Abstract
OBJECTIVES:
To evaluate the preliminary results obtained using diffusion-weighted magnetic resonance imaging and the apparent diffusion coefficient for planning computed tomography-guided biopsies of selected mediastinal lesions.
METHODS:
Eight patients with mediastinal lesions suspicious for malignancy were referred for computed tomography-guided biopsy. Diffusion-weighted magnetic resonance imaging and apparent diffusion coefficient measurement were performed to assist in biopsy planning with diffusion/computed tomography fused images. We selected mediastinal lesions that could provide discordant diagnoses depending on the biopsy site, including large heterogeneous masses, lesions associated with lung atelectasis or consolidation, lesions involving large mediastinal vessels and lesions for which the results of biopsy using other methods and histopathological examination were divergent from the clinical and radiological suspicion.
RESULTS:
In all cases, the biopsy needle was successfully directed to areas of higher signal intensity on diffusion-weighted sequences and the lowest apparent diffusion coefficient within the lesion (mean, 0.8 [range, 0.6–1.1]×10-3 mm2/s), suggesting high cellularity. All biopsies provided adequate material for specific histopathological diagnoses of four lymphomas, two sarcomas and two thymomas.
CONCLUSION:
Functional imaging tools, such as diffusion-weighted imaging and the apparent diffusion coefficient, are promising for implementation in noninvasive and imaging-guided procedures. However, additional studies are needed to confirm that mediastinal biopsy can be improved with these techniques.
Keywords: MRI-Guided Biopsy, DWI-Guided Biopsy, Mediastinal Lesion, Diagnosis, Malignancy, Tomography
INTRODUCTION
Imaging evaluation of mediastinal tumors is essential for accurately assessing their location, extent and anatomical relationships with adjacent structures. However, imaging findings alone cannot be used to accurately determine the etiology of mediastinal lesions and specific diagnosis is critical for proper treatment planning and prognosis. Excellent diagnostic accuracy and a low complication rate have established computed tomography (CT)-guided percutaneous needle biopsy as an efficient and safe tool for the specific diagnosis of thoracic lesions 1.
Because needle biopsy supplies only small tumor fragments, selection of the appropriate location within the lesion is necessary to provide adequate material that represents the actual behavior of the tumor for analysis 2. This process can be difficult, especially for large heterogeneous lesions or those with cystic or necrotic areas. Functional and metabolic methods have been used to identify regions within the lesion that truly represent its aggressiveness for therapeutic purposes. Contrast-enhanced CT and positron emission tomography (PET)/CT with 18-fluorodeoxyglucose are examples of modalities that are routinely used for this purpose 3–5.
The value of magnetic resonance (MR) imaging in mediastinal tumor evaluation, especially in the assessment of vascular and chest wall invasion, has long been considered to be well established 6. However, recent MR advances, such as diffusion-weighted (DW) imaging and the apparent diffusion coefficient (ADC), have made this method useful for staging, therapeutic planning and response evaluation, thereby improving approaches for the diagnosis and treatment of patients with mediastinal lesions 7–10. DW techniques are related to the diffusion of water inside a voxel (tridimensional pixel) that is contained within the cell membrane boundaries and greater cellularity thus results in greater diffusion restriction in tumors and other tissues. The DW approach was apparently created initially for examination of the central nervous system 11,12; in this context, it has been used not only for the detection of acute ischemia but also for the characterization and differentiation of brain tumors and intracranial infections, such as abscesses. DW imaging is based on the random Brownian motion of water molecules and the extent of tissue cellularity and intactness of the cell membrane help to determine the extent to which water molecule diffusion is impeded, which can be assessed quantitatively using the ADC.
Advanced oncological treatment is based on targeted therapy, molecular profiling and translational research and DW MR imaging with the ADC can provide functional information about tissue cellularity and the integrity of cellular membranes. Investigators have recently demonstrated the diagnostic potential of DW MR images and ADC in oncology 13–16 and several studies have shown that these sequences are useful in the differential diagnosis of benign and malignant lesions, including mediastinal lesions 17,18. Thus, MR imaging can also assist in determining the most appropriate site for biopsy within a mediastinal lesion. This application is very important in patients with cancer, especially those with severe disease, as the avoidance of inappropriate material collection leading to the need for a new biopsy is extremely desirable in these patients.
Thus, the purpose of our study was to evaluate the utility of DW MR imaging and the ADC in planning transthoracic CT-guided biopsies of mediastinal lesions suspicious for malignancy.
PATIENTS AND METHODS
In this prospective study, eight patients with mediastinal lesions that were suspicious for malignancy based on clinical and imaging findings and who were referred for initial transthoracic CT-guided biopsy at our institution between February 2011 and September 2012 were evaluated. All of these patients required further evaluation by MR imaging, as determined by the following criteria: 1) the presence of multiple or large heterogeneous masses, 2) the association of lesions with lung atelectasis or consolidation in the adjacent lung parenchyma, 3) invasion of the chest wall or mediastinal structures, 4) large vessel involvement and/or 5) biopsy results obtained via another method that diverged from the clinical and radiological suspicion. None of the patients had previously undergone CT-guided biopsy that yielded inconclusive results. Screening coagulation tests were ordered routinely and all patients underwent chest MR imaging 1 h prior to biopsy to assist in procedure planning. The institutional review board of AC Camargo Cancer Center approved this study and all patients provided written informed consent. This investigation was conducted according to the principles expressed in the Declaration of Helsinki.
MR imaging was performed with the patients in the supine position using a 1.5-T unit (Signa HDxt; General Electric Medical Systems, Milwaukee, WI, USA) with a body phased-array coil. Before DW imaging, axial T1-weighted and T2-weighted fast spin-echo images (repetition time/echo time: 6.3/2.7 ms and 3200/40 ms, respectively) with fat signal suppression, 6-mm slice thickness, a 30-cm field of view and a 256 acquisition matrix were obtained.
The DW imaging was performed using a multislice, single-shot, spin-echo and echo-planar imaging sequence in the transverse plane. DW images were acquired with no gated sequence on the basis of prior T1- and T2-weighted images; this sequence was performed only in the sections in which the pulmonary lesion was located during breath holding. Pairs of motion-probing gradients were applied before and after the 180° radiofrequency pulse of the spin-echo T2-weighted sequence in three orthogonal directions (X, Y and Z). The DW images were obtained at diffusion factor (b) values of 0 and 600 s/mm2.
ADC maps were automatically reconstructed for all DW images and ADCs were measured after defining regions of interest. Regions within the lesions with the lowest ADC values were identified for CT-guided biopsy.
Post-contrast T1-weighted imaging was performed using the same (pre-contrast) parameters and gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ, USA) paramagnetic contrast. A 20-ml dose was administered at a rate of 2 ml/s using a power injector.
For each patient, panoramic acquisition of a scout image was performed with 5–10-mm cuts for biopsy planning purposes. Then, the DW and CT images were fused using AquariusNET software (TeraRecon, Foster City, CA, USA) to obtain the corresponding plans. All image fusion procedures were very accurate, with millimetric adjustments made by overlapping of images in the same plane and in the same cut, considering at least three points of common anatomical reference. An approach enabling CT-guided direction of the needle tip to the region of greatest cellularity, as identified by MR imaging performed 1 h prior to the procedure, was chosen.
All biopsies were performed with a helical CT unit (HiSpeed; General Electric Medical Systems) using the cutting needle biopsy technique and an automated 20-gauge coaxial system (Angiotech, Vancouver, Canada). The needles used were either 10 cm or 15 cm in length, depending on the distance between the skin and the lesion. Local anesthesia was administered routinely and sedation was not necessary in any case. At least five specimens were collected from the single needle location chosen under DW image and ADC guidance in each lesion. The biopsy specimens were subjected to histological evaluation for specific diagnosis.
RESULTS
Five patients in the study sample were male and three were female; the mean age was 44 (range, 19–63) years. Table 1 summarizes the patients' demographic characteristics and the radiological and histological characteristics of the lesions.
Table 1.
-Characteristics of patients with mediastinal lesions who underwent transthoracic biopsy based on magnetic resonance imaging findings.
| Age (years) | Gender | Mediastinal location | Indication | Size (mm) | ADC (×10-3 mm2/s) | Histopathological diagnosis |
| 22 | Male | Anterior | L, H, CI, MI, VI | 170 | 1.1 | Classical Hodgkin lymphoma, nodular sclerosis |
| 62 | Female | Right posterior | L, H | 98 | 0.89 | Pleomorphic leiomyosarcoma |
| 63 | Male | Anterior | L, H, MI, VI | 90 | 0.77 | B3 thymoma |
| 35 | Male | Superior | L, H, MI, VI | 150 | 0.61 | Gray zone lymphoma |
| 32 | Male | Superior | L, H, MI, VI | 83 | 0.65 | High-grade peripheral nerve sheath sarcoma |
| 19 | Female | Anterior | L, H, CI, MI, VI | 120 | 0.72 | Non-Hodgkin lymphoma |
| 58 | Male | Anterior | L, H, CI, MI | 78 | 0.81 | B1 thymoma |
| 61 | Female | Anterior | L, H, CI, MI, VI | 92 | 0.91 | Non-Hodgkin lymphoma |
ADC: apparent diffusion coefficient; L: large; H: heterogeneous; CI: chest infiltration; MI: mediastinal infiltration; VI: vascular involvement.
All mediastinal biopsies provided adequate material, enabling specific diagnoses: four cases of lymphoma (one classical Hodgkin, one gray zone and two classical non-Hodgkin lymphomas), two cases of sarcoma (one pleomorphic and one high-grade peripheral nerve sheath sarcoma) and two cases of thymoma (one B1 thymoma and one B3 thymoma). No major complications were reported after the procedure.
All the biopsied areas showed higher signal intensity on DW sequences and correspondingly lower ADCs (mean, 0.8 [range, 0.6–1.1]×10-3 mm2/s) compared with other areas within the lesion, suggesting high cellularity (Figures 1 and 2).
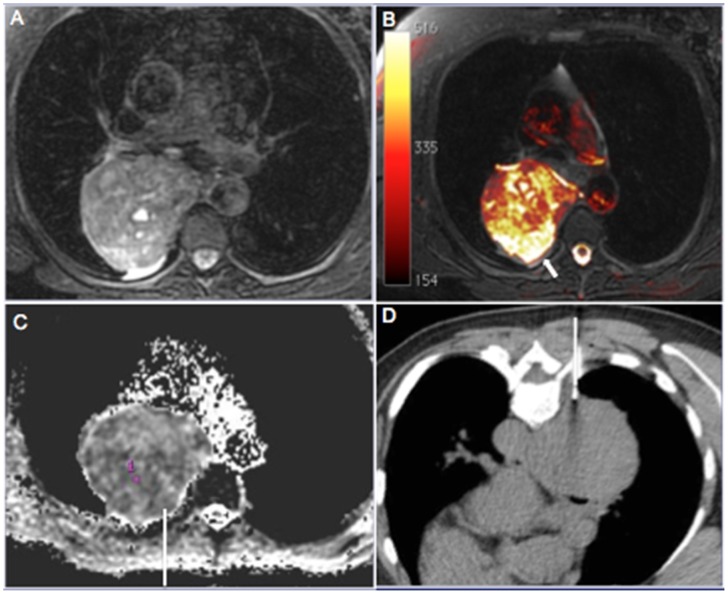
Figure 1.
A 62-year-old woman who presented with a heterogeneous mass in the right posterior mediastinum. The entire mass showed heterogeneous signal hyperintensity on T2-weighted magnetic resonance images (a) and fused T1- and diffusion-weighted images. (b) Note the remarkable signal hyperintensity in the posterior periphery of the lesion (arrow) compared with other areas, with definitive signs of restriction on the apparent diffusion coefficient (ADC) map. (c) The ADC value in the target area was 0.89×10-3 mm2/s. The needle was directed to this area during computed tomography–guided biopsy. (d) Histopathological analysis yielded a diagnosis of pleomorphic leiomyosarcoma.
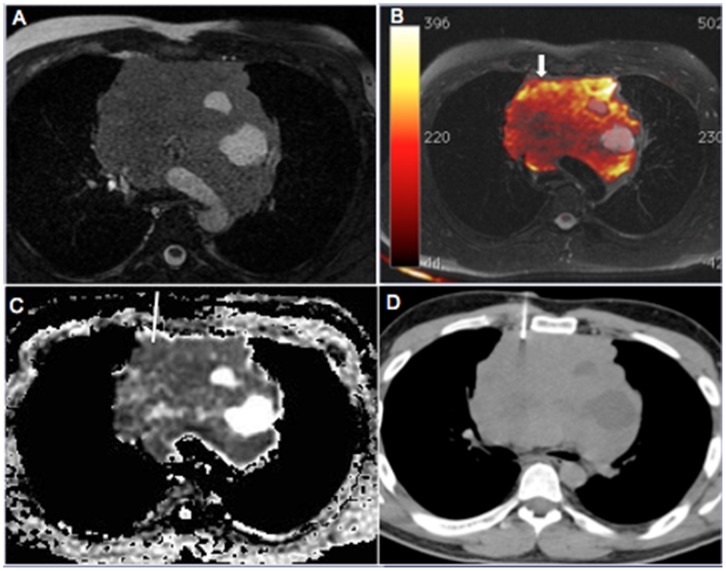
Figure 2.
A 35-year-old man with a large mass in the anterior mediastinum. The mass showed heterogeneous signal intensity on T2-weighted images (a) and fused T1- and diffusion-weighted images. (b) Note the circumscribed areas of signal hyperintensity suggestive of cysts but also the remarkable signal hyperintensity (arrow) away from the cystic areas near the right anterior lesion margin, with definitive signs of restriction on the apparent diffusion coefficient (ADC) map. (c) The ADC value in the target area was 0.61×10-3 mm2/s. This mass was subjected to computed tomography–guided biopsy (d), and histopathological examination yielded a diagnosis of gray zone lymphoma.
DISCUSSION
Contemporary oncological treatment is based on targeted therapy and molecular profiling. Within this context, the ability of the interventional radiologist to obtain tissue specimens that represent tumor aggressiveness has become increasingly important 19. Indeed, inadequate biopsies can cause misdiagnosis and delay appropriate treatment. Functional imaging methods are essential for effective image-guided percutaneous biopsy and obviate the need for more invasive procedures for proper diagnosis and therapeutic planning 20,21.
The aim of MR image planning is to avoid re-biopsy and the risks of invasive diagnostic procedures, such as video-assisted thoracoscopic and open surgical biopsies. The great advantage of imaging-guided procedures is that they improves diagnostic yield and are associated with a low morbidity. Our initial results demonstrated that DW imaging and the ADC are helpful in determining the most appropriate biopsy site, thus improving pre-procedural planning for transthoracic mediastinal biopsy with no major complications.
DW imaging is a noninvasive modality that can be used to differentiate malignancies from benign mediastinal tumors; it also provides useful information for the grading of mediastinal malignancies 12,13. Images obtained by DW sequences differ from those acquired by traditional MR imaging. DW imaging essentially depends on the random movement (Brownian motion) of water protons through biological tissue 19, which causes phasic dispersion of the spins, resulting in signal loss in the diffusion-sensitive sequence. This sequence enables qualitative analysis of the diffusion of water molecules through tissues via the interpretation of the signal strength in the studied region. Quantitative analysis is possible through ADC calculation and the assignment of absolute values describing the signal intensity in the studied region 20. These qualitative and quantitative analyses are mainly influenced by the presence of barriers that restrict the diffusion of water molecules in their microenvironment, producing different contrast levels in different tissues. The movement of water molecules in the intracellular, extracellular and intravascular spaces ultimately reflects the barrier integrity in these structures. The best known example of a diffusion barrier to water molecules is the cell membrane; other examples of barriers include cell structural components and cellular and tissue connections, such as the cytoskeleton, macromolecules, organelles and tight junctions. Consequently, different tissues display unique signal intensity levels and ADCs depending on their particular structures 19,20. A malignancy typically has a high cell density and reduced extracellular space, both of which decrease the diffusion of water and result in a higher DW signal intensity and a lower ADC value. In the present study, areas of hyperintensity on DW images with lower ADC values were distinct from other areas within all the lesions.
MR imaging has several advantages over other imaging techniques for biopsy planning. First, MR imaging provides unparalleled soft-tissue contrast and exquisite anatomical detail, enabling the identification of target lesions that cannot be viewed on ultrasound or CT and the detailed visualization of the anatomy surrounding the target. This level of detail aids in preventing collateral damage to delicate structures that are not typically visible on images produced using other modalities. Additionally, MR imaging provides the unique ability to elucidate different tissue characteristics during a procedure through the use of different pulse sequences, ranging from simple T1 or T2 weighting to more advanced functions such as flow, perfusion and diffusion 18,22,23.
Hoffmann et al. 23 reported on the accuracy, duration and factors influencing MR-guided liver or soft-tissue biopsy. The major disadvantages of this procedure are the cost and time requirement. Despite these limitations, MR imaging should be considered and its use may be justified in complex cases, including large, heterogeneous and mixed-pattern lesions, because the failure to collect adequate material and the resulting need for repeat biopsy increase the risks and costs of the procedure. MR imaging can also be used to avoid the risks of ionizing radiation in health professionals and patients undergoing percutaneous procedures 24. Moreover, given the high success rate of cancer treatment in children, these patients are followed for a long time using imaging methods that expose them to ionizing radiation, increasing the risk of neoplastic development 25. Thus, MR imaging is an alternative method that can be used to follow these patients, reducing the risk of second primary tumor development induced by exposure to radiation from diagnostic methods. MR imaging can also be advantageous in the evaluation of patients with adverse reactions to intravenous contrast or impaired renal function 26.
MR/CT image fusion can be performed readily using commercially available software designed for this purpose 3–5. This methodology has been used with promising results for lesions identified by PET/CT 3,4. Tatli et al. 3 recently described the use of specialized software to fuse previously acquired PET/CT images with CT images acquired at the time of biopsy.
Some limitations of this study should be considered. The small number of cases and the lack of a control arm prevented us from definitively concluding that MR imaging made a significant difference in our patients' biopsies and no patient underwent control MR examination post-biopsy. Additionally, a wide and distinct range of ADC values was identified among the patients, impairing the establishment of an ADC cutoff value; thus, each lesion was evaluated separately. However, to our knowledge, this preliminary study is the first to demonstrate the feasibility of using DW MR imaging and the ADC for mediastinal procedure planning. Additional studies, including those comparing the results of MR- and CT-guided biopsies, are necessary to further evaluate the impact of this technique on the diagnostic yield of transthoracic mediastinal biopsy, particularly in patients with large lesions and/or severe illnesses.
In conclusion, MR imaging has several advantages in clinical and oncological practice. Functional imaging tools, such as DW imaging and the ADC, are promising for implementation in noninvasive and imaging-guided procedures. However, additional studies are needed to confirm whether mediastinal biopsy can be improved with these techniques.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Lal H, Neyaz Z, Nath A, Borah S. CT-guided percutaneous biopsy of intrathoracic lesions. Korean J Radiol. 2012;13(2):210–26. doi: 10.3348/kjr.2012.13.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guimaraes MD, de Andrade MQ, da Fonte AC, Chojniak R, Gross JL. CT-guided cutting needle biopsy of lung lesions - an effective procedure for adequate material and specific diagnose. Eur J Radiol. 2011;80(3):e488–90. doi: 10.1016/j.ejrad.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 3.O′Sullivan PJ, Rohren EM, Madewell JE. Positron emission tomography-CT imaging in guiding musculoskeletal biopsy. Radiol Clin North Am. 2008;46(3):475–86. doi: 10.1016/j.rcl.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Tatli S, Gerbaudo VH, Feeley CM, Shyn PB, Tuncali K, Silverman SG. PET/CT-guided percutaneous biopsy of abdominal masses: initial experience. J Vasc Interv Radiol. 2011;22(4):507–14. doi: 10.1016/j.jvir.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Bitencourt AG, Tyng CJ, Pinto PN, Almeida MFA, Meyrelles LC, Pinheiro RP, et al. Percutaneous biopsy based on PET/CT findings in cancer patients: technique, indications, and results. Clin Nucl Med. 2012;37(5):e95–7. doi: 10.1097/RLU.0b013e3182443b78. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Al-Janabi NJ. Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging. 2010;32(6):1325–39. doi: 10.1002/jmri.22377. [DOI] [PubMed] [Google Scholar]
- 7.Sinkus R, Van Beers BE, Vilgrain V, DeSouza N, Waterton JC. Apparent diffusion coefficient from magnetic resonance imaging as a biomarker in oncology drug development. Eur J Cancer. 2012;48(4):425–31. doi: 10.1016/j.ejca.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Colagrande S, Carbone SF, Carusi LM, Cova M, Villari N. Magnetic resonance diffusion-weighted imaging: extraneurological applications. Radiol Med. 2006;111(3):392–419. doi: 10.1007/s11547-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 9.Türkbey B, Aras O, Karabulut N, Turgut AT, Akpinar E, Alibek S, et al. Diffusion-weighted MRI for detecting and monitoring cancer: a review of current applications in body imaging. Diagn Interv Radiol. 2012;18(1):46–59. doi: 10.4261/1305-3825.DIR.4708-11.2. [DOI] [PubMed] [Google Scholar]
- 10.Gümüştaş S, Inan N, Sarisoy HT, Anik Y, Arslan A, Ciftçi E, et al. Malignant versus benign mediastinal lesions: quantitative assessment with diffusion weighted MR imaging. Eur Radiol. 2011;21(11):2255–60. doi: 10.1007/s00330-011-2180-9. [DOI] [PubMed] [Google Scholar]
- 11.Rana S, Albayram S, Lin DD, Yousem DM. Diffusion-weighted imaging and apparent diffusion coefficient maps in a case of intracerebral abscess with ventricular extension. AJNR Am J Neuroradiol. 2002;23(1):109–12. [PMC free article] [PubMed] [Google Scholar]
- 12.Sener RN. Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph. 2001;25(4):299–326. doi: 10.1016/s0895-6111(00)00083-5. [DOI] [PubMed] [Google Scholar]
- 13.Tondo F, Saponaro A, Stecco A, Lombardi M, Casadio C, Carriero A. Role of diffusion-weighted imaging in the differential diagnosis of benign and malignant lesions of the chest-mediastinum. Radiol Med. 2011;116(5):720–33. doi: 10.1007/s11547-011-0629-1. [DOI] [PubMed] [Google Scholar]
- 14.Hochhegger B, Marchiori E, Irion K, Moreira J, Zanetti G. MRI in assessment of lung cancer. Thorax. 2011;66(4):357. doi: 10.1136/thx.2011.159111. [DOI] [PubMed] [Google Scholar]
- 15.Koşucu P, Tekinbaş C, Erol M, Sari A, Kavgaci H, Oztuna F, et al. Mediastinal lymph nodes: assessment with diffusion-weighted MR imaging. J Magn Reson Imaging. 2009;30(2):292–7. doi: 10.1002/jmri.21850. [DOI] [PubMed] [Google Scholar]
- 16.Sadohara J, Fujimoto K, Müller NL, Kato S, Takamori S, Ohkuma K, et al. Thymic epithelial tumors: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol. 2006;60(1):70–9. doi: 10.1016/j.ejrad.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Sun YS, Cui Y, Tang L, Qi LP, Wang N, Zhang XY, et al. Early evaluation of cancer response by a new functional biomarker: apparent diffusion coefficient. AJR Am J Roentgenol. 2011;197(1):W23–9. doi: 10.2214/AJR.10.4912. [DOI] [PubMed] [Google Scholar]
- 18.Sieren JC, Ohno Y, Koyama H, Sugimura K, McLennan G. Recent technological and application developments in computed tomography and magnetic resonance imaging for improved pulmonary nodule detection and lung cancer staging. J Magn Reson Imaging. 2010;32(6):1353–69. doi: 10.1002/jmri.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–25. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padhani AR. Diffusion magnetic resonance imaging in cancer patients management. Semin Radiat Oncol. 2011;21(2):119–40. doi: 10.1016/j.semradonc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Guimaraes MD, Marchiori E, Hochhegger B, Chojniak R, Gross JL. CT-guided biopsy of lung lesions: defining the best needle option for a specific diagnosis. Clinics. 2014;69(5):335–40. doi: 10.6061/clinics/2014(05)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss CR, Nour SG, Lewin JS. MR-guided biopsy: a review of current techniques and applications. J Magn Reson Imaging. 2008;27(2):311–25. doi: 10.1002/jmri.21270. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann R, Thomas C, Rempp H, Schmidt D, Pereira PL, Claussen CD, et al. Performing MR-guided biopsies in clinical routine: factors that influence accuracy and procedure time. Eur Radiol. 2011;22(3):663–71. doi: 10.1007/s00330-011-2297-x. [DOI] [PubMed] [Google Scholar]
- 24.Guimarães MD, Gross JL, Chojniak R, Marchiori E. MRI-guided biopsy: a valuable procedure alternative do avoid the risks of ionizing radiation from diagnostic imaging methods. Cardiovasc Intervent Radiol. 2014;37(3):858–60. doi: 10.1007/s00270-013-0677-0. [DOI] [PubMed] [Google Scholar]
- 25.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amet S, Deray G. Renal toxicity of contrast agents in oncologic patients. Bull Cancer. 2012;99(3):295–307. doi: 10.1684/bdc.2011.1477. [DOI] [PubMed] [Google Scholar]