Abstract
Context:
Osteocyte activity is crucial to the maintenance of bone quality. Sclerostin, an osteocyte product, inhibits bone formation, yet higher circulating sclerostin is associated with higher bone density. Bone marrow fat (MF) is associated with osteoporosis, but little is known about the relationship between osteocyte activity and MF.
Objective:
Our objective was to assess the relationships between circulating sclerostin, vertebral MF, volumetric bone mineral density (vBMD), and other fat depots in older adults.
Design, Setting, and Participants:
We conducted a cross-sectional study in the Age Gene/Environment Susceptibility-Reykjavik cohort.
Main Outcome Measures:
Outcome measures included vertebral MF (L1-L4) measured with magnetic resonance spectroscopy and vBMD (spine and hip) and abdominal fat measured with quantitative computed tomography.
Results:
After excluding subjects with bone-active medication use (n = 50), inadequate serum (n = 2), or inadequate magnetic resonance spectroscopy (n = 1), analyses included 115 men and 134 women (mean age 79 y, mean body mass index 27.7 kg/m2). In men, but not women, vertebral MF was greater in those with higher serum sclerostin levels. MF was 52.2 % in the lowest tertile of serum sclerostin and 56.3% in the highest tertile in men (P for trend <.01) in models adjusted for age, body mass index, and diabetes. Sclerostin was positively associated with cortical and trabecular total hip vBMD, weight in men and women, and total fat mass in men but was not associated with total lean mass or abdominal fat depots.
Conclusion:
Circulating sclerostin levels are associated with higher vertebral marrow fat in men, suggesting a relationship between osteocyte function and marrow adipogenesis.
Osteoporosis, characterized by low bone mineral density (BMD), poor bone quality, and increased fracture risk, is a significant public health concern in older adults. In the United States, the lifetime risk of a major osteoporotic fracture at age 50 years is approximately 40% for white women and 13% for men (1). Osteocytes, cells within the mineralized matrix that are crucial for bone remodeling, play an important role in osteoporosis, but our ability to measure osteocyte activity is currently limited. Assays for circulating sclerostin have recently become available, providing an indirect assessment of osteocyte function. Sclerostin is a glycoprotein expressed by osteocytes as an inhibitor of the Wnt/B-catenin pathway and a negative regulator of bone formation (2).
Surprisingly, studies have demonstrated positive correlations between circulating sclerostin levels and areal BMD (aBMD) measured by dual-energy X-ray absorptiometry (DXA) (3–10). One proposed explanation is that circulating sclerostin levels depend on the number of osteocytes, in theory proportional to the total amount of bone, in addition to their activity (5). In the studies that have reported results for volumetric BMD (vBMD), there also appears to be a positive relationship between circulating sclerostin levels and vBMD at some, but not all, skeletal sites (4, 11, 12). In older women, higher sclerostin levels were associated with greater vBMD, cross-sectional area (CSA), and estimated bone strength at the 66% tibia but not at the 33% tibia (12).
Body mass index (BMI) is positively associated with BMD, in part because of the greater load on bone. However, previous studies have provided inconsistent results for the relationship between sclerostin and BMI: some reported positive association (8, 11, 12), whereas others reported no (7, 10) association between sclerostin and weight or BMI. The separate effects of fat mass and lean mass on bone remain controversial, and specific fat depots appear to have different relationships with bone. Investigations into the association of these components with circulating sclerostin have been limited.
In particular, the bone marrow fat (MF) depot appears to have a distinct role in the relationship to bone. Higher levels of vertebral MF are observed with osteoporosis, including prevalent vertebral fracture (13–15). This association may be explained in part by shifts in stem cell lineage allocation toward adipocytes and away from osteoblastogenesis in osteoporosis (16). Stem cell lineage allocation is influenced by the Wnt pathway, leading to the hypothesis that higher levels of sclerostin might be associated with increased MF as well as reduced osteoblastogenesis. The relationship between MF and osteocyte function has not been clarified, and no previous clinical studies are available on the relationship between sclerostin and MF. In this analysis, we use cross-sectional data from older men and women in the Iceland Age, Gene/Environment Susceptibility (AGES)-Reykjavik cohort to characterize the relationship between circulating sclerostin level and MF, vBMD, lean mass, and several fat depots.
Materials and Methods
Cohort
The analyses in this study included 134 women and 115 men who attended the follow-up visit for the AGES study in Reykjavik, Iceland, and participated in an ancillary study of MF (17). The original Reykjavik study of cardiovascular disease, started in 1967, included 30 795 participants (18, 19). The subsequent AGES-Reykjavik study, initiated in 2002, included 5764 participants randomly selected from those still alive in the original study, whose age ranged from 67 to 93 years (20). Approximately 5 years later, 3411 participants attended a follow-up visit. Participants were queried regarding diabetes status, medication use, and moderate or vigorous physical activity in the previous year. Height and weight were measured, and a fasting blood specimen was obtained. At this follow-up visit, eligible participants were invited to participate in this ancillary study with the goal of recruiting at least 300 participants. A total of 303 participants were included in this ancillary study with the following criteria: completion of quantitative computed tomography (QCT) scans at the follow-up visit, no contraindications for magnetic resonance spectroscopy (MRS) scans, and patient consent. The ancillary study was approved by the institutional review boards of the National Bioethics Committee, VSN: 00-063 in Iceland, the National Institute on Aging, and the University of California San Francisco (UCSF). Participants with inadequate serum (n = 2), no MRS (n = 1), or use of bone-active medication (n = 50) are excluded from analyses. Bone-active medications include bisphosphonate, raloxifene, calcitonin, PTH, hormone replacement therapy, thiazolidinedione, tibolone, antiepileptics, antiandrogen therapy, aromatase inhibitors, and glucocorticoids.
Quantitative computed tomography
QCT scans were obtained at lumbar spine and hip for bone composition parameters using a four-detector computed tomography system (Sensation; Siemens Medical Systems) as previously described (20). First, bone mineral reference standards (three sample calibration phantom; Image Analysis) were placed under the participants' spine and hips and scanned simultaneously for calibration. Helical studies were done at the lumbar spine (L1 and L2 vertebrae) and hip (proximal femur from 1 cm superior to acetabulum to 3–5 mm inferior to lesser trochanter) at the following parameters: 120 kVp; 150 mAs for spine and 140 mAs for hip; 1-mm slice thickness; and pitch = 1. A single axial section was obtained at midthigh at the following parameters: 120 kVp and 10-mm slice thickness.
For each trabecular, cortical, and integral region of interest, vBMD (grams per cubic centimeter), bone mineral content (grams), and bone volume (cubic centimeters) were calculated from QCT data. Spine trabecular vBMD was calculated from an elliptical region in the anterior midvertebra, whereas integral vBMD of the spine used the entire midvertebra excluding transverse elements. Cross-sectional areas and vertebral compressive strength were determined as previously described (20).
To obtain information on visceral fat and abdominal musculature, a 10-mm cross-section was obtained through the L4/L5 intervertebral space at 140 kVp, 330 mAs. Analyses of abdomen images were carried out using a program adapted to characterize the visceral and sc fat compartments. The visceral compartment and muscle groups are first manually outlined. Calculation of the outer body contour, the sc fat, and the partition of the muscle compartments into adipose and lean components is carried out automatically.
Proton magnetic resonance spectroscopy (1H-MRS)
Single-voxel 1H-MRS was acquired at vertebral bodies from L1 to L4 using a 1.5 Tesla scanner (GE Healthcare) with an eight-channel cervical-thoracic-lumbar spine coil (using the lower three elements; GE Healthcare). Prior to the scan, imaging with standard clinical sagittal T2-weighted FSE sequence (repetition time/echo time = 5000 per 87 msec, echo train length = 32, field of view = 22 cm, slice thickness = 6 mm) was done to visualize the assessment of lumbar vertebrae for the prescription of the spectral acquisition box. The point-resolved spectroscopy box, with size kept constant throughout the study, was placed in the middle of the vertebral body. Outer volume saturation bands were used to eliminate the potential contamination of outside signals. 1H-MRS was performed using point-resolved spectroscopy (PRESS) sequence with following parameters: repetition time/echo time = 2000 per 37 milliseconds, 64 averages without water suppression, sweep width = 5000 Hz, data point = 2048, and voxel size = 12 × 12 × 20 mm3 = 2.88 cm3.
The spectral data obtained were analyzed using GE SAGE software. After the spectra were corrected for phase, baseline, and frequency shift, the water peak (4.67 ppm) and fat peak (1.3 ppm) were fitted using Marquardt Fit. MF was then calculated as fat/(fat + water)100% using the two peak areas. The average MFs from all four vertebral levels were used in the analyses.
To evaluate reproducibility, five healthy controls were scanned from L1 to L4 twice with repositioning between two scans. The root mean square coefficient of variation of MF was 5.9%. Furthermore, spectral data from 67 subjects were transferred to UCSF Radiology for postprocessing quality control. The root mean square coefficient of variation of MF quantified by two different sites (AGES and UCSF Radiology) was less than 1%.
Dual-energy X-ray absorptiometry
DXA scans of the lumbar spine (anterior-posterior and lateral), proximal femur, and whole body were obtained using a GE Healthcare iDXA scanner, software version 11.4. The presence of vertebral fractures was assessed on lateral spine DXA scans based on vertebral height measured at each evaluable level using six-point morphometry. Each level was assigned a grade of none, mild, moderate, or severe deformity (21). A grade of moderate or severe was considered evidence of a vertebral fracture.
Assays for sclerostin and bone turnover markers
Serum was collected fasting and stored at −70°C. Sclerostin and markers for bone formation [amino-terminal propeptide of type 1 procollagen (P1NP)] and bone resorption [serum C-terminal cross-linking telopeptide of type I collagen (CTX)] were assayed in one batch at the Maine Medical Center Research Institute Laboratory (Scarborough, Maine). The circulating sclerostin level was measured by an ELISA (TECOmedical AG) (22). P1NP and CTX-1 were measured using the IDS-iSYS (Immunodiagnostic Systems Ltd) assays.
Statistical analyses
Analyses were run separately for men and women, given previous findings in this cohort suggesting gender differences in the relationship between marrow fat and bone (17). To avoid assumptions regarding linearity, least square means of selected bone and body composition parameters were estimated by tertiles of serum sclerostin levels in linear regression models and examined for evidence of nonlinearity. Models were adjusted for age, diabetes status, and BMI as previous studies have reported associations with sclerostin levels (11, 23). Adjusting for physical activity level was considered but not performed because moderate/vigorous physical activity was not associated with sclerostin in this study (P = .99 in men; P = .46 in women). We also used linear models for the association of sclerostin as a continuous variable with all study end points, adjusting for age and also for age, diabetes, and BMI. We checked for departures from linearity using a three-knot cubic spline transformation of sclerostin and where nonlinearity was detected (P < .05) report the P value for the 2-df test of the null hypothesis that the coefficients for both spline components are zero (24); otherwise, we report the P value for the sclerostin coefficient from the simpler model assuming linearity. All analyses were performed in SAS version 9.1 (SAS Institute).
Results
Baseline characteristics of participants included in analyses are shown in Table 1. The mean ages of the cohort were 80 years (range 75–91 y) for men and 79 years (range 74–87 y) for women. The mean circulating sclerostin levels were 1.27 ng/mL for men and 0.84 ng/mL for women.
Table 1.
Baseline Characteristics
| Men | Women | |
|---|---|---|
| n | 115 | 134 |
| Age at MRS visit, y | 80.0 ± 3.1 | 78.6 ± 3.0 |
| Diabetic participants, n | 7 (6.1) | 10 (7.5) |
| Prevalent vertebral fracture, n | 20 (17.5) | 31 (23.1) |
| Marrow fat, % | 53.3 ± 8.1 | 54.9 ± 8.4 |
| BMI, kg/m2 | 27.3 ± 3.6 | 28.0 ± 4.0 |
| Sclerostin, ng/mL | 1.27 ± 0.41 | 0.84 ± 0.27 |
Results are shown either as n (percentage) or mean ± SD.
Marrow fat increased with higher sclerostin in men in models adjusted for age [β = 2.7% (95% confidence interval [CI] 1.2%, 4.1%), for 1 SD increase in sclerostin (P < .01)] and in models additionally adjusted for diabetes status and BMI [β = 2.5% (95% CI 1.0%, 4.0%), for 1 SD increase in sclerostin (P < .01)]. In women, however, marrow fat was not associated with sclerostin in age-adjusted models [β = 0.4% (95% CI −1.0%, 1.9%) (P = .54)] or with additional adjustment for diabetes status and BMI [β = .3% (95% CI −1.2%, 1.8%) (P = .67)]. Tests for nonlinearity were not statistically significant in men (P = .87) or women (P = .99). In analyses adjusted for age, diabetes status, and BMI, mean levels of marrow fat by tertiles of sclerostin in men were 52.2% (low tertile), 53.9% (middle tertile), and 56.3% (high tertile) (Figure 1). In women, the adjusted mean levels of marrow fat were 56.9%, 55.3%, and 56.6%.
Figure 1.
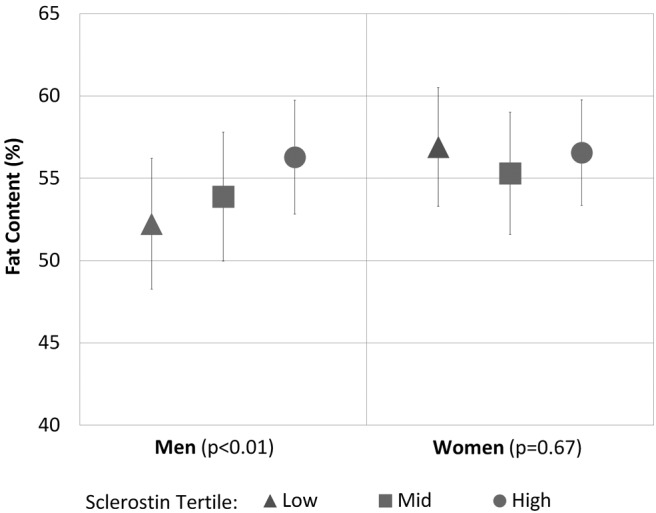
Mean and 95% CI of vertebral marrow fat (L1–L4) by sclerostin tertiles in older men (n = 115) and women (n = 134), adjusted for age, diabetes status, and BMI.
The aBMD by DXA increased significantly with higher sclerostin levels at the spine, femoral neck, and total hip for both men (Table 2) and women (Table 3). QCT measurements of integral vBMD at spine, femoral neck, and total hip also increased significantly with higher sclerostin levels for men and women. Both cortical and trabecular vBMD tended to increase with higher sclerostin levels. Vertebral compressive strength increased significantly with higher sclerostin levels in women and tended to increase in men (P = .06). The CSA was not associated with sclerostin in either men or women. P1NP and CTX were both significantly negatively associated with sclerostin in women but not in men.
Table 2.
Association of Sclerostin Levels With Bone and Body Composition Parameters in Men
| Adjusted Meana Sclerostin Tertiles |
P Valueb |
||||
|---|---|---|---|---|---|
| Low | Middle | High | Age Adjusted | Multivariable Adjusteda | |
| Sclerostin, ng/mL, range | 0.60–1.03 | 1.04–1.39 | 1.40–2.87 | ||
| Spine | |||||
| Trabecular vBMD, g/cm3 | 0.07 | 0.07 | 0.08 | .03c | .10c |
| Integral vBMD, g/cm3 | 0.18 | 0.19 | 0.20 | <.01c | <.01c |
| Compressive strength, kN | 0.16 | 0.19 | 0.21 | .01c | .06c |
| CSA, mm2 | 11.9 | 11.8 | 12.3 | .27 | .37 |
| aBMD, g/cm2 | 1.20 | 1.32 | 1.36 | <.01c | <.01c |
| Femoral neck | |||||
| Trabecular vBMD, g/cm3 | 0.10 | 0.11 | 0.13 | <.01 | <.01 |
| Cortical vBMD, g/cm3 | 0.48 | 0.48 | 0.49 | .14 | .11 |
| Integral vBMD, g/cm3 | 0.20 | 0.21 | 0.24 | <.01 | <.01 |
| aBMD, g/cm2 | 0.81 | 0.87 | 0.94 | <.01 | <.01 |
| Total hip | |||||
| Trabecular vBMD, g/cm3 | 0.13 | 0.14 | 0.16 | <.01 | <.01 |
| Cortical vBMD, g/cm3 | 0.46 | 0.48 | 0.49 | <.01c | <.01c |
| Integral vBMD, g/cm3 | 0.20 | 0.21 | 0.23 | <.01 | <.01 |
| aBMD, g/cm2 | 0.88 | 0.93 | 1.01 | <.01c | <.01c |
| Bone turnover markers | |||||
| P1NP, ng/mL | 48.7 | 41.6 | 45.3 | .38 | .58 |
| CTX, ng/mL | 0.58 | 0.46 | 0.55 | .10 | .91 |
| Body composition | |||||
| Weight, kga | 82.0 | 81.6 | 86.5 | .04 | .03 |
| BMI, kg/m2a | 27.4 | 26.9 | 28.2 | .15 | .19 |
| Total lean mass by DXA, kg | 52.0 | 51.8 | 52.7 | .48 | .65 |
| Total fat by DXA, kg | 26.4 | 27.6 | 28.1 | .03 | .04 |
| Abdominal visceral fat, g | 183.5 | 197.7 | 200.6 | .08 | .24 |
| Abdominal sc fat, g | 195.9 | 204.2 | 206.5 | .02 | .07 |
| Muscle attenuation, HU | 42.4 | 40.8 | 41.6 | .40 | .61 |
Adjusted for age, diabetes status, and BMI, except weight and BMI are adjusted for age and diabetes status.
P value for sclerostin as a continuous variable in linear regression.
Evidence of nonlinearity (P value for joint effect of two spline components).
Table 3.
Association of Sclerostin Levels With Bone and Body Composition Parameters in Women
| Adjusted Meana Sclerostin Tertiles |
P Valueb |
||||
|---|---|---|---|---|---|
| Low | Middle | High | Age Adjusted | Multivariable Adjusteda | |
| Sclerostin, ng/mL, range | 0.29–0.71 | 0.72–0.89 | 0.90–2.18 | ||
| Spine | |||||
| Trabecular vBMD, g/cm3 | 0.05 | 0.07 | 0.08 | <.01 | <.01 |
| Integral vBMD, g/cm3 | 0.16 | 0.18 | 0.20 | <.01 | <.01 |
| Compressive strength, kN | 0.08 | 0.12 | 0.16 | <.01 | <.01 |
| CSA, mm2 | 9.5 | 9.3 | 9.7 | .06 | .30 |
| aBMD, g/cm2 | 1.06 | 1.13 | 1.24 | <.01 | <.01 |
| Femoral neck | |||||
| Trabecular vBMD, g/cm3 | 0.10 | 0.12 | 0.12 | .01 | .02 |
| Cortical vBMD, g/cm3 | 0.46 | 0.48 | 0.48 | .01 | .01 |
| Integral vBMD, g/cm3 | 0.20 | 0.22 | 0.23 | <.01 | <.01 |
| aBMD, g/cm2 | 0.75 | 0.79 | 0.84 | <.01 | <.01 |
| Total hip | |||||
| Trabecular vBMD, g/cm3 | 0.13 | 0.14 | 0.14 | .01 | .01 |
| Cortical vBMD, g/cm3 | 0.44 | 0.46 | 0.47 | <.01c | <.01c |
| Integral vBMD, g/cm3 | 0.19 | 0.21 | 0.22 | <.01c | <.01c |
| aBMD, g/cm2 | 0.80 | 0.85 | 0.89 | <.01 | <.01 |
| Bone turnover markers | |||||
| P1NP, ng/mL | 65.2 | 61.0 | 52.7 | <.01 | <.01 |
| CTX, ng/mL | 0.72 | 0.72 | 0.56 | <.01 | .02 |
| Body composition | |||||
| Weight, kga | 74.0 | 70.1 | 77.4 | .01 | .02 |
| BMI, kg/m2a | 28.3 | 26.7 | 29.1 | .04 | .04 |
| Total lean mass by DXA, kg | 41.4 | 40.7 | 41.2 | .23 | .55 |
| Total fat by DXA, kg | 29.6 | 30.1 | 30.9 | <.01 | .07 |
| Abdominal visceral fat, g | 180.8 | 183.6 | 169.7 | .52 | .19 |
| Abdominal sc fat, g | 264.4 | 262.2 | 257.1 | .50 | .15 |
| Muscle attenuation, HU | 39.0 | 40.2 | 40.1 | .72 | .17 |
Adjusted for age, diabetes status, and BMI, except weight and BMI are adjusted for age and diabetes status.
P value for sclerostin as a continuous variable in linear regression.
Evidence of nonlinearity (P value for joint effect of two spline components).
Circulating sclerostin levels were not associated with prevalent vertebral fracture status. The geometric means of circulating sclerostin level, adjusted for age, BMI, and diabetes, were 1.26 ng/mL and 1.20 ng/mL (P = .55) for men with and without vertebral fracture, respectively; in women, the geometric means were 0.79 ng/mL and 0.81 ng/mL (P = .69) for those with and without fracture.
Weight was positively associated with sclerostin in men and women, and BMI was positively associated in women (Tables 2 and 3). Total fat mass measured by DXA was also positively associated with sclerostin in men but not women. Total lean mass did not show any association with sclerostin in men or women. QCT measurements of muscle attenuation and abdominal visceral and sc fat were not associated with sclerostin in men or women in adjusted models.
Discussion
In this study comprised of older participants, circulating sclerostin was positively associated with MF in men but not in women. In addition, serum sclerostin levels were positively associated with both cortical and trabecular volumetric bone density in men and women. Previous studies of sclerostin and MF are not available. Our results are consistent with findings that Wnt signaling, a target of sclerostin, influences marrow adipogenesis (25, 26) and suggest a relationship between osteocyte function and MF levels. In mice, osterix is expressed in marrow fat cells as well as bone-lining cells and osteocytes, indicating that adipocytes and osteocytes may share a common lineage (27). If adipocytes and osteocytes arise from a common progenitor, this could be a mechanism underlying the positive relationship observed between marrow fat and sclerostin.
Further investigation is needed to understand the gender difference in the association between MF and sclerostin. Estrogen therapy is associated with lower sclerostin levels (4) and with lower MF (28). Rodent models provide evidence that higher estrogen levels reduce the amount of MF through stimulation of increased lipolysis in marrow adipocytes and mesenchymal stem cells (29). Low estrogen levels may be the predominant factor determining marrow fat levels in postmenopausal women who have lower estrogen levels than men of a similar age (30).
As with previous studies, we found positive associations between sclerostin and aBMD. We also found evidence that sclerostin levels are associated with both trabecular and cortical vBMD. This is consistent with the only previous study using central QCT (4). Previous studies using peripheral QCT (12) and high-resolution peripheral QCT (11) also found positive associations between sclerostin and vBMD at the distal radius and tibia in older adults. In our study, vertebral compressive strength increased with sclerostin level in women, whereas CSA was not correlated with sclerostin. This suggests that the association between compressive strength and sclerostin in women was mainly due to an association with higher BMD rather than CSA. The only available previous study found bone strength to be positively associated with sclerostin at 66% of total tibia length but not 33% of the total tibia length (12). Previous studies also found a positive association between sclerostin and CSA at distal radius (11) and cortical area at 33% of the total tibia length and 66% of the total tibia length (12).
The positive associations of sclerostin with BMD, measured by DXA or QCT, and with QCT estimates of bone strength are not clearly understood because the antisclerostin antibody is known to increase BMD. Furthermore, in the AGES-Reykjavik cohort (17) and in other studies (13–15), a negative association between MF and BMD has been reported. Therefore, it seems surprising that sclerostin is positively associated with both MF and BMD in men. However, higher MF was also associated with prevalent vertebral fracture in the same cohort, independent of BMD (17). Some previous studies have reported that sclerostin is associated with increased fracture risk (10, 31), although this remains controversial (3, 5). We did not find an association between sclerostin and prevalent vertebral fracture, but our sample size was limited. It is possible that MF and sclerostin are both markers of decreased bone quality independent of BMD.
Weight was significantly associated with sclerostin in men and women. BMI was associated with sclerostin only in women. Some previous studies have reported positive associations between weight or BMI and sclerostin (7, 8, 11), whereas others have reported no association (10, 12). In contrast to these cross-sectional results, sclerostin levels appear to increase in response to weight loss (6). Longitudinal studies are needed to fully understand the relationship between sclerostin and body size.
In our cohort, total fat mass, but not total lean mass, was associated with sclerostin in men. In women neither fat nor lean mass was associated with sclerostin. One previous study also found no significant association between sclerostin and lean mass or fat mass in women (8). Our study found no significant association between sclerostin and abdominal visceral or sc fat mass. Previous reports using QCT measurements of abdominal fat are not available. A previous study in postmenopausal women found that abdominal and gynoid fat mass, assessed by DXA, were positively associated with sclerostin (9). Lean mass strongly influences bone density, but our results suggest that this is not mediated through levels of circulating sclerostin. Our results for fat mass, however, suggest that common pathways may link sclerostin, fat mass, and bone.
Strengths of this study include high-quality assessments of bone, body composition, and MF in an established cohort of older men and women. An important limitation of this study is the cross-sectional design that precludes the assessment of temporality. Our sample size limited our ability to identify differences in sclerostin by vertebral fracture status. In addition, we measured serum sclerostin, not tissue levels. Sclerostin levels in peripheral serum and in bone marrow plasma are correlated (31), but serum sclerostin may not fully reflect tissue levels. Serum hormone levels are not available in the AGES cohort, so we were not able to test the effects of estrogen on the relationship between sclerostin and MF. Because this study consists of participants from the AGES-Reykjavik study in Iceland, this study includes only older white adults. Thus, the results may not apply to other race/ethnic groups or to younger age groups.
Previous studies have demonstrated that bone marrow fat is associated with lower BMD and with prevalent vertebral fractures. In the Iceland AGES cohort, we found that higher circulating sclerostin was associated with higher marrow fat in men but not women, suggesting that osteocyte activity may influence marrow fat. Longitudinal studies are needed to determine the temporal relationship between circulating sclerostin and marrow fat levels.
Acknowledgments
The researchers are indebted to the participants for their willingness to participate in the study.
The ancillary study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant R01AR057819). The AGES Reykjavik Study is supported by funding from the National Institutes of Health (Contract N01-AG-12100), the National Institute on Aging Intramural Research Program, Hjartavernd (The Icelandic Heart Association), and the Althingi (The Icelandic Parliament). C.J.R. received support from the National Institute of Diabetes and Digestive and Kidney Diseases (Grant R24DK092759).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aBMD
- areal BMD
- AGES
- Age, Gene/Environment Susceptibility
- BMD
- bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- CSA
- cross-sectional area
- CTX
- C-terminal cross-linking telopeptide of type I collagen
- DXA
- dual-energy X-ray absorptiometry
- 1H-MRS
- proton MRS
- MF
- marrow fat
- MRS
- magnetic resonance spectroscopy
- P1NP
- amino-terminal propeptide of type 1 procollagen
- QCT
- quantitative computed tomography
- UCSF
- University of California San Francisco
- vBMD
- volumetric BMD.
References
- 1. Riggs BL, Melton LJ, III, eds. Osteoporosis Etiology, Diagnosis, and Management. 2nd ed Philadelphia: Lippincott-Raven; 1995. [Google Scholar]
- 2. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. [DOI] [PubMed] [Google Scholar]
- 3. Szulc P, Bertholon C, Borel O, Marchand F, Chapurlat R. Lower fracture risk in older men with higher sclerostin concentration: a prospective analysis from the MINOS study. J Bone Miner Res. 2013;28:855–864. [DOI] [PubMed] [Google Scholar]
- 4. Modder UI, Hoey KA, Amin S, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int. 2013;24:489–494. [DOI] [PubMed] [Google Scholar]
- 6. Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amrein K, Amrein S, Drexler C, et al. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab. 2012;97:148–154. [DOI] [PubMed] [Google Scholar]
- 8. Sheng Z, Tong D, Ou Y, et al. Serum sclerostin levels were positively correlated with fat mass and bone mineral density in central south Chinese postmenopausal women. Clin Endocrinol (Oxf). 2012;76:797–801. [DOI] [PubMed] [Google Scholar]
- 9. Urano T, Shiraki M, Ouchi Y, Inoue S. Association of circulating sclerostin levels with fat mass and metabolic disease-related markers in Japanese postmenopausal women. J Clin Endocrinol Metab. 2012;97:E1473–E1477. [DOI] [PubMed] [Google Scholar]
- 10. Arasu A, Cawthon PM, Lui LY, et al. Serum sclerostin and risk of hip fracture in older Caucasian women. J Clin Endocrinol Metab. 2012;97:2027–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szulc P, Boutroy S, Vilayphiou N, et al. Correlates of bone microarchitectural parameters and serum sclerostin levels in men: the STRAMBO study. J Bone Miner Res. 2013;28:1760–70. [DOI] [PubMed] [Google Scholar]
- 12. Thorson S, Prasad T, Sheu Y, et al. Sclerostin and bone strength in women in their 10th decade of life. J Bone Miner Res. 2013;28:2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. [DOI] [PubMed] [Google Scholar]
- 14. Griffith JF, Yeung DK, Antonio GE, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–951. [DOI] [PubMed] [Google Scholar]
- 15. Griffith JF, Yeung DK, Antonio GE, et al. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241:831–838. [DOI] [PubMed] [Google Scholar]
- 16. Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Eurkaryotic Gene Expression. 2009;19:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98:2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bjornsson G, Bjornnsson O, Davidsson D. 1982 Report ABC XXIV Health Survey in the Reykjavik area: women, stages I–III, 1968–1969, 1971–1972, and 1976–1978. Participants invitation response, etc. Reykjavik, Iceland: Icelandic Heart Association. [Google Scholar]
- 19. Bjornsson O, Davidsson D, Olafsson H. 1979 Report ABC XVIII Health Survey in the Reykjavik area: men, stages I-III, 1967–1969, 1970–1971, and 1974–1976. Participants invitation response, etc. Reykjavik, Iceland: Icelandic Heart Association. [Google Scholar]
- 20. Sigurdsson G, Aspelund T, Chang M, et al. Increasing sex difference in bone strength in old age: the Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK). Bone. 2006;39:644–651. [DOI] [PubMed] [Google Scholar]
- 21. Binkley N, Krueger D, Gangnon R, Genant HK, Drezner MK. Lateral vertebral assessment: a valuable technique to detect clinically significant vertebral fractures. Osteoporos Int. 2005;16:1513–1518. [DOI] [PubMed] [Google Scholar]
- 22. McNulty M, Singh RJ, Li X, Bergstralh EJ, Kumar R. Determination of serum and plasma sclerostin concentrations by enzyme-linked immunoassays. J Clin Endocrinol Metab. 2011;96:E1159–E1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia-Martin A, Rozas-Moreno P, Reyes-Garcia R, et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:234–241. [DOI] [PubMed] [Google Scholar]
- 24. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE, eds. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed San Francisco: Springer; 2011. [Google Scholar]
- 25. Kennell JA, MacDougald OA. Wnt signaling inhibits adipogenesis through β-catenin-dependent and -independent mechanisms. J Biol Chem. 2005;280:24004–24010. [DOI] [PubMed] [Google Scholar]
- 26. Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22:1720–1731. [DOI] [PubMed] [Google Scholar]
- 27. Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One. 2014;9:e85161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wend K, Wend P, Drew BG, Hevener AL, Miranda-Carboni GA, Krum SA. ERα regulates lipid metabolism in bone through ATGL and perilipin. J Cell Biochem. 2013;114:1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greendale GA, Edelstein S, Barrett-Connor E. Endogoneous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res. 1997;12:1833–1843. [DOI] [PubMed] [Google Scholar]
- 31. Drake MT, Srinivasan B, Modder UI, et al. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5056–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]


