Summary
Background
Small studies suggest peanut oral immunotherapy (OIT) might be effective in the treatment of peanut allergy. We aimed to establish the efficacy of OIT for the desensitisation of children with allergy to peanuts.
Methods
We did a randomised controlled crossover trial to compare the efficacy of active OIT (using characterised peanut flour; protein doses of 2–800 mg/day) with control (peanut avoidance, the present standard of care) at the NIHR/Wellcome Trust Cambridge Clinical Research Facility (Cambridge, UK). Randomisation (1:1) was by use of an audited online system; group allocation was not masked. Eligible participants were aged 7–16 years with an immediate hypersensitivity reaction after peanut ingestion, positive skin prick test to peanuts, and positive by double-blind placebo-controlled food challenge (DBPCFC). We excluded participants if they had a major chronic illness, if the care provider or a present household member had suspected or diagnosed allergy to peanuts, or if there was an unwillingness or inability to comply with study procedures. Our primary outcome was desensitisation, defined as negative peanut challenge (1400 mg protein in DBPCFC) at 6 months (first phase). Control participants underwent OIT during the second phase, with subsequent DBPCFC. Immunological parameters and disease-specific quality-of-life scores were measured. Analysis was by intention to treat. Fisher's exact test was used to compare the proportion of those with desensitisation to peanut after 6 months between the active and control group at the end of the first phase. This trial is registered with Current Controlled Trials, number ISRCTN62416244.
Findings
The primary outcome, desensitisation, was recorded for 62% (24 of 39 participants; 95% CI 45–78) in the active group and none of the control group after the first phase (0 of 46; 95% CI 0–9; p<0·001). 84% (95% CI 70–93) of the active group tolerated daily ingestion of 800 mg protein (equivalent to roughly five peanuts). Median increase in peanut threshold after OIT was 1345 mg (range 45–1400; p<0·001) or 25·5 times (range 1·82–280; p<0·001). After the second phase, 54% (95% CI 35–72) tolerated 1400 mg challenge (equivalent to roughly ten peanuts) and 91% (79–98) tolerated daily ingestion of 800 mg protein. Quality-of-life scores improved (decreased) after OIT (median change −1·61; p<0·001). Side-effects were mild in most participants. Gastrointestinal symptoms were, collectively, most common (31 participants with nausea, 31 with vomiting, and one with diarrhoea), then oral pruritus after 6·3% of doses (76 participants) and wheeze after 0·41% of doses (21 participants). Intramuscular adrenaline was used after 0·01% of doses (one participant).
Interpretation
OIT successfully induced desensitisation in most children within the study population with peanut allergy of any severity, with a clinically meaningful increase in peanut threshold. Quality of life improved after intervention and there was a good safety profile. Immunological changes corresponded with clinical desensitisation. Further studies in wider populations are recommended; peanut OIT should not be done in non-specialist settings, but it is effective and well tolerated in the studied age group.
Funding
MRC-NIHR partnership.
Introduction
Allergy to peanuts is an increasingly common and important medical disorder, affecting 0·5–1·4% of children in high-income countries.1, 2 Peanut allergy is the most common cause of severe and fatal allergic reactions related to food, it is difficult to identify people at highest risk,3 and resolution is uncommon.4 Quality of life is reduced because of the likelihood of anaphylaxis, causing constant fear over food choices.5, 6 Despite present management, families have poor knowledge of how to avoid and treat food allergy emergencies.7 Accidental reactions are common, with annual incidences of 14–55%.8, 9, 10
Therefore, there is a need for a disease-modifying therapy. Immunotherapy is an established treatment for inhalant allergies11, 12 and insect-venom anaphylaxis.13 Early studies of subcutaneous immunotherapy for peanut allergy were associated with severe adverse reactions, possibly due to the route of administration.14 The oral route might be associated with greater safety, and has been studied in egg and milk allergy.15, 16
There is a need for systematic study of oral immunotherapy (OIT) for the treatment peanut allergy. Therefore, after a phase 1 study that showed good tolerability,17, 18 our aim was to establish the efficacy of OIT for the desensitisation of children with allergy to peanuts.
Method
Participants
Between January, 2010, and March, 2013, we did a single-centre phase 2 randomised controlled two-phase trial at the NIHR/Wellcome Trust Cambridge Clinical Research Facility (Cambridge, UK). During the first phase the active group underwent 26 weeks of peanut OIT, and the control group underwent 26 weeks of standard care (peanut avoidance). At the end of the first phase (26 weeks) all participants were assessed for peanut allergy by double-blind placebo-controlled food challenge (DBPCFC). During the second phase, participants in the control group still allergic to peanuts were offered peanut OIT, with a subsequent further DBPCFC.
Participants were recruited both locally (allergy clinic) and nationally (through national patient support group Anaphylaxis Campaign). Eligible participants were aged 7–16 years with an immediate hypersensitivity reaction after peanut ingestion, positive skin prick test to peanut (extract ALK-Abello, Hørsholm, Denmark) defined by weal of 3 mm or larger in the presence of a negative saline and positive histamine control, and positive DBPCFC.19 We excluded participants if they had a major chronic illness (except for eczema, rhinitis, or asthma) since this was an immunomodulatory therapy, if the care provider or present household member had suspected or diagnosed allergy to peanuts, or if there was an unwillingness or inability to comply with study procedures. We did not exclude participants who had a previous life-threatening reaction, tree-nut allergy, or a history of severe asthma.
The Cambridge Central Ethics Committee approved the study (09/H0308/154) and the guardian of each participant gave written informed consent. Children of an appropriate age were encouraged to provide their own assent. The University of Cambridge and Cambridge University Hospitals NHS Trust (RD authorisation A091686) jointly sponsored the study.
Randomisation and masking
Participants were randomly assigned (1:1) via an audited online system (Randomizer, Medical University of Graz, Austria) to receive either the active therapy or the control intervention. Minimisation was used to reduce imbalance of baseline covariates, with a random element using a weighting probability of 0·8. Factors were sex, age, challenge threshold, peanut specific serum IgE, severity from history, and presence of asthma or other food allergy. Group allocation was not masked.
Procedures
The active intervention (OIT) was given in daily doses of characterised peanut flour (light roast flour; Golden Peanut Company, Alphretta, GA, USA). First, there was a gradual updosing phase with 2 week increments to protein doses of 800 mg/day, and subsequently a maintenance period where the highest tolerated dose (with a target of 800 mg/day) was taken daily to complete a total of 26 weeks OIT. We devised a novel updosing regime, different from other published protocols (patent pending): doses were 2 mg, 5 mg, 12·5 mg, 25 mg, 50 mg, 100 mg, 200 mg, 400 mg, and 800 mg of peanut protein.18 The rationale for choosing a daily maintenance dose of 800 mg was on the basis of the highest amount of peanut protein used in the pilot study that participants were able to ingest on an ongoing daily basis, which was also associated with a suggestion of efficacy. Peanut protein was presented as a finely ground defatted powder that was mixed into food before ingestion. Dose increments took place in the NIHR/Wellcome Trust Clinical Research Facility and participants were observed for 2 h. The same dose was then given at home daily for 2–3 weeks. Participants were asked to take their dose with food and instructed not to exercise for 2 h after taking a dose. Participants were asked to complete symptom diaries and were provided with adrenaline autoinjectors. At the end of the study participants were encouraged to continue daily consumption of 800 mg peanut protein.
Outcomes
The primary outcome was the proportion of desensitised participants in each group at the end of the first phase. Desensitisation was defined as no reaction during peanut DBPCFC with a cumulative dose of 1400 mg peanut protein. The primary outcome was assessed for all participants together and also by recruitment group (local or national).
Secondary outcomes were the proportion of participants who tolerated daily ingestion of 800 mg protein (equivalent to five peanuts) up to 26 weeks; the proportion of the control group who were desensitised or tolerated daily ingestion of 800 mg protein during the second phase; the fold and absolute increase in threshold (maximum tolerated peanut protein in mg) after OIT—defined as no observed adverse effect level (NOAEL; highest dose of peanut protein tolerated in mg protein during challenge or immunotherapy); the change in quality-of-life score; the number and type of adverse events; the change in immunological outcomes (basophil area under curve of CD63% and mean fluorescent intensity [MFI], peanut specific IgE, total IgE, and skin prick test weal diameter).
All peanut challenges were done as DBPCFCs in accordance with best practice,19 using separate active and placebo phases and masked with the validated EuroPrevall dessert food carrier recipe (range of doses from 5, 50, 100, 300, and 1000 mg peanut protein).20 We chose a cumulative challenge dose equivalent to roughly ten peanuts to show desensitisation to an amount of peanut that we judged unlikely to be encountered accidentally after OIT. Random number lists established the order of DBPCFC placebo and active arms. All study personnel were masked to the challenge assignment except the scientist who prepared the challenge material; they had no interaction with the participant or study team.
Quality of life was measured by a disease-specific questionnaire, the Food Allergy Quality of Life—Parent Form (FAQLQ-PF), available for 0–12 years.21 Hence this questionnaire was only offered to families with a child aged 7–12 years. The questionnaire was scored on a six-point scale, averaged over three areas with equal weighting: emotional impact, food anxiety, and social and dietary limitation. Scores were obtained from baseline and the end of each phase.
Flow cytometric analysis (fluorescence-activated cell sorting) of patient samples was done on whole-blood specimens to quantify the proportion and MFI of CD63 basophils. Skin prick tests were done with standardised peanut extract from ALK-Abello, (Hørsholm, Denmark) and a single point lancet. Peanut specific IgE was measured with the ImmunoCap system (Thermo Scientific, Waltham, MA, USA). Measurements were made at baseline and after each phase.
Statistical analyses
All analyses were planned prospectively and detailed in a statistical analysis plan. Fisher's exact test was used to compare the proportion of participants with desensitisation to peanut after 6 months between the active and control group at the end of the first phase. Exact unconditional confidence limits for the absolute risk difference were calculated. Secondary analyses tested for treatment differences with Fisher's exact test (proportion response to treatment in active group and control group at end of the second phase), Wilcoxon signed rank tests (absolute and fold change in threshold), and Mann-Whitney U test (difference between groups in quality-of-life scores, basophil area under curve of CD63% and MFI, peanut-specific IgE, and skin prick test weal diameter).
Tobit regression22 was used in preference to linear regression, because it is better suited to analysis of censored dependent variables. We expected a large number of NOAEL values to be right censored at 1400 mg, as successfully desensitised participants would not react to this top dose during challenge. Estimates were provided for treatment on 6 month NOAEL adjusted for baseline covariates. All statistical tests described use a two-sided 5% significance level. Intention-to-treat analysis was done on the full analysis population that included all participants who were randomised and participated in at least one post-baseline assessment (DBPCFC).
Sample size was based on Fisher's exact test with 90% power and 5% significance (two-sided). A sample size of 49 in each group is sufficient to detect proportions of participants with desensitisation to peanut of 0·64 and 0·30 in the active and control groups respectively at the end of the first phase. Allowing for 5% dropout increased the sample size to 52 participants in each group and 104 overall. Based on the above we would expect 35 waiting list group participants to proceed to the active intervention in the second phase.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
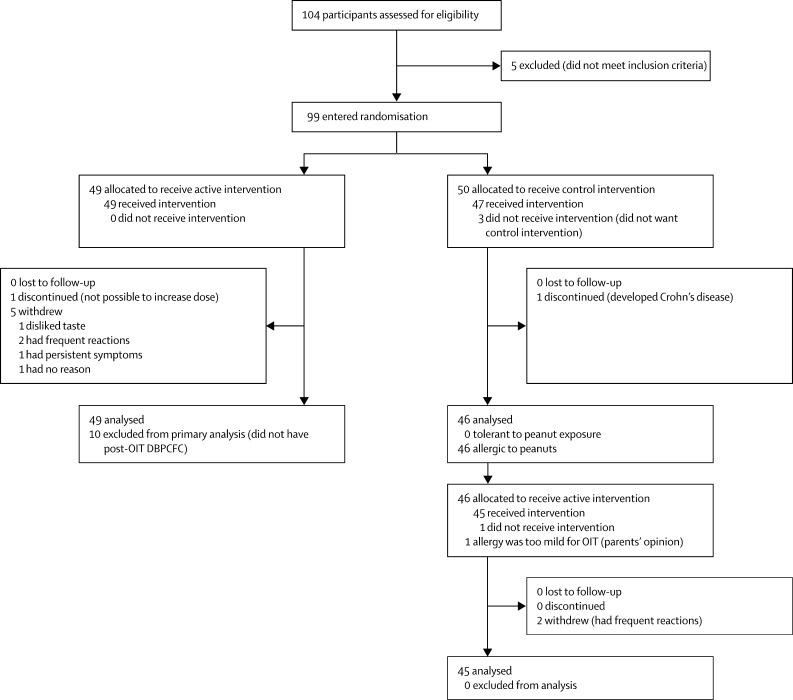
Figure 1 shows the trial profile. We enrolled 104 children, aged 7–16 years (median 12·4 years). The baseline characteristics of the participants are listed in the appendix. Five children did not react during their baseline peanut challenge. Therefore 99 children were randomly assigned to the study groups: 49 active and 50 control (figure 1). One child discontinued and five withdrew from the active group during the first phase. Four further participants were excluded from the primary analysis because they had not reached the target maintenance OIT dose at 6 months. In the control group, four children withdrew and one discontinued during the first phase.
Figure 1.
Trial profile
There was a significant difference between the proportions of participants who had no reaction to 1400 mg peanut protein during DBPCFC at the end of the first phase in the active (24 of 39 participants; 62%, 95% CI 45–78%) compared with the control group (0 of 46; 0%, 95% CI 0–9·1; p<0·001; table 1). The absolute risk difference was estimated as 62% with a conservative 95% CI of 43–77. There was no significant difference in primary outcome between locally and nationally recruited participants (data not shown).
Table 1.
Clinical endpoints for first and second phases
| Control (n=46) | Active (n=39) | p value | |
|---|---|---|---|
| First phase | |||
| Number desensitised | 0 | 24 | <0·001* |
| Number not desensitised | 46 | 15 | |
| Proportion desensitised | 0 (0 to 0·091) | 0·62 (0·45 to 0·78) | .. |
| Proportion able to tolerate daily ingestion | 0 | 0·84 (0·70 to 0·93) | .. |
| Median absolute change in NOAEL, mg | 0 (−95 to 45) | 1345 (45 to 1400) | 0·002, <0·001† |
| Median fold change in NOAEL, mg | 0·81 (0·05 to 1·82) | 25·5 (1·82 to 280) | 0·003, <0·001† |
| Median NOAEL after first phase, mg‡ | 5 (5 to 400) | 1400 (100 to 1400) | <0·001§ |
| Second phase | |||
| Proportion desensitised | 0·54 (0·35 to 0·72) | .. | .. |
| Proportion able to tolerate daily ingestion | 0·91 (0·79 to 0·98) | .. | .. |
| Median change in FAQLQ-PF score from baseline to post-treatment | −1·41 (−4·83 to 1·38)¶ | −1·61 (−4·87 to 0·24)‖ | <0·001, <0·001** |
Data are proportion (95% CI) or median (range). NOAEL=no observed adverse effect level. FAQLQ-PF=Food Allergy Quality of Life Questionnaire—Parent Form for 5–12 years.
From Fisher's exact test.
From Wilcoxon signed rank tests.
Median difference in NOAEL between groups was 1395 mg (95% CI 395 to 1395); p<0·001 (from Mann Whitney U test).
From Mann Whitney U test.
n=20.
n=19.
From Wilcoxon signed rank tests.
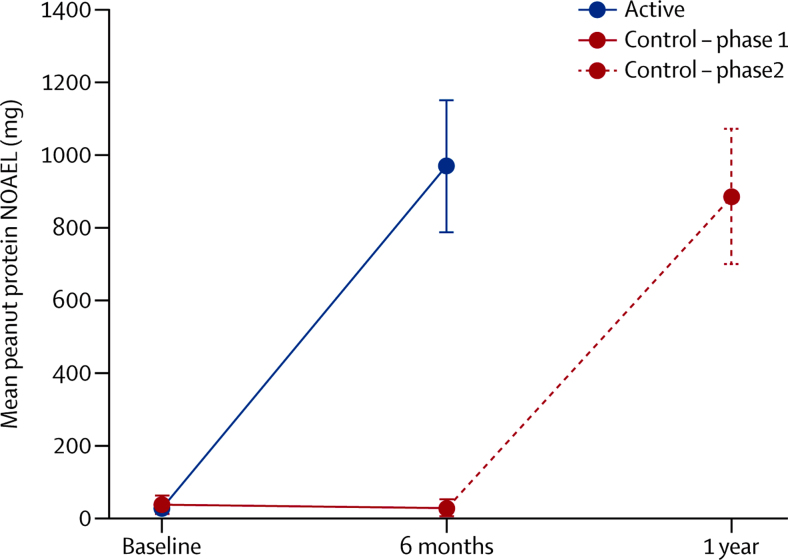
84% (95% CI 70–93) of the active group at the end of the first phase, and 91% (79–98) of the control group at the end of the second phase (after OIT) were able to tolerate daily ingestion of 800 mg protein for 26 weeks. There was a significant increase in peanut NOAEL in the active group after the first phase, with a median change in threshold of 25·5 times (p<0·001; table 1) compared with a small negative change in peanut threshold (NOAEL) in the control group during the first phase (0·81, range 0·05–1·82; figure 2).
Figure 2.
Peanut protein NOAEL by treatment group
Points show the mean and whiskers the 95% CIs. Difference at 6 months p<0·001 (from Mann Whitney U test). NOAEL=no observed adverse effect level.
Quality-of-life scores assessed by the FAQLQ-PF instrument21 were similar in active and control groups at baseline. Both groups showed a similar and clinically meaningful improvement (decrease) in quality of life scores after treatment (table 1).
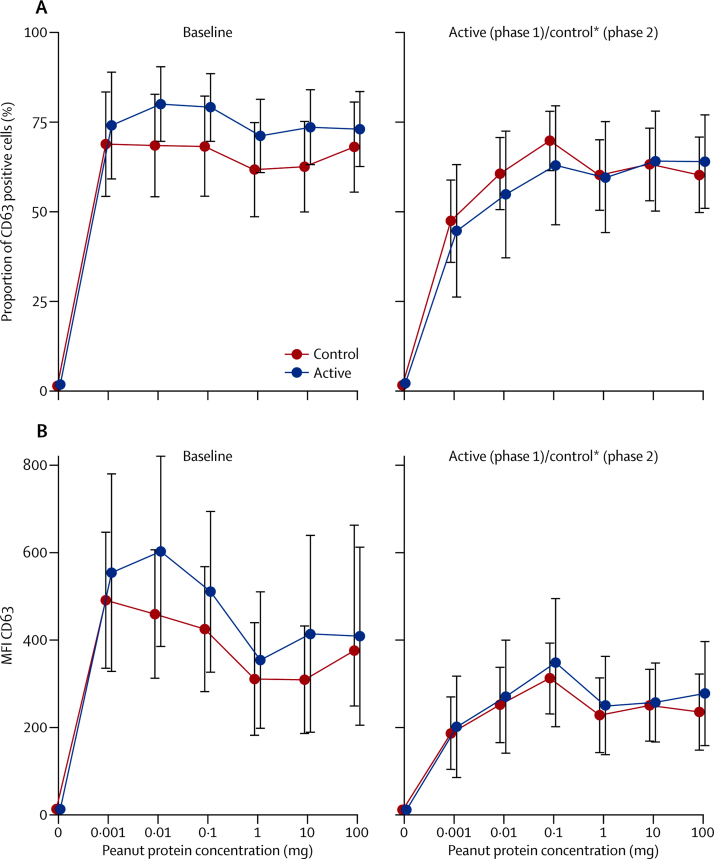
Immunological assessments showed a significant small reduction in median skin prick test weal diameter and increase in peanut-specific IgE after 24 weeks OIT in the active group (appendix). Basophil stimulation test data were expressed as the area under the curve (AUC) of plots of MFI and proportion of CD63-positive cells against concentration of peanut protein. No significant within-patient differences were identified after treatment for AUC of MFI or proportion of CD63, although there was a reduction in MFI and proportion of CD63 at lower peanut concentrations after OIT (figure 3).
Figure 3.
In-vitro basophil activation by peanut before and after desensitisation
Heparinised whole blood was stimulated with a range of peanut protein concentrations (0·001–100 μg/mL) and flow cytometry was used to assess expression as a proportion of CD63 positive cells (A) or by MFI (B) within the basophil population. Differences in areas under the curve were not significant. MFI=mean fluorescent intensity. *After desensitisation.
Tobit regression suggested several baseline covariates could affect the final NOAEL (table 2). Baseline NOAEL, World Allergy Organization grade 2 reaction, and total IgE were also associated with higher post-OIT NOAEL. Age, family history, weight, and peanut-specific IgE were associated with lower final NOAEL.
Table 2.
Tobit regression model exponentiated estimates—dependent variable natural log 6-month NOAEL
| Estimate (95% CI) | p value | |
|---|---|---|
| Oral immunotherapy | 105·5 (67·73–164·40) | <0·001 |
| Log (baseline NOAEL + 1) | 1·40 (1·15–1·69) | <0·001 |
| Age | 0·76 (0·65–0·89) | <0·001 |
| Female | 1·42 (0·87–2·31) | 0·16 |
| Weight | 1·04 (1·01–1·06) | 0·004 |
| Quality of life | 1·12 (0·89–1·42) | 0·32 |
| Asthma | 1·09 (0·65–1·74) | 0·79 |
| Eczema | 0·82 (0·53–1·26) | 0·36 |
| Rhinitis | 0·69 (0·46–1·05) | 0·09 |
| Other food allergy | 0·84 (0·52–1·36) | 0·48 |
| Family history of peanut allergy | 0·41 (0·24–0·71) | 0·001 |
| WAO grade 2 | 2·88 (1·65–5·01) | <0·001 |
| WAO grade 3 | 0·86 (0·44–1·68) | 0·66 |
| WAO grade 4 | 0·62 (0·21–1·82) | 0·40 |
| Peanut SPT wheal diameter | 1·02 (0·94–1·11) | 0·60 |
| Other nut SPT wheal diameter >3 mm | 1·37 (0·81–2·31) | 0·23 |
| Tryptase | 1·06 (0·95–1·19) | 0·32 |
| Log (peanut specific IgE + 1) | 0·60 (0·51–0·71) | <0·001 |
| Log (total IgE + 1) | 1·74 (1·35–2·23) | <0·001 |
| Log (basophil activation CD63 MFI AUC) | 0·98 (0·73–1·32) | 0·91 |
NOAEL is the highest amount of peanut protein (mg) tolerated after OIT. The continuous variables can be interpreted as the percentage change in NOAEL expected from a unit increase of that variable when all other covariates are fixed. The categorical variable estimates can be interpreted as the percentage change compared with the reference group when all other covariates are fixed. For logged covariates the expected percentage change in 6 month NOAEL with a 10% increase in the logged covariate can be calculated as 1·10⁁log (exponentiated estimate). For example, for a 10% increase in baseline NOAEL (mg) we would expect a [1·1⁁log(1·40)=1·032] 3·2% increase in 6 month NOAEL (mg). Similarly, for a 10% increase in baseline peanut specific IgE (kU/L) we would expect a 4·8% decrease in 6 month NOAEL (mg). NOAEL=no observed adverse effect level. OIT=oral immunotherapy. WAO=World Allergy Organization. SPT=skin prick test. MFI=mean fluorescent intensity. AUC=area under the curve.
The number and nature of adverse events was similar in both groups after treatment. Most events were mild with oral itching being the most common (occurring after 6·3% of all doses; table 3). Cutaneous events were uncommon, present after only 0·16% of doses. Wheezing occurred after 0·41% of doses in 22% of participants and was treated with inhaled β2 agonists and oral antihistamines alone in all cases except for one participant who, additionally, self-administered intramuscular adrenaline on two occasions, with rapid resolution of his symptoms. There were no serious adverse reactions and no cardiovascular events.
Table 3.
Adverse events during treatment presented
| Participants who experience an adverse event | Adverse events per dose of OIT | |
|---|---|---|
| Symptoms | ||
| Mouth itch | 76 (81%) | 1121 (6·30%) |
| Abdominal pain | 54 (57%) | 460 (2·59%) |
| Nausea | 31 (33%) | 393 (2·21%) |
| Vomiting | 31 (33%) | 134 (0·75%) |
| Diarrhoea | 1 (1%) | 5 (0·03%) |
| Urticaria | 12 (13%) | 29 (0·16%) |
| Angio-oedema | 18 (19%) | 71 (0·40%) |
| Erythema | 20 (21%) | 41 (0·23%) |
| Rhinitis | 23 (24%) | 65 (0·37%) |
| Wheezing | 21 (22%) | 73 (0·41%) |
| Laryngeal oedema | 1 (1%) | 1 (0·01%) |
| Cardiovascular collapse or fainting | 0 | 0 |
| Outcome | ||
| Admission to intensive-care unit, serious adverse reaction, or serious unexpected suspected adverse reaction | 0 | 0 |
| Use of inhaled β2 agonist | 18 (19%) | 63 (0·35%) |
| Use of intramuscular adrenaline | 1 (1%) | 2 (0·01%) |
Data are n (%). Total doses were 17 793. OIT=oral immunotherapy.
Discussion
Daily doses of peanut OIT of up to 800 mg protein had a clinically meaningful effect, shown by a high incidence of desensitisation, large absolute and fold increases in threshold (NOAEL), and a significant improvement in quality-of-life score; 84% of participants in first phase and 91% in the second phase could tolerate daily ingestion roughly equivalent to five peanuts per day. To our knowledge, our findings provide the first well controlled and accurate estimate of the effect size, benefits, and risks of desensitisation with peanut OIT (panel).
Panel. Research in context.
Systematic review
We searched PubMed and the Cochrane database for English language publications with the MeSH terms “peanut oral immunotherapy”, “peanut desensitization”, and “peanut tolerance”. Date limits for our search were from January, 1980, to August, 2013. A recent systematic review of studies of peanut oral immunotherapy (OIT) identified a single small randomised controlled study in 28 children.23 It suggested a positive effect of peanut OIT but was not powered to estimate efficacy. Interpretation of published studies has also been hampered by small size, exclusion of severely allergic children, and failure to confirm allergic status on enrolment by food challenge.
Interpretation
To our knowledge, we did the first phase 2 study appropriately powered to derive an accurate estimate of the effect size of the treatment in children aged 7–15 years. By contrast with other studies, we assessed a representative UK population including children with severe reactions, with allergy confirmed by DBPCFC. 84% and 91% of participants could tolerate the equivalent of five peanuts per day, and a high rate of desensitisation (ability to tolerate the equivalent of roughly ten peanuts) was shown. There was a substantial effect size, and improvement in quality of life with a good safety profile. Tolerance after cessation of OIT was not assessed. Our data apply to the population studied and the doses employed, and there is a need for replication and expansion of populations studied. This study shows that peanut immunotherapy is an effective and well tolerated treatment in this age group.
Raising the reactive threshold for patients is a key outcome of this treatment. Without OIT, patient ability to avoid hidden peanuts is based on previous experience and inconsistent labelling and advice from food providers.3, 24, 25, 26 Consequently, accidental reactions often happen (14–55% per year8, 9, 10) and involve the use of health-care resources. Fear of death and severe reactions drives down quality of life.5, 6
Peanut OIT raises the reactive threshold at least 25-times so that 84–91% of participants can tolerate daily ingestion of 800 mg protein. Furthermore, 54–62% of children can tolerate a challenge with 1400 mg protein, roughly equivalent to ten peanuts. We showed previously that two-thirds of individuals who can tolerate 1400 mg could also tolerate 6000 mg protein (equivalent to 38 peanuts). Those who could not had only mild reactions.18 These calculations are based on an average peanut containing 160 mg protein, although variation occurs up to 260 mg. This treatment therefore allows participants to eat large quantities of peanuts, above the levels present in contaminated snacks and meals, allowing them to eat more freely and enjoy a reduction in social restrictions. Consequently, our study confirms an improvement in food-allergy specific quality of life, suggested by an earlier uncontrolled study of peanut OIT.27 Quality of life was measured on a validated disease-specific six-point scale21 and the improvements evident in the active and control groups after intervention (–1·61, −1·41) are clinically meaningful, although participants were not masked to treatment allocation and this might have introduced bias.
Our findings should be interpreted in the context of the study limitations. We did not mask treatment allocation. Instead we used a masked objective test to measure the primary outcome (ie, no reaction to peanut during DBPCFC). There was a small risk of bias as participants who knew they were receiving active treatment might have under-reported minor symptoms at the higher challenge doses, although these symptoms would have been uncommon and subjective. Additionally, during first phase, ten participants did not undergo a post-OIT challenge because they had withdrawn or not reached the target maintenance OIT dose at 6 months. It is probable that in the first phase the true response rate is lower than estimated; however, in the second phase, where there were few dropouts, we still noted a large effect. A very conservative sensitivity analysis was done in which all the unobserved participants in the active group were imputed as not desensitised and all the unobserved participants in the control group were imputed as desensitised. The analysis gave a risk difference of 0·41 (95% CI 0·21–0·58), which supports the conclusions of our main analysis.
Since, to our knowledge, this is the first study of its type, our findings are relevant to the population studied, but will need confirmation in other subgroups of patients. Because of the significant risks involved, OIT should be restricted to specialist centres.
We studied desensitisation over a 6 month period, rather than tolerance after cessation of treatment, because stopping OIT after a median of 9 months has been shown to lead to loss of desensitisation.28 It is probable that long-term peanut protein ingestion will be needed to provide continued protection from accidental exposure, perhaps for several years. This view is supported by immunological investigation; during peanut OIT, we showed evidence of basophil and mast-cell desensitisation and other groups have shown a gradual change in peanut-specific T-cell-surface markers from Th2 to Th1 phenotype.29 Long-lived terminally differentiated IgE secreting plasma cells survive in the bone marrow in this environment for years, consequently, peanut specific IgE levels persist for several years after starting OIT, despite apparent clinical desensitisation.29
Unsurprisingly there were many more allergic events during active treatment than during periods of peanut avoidance. The safety data in this trial show most adverse events were mild and due to gastrointestinal symptoms (eg, oral itching), as expected from the route of administration. Skin reactions were uncommon (urticaria after 0·16% of doses). Reactions involving wheezing occurred after 0·41% of doses, or roughly one fifth of participants. Although wheezing could be taken as a sign of a more severe reaction, in all but one participant it was mild and responded to standard doses of inhaled bronchodilator drugs. One participant self-administered adrenaline at home with good effect on two occasions for wheezing after his peanut OIT doses—he was withdrawn from the study. No participants had hypotension.
We previously devised a new regimen18 with a standard starting dose for all participants, because our pilot study17 showed that tailoring the starting dose to individual thresholds commonly resulted in adverse events, even at low doses. This regime is therefore better tolerated and an improvement. From the limited data available in published work it is apparent that the speed of the updosing schedule has a greater negative effect on safety and efficacy than the size of the maintenance dose, with semi-rush regimens showing less efficacy and more common reactions.28, 29 We therefore used a gradual updosing regimen.
Prognostic factors were explored with Tobit regression.22 Treatment with OIT was, unsurprisingly, the most influential positive factor. Serum peanut-specific IgE, age, and family history were also associated with a negative effect on NOAEL (weight had a weak effect). These are all prognostic factors that one would intuitively think would make immunotherapy more difficult, and need further study.
Acknowledgments
Acknowledgments
This project was awarded by the Efficacy and Mechanism Evaluation Programme (08/99/18), and is funded by the Medical Research Council (MRC) and managed by the National Institute for Health Research (NIHR) on behalf of the MRC-NIHR partnership, and jointly sponsored by the University of Cambridge and Addenbrooke's Hospital (Cambridge University Hospital Foundation Trust). The NIHR/Wellcome Trust Cambridge Clinical Research Facility on the Cambridge Biomedical Campus supported the research. We thank Gareth Jones, headteacher at The Perse Prep School in Cambridge, and the Perse Prep Parents for fundraising to buy equipment used during this work. We thank our data monitoring committee (Nicola Brathwaite [chair], Ken Ong, Carlo Acerini), and members of our Trial Steering Committee (Robert Boyle [Chair], Chris Palmer, Hazel Gowland). We thank the Anaphylaxis Campaign who reviewed the study protocol, funding application, and patient information sheets, and provided a patient representative (Hazel Gowland) as a member of the Trial Steering Committee.
Contributors
PE and AC conceived, designed, and managed the study. CP contributed to the statistical design of the study and was on the trial steering committee. KA, PE, and AC drafted the report. KA and YK did the clinical procedures. SI, LF, and JD did the mechanistic work. LP, SB, and CP provided statistical services to the study. All authors contributed to and reviewed the final report.
Conflicts of interest
PE and AC are inventors on a patent application that covers the protocol described in this study. The other authors declare that they have no conflicts of interest.
Supplementary Material
References
- 1.Venter C, Arshad H, Grundy J. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65:103–108. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer S, Munoz-Furlong A, Sampson H. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 3.Pumphrey R, Gowland H. Further fatal allergic reactions to food in the United Kingdom, 1999–2006. J Allergy Clin Immunol. 2007;119:1018–1019. doi: 10.1016/j.jaci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Ho M, Wong W, Heine R, Hosking C, Hill D, Allen K. Early clinical predictors of remission of peanut allergy in children. J Allergy Clin Immunol. 2008;121:731–736. doi: 10.1016/j.jaci.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Primeau M, Kagan R, Joseph L. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin Exp Allergy. 2000;30:1135–1143. doi: 10.1046/j.1365-2222.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 6.Avery N, King R, Knight S, Hourihane J. Assessment of quality of life in children with peanut allergy. Ped Allergy Immunol. 2003;14:378–382. doi: 10.1034/j.1399-3038.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor S, Roberts G, Bynoe Y, Gaughan M, Habibi P, Lack G. Influence of a multidisciplinary paediatric allergy clinic on parental knowledge and rate of subsequent allergic reactions. Allergy. 2004;59:185–191. doi: 10.1046/j.1398-9995.2003.00365.x. [DOI] [PubMed] [Google Scholar]
- 8.Ewan P, Clark A. Long-term prospective observational study of the outcome of a management plan in participants with peanut and nut allergy referred to a regional allergy centre. Lancet. 2001;357:111–115. doi: 10.1016/s0140-6736(00)03543-1. [DOI] [PubMed] [Google Scholar]
- 9.Sicherer S, Burks A, Sampson H. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics. 1998;102:e6. doi: 10.1542/peds.102.1.e6. [DOI] [PubMed] [Google Scholar]
- 10.Clark A, Ewan P. Good prognosis, clinical features and circumstances of peanut and tree nut reactions in children treated by a specialist allergy centre. J Allergy Clin Immunol. 2008;122:286–289. doi: 10.1016/j.jaci.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Durham S, Emminger W, Kapp A. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 12.Calderon M, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;24 doi: 10.1002/14651858.CD001936.pub2. CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle R, Elremeli M, Hockenhull J. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD008838.pub2. CD008838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson H, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–751. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 15.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–1269. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 16.Longo G, Barbi E, Berti I. Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol. 2008;121:343–347. doi: 10.1016/j.jaci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Clark A, Islam S, King Y, Deighton J, Anagnostou K, Ewan P. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009;64:1218–1220. doi: 10.1111/j.1398-9995.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 18.Anagnostou K, Clark A, King Y, Islam S, Deighton J, Ewan P. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin Exp Allergy. 2011;41:1273–1281. doi: 10.1111/j.1365-2222.2011.03699.x. [DOI] [PubMed] [Google Scholar]
- 19.Niggemann B. When is an oral food challenge positive? Allergy. 2010;65:2–6. doi: 10.1111/j.1398-9995.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- 20.Keil T, McBride D, Grimshaw K. The multinational birth cohort of EuroPrevall: background, aims and methods. Allergy. 2010;65:482–490. doi: 10.1111/j.1398-9995.2009.02171.x. [DOI] [PubMed] [Google Scholar]
- 21.Flokstra-de Blok B, DunnGalvin A, Vlieg-Boerstra B. Development and validation of a self-administered Food Allergy Quality of Life Questionnaire for children. Clin Exp Allergy. 2009;39:127–137. doi: 10.1111/j.1365-2222.2008.03120.x. [DOI] [PubMed] [Google Scholar]
- 22.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26:24–36. [Google Scholar]
- 23.Nurmatov U, Venderbosch I, Devereux G, Simons F, Sheikh A. Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Syst Rev. 2012;9 doi: 10.1002/14651858.CD009014.pub2. CD009014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnett J, Leftwich J, Muncer K. How do peanut and nut-allergic consumers use information on the packaging to avoid allergens? Allergy. 2011;66:969–978. doi: 10.1111/j.1398-9995.2011.02563.x. [DOI] [PubMed] [Google Scholar]
- 25.Rimbaud L, Heraud F, La Vieille S, Leblanc J, Crepet A. Quantitative risk assessment relating to adventitious presence of allergens in food: a probabilistic model applied to peanut in chocolate. Risk Analysis. 2010;30:7–19. doi: 10.1111/j.1539-6924.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- 26.Leitch I, Walker M, Davey R. Food allergy: gambling your life on a take-away meal. Int J Env Health Res. 2005;15:79–87. doi: 10.1080/09603120500062052. [DOI] [PubMed] [Google Scholar]
- 27.Factor J, Mendelson L, Lee J, Nouman G, Lester MR. Effect of oral immunotherapy to peanut on food-specific quality of life. Ann Allergy Asthma Immunol. 2012;109:348–352. doi: 10.1016/j.anai.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Blumchen K, Ulbricht H, Staden U. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 29.Jones S, Pons L, Roberts J. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.