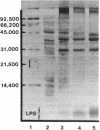
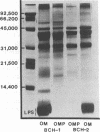
Abstract
Major outer membrane antigens, proteins, and lipopolysaccharides (LPSs), from nontypable Haemophilus influenzae were characterized and examined as targets for complement-dependent human bactericidal antibodies. Outer membranes from two nontypable H. influenzae isolates that caused otitis media and pneumonia (middle ear and transtracheal aspirates) were prepared by shearing organisms in EDTA. These membranes were compared with membranes prepared independently by spheroplasting and lysozyme treatment of whole cells and found to have: similar sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) patterns of the proteins; identical densities (rho = 1.22 g/cm3); and minimal d-lactose dehydrogenase activity indicating purity from cytoplasmic membranes. Outer membranes were solubilized in an LPS-disaggregating buffer and proteins were separated from LPS by molecular sieve chromatography. The SDS-PAGE patterns of outer membrane proteins (OMPs) from the two strains differed in the major band although other prominent bands appeared similar in molecular weight. LPS prepared by hot phenol water extraction of each of the strains contained 45% (pneumonia isolate) and 60% (otitis isolate) lipid (wt/wt), 49% and 50% carbohydrate (wt/wt), respectively, and less than 1%, 3-deoxy-manno octulosonic acid. Immunoglobulin M (IgM) purified from normal human serum (NHS) plus complement was bactericidal for both strains. Purified immunoglobulin G (IgG) from NHS killed the middle ear isolate and immune convalescent IgM from the serum of the patient with pneumonia killed his isolate. NHS or convalescent serum were absorbed with OMPs and LPS (0.6-110 micrograms) from each of the strains and immune specific inhibition of bactericidal antibody activity by each antigen was determined. OMPs from the pulmonary isolate inhibited bactericidal antibody activity directed against the isolate in both NHS (1.5 microgram of antigen) and immune serum (0.75 microgram of antigen). OMPs (60 micrograms) from the ear isolate also inhibited bactericidal activity in the respective immune serum. LPSs exhibited minimal inhibition (greater than 110 micrograms). Three human sera (two normal, one immune) were selectively depleted of 80% of antibody activity against OMPs (measured by enzyme-linked immunosorbent assay) by affinity chromatography using OMPs from the pulmonary isolate coupled to a solid phase. These OMP antibody-depleted sera also showed an 88% reduction of bactericidal activity against this strain. Immunopurified antibody against OMPs eluted from the solid phase was bactericidal.
Full text
PDF




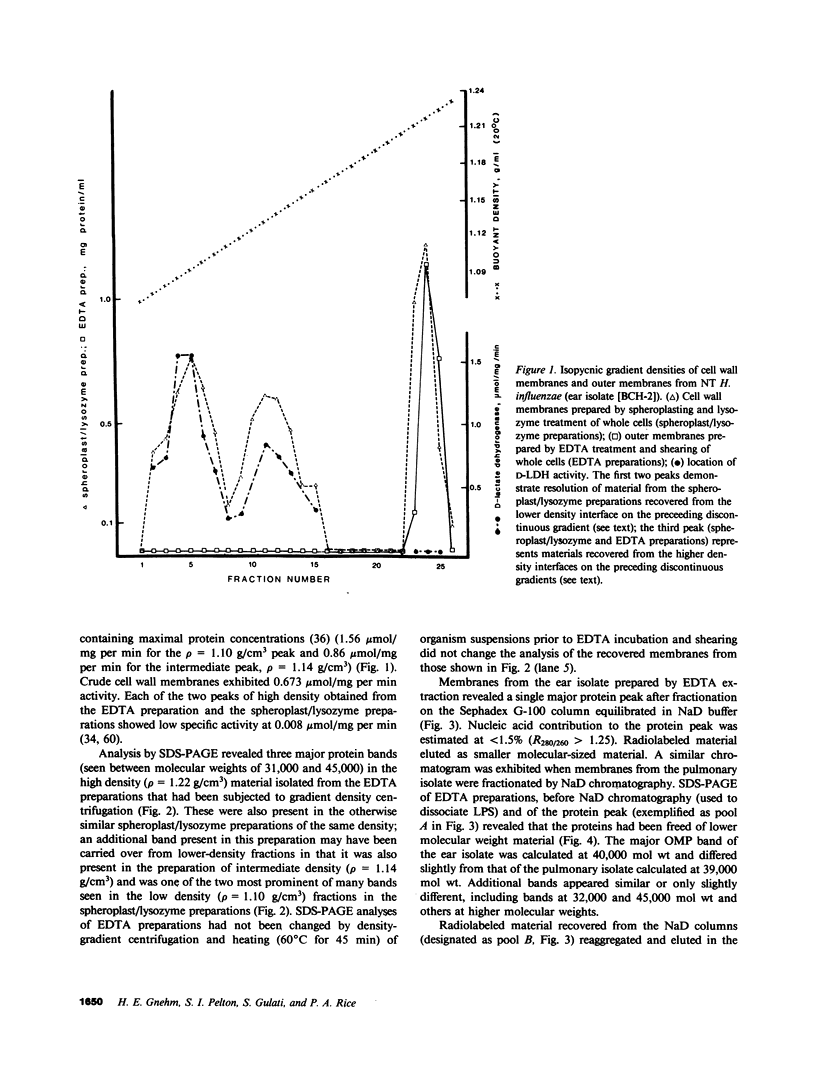
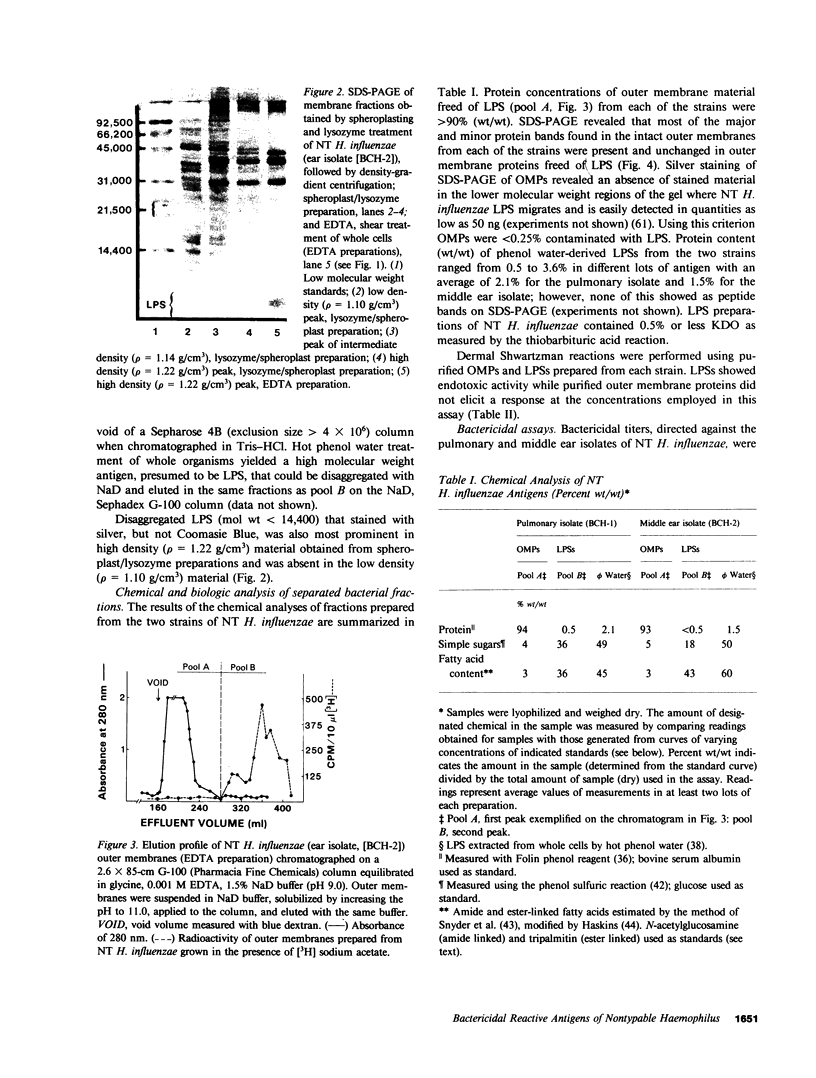
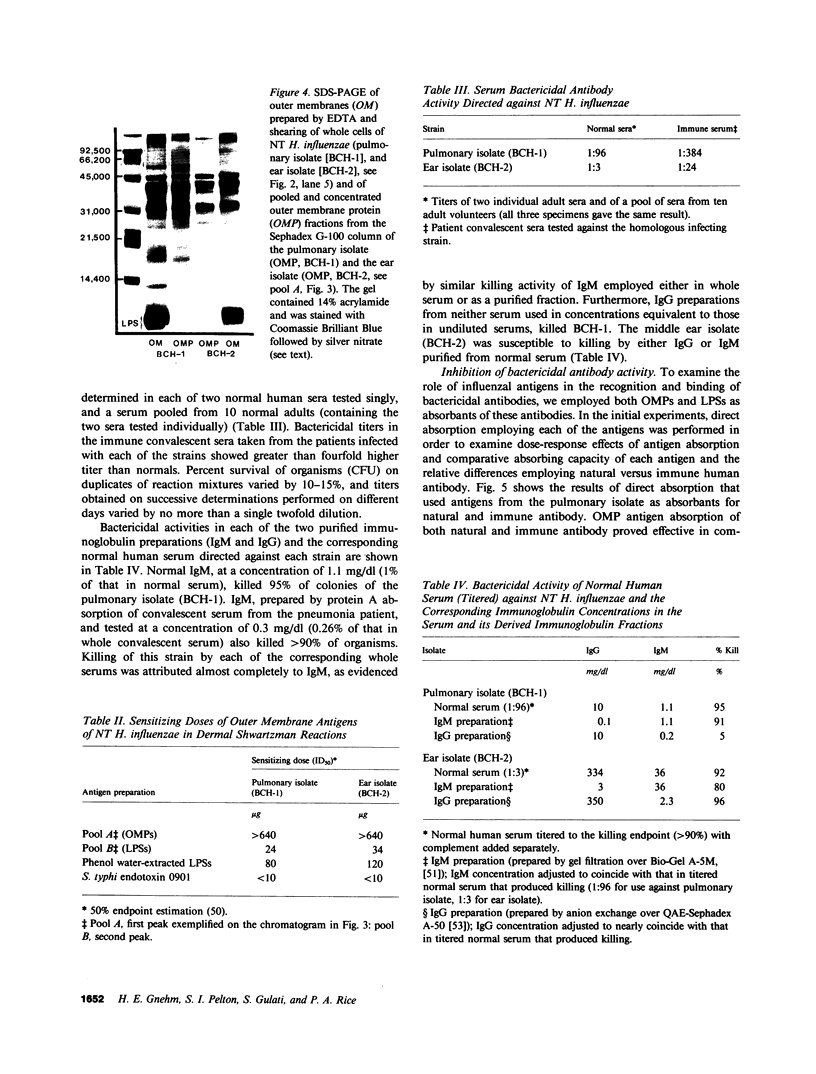
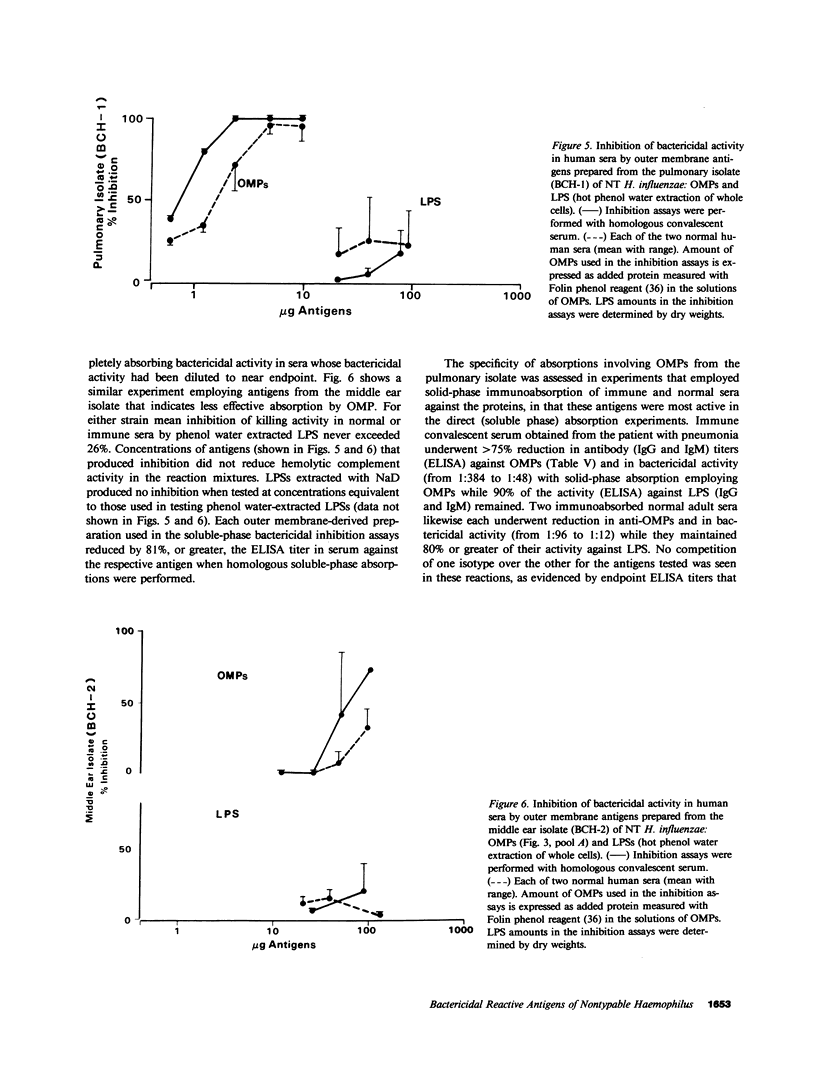





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Granoff D. M., Munson R. S., Jr Outer-membrane protein subtypes of Haemophilus influenzae type b and spread of disease in day-care centers. J Infect Dis. 1981 Sep;144(3):210–217. doi: 10.1093/infdis/144.3.210. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982 May;36(2):535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Berk S. L., Holtsclaw S. A., Wiener S. L., Smith J. K. Nontypeable Haemophilus influenzae in the elderly. Arch Intern Med. 1982 Mar;142(3):537–539. [PubMed] [Google Scholar]
- Branefors-Helander P. Serological studies of Haemophilus influenzae. IV. The antibody response in rabbits against capsular and O antigens of H. influenzae. Int Arch Allergy Appl Immunol. 1973;45(5):657–674. [PubMed] [Google Scholar]
- Branefors P., Dahlberg T. Serum bactericidal effect on capsulated and non-capsulated Haemophilus influenzae. Acta Pathol Microbiol Scand C. 1980 Feb;88(1):47–56. doi: 10.1111/j.1699-0463.1980.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Burans J. P., Lynn M., Solotorovsky M. Induction of active immunity with membrane fractions from Haemophilus influenzae type b. Infect Immun. 1983 Jul;41(1):285–293. doi: 10.1128/iai.41.1.285-293.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgin-Wolff A., Hernandez R., Just M. Separation of rubella IgM, IgA, and IgG antibodies by gel filtration on agarose. Lancet. 1971 Dec 11;2(7737):1278–1280. doi: 10.1016/s0140-6736(71)90600-3. [DOI] [PubMed] [Google Scholar]
- Dahlberg T., Branefors P. Characterization of the bactericidal antibody response against Haemophilus influenzae. Acta Pathol Microbiol Scand C. 1980 Jun;88(3):115–120. doi: 10.1111/j.1699-0463.1980.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner P. D., Shahidi A. Postpartum bacteremia due to Haemophilus influenzae. Report of two cases. Obstet Gynecol. 1969 Sep;34(3):403–405. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Evans F. O., Jr, Sydnor J. B., Moore W. E., Moore G. R., Manwaring J. L., Brill A. H., Jackson R. T., Hanna S., Skaar J. S., Holdeman L. V. Sinusitis of the maxillary antrum. N Engl J Med. 1975 Oct 9;293(15):735–739. doi: 10.1056/NEJM197510092931502. [DOI] [PubMed] [Google Scholar]
- Flesher A. R., Insel R. A. Characterization of lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1978 Dec;138(6):719–730. doi: 10.1093/infdis/138.6.719. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Bertram M. A. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J Infect Dis. 1977 Dec;136(6):733–739. doi: 10.1093/infdis/136.6.733. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump D. W., Christmas W. A., Forsyth B. R., Phillips C. A., Stouch W. H. Serum and secretory antibodies in patients with chronic bronchitis. Arch Intern Med. 1973 Dec;132(6):847–851. [PubMed] [Google Scholar]
- Gyorkey F., Musher D., Gyorkey P., Goree A., Baughn R. Nontypable Haemophilus influenzae are unencapsulated both in vivo and in vitro. J Infect Dis. 1984 Apr;149(4):518–522. doi: 10.1093/infdis/149.4.518. [DOI] [PubMed] [Google Scholar]
- HJORT P. F., RAPAPORT S. I. THE SHWARTZMAN REACTION: PATHOGENETIC MECHANISMS AND CLINICAL MANIFESTATIONS. Annu Rev Med. 1965;16:135–168. doi: 10.1146/annurev.me.16.020165.001031. [DOI] [PubMed] [Google Scholar]
- HOOK W. A., MUSCHEL L. H. ANTICOMPLEMENTARY EFFECTS AND COMPLEMENT ACTIVITY OF HUMAN SERA. Proc Soc Exp Biol Med. 1964 Oct;117:292–297. doi: 10.3181/00379727-117-29561. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Insel R. A., Anderson P., Loeb M. R., Smith D. H. A polysaccharide-protein complex from Haemophilus influenzae type b. II. Human antibodies to its somatic components. J Infect Dis. 1981 Dec;144(6):521–529. doi: 10.1093/infdis/144.6.521. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Seiler M. W. Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1975 Oct;132(4):440–450. doi: 10.1093/infdis/132.4.440. [DOI] [PubMed] [Google Scholar]
- Klein J. O. Microbiology and antimicrobial treatment of otitis media. Ann Otol Rhinol Laryngol Suppl. 1981 May-Jun;90(3 Pt 3):30–36. doi: 10.1177/00034894810903s209. [DOI] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Sarvas M. Immunochemical characterization of major outer membrane components from Salmonella typhimurium. Infect Immun. 1981 Sep;33(3):750–757. doi: 10.1128/iai.33.3.750-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Zachary A. L., Smith D. H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981 Jan;145(1):596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. R. Haemophilus influenzae antigen-antibody reactions. J Pathol Bacteriol. 1965 Oct;90(2):379–392. doi: 10.1002/path.1700900204. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mintz C. S., Apicella M. A., Morse S. A. Electrophoretic and serological characterization of the lipopolysaccharide produced by Neisseria gonorrhoeae. J Infect Dis. 1984 Apr;149(4):544–552. doi: 10.1093/infdis/149.4.544. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Dudas K. C., Mylotte J. M., Apicella M. A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983 May;147(5):838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Hague-Park M., Baughn R. E., Wallace R. J., Jr, Cowley B. Opsonizing and bactericidal effects of normal human serum on nontypable Haemophilus influenzae. Infect Immun. 1983 Jan;39(1):297–304. doi: 10.1128/iai.39.1.297-304.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Norden C. W., Demchak T. A. Significance of noncapsular antigens in protection against experimental Haemophilus influenzae type b disease: cross-reactivity. Infect Immun. 1978 Aug;21(2):619–626. doi: 10.1128/iai.21.2.619-626.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Raichvarg D., Brossard C., Agneray J. Chemical composition and biological activities of a phenol-water extract from Haemophilus influenzae type a. Infect Immun. 1979 Nov;26(2):415–421. doi: 10.1128/iai.26.2.415-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichvarg D., Brossard C., Agneray J. Preparation of a nontoxic and immunogenic polysaccharide fraction from a Haemophilus influenzae phenol-water extract. Infect Immun. 1980 Jul;29(1):171–174. doi: 10.1128/iai.29.1.171-174.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brown R., Haskins W. T., Malmgren B., Milner K. C., Rudbach J. A. Reaction of endotoxin and surfactants. I. Physical and biological properties of endotoxin treated with sodium deoxycholate. J Bacteriol. 1966 Nov;92(5):1493–1509. doi: 10.1128/jb.92.5.1493-1509.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. 1982 Jul;70(1):157–167. doi: 10.1172/JCI110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Wollenweber H. W., Russa R., Brade H., Zähringer U. Concepts of the chemical structure of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):432–438. doi: 10.1093/clinids/6.4.432. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Schoolnik G. K., Ochs H. D., Buchanan T. M. Immunoglobulin class responsible for gonococcal bactericidal activity of normal human sera. J Immunol. 1979 May;122(5):1771–1779. [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Granoff D. M. Human antibody responses to lipopolysaccharide after meningitis due to Haemophilus influenzae type b. J Infect Dis. 1982 Feb;145(2):181–190. doi: 10.1093/infdis/145.2.181. [DOI] [PubMed] [Google Scholar]
- Shurin P. A., Pelton S. I., Tager I. B., Kasper D. L. Bactericidal antibody and susceptibility to otitis media caused by nontypable strains of Haemophilus influenzae. J Pediatr. 1980 Sep;97(3):364–369. doi: 10.1016/s0022-3476(80)80182-x. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Southwick F. S., Moellering R. C., Jr, Sherman E. Hemophilus influenzae in hospitalized adults: current perspectives. Am J Med. 1980 Aug;69(2):219–226. doi: 10.1016/0002-9343(80)90381-2. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Golden C. A., Kanner R. E., Renzetti A. D. Haemophilus influenzae and haemophilus parainfluenzae in chronic obstructive pulmonary disease. Lancet. 1976 Jun 12;1(7972):1253–1255. doi: 10.1016/s0140-6736(76)91733-5. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. On the nature of nontypable Haemophilus influenzae. Antonie Van Leeuwenhoek. 1978;44(3-4):367–376. doi: 10.1007/BF00394313. [DOI] [PubMed] [Google Scholar]
- Sweet R. L., Mills J., Hadley K. W., Blumenstock E., Schachter J., Robbie M. O., Draper D. L. Use of laparoscopy to determine the microbiologic etiology of acute salpingitis. Am J Obstet Gynecol. 1979 May 1;134(1):68–74. doi: 10.1016/0002-9378(79)90798-1. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Artenstein M. S. Cross-reactivity of Neisseria gonorrhoeae and Neisseria meningitidis and the nature of antigens involved in the bactericidal reaction. J Infect Dis. 1974 Sep;130(3):240–247. doi: 10.1093/infdis/130.3.240. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Verghese A., Berk S. L. Bacterial pneumonia in the elderly. Medicine (Baltimore) 1983 Sep;62(5):271–285. doi: 10.1097/00005792-198309000-00002. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Wald E. R., Milmoe G. J., Bowen A., Ledesma-Medina J., Salamon N., Bluestone C. D. Acute maxillary sinusitis in children. N Engl J Med. 1981 Mar 26;304(13):749–754. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Baker C. J., Quinones F. J., Hollis D. G., Weaver R. E., Wiss K. Nontypable Haemophilus influenzae (biotype 4) as a neonatal, maternal, and genital pathogen. Rev Infect Dis. 1983 Jan-Feb;5(1):123–136. doi: 10.1093/clinids/5.1.123. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musher D. M., Septimus E. J., McGowan J. E., Jr, Quinones F. J., Wiss K., Vance P. H., Trier P. A. Haemophilus influenzae infections in adults: characterization of strains by serotypes, biotypes, and beta-lactamase production. J Infect Dis. 1981 Aug;144(2):101–106. doi: 10.1093/infdis/144.2.101. [DOI] [PubMed] [Google Scholar]
- Ward J. I., Siber G. R., Scheifele D. W., Smith D. H. Rapid diagnosis of Hemophilus influenzae type b infections by latex particle agglutination and counterimmunoelectrophoresis. J Pediatr. 1978 Jul;93(1):37–42. doi: 10.1016/s0022-3476(78)80596-4. [DOI] [PubMed] [Google Scholar]
- Winkelhake J. L., Kasper D. L. Affinity chromatography of anti-meningococcal antiserum. J Immunol. 1972 Oct;109(4):824–833. [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Scocca J. J. Constitution of the cell envelope of Haemophilus influenzae in relation to competence for genetic transformation. J Bacteriol. 1975 Aug;123(2):666–677. doi: 10.1128/jb.123.2.666-677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]