadar, Uday Chavan, and Minakshi Pisal at Satara; Shobhna Lakshman, Agnes Shironmanii, Andrea Bernard, and K Suganya at Chennai, and Jesina Pereira); the collaborating psychiatrists (T C Ramesh Kumar, R Padmavati, H Dabholkar, P Dabholkar, A Chavan, R Deshpande, R Hegde, P Pai Kakode, M Chagas Silva, and P Castelino) for their role in coordination of the various aspects of the study at the three sites; Morven Leese who designed the statistical analysis plan and Kimberley Goldsmith who aided in the drafting of the final analysis protocol; Michael Dewey for statistical contributions; the ethics committees at King's College London and the London School of Hygiene & Tropical Medicine, and the institutional review boards of Sangath and SCARF for providing a crucial and independent overview of the ethical and procedural aspects of the trial; and the members of the trial monitoring committee (Mohan Isaac [Chairperson], Baljeet Ahluwalia, P Chandra, R Raguram, and K Thennarasu) for their active and valuable contributions in development and monitoring of the trial. GT is funded in relation to a National Institute for Health Research (NIHR) applied programme grant awarded to the South London and Maudsley National Health Service (NHS) Foundation Trust, and in relation to the NIHR Specialist Mental Health Biomedical Research Centre at the Institute of Psychiatry (King's College London) and the South London and Maudsley NHS Foundation Trust. VP is supported by a Wellcome Trust Senior Research Fellowship in clinical science. MK was supported by a Wellcome Trust Clinical PhD Fellowship during her involvement in the trial.
Contributors
GT, VP, RT, and SC designed the study and SN, SJ, HD, MB, and MK administered interventions. SC was the trial manager. HAW took overall lead in the statistical analysis of the data. All authors contributed to the interpretation of the data, writing of the report, and approved the final manuscript.
Declaration of interests
We declare that we have no competing interests.
Supplementary Material
References
- 1.Lund C, De Silva M, Plagerson S. Poverty and mental disorders: breaking the cycle in low-income and middle-income countries. Lancet. 2011;378:1502–1514. doi: 10.1016/S0140-6736(11)60754-X. [DOI] [PubMed] [Google Scholar]
- 2.Thornicroft G, Brohan E, Rose D, Sartorius N, Leese M, the INDIGO Study Group Global pattern of experienced and anticipated discrimination against people with schizophrenia: a cross-sectional survey. Lancet. 2009;373:408–415. doi: 10.1016/S0140-6736(08)61817-6. [DOI] [PubMed] [Google Scholar]
- 3.Collins PYPV, Patel V, Joestl SS, the Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee S, Pillai A, Jain S, Cohen A, Patel V. Outcomes of people with psychotic disorders in a community-based rehabilitation programme in rural India. Br J Psychiatry. 2009;195:433–439. doi: 10.1192/bjp.bp.108.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee S, Leese M, Koschorke M, the COmmunity care for People with Schizophrenia in India (COPSI) group Collaborative community based care for people and their families living with schizophrenia in India: protocol for a randomised controlled trial. Trials. 2011;12:12. doi: 10.1186/1745-6215-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haro JM, Kamath SA, Ochoa S. The Clinical Global Impression-Schizophrenia Scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl. 2003;416:16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- 7.Balaji M, Chatterjee S, Koschorke M. The development of a lay health worker delivered collaborative community based intervention for people with schizophrenia in India. BMC Health Serv Res. 2012;12:42. doi: 10.1186/1472-6963-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 9.Thara R. IDEAS (Indian Disability Evaluation and Assessment Scale)—a scale for measuring and quantifying disability in mental disorders. the Indian Psychiatric Society; India: 2002. [Google Scholar]
- 10.Chaudhury PK, Deka K, Chetia D. Disability associated with mental disorders. Indian J Psychiatry. 2006;48:95–101. doi: 10.4103/0019-5545.31597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brohan E, Clement S, Rose D, Sartorius N, Slade M, Thornicroft G. Development and psychometric evaluation of the Discrimination and Stigma Scale (DISC) Psychiatry Res. 2013;208:33–40. doi: 10.1016/j.psychres.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Ritsher JB, Otilingam PG, Grajales M. Internalized stigma of mental illness: psychometric properties of a new measure. Psychiatry Res. 2003;121:31–49. doi: 10.1016/j.psychres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton S, Pinfold V, Rose D. The effect of disclosure of mental illness by interviewers on reports of discrimination experienced by service users: a randomized study. Int Rev Psychiatry. 2011;23:47–54. doi: 10.3109/09540261.2010.545367. [DOI] [PubMed] [Google Scholar]
- 14.Brohan E, Henderson C, Little K, Thornicroft G. Employees with mental health problems: Survey of U.K. employers' knowledge, attitudes and workplace practices. Epidemiol Psichiatr Soc. 2010;19:326–332. [PubMed] [Google Scholar]
- 15.Sartorius N, Gulbinat W, Harrison G, Laska E, Siegel C. Long-term follow-up of schizophrenia in 16 countries. A description of the International Study of Schizophrenia conducted by the World Health Organization. Soc Psychiatry Psychiatr Epidemiol. 1996;31:249–258. doi: 10.1007/BF00787917. [DOI] [PubMed] [Google Scholar]
- 16.Chisholm D, Sekar K, Kumar KK. Integration of mental health care into primary care. Demonstration cost-outcome study in India and Pakistan. Br J Psychiatry. 2000;176:581–588. doi: 10.1192/bjp.176.6.581. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee S, Patel V, Chatterjee A, Weiss HA. Evaluation of a community-based rehabilitation model for chronic schizophrenia in rural India. Br J Psychiatry. 2003;182:57–62. doi: 10.1192/bjp.182.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol. 2004;57:785–794. doi: 10.1016/j.jclinepi.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials. 2005;2:152–162. doi: 10.1191/1740774505cn076oa. [DOI] [PubMed] [Google Scholar]
- 21.Hackshaw A, Kirkwood A. Interpreting and reporting clinical trials with results of borderline significance. BMJ. 2011;343:d3340. doi: 10.1136/bmj.d3340. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D, the CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7:e1000251. doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornicroft G, Wykes T, Holloway F, Johnson S, Szmukler G. From efficacy to effectiveness in community mental health services. PRiSM Psychosis Study. 10. Br J Psychiatry. 1998;173:423–427. doi: 10.1192/bjp.173.5.423. [DOI] [PubMed] [Google Scholar]
- 24.Kulhara P, Chakrabarti S, Avasthi A, Sharma A, Sharma S. Psychoeducational intervention for caregivers of Indian patients with schizophrenia: a randomised-controlled trial. Acta Psychiatr Scand. 2009;119:472–483. doi: 10.1111/j.1600-0447.2008.01304.x. [DOI] [PubMed] [Google Scholar]
- 25.Parantahaman V, Satnam K, Lim J-L. Effective implementation of a structured psychoeducation programme among caregivers of patients with schizophrenia in the community. Asian J Psychiatry. 2010;3:206–212. doi: 10.1016/j.ajp.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Sharif F, Shaygan M, Mani A. Effect of a psycho-educational intervention for family members on caregiver burdens and psychiatric symptoms in patients with schizophrenia in Shiraz, Iran. BMC Psychiatry. 2012;12:48. doi: 10.1186/1471-244X-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farooq S, Nazar Z, Irfan M. Schizophrenia medication adherence in a resource-poor setting: randomised controlled trial of supervised treatment in out-patients for schizophrenia (STOPS) Br J Psychiatry. 2011;199:467–472. doi: 10.1192/bjp.bp.110.085340. [DOI] [PubMed] [Google Scholar]
- 28.Thornicroft G, Tansella M. The balanced care model for global mental health. Psychol Med. 2013;43:849–863. doi: 10.1017/S0033291712001420. [DOI] [PubMed] [Google Scholar]
- 29.Dua T, Barbui C, Clark N. Evidence-based guidelines for mental, neurological, and substance use disorders in low- and middle-income countries: summary of WHO recommendations. PLoS Med. 2011;8:e1001122. doi: 10.1371/journal.pmed.1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaebel W, Becker T, Janssen B, the European Psychiatric Association EPA guidance on the quality of mental health services. Eur Psychiatry. 2012;27:87–113. doi: 10.1016/j.eurpsy.2011.12.001. [DOI] [PubMed] [Google Scholar]
Uncited Reference
- 20.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, the CONSORT Group Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.