Abstract
Context:
Thyroid hormone (TH) is essential for normal development; therefore, disruption of TH action by a number of industrial chemicals is critical to identify. Several chemicals including polychlorinated biphenyls are metabolized by the dioxin-inducible enzyme CYP1A1; some of their metabolites can interact with the TH receptor. In animals, this mechanism is reflected by a strong correlation between the expression of CYP1A1 mRNA and TH-regulated mRNAs. If this mechanism occurs in humans, we expect that CYP1A1 expression will be positively correlated with the expression of genes regulated by TH.
Objective:
The objective of the study was to test the hypothesis that CYP1A1 mRNA expression is correlated with TH-regulated mRNAs in human placenta.
Methods:
One hundred sixty-four placental samples from pregnancies with no thyroid disease were obtained from the GESTE study (Sherbrooke, Québec, Canada). Maternal and cord blood TH levels were measured at birth. The mRNA levels of CYP1A1 and placental TH receptor targets [placental lactogen (PL) and GH-V] were quantitated by quantitative PCR.
Results:
CYP1A1 mRNA abundance varied 5-fold across 132 placental samples that had detectable CYP1A1 mRNA. CYP1A1 mRNA was positively correlated with PL (r = 0.64; P < .0001) and GH-V (P < .0001, r = 0.62) mRNA. PL and GH-V mRNA were correlated with each other (r = 0.95; P < .0001), suggesting a common activator. The mRNAs not regulated by TH were not correlated with CYP1A1 expression.
Conclusions:
CYP1A1 mRNA expression is strongly associated with the expression of TH-regulated target gene mRNAs in human placenta, consistent with the endocrine-disrupting action of metabolites produced by CYP1A1.
The Endocrine Society defines an endocrine disruptor as an exogenous chemical or mixture of chemicals that interferes with any aspect of hormone action (1). Thyroid hormone (TH) disruption is a particularly challenging issue to address because there are few unambiguous measures of TH action at the receptor level that can be easily captured in human population studies. TH action is regulated by a complex network of processes that provide tissues some autonomy in regulating TH action (2). Thus, disruption to TH action in a tissue may be measureable by changes in the expression of TH-regulated genes in that tissue but may not be reflected by changes in circulating levels of TH (eg, reference 3). It is therefore important to begin to address the impacts of environmental chemicals on TH action at the receptor level in the human population.
The human population is exposed continuously to a large number of chemicals (4) that may disrupt TH action at different points of regulation (5). These chemicals include, but are not limited to, several classes of persistent organic pollutants to which the human population is chronically exposed such as 2,3,7,8-tetrachlorodibenzo dioxin, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers, and perfluorinated compounds (6). Because there are no unexposed individuals or subgroups within the human population to serve as controls, it is important to have both biomarkers of TH action at the receptor and biomarkers of chemical exposure.
Recent experimental studies in rodents indicate that the inducible enzyme CYP1A1 is an important component of at least one mechanism by which some ubiquitous environmental chemicals can directly interact with the TH receptor (THR) and that we determined to exploit in an epidemiological paradigm. We used human placenta for this purpose both because this tissue is accessible and because it has implications for impacts of chemical exposures on fetal development.
Our reasoning is as follows. First, CYP1A1 is a major xenobiotic metabolizing enzyme in the human placenta, induced by the array of aryl hydrocarbon receptor (AhR) ligands including products of cigarette smoke (7, 8), and that exhibits broad variability of expression in the human placenta (8). Thus, CYP1A1 can reasonably be interpreted to be a biomarker for the differential exposure to AhR ligands (9). Second, CYP1A1 induction and activity is required for specific PCB congeners to activate THR in a luciferase assay using a rat pituitary cell line (10), indicating that hydroxylation is a key step in the formation of THR ligands. This observation is consistent with several reports that hydroxylated PCBs can directly interfere with THR function (eg, references 11–13). Thus, by hydroxylating some ubiquitous chemicals such as PCBs, CYP1A1 appears to convert these xenobiotics into THR ligands. And third, CYP1A1 mRNA levels in rat liver are positively correlated with the expression of mRNAs known to be directly regulated by TH (14). Thus, in the absence of a control group, CYP1A1 expression may be a biomarker of human exposure to chemicals (ie, AhR ligands) that result in the production of xenobiotic metabolites that can interact directly with the THR even in the absence of changes in TH circulating levels. If this mechanism is relevant for humans, then we predicted that CYP1A1 mRNA levels would be correlated with the expression of TH regulated mRNA abundance in human placenta.
We tested this prediction in placental samples obtained as part of a large epidemiological study of pregnancy women (15). We used human placental lactogen (PL) and human GH variant (GH-V) as a measure of TH action because these are known to be direct targets of TH action (16). We also measured the expression of genes known to be unaffected by TH as negative controls. Although correlative, our findings are the first to document the association of CYP1A1 expression and that of TH-regulated genes, consistent with the hypothesis that CYP1A1 metabolites activate the THR.
Materials and Methods
Placental cell culture experiments
A human placental cell line (BeWo) was obtained from the American Type Tissue Collection. Cells were grown for 24 hours in six-well plates with F-12K medium without phenol red, supplemented with 1 mM L-glutamine (Mediatech) and 10% fetal bovine serum (Hyclone) in a 37°C humidified incubator with 5% CO2. Cells were washed and cultured for 24 hours in F-12K medium with 1 mM L-glutamine and 10% fetal bovine serum that had previously been treated with AG 1-X8 resin (analytical grade; Bio-Rad Laboratories) to remove thyroid hormone from the serum (17). They were then washed again and cultured for 24 hours in AG 1-X8-treated F-12K medium with the addition of varying T3 concentrations. T3 was dissolved in ethanol and added to media for final molar concentrations of 1 × 10−11 M to 1 × 10−5 M, which corresponds to 6.5 × 10−1 ng/dL to 6.5 × 10+5 ng/dL; the final concentration of vehicle was always less than 0.168%. After the 24-hour treatment period, the media were removed, cells were washed in 1× PBS, and RNA was isolated with Trizol (Invitrogen Corp). Total RNA was isolated from each well. One microgram of RNA from each sample was reverse transcribed to cDNA using reverse transcriptase (Applied Biosystems, Inc) and random primers with the manufacturer's standard protocol. Quantitative PCR (qPCR) and statistical analysis of the results were performed as described below.
Placental samples
The “Pregnancy and Healthy Child: Study on Thyroid and Environment” (GESTE) study is a Canadian prospective birth cohort study evaluating low-dose environmental contaminants and their health effects (15). This study was performed at the Research Center of the Sherbrooke University Hospital, Québec, Canada, with the protocol being approved by the Human Research Ethics Committee, and informed consent given by each participant. Pregnant women (n = 380) without thyroid-related diagnoses were enrolled for this study in early pregnancy (<20 wk gestation). Demographic data including smoking behavior were obtained using a questionnaire at enrollment and were updated at delivery. Birth weight and gestational age were obtained from medical records and used to characterize the pregnancies from which the placentas were obtained.
Thyroid hormone levels and placental tissue
Blood was collected from the mother at enrollment and delivery and from the umbilical cord to characterize thyroid function (free and total T4 and T3, and TSH). Placental tissue was sampled immediately after expulsion. Two cotyledons were taken on the maternal side, specifically opposite to the umbilical cord. One hundred eighty-nine placental samples were available from the GESTE study. Ten samples were from pregnancies in which the mother was found to have hypothyroidism during that pregnancy and were removed from further analysis. Fifteen placental samples were from twins and were excluded from further analysis. This left 164 unique placental samples in which RNA was isolated, and qPCR was used for relative quantitation of mRNA levels as described below.
Placental mRNA isolation
Approximately 100 mg of each placental sample was used to isolate RNA. Five hundred microliters of Trizol was added to each sample and homogenized using a Bullet Blender (Next Advance Inc) with two scoops of mixed-size stainless steel beads. Another 500 μL of Trizol was added to each sample, and RNA was extracted according to the manufacturer's instructions. To exclude the presence of genomic DNA, samples were treated with deoxyribonuclease (Applied Biosystems, Inc) using the standard protocol. Total RNA was quantified by UV spectrophotometry (Nanodrop 1000; Thermo Scientific). One microgram of RNA from each sample was reverse transcribed to cDNA using reverse transcriptase (Applied Biosystems, Inc) and random primers with the manufacturer's standard protocol.
Quantitative PCR
The FastStart Universal SYBR Green Master kit (Roche Diagnostics Corp) was used for the qPCRs along with 1 μL of cDNA and 300 nM forward and 300 nM reverse primers for each target gene. Because of the low abundance of CYP1A1 transcripts, 4 μL of cDNA was used in CYP1A1 qPCRs. Each sample was run in duplicate for each gene target. A sample from a single pool of cDNA prepared from total RNA from BeWo cells was run in triplicate for each gene on each plate to correct for plate-to-plate variation. The thermal profile was as follows: 10 minutes at 95°C; 40 cycles of 15 seconds at 95°C, 30 seconds at 60°C, and 15 seconds at 72°C; and a melting-curve analysis to identify nonspecific products. Relative levels of target gene mRNA were determined by the method of Pfaffl (18) using the calibrator pool to obtain relative fold change. Plates were normalized using a triplicate measure of β-actin for the calibrator pool. For a negative control, a no-reverse transcriptase sample was used for each target gene, on each plate. Primer sequences are shown in Supplemental Table 1.
Statistical analysis
Prism statistical software (GraphPad Software) was used for statistical analysis. Unpaired t tests were used to test for differences between two means in cell culture (Figure 1, C and D). One-way ANOVA was used to test for differences among means in Figure 1, A and B. Pearson's correlation test and linear regression were used to test the strength of association between relative gene mRNA expression levels as well as between relative gene mRNA expression levels and thyroid hormone levels.
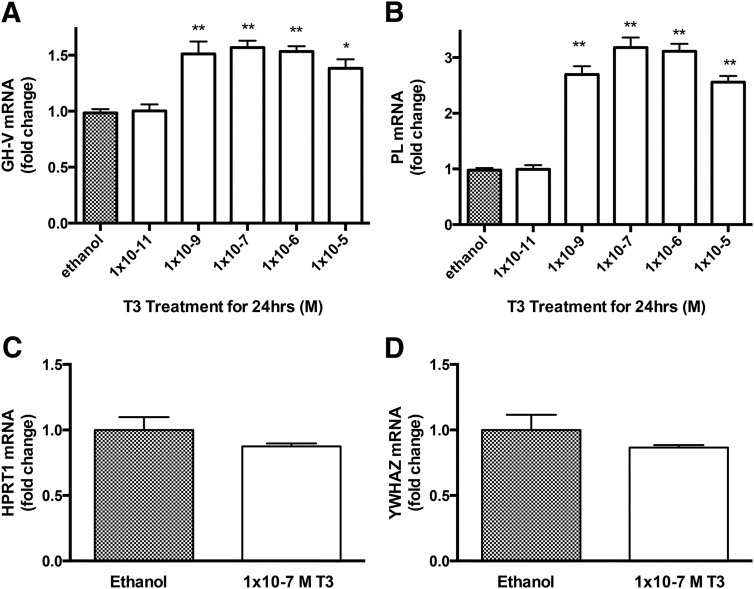
Figure 1.
T3 up-regulates mRNAs coding for GH-V and PL but not for HPRT1 and YWHAZ in BeWo cells. A and B, T3 at 1 × 10−9 M and greater significantly increased mRNAs coding for GH-V (A) and PL (B) compared with control, with a maximal response at 1 × 10−7 M. C and D, 1 × 10−7 M T3 did not significantly affect the abundance of mRNAs coding for HPRT1 (C) or YWHAZ (D). Bars represent the mean (±SEM) (n = 9–12 per treatment). *, P < .05; **, P < .01.
Results
TH-responsive and nonresponsive genes in cell culture
mRNAs encoding GH-V and PL were significantly more abundant in BeWo cells treated with T3 than with vehicle (Figure 1, A and B), as previously reported (16). The expression of these genes was increased by T3 treatment at 10−9 M and greater. To identify negative control genes in placenta (ie, genes not regulated by TH), we chose two genes noted to be placental specific housekeeping genes in the literature and tested for T3 responsiveness: YWHAZ coding for tyrosine 3 monooxygenase/tryptophan 5 monooxygenase activation protein, delta polypeptide and HPRT1 coding for hypoxanthine phosphoribosyl-transferase I (19). The mRNA expression of these genes was not affected by T3 treatment (Figure 1, C and D).
Demographic information related to human placental tissue
Of the 164 placental samples, 132 exhibited detectable CYP1A1 levels by qPCR, and 32 did not. We characterized the subjects based on this criterion and Table 1 describes the demographics of these two groups. The populations were made up of primarily Canadian-born women, the mean age was approximately 28 years, and they were recruited into the study between 3 and 20 weeks of gestation. The CYP1A1-positive and CYP1A1-negative groups had similar means for weight and body mass index (BMI), but the means for the CYP1A1-positive group were lower than typical for Canadian women aged 20–39 years (20). Serum TH levels both at the time of recruitment and at the time of delivery for both of these groups were all within cord blood and trimester-specific reference ranges (22, 23).
Table 1.
Demographic and TH Data for the Pregnancies With Detectable CYP1A1 Levels and Undetectable CYP1A1 Levels
| CYP1A1-Positive Population |
CYP1A1-Negative Population |
Reference Range | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | # | % | Mean (SD) | n | # | % | Mean (SD) | ||
| Maternal age, y | 132 | 27.8 (4.5) | 32 | 28.6 (4.9) | |||||
| Canadian born | 132 | 125 | 95 | 32 | 31 | 97 | |||
| Weight, kg | 131 | 62.7 (11.4) | 32 | 65.6 (11.0) | 65.0–72.4a | ||||
| Height, m | 132 | 1.61 (0.07) | 32 | 1.62 (0.07) | 1.61–1.65a | ||||
| BMI | 131 | 24.1 (4.5) | 32 | 25.0 (4.7) | 24.7–26.6a | ||||
| Gestational age at recruitment, wk | 132 | 11.3 (2.7) | 32 | 11.1 (2.9) | |||||
| Smoking during pregnancy | 129 | 19 | 14 | 32 | 3 | 9 | |||
| Gestational age at delivery, wk | 132 | 39.3 (1.6) | 32 | 39.4 (1.4) | |||||
| Premature (<37 wk gestation) | 132 | 8 | 6 | 32 | 1 | 3 | |||
| Weight of baby, g | 131 | 3417 (443) | 32 | 3349 (474) | |||||
| Maternal thyroid levels at recruitment | |||||||||
| Total T4, μg/dL | 132 | 9.2 (2.2) | 32 | 8.7 (2.0) | 6.5–10.1b | ||||
| Free T4, ng/dL | 131 | 1.12 (0.14) | 32 | 1.13 (0.16) | 0.8–1.2b | ||||
| TSH, μIU/mL | 132 | 1.37 (0.79) | 32 | 1.52 (1.06) | 0.6–3.4b | ||||
| Total T3, ng/dL | 132 | 118.7 (39.8) | 32 | 121 (32.7) | 97–149b | ||||
| Maternal thyroid levels at delivery | |||||||||
| Total T4, μg/dL | 117 | 8.8 (1.8) | 29 | 9.0 (2.1) | 6.3–9.7c | ||||
| Free T4, μg/dL | 115 | 0.78 (0.11) | 29 | 0.82 (0.11) | 0.5–0.8c | ||||
| TSH, μΙU/mL | 115 | 2.3 (1.3) | 29 | 2.0 (1.1) | 0.38–4.04c | ||||
| Total T3, ng/dL | 117 | 127.3 (31.5) | 29 | 134 (20.8) | 123–162c | ||||
| Cord blood thyroid levels | |||||||||
| Total T4, μg/dL | 124 | 8.9 (1.8) | 26 | 9.2 (1.4) | 7.3–14.1d | ||||
| Free T4, ng/dL | 124 | 0.99 (0.12) | 27 | 1.00 (0.19) | |||||
| TSH, μIU/mL | 124 | 9.91 (7.24) | 27 | 9.87 (5.98) | |||||
| Total T3, ng/dL | 124 | 36.2 (13.1) | 26 | 43.8 (21.3) | 25.3–99.0d | ||||
Reference confidence intervals for the mean weight, height, and BMI of 20- to 39-year-old women from the Canadian Health Measures Survey (20).
Reference ranges for thyroid levels are for first trimester (21).
Reference ranges for thyroid levels are for third trimester (21).
Reference ranges for thyroid levels are for cord blood (22).
Smoking associated with elevation of placental CYP1A1 mRNA levels
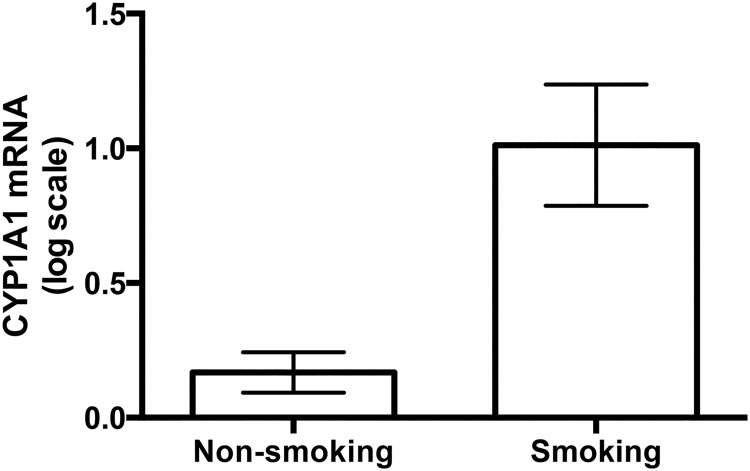
In the CYP1A1-positive group, there were 19 women who reported smoking during pregnancy. The mean CYP1A1 mRNA level for those individuals reporting smoking during pregnancy was significantly higher than in those reporting that they did not smoke during pregnancy (Figure 2, P = .001).
Figure 2.
CYP1A1 mRNA levels in placental samples taken from women who reported smoking (n = 19) or not (n = 110). Three women who did not report on smoking were not included in this analysis. CYP1A1 mRNA levels of smokers were significantly higher than those of nonsmokers using an unpaired t test (t = 4.2, df = 127, P < .0001). Zero on this graph is equivalent to the CYP1A1 mRNA levels in unstimulated BeWo cells. Bars represent mean ± SEM.
CYP1A1 associated with TH-regulated genes
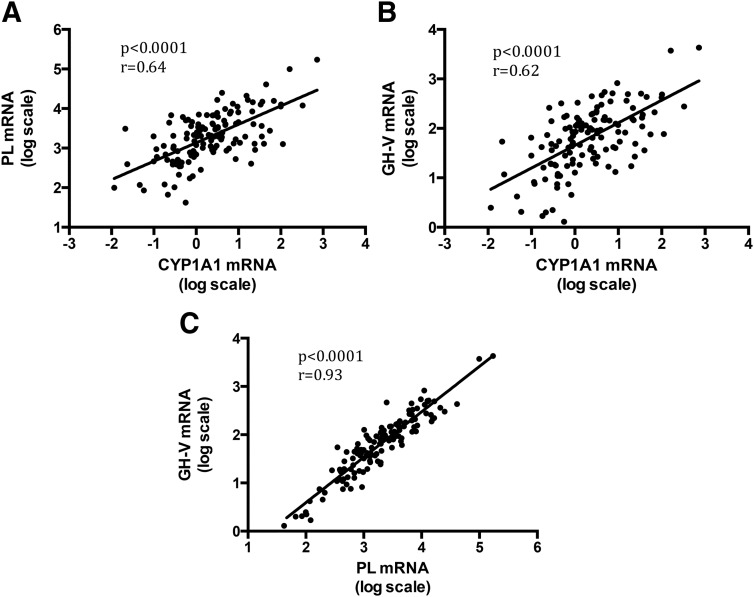
In the CYP1A1-positive group, we demonstrated a positive correlation between CYP1A1 and PL mRNA levels (Figure 3A, n = 132, r = 0.64, r2 = 0.41; P < .0001), and between CYP1A1 and GH-V mRNA levels (Figure 3B, n = 132, r = 0.62, r2 = 0.38; P < .0001). GH-V mRNA levels were very tightly correlated with those of PL (Figure 3C, n = 132, r = 0.93, r2 = 0.87; P < .0001). CYP1A1 mRNA levels were not correlated with either YWHAZ mRNA levels (Supplemental Figure 1A, n = 131, P = .49) or with HPRT1 mRNA levels (Supplemental Figure 1B, n = 132, P = .11). Also, the significant correlation between CYP1A1 mRNA levels and PL or GH-V mRNA levels persisted when these values were normalized to either YWHAZ or HPRT1 mRNA levels instead of β-actin mRNA levels (Supplemental Figures 2 and 3).
Figure 3.
CYP1A1 mRNA levels correlate with TH-regulated gene (PL and GH-V) mRNA levels in placental tissue samples (n = 132). 0 on the x-axis and y-axis is equivalent to the expression level in unstimulated BeWo cells. Linear regression plots are shown for the following: PL vs CYP1A1 mRNA levels (A), GH-V vs CYP1A1 mRNA levels (B), and GH-V vs PL mRNA levels (C).
CYP1A1 is not correlated with TH levels
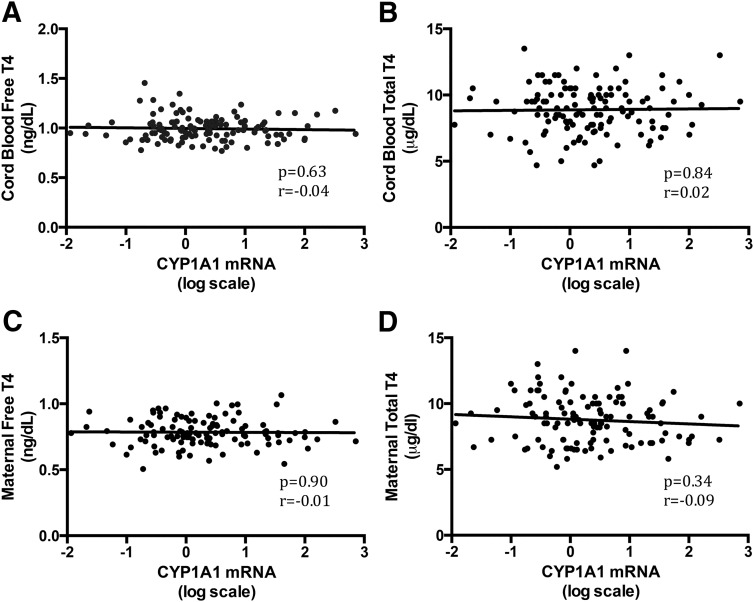
In the CYP1A1-positive group, Pearson's correlation test revealed no significant correlation between maternal or cord blood levels of TH or TSH and CYP1A1 mRNA levels (P > .1). Figure 4, A–D, shows the distribution of these data with regression lines for cord blood free T4 (n = 124), cord blood total T4 (n = 124), maternal free T4 (n = 115), and maternal total T4 (n = 117) vs CYP1A1 mRNA levels.
Figure 4.
CYP1A1 mRNA is not correlated with TH levels in maternal or cord blood. A and B, Cord blood free (A) and total (B) T4 regressed against CYP1A1 mRNA. C and D, Maternal free (C) and total (D) T4 regressed against CYP1A1 mRNA. Note the log scale for CYP1A1 mRNA [n = 124 (cord blood total T4 and free T4), n = 115 (maternal free T4), and n = 117 (maternal total T4)]. CYP1A1 mRNA levels are not correlated with total T3 or TSH (data not shown).
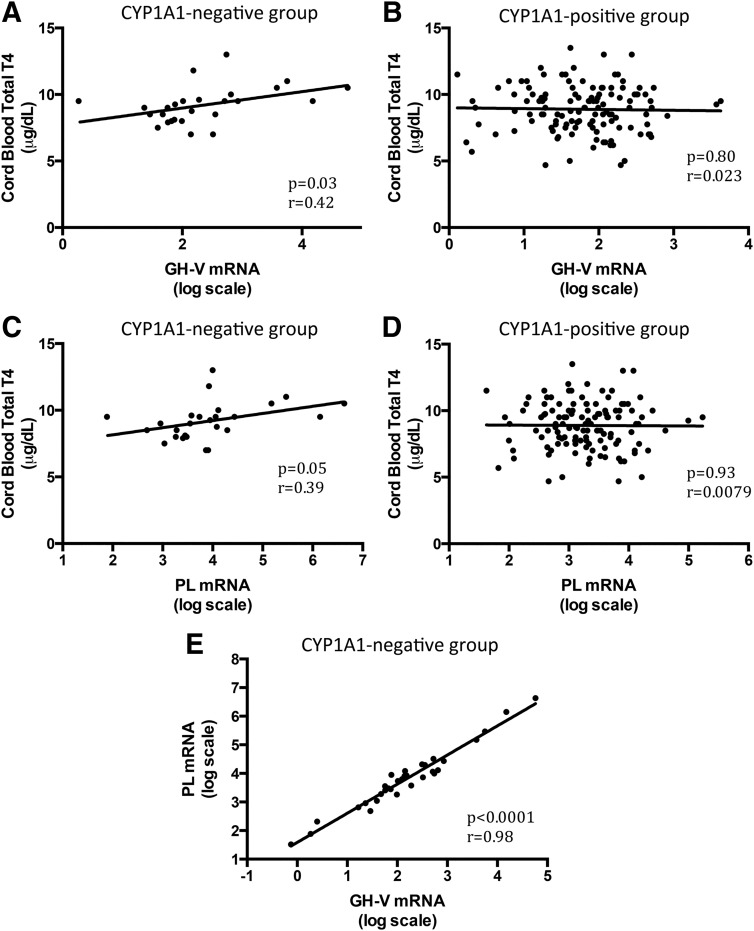
TH-regulated genes correlated with cord blood TH levels only in CYP1A1-negative group
In the CYP1A1-negative group (n = 32), there were significant correlations between fetal TH levels and placental TH-regulated gene mRNA levels: cord blood total T4 and GH-V (Figure 5A, n = 26, r = 0.41, r2 = 0.17; P = .03), cord blood total T4 and PL (Figure 5C, n = 26, r = 0.39, r2 = 0.15; P = .05), and cord blood free T4 and PL (n = 27, r = 0.42, r2 = 0.18; P = .03). PL and GH-V mRNAs were also tightly associated as was seen in the CYP1A1-positive group (Figure 5E, n = 32, r = 0.98, r2 = 0.96; P < .0001). No associations were noted between GH-V or PL and any of the TH levels, maternal or fetal, in the CYP1A1-positive group (examples in Figure 5, B and D, P > .3).
Figure 5.
TH-regulated mRNA levels correlated with cord blood TH levels in placental samples with undetectable CYP1A1 mRNA (CYP1A1 negative group, n = 26) but not in placental samples with detectable CYP1A1 mRNA (CYP1A1 positive group, n = 124). A and B, GH-V mRNA correlates with cord blood total T4 in the CYP1A1-negative group (A) but not in the CYP1A1-positive group (B). C and D, PL mRNA correlates with cord blood total T4 in CYP1A1-negative group (C) but not in the CYP1A1-positive group. E, In the small CYP1A1-negative group, the GH-V mRNA and PL mRNA levels correlate very well with each other.
Discussion
We found that CYP1A1 mRNA abundance was strongly correlated with that of two separate TH-regulated genes, PL (r = 0.64) and GH-V (r = 0.62). PL and GH-V expression levels were also tightly correlated with each other (r = 0.93), suggesting that both are regulated by a common factor. In contrast, CYP1A1 expression was not correlated with the expression of two mRNAs that are not regulated by THR, YWHAZ and HPRT1, nor was it correlated with maternal or cord serum total or free T4 or TSH. These results were predicted based on the observations that AhR activation and CYP1A1 induction and activity are required for specific PCB congeners (PCBs 105 and 118) to activate the THR in a GH3 cell-based luciferase assay (10). In the presence of these same PCB congeners, CYP1A1 mRNA levels in rat liver are positively correlated with TH-regulated genes (S14 and malic enzyme) (14). The relationship of CYP1A1 with TH-regulated genes in the human placenta that we now report is correlative but supports the hypothesis that the dioxin-inducible enzyme CYP1A1 is activating some environmental chemicals in the human placenta that can interact with the THR.
Although the correlation among mRNAs coding for CYP1A1, PL, and GH-V were predicted, we explored several possible alternative explanations for these observations. First, both PL and GH-V were up-regulated by T3 in BeWo cells (Figure 1), indicating these genes can be regulated by T3. The responses appeared to be physiologically appropriate for the effective dose of T3, relative to the reference ranges for total T3 in an adult nonpregnant population (1.18 × 10−9 to 2.07 × 10−9 M or 77–135 ng/dL), in a first-trimester pregnant population (1.49 × 10−9 to 2.29 × 10−9 M or 97–149 ng/dL), in a third-trimester pregnant population (1.89 × 10−9 to 2.49 × 10−9 M or 123–162 ng/dL) and in cord blood (3.84 × 10−10 to 1.52 × 10−9 M or 25–99 ng/dL) (22, 23). Both PL and GH-V had similar response curves; the maximal response was at 1 × 10−7 M or 6510 ng/dL T3 treatment, which is well above human serum T3 reference ranges. At even higher T3 concentrations, the expression of GH-V and PL trended downward, suggesting overstimulation and down-regulation of the signaling pathway. Neither YWHAZ nor HPRT1 responded to T3 treatment in BeWo cells. Thus, we obtained a reasonable assurance GH-V and PL mRNAs were appropriate biomarkers of T3 action, and YWHAZ and HPRT1 were appropriate control genes in human placenta.
Second, we considered the possibility that β-actin expression, to which CYP1A1, GH-V, and PL were normalized, was driving their correlations. We tested for this using two placental specific housekeeping genes, YWHAZ and HPRT1 (19). First, YWHAZ and HPRT1 mRNA levels were not correlated with CYP1A1 mRNA levels using β-actin as a reference gene (Supplemental Figure 1). In addition, the correlations between CYP1A1, PL, and GH-V in the placental samples were retained when using either YWHAZ or HPRT1 as reference genes (Supplemental Figures 2 and 3). Thus, we concluded that variability in β-actin expression in the placenta was not driving the observed correlations between CYP1A1, PL, and GH-V mRNA.
Third, we also considered the possibility that the 19 samples from women who reported smoking had elevated CYP1A1 levels and that this drove the correlation in the full collection of tissues. We demonstrated that, as previously reported (7, 23), CYP1A1 expression was higher in placentas from women who reported smoking (Figure 2). However, CYP1A1 expression remained highly and significantly correlated with the expression of PL (r = 0.68, r2 = 0.41; P < .0001) and GH-V (r = 0.65, r2 = 0.38; P < .0001) in placentas from women who reported not smoking (data not shown). Thus, we concluded that the smoking subpopulation was not driving the correlation between CYP1A1- and TH-regulated genes.
Lastly, we considered the possibility that correlations between CYP1A1, PL, and GH-V mRNAs were being driven by TH itself and that this would be reflected by variability in maternal or cord serum hormone levels. However, CYP1A1 mRNA levels were not correlated with TH levels in the cord blood or maternal serum (Figure 5). Moreover, TH levels were not correlated with the expression of PL or GH-V in placental samples with detectable CYP1A1 mRNA. These observations are not consistent with the hypothesis that serum TH levels are driving the correlations between CYP1A1, PL, and GH-V mRNAs.
There are several important implications of the findings presented here. Perhaps the most important is that the present data support the concept that environmental chemicals may interfere with TH action by a mechanism that is not limited to or revealed by changes in serum TH levels. Animal studies clearly demonstrate the plausibility of this. Early studies in rats showed that PCBs could simultaneously decrease serum TH and increase the expression of TH-regulated genes in the developing brain in a manner that was temporally and spatially consistent with a TH-like effect (24, 25). Further work demonstrated that PCB exposure during development did not uniformly produce TH-like effects on all measures of TH action in the developing brain (26), indicating that even within the developing brain, the impact of PCB exposure on TH action was cell specific and not fully revealed by serum TH levels. Our current study demonstrated that in placental samples with detectable CYP1A1 mRNA levels, TH-regulated genes were not correlated with either maternal or cord blood TH but were strongly correlated with CYP1A1 mRNA. If this reflects similar events in other tissues such as the fetal brain, it could explain the relationship between prenatal exposure to PCBs and cognitive deficits in the offspring (27).
The next step in dissecting this mechanism of endocrine disruption will be to identify the chemical species that may be converted by CYP1A1 to produce these TH-like effects. In doing so, it will be important to address the complexity of xenobiotic chemical effects on hormone actions. For example, CYP1A1 may produce a hydroxylated species of PCB (eg, 4-OH-PCB 47) that directly activates the THR. However, this PCB metabolite may block the action of D3, increasing intracellular free T3 or may alter T3 transport, resulting in an increase in intracellular free T3. Xenobiotic metabolites may have very complex actions, simultaneously affecting several processes that can impact cellular THR activation levels.
Discussing placental samples that have detectable CYP1A1 mRNA begets a discussion of those samples without detectable CYP1A1 mRNA. Although small, this group represents nearly 20% of the samples used in this study and may be an important group to consider in relation to gene-environment interactions. There is significant genetic polymorphism in the human CYP1A1 gene (28), although it does not appear to be related to expression level (29). In the current situation, a lack of CYP1A1 expression is an indicator of both genetic and environmental regulators of its expression. Most importantly, we noted a weak, but significant, correlation between cord blood thyroid hormone and the TH-regulated genes, PL and GH-V, but no correlation with maternal serum TH levels. These results are consistent with the hypothesis that in the absence of, or very low, CYP1A1 expression to convert ubiquitous xenobiotic chemicals into thyroid hormone receptor agonists, TH itself regulates the expression of PL and GH-V.
The correlation between TH-regulated gene expression and cord blood, but not maternal, TH levels suggests a potentially interesting concept about placental physiology. Specifically, the placenta plays an important role in transporting maternal thyroid hormone across to the fetus, particularly early in pregnancy when the fetus does not produce TH but requires TH for normal development (30). TH is also important for placental growth and development (31). This very limited data set on placental samples without detectable CYP1A1 suggests the intriguing idea that at the time of birth or at the end of pregnancy, TH signaling in the placenta is related to fetal TH and not maternal TH.
In summary, we report a robust correlation in human placenta between CYP1A1 mRNA and mRNA levels of two TH-regulated genes (PL and GH-V) and an even tighter correlation between these two TH-regulated genes, suggesting a common stimulus. Because CYP1A1 was not otherwise associated with TH levels, these findings are consistent with the action of xenobiotic chemical (eg, PCBs) being metabolized by CYP1A1 in these placental samples and acting in a manner that increases the TH activity within the placenta. We put forward that the relationship between CYP1A1 expression and TH-regulated genes may be an important biomarker of environmental impacts on TH action that can be used in epidemiological studies.
Acknowledgments
We thank Dr Cherniak (Centre Hospitalier Universitaire de Sherbrooke maternity service and Centre Hospitalier Universitaire de Sherbrooke Department of Family Medicine) in recruiting pregnant women and in the collection of samples at delivery. Special thanks to Maria Suvorova and her team for quality placental dissections. Katherine Geromini's work on the placental qPCR data was instrumental in this study and performed as part of an MS degree in the Molecular and Cellular Biology Graduate Program at the University of Massachusetts, Amherst.
This work was supported by National Institutes of Health Grant RO1 ES010026 and Passport Foundation grants (to R.T.Z.) and Fonds de Recherche du Québec-Santé Grant 12397 and Canadian Institutes of Health Research Grant MOP-84551 (to L.T.). The GESTE study was supported by Grant 12397 from the Fonds de Recherche du Québec-Santé and Grant MOP-84551 from the Canadian Institutes of Health Research (to L.T.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AhR
- aryl hydrocarbon receptor
- BMI
- body mass index
- GH-V
- human GH variant
- PCB
- polychlorinated biphenyl
- PL
- placental lactogen
- qPCR
- quantitative PCR
- TH
- thyroid hormone
- THR
- TH receptor.
References
- 1. Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from the endocrine society. Endocrinology. 2012;153:4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33:842–852. [DOI] [PubMed] [Google Scholar]
- 3. Arrojo EDR, Fonseca TL, Werneck-de-Castro JP, Bianco AC. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim Biophys Acta. 2013;1830:3956–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 5. Brucker-Davis F. Effects of environmental synthetic chemicals on thyroid function. Thyroid. 1998;8:827–856. [DOI] [PubMed] [Google Scholar]
- 6. Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012;355:240–248. [DOI] [PubMed] [Google Scholar]
- 7. Huuskonen P, Storvik M, Reinisalo M, et al. Microarray analysis of the global alterations in the gene expression in the placentas from cigarette-smoking mothers. Clin Pharmacol Ther. 2008;83:542–550. [DOI] [PubMed] [Google Scholar]
- 8. Stejskalova L, Pavek P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr Pharm Biotechnol. 2011;12:715–730. [DOI] [PubMed] [Google Scholar]
- 9. Whyatt RM, Garte SJ, Cosma G, et al. CYP1A1 messenger RNA levels in placental tissue as a biomarker of environmental exposure. Cancer Epidemiol Biomarkers Prev. 1995;4:147–153. [PubMed] [Google Scholar]
- 10. Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, Zoeller RT. Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ Health Perspect. 2007;115:1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freitas J, Cano P, Craig-Veit C, Goodson ML, Furlow JD, Murk AJ. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro. 2011;25:257–266. [DOI] [PubMed] [Google Scholar]
- 12. Amano I, Miyazaki W, Iwasaki T, Shimokawa N, Koibuchi N. The effect of hydroxylated polychlorinated biphenyl (OH-PCB) on thyroid hormone receptor (TR)-mediated transcription through native-thyroid hormone response element (TRE). Ind Health. 2010;48:115–118. [DOI] [PubMed] [Google Scholar]
- 13. You SH, Gauger KJ, Bansal R, Zoeller RT. 4-Hydroxy-PCB106 acts as a direct thyroid hormone receptor agonist in rat GH3 cells. Mol Cell Endocrinol. 2006;257–258:26–34. [DOI] [PubMed] [Google Scholar]
- 14. Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology. 2011;152:2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdelouahab N, Langlois MF, Lavoie L, Corbin F, Pasquier JC, Takser L. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol. 2013;178:701–713. [DOI] [PubMed] [Google Scholar]
- 16. Nickel BE, Cattini PA. Tissue-specific expression and thyroid hormone regulation of the endogenous placental growth hormone variant and chorionic somatomammotropin genes in a human choriocarcinoma cell line. Endocrinology. 1991;128:2353–2359. [DOI] [PubMed] [Google Scholar]
- 17. Samuels HH, Stanley F, Casanova J. Depletion of L-3,5,3′-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979;105:80–85. [DOI] [PubMed] [Google Scholar]
- 18. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta. 2005;26:601–607. [DOI] [PubMed] [Google Scholar]
- 20. Statistics Canada. Canadian Health Measures Survey: Cycle 2 Data Tables, 2009 to 2011. Industry Division, ed. 1st ed Ottawa: Statistics Canada; 2013. [Google Scholar]
- 21. Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114:1326–1331. [DOI] [PubMed] [Google Scholar]
- 22. Santini F, Chiovato L, Ghirri P, et al. Serum iodothyronines in the human fetus and the newborn: evidence for an important role of placenta in fetal thyroid hormone homeostasis. J Clin Endocrinol Metab. 1999;84:493–498. [DOI] [PubMed] [Google Scholar]
- 23. Pasanen M, Pelkonen O. The expression and environmental regulation of P450 enzymes in human placenta. Crit Rev Toxicol. 1994;24:211–229. [DOI] [PubMed] [Google Scholar]
- 24. Bansal R, You SH, Herzig CT, Zoeller RT. Maternal thyroid hormone increases HES expression in the fetal rat brain: an effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs). Brain Res Dev Brain Res. 2005;156:13–22. [DOI] [PubMed] [Google Scholar]
- 25. Zoeller RT, Dowling AL, Vas AA. Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology. 2000;141:181–189. [DOI] [PubMed] [Google Scholar]
- 26. Bansal R, Zoeller RT. Polychlorinated biphenyls (Aroclor 1254) do not uniformly produce agonist actions on thyroid hormone responses in the developing rat brain. Endocrinology. 2008;149:4001–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111:357–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsai PC, Huang W, Lee YC, Chan SH, Guo YL. Genetic polymorphisms in CYP1A1 and GSTM1 predispose humans to PCBs/PCDFs-induced skin lesions. Chemosphere. 2006;63(8):1410–1418. [DOI] [PubMed] [Google Scholar]
- 29. Goth-Goldstein R, Stampfer MR, Erdmann CA, Russell M. Interindividual variation in CYP1A1 expression in breast tissue and the role of genetic polymorphism. Carcinogenesis. 2000;21:2119–2122. [DOI] [PubMed] [Google Scholar]
- 30. Patel J, Landers K, Li H, Mortimer RH, Richard K. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 2011;22:164–170. [DOI] [PubMed] [Google Scholar]
- 31. Barber KJ, Franklyn JA, McCabe CJ, et al. The in vitro effects of triiodothyronine on epidermal growth factor-induced trophoblast function. J Clin Endocrinol Metab. 2005;90:1655–1661. [DOI] [PubMed] [Google Scholar]