Abstract
Background:
Cytokines and growth factors play important roles in endometrial function and the pathogenesis of endometriosis. mRNAs encoding cytokines and growth factors undergo rapid turnover; primarily mediated by adenosine- and uridine-rich elements (AREs) located in their 3′-untranslated regions. T-cell intracellular antigen (TIA-1), an mRNA-binding protein, binds to AREs in target transcripts, leading to decreased gene expression.
Objective:
The purpose of this article was to determine whether TIA-1 plays a role in the regulation of endometrial cytokine and growth factor expression during the normal menstrual cycle and whether TIA-1 expression is altered in women with endometriosis.
Methods:
Eutopic endometrial tissue obtained from women without endometriosis (n = 30) and eutopic and ectopic endometrial tissues from women with endometriosis (n = 17) were immunostained for TIA-1. Staining intensities were evaluated by histological scores (HSCOREs). The regulation of endometrial TIA-1 expression by immune factors and steroid hormones was studied by treating primary cultured human endometrial stromal cells (HESCs) with vehicle, lipopolysaccharide, TNF-α, IL-6, estradiol, or progesterone, followed by protein blot analyses. HESCs were engineered to over- or underexpress TIA-1 to test whether TIA-1 regulates IL-6 or TNF-α expression in these cells.
Results:
We found that TIA-1 is expressed in endometrial stromal and glandular cells throughout the menstrual cycle and that this expression is significantly higher in the perimenstrual phase. In women with endometriosis, TIA-1 expression in eutopic and ectopic endometrium was reduced compared with TIA-1 expression in eutopic endometrium of unaffected control women. Lipopolysaccharide and TNF-α increased TIA-1 expression in HESCs in vitro, whereas IL-6 or steroid hormones had no effect. In HESCs, down-regulation of TIA-1 resulted in elevated IL-6 and TNF-α expression, whereas TIA-1 overexpression resulted in decreased IL-6 and TNF-α expression.
Conclusions:
Endometrial TIA-1 is regulated throughout the menstrual cycle, TIA-1 modulates the expression of immune factors in endometrial cells, and downregulation of TIA-1 may contribute to the pathogenesis of endometriosis.
The endometrium is composed of glandular epithelium and stroma that respond to sex steroids, principally estradiol (E2) and progesterone, to undergo growth and regression in a cyclic manner throughout the reproductive life of a woman. In addition to steroid hormones, endometrial function relies on timely recruitment, activation, and disposal of immune cells. Macrophages serve as endometrial scavengers, helping to clear cellular debris and apoptotic cells from the cycling endometrium. T lymphocytes regulate and promote B-cell production of antibodies and also have an impact on macrophage functions. In addition, a large number of cytokines and growth factors are produced by stromal, glandular, and immune cells within the endometrium and play important roles in mediating multiple aspects of endometrial function (for a review, see Ref. 1).
Endometriosis is a pathologic condition characterized by the presence of endometrial stromal and glandular cells outside of the uterus. Endometriosis most commonly presents with infertility and/or pelvic pain and is estimated to affect 5% to 10% of women in the general population with a prevalence of 25% to 40% in infertile women (2). The infertility and chronic pelvic pain associated with endometriosis lead to significant morbidity and financial burden for the individual and for the society (3); US annual health care costs and costs associated with loss of productivity stemming from endometriosis were estimated to be $22 billion in 2002 (4).
A number of theories have been proposed to explain the pathogenesis of endometriosis (for reviews, see Refs. 1, 5). Among these, the most commonly accepted is the theory of retrograde menstruation proposed by Sampson in 1927 (6). Although many aspects of endometriosis support this theory, retrograde menstruation occurs in 76% to 90% of women (7, 8), a prevalence much higher than that of endometriosis, suggesting that additional factors are likely to determine individual susceptibility to endometriosis.
Immunologic abnormalities have been reported in women with endometriosis and in animal models of the disease (for a review, see. Ref. 1). It has been postulated that an altered immune response could underlie implantation and/or growth of ectopic endometrium in susceptible women. In addition to perturbations in cell-mediated and humoral components of innate and acquired immunity, endometriosis has been associated with altered expression of cytokines and growth factors, which may promote implantation and growth of ectopic endometrium by inducing cellular proliferation and angiogenesis (1). Indeed, the peritoneal fluid of women with endometriosis demonstrates elevated levels of several cytokines and growth factors, which include TNF-α (9–12), IL-1β (10–14), IL-6 (12, 15–20), IL-8 (12, 20–24), IL-10 (12, 16, 25, 26), monocyte chemotactic protein-1 (12, 23, 27), regulated upon activation, normal T cell expressed and secreted (12, 28), TGF-β (23), and vascular endothelial growth factor (12, 19, 29).
Posttranscriptional regulation of mRNA stability is a common mechanism governing the expression of many cytokines and growth factors (30). Proteins that recognize adenosine- and uridine-rich elements (ie, AU-rich elements [AREs]) in 3′-untranslated regions of target transcripts mediate many of these processes (31, 32). These ARE-binding proteins (ARE-BPs) regulate gene expression by modulating translation of bound transcripts and/or by having an impact on their stability (ie, mRNA half-life) (33). T-cell intracellular antigen (TIA-1) is an ARE-BP that acts as a translational silencer for proinflammatory genes including TNF-α, IL-1β, IL-6 (34), and cyclooxygenase 2 (COX-2) (35). TIA-1 has also been implicated in the down-regulation of IGF-binding protein-3 in human hepatocellular carcinomas (36) and is overexpressed in the thyroid tissue of adolescents with immune thyroid diseases (37). Importantly, mice lacking TIA-1 develop arthritis, an inflammatory disorder, a phenotype consistent with a role for TIA-1 in suppressing inflammation (38). In summary, TIA-1 is a destabilizing factor that selectively binds and down-regulates the expression of target mRNAs, including many that encode for immune mediators that are expressed in the endometrium and are elevated in the peritoneal fluid of women with endometriosis.
In this study, we first determined that TIA-1 is expressed in the human endometrium. We then hypothesized that TIA-1 expression would be regulated throughout the menstrual cycle, consistent with a role for this ARE-BP in modulating cytokine and growth factor expression necessary for embryo receptivity in conception cycles and essential to normal menstrual cyclicity in nonconception cycles. Because cytokines and growth factors are strongly implicated in the establishment and growth of endometriotic implants, we also hypothesized that aberrant expression of TIA-1 would be associated with this condition. To this end, we characterized the expression of TIA-1 in eutopic and ectopic human endometrium in vivo and studied the role and regulation of TIA-1 using primary human endometrial stromal cell (HESC) cultures.
Materials and Methods
Tissue collection
Normal endometrial tissue samples were procured from patients at Yale-New Haven Hospital (New Haven, Connecticut), after hysterectomies or from endometrial biopsies that were being performed for treatment/diagnostic evaluation of a clinical indication relevant to the patient's primary gynecological complaint; specimens were only included in the study if there was no documented “suspicion of endometrial disease.” The study was approved by the institutional review board at Yale University School of Medicine under the protocol titled “Endometrial Evaluation” (HIC no. 1004006657). Consents for sample collection were obtained before surgical procedures. To establish menstrual cycle dating, the subject's menstrual history was obtained, and the endometrial histology was assessed using the criteria described by Noyes et al (39). Normal eutopic endometrial tissue samples were obtained from 30 reproductive age women and were grouped according to menstrual cycle phase: early proliferative (EP) (days 1–5, n = 4), midproliferative (MP) (days 6–10, n = 4), late proliferative (LP) (days 11–14, n = 4), early secretory (ES) (days 15–18, n = 5), midsecretory (MS) (days 19–23, n = 6), and late secretory (LS) (days 24–28, n = 7). Samples were assessed by immunohistochemistry.
To isolate primary endometrial stromal cells for culture, endometrial samples (n = 5) were obtained from fertile women who did not receive hormonal treatment in the preceding 3 months. These samples were placed in Hanks' balanced salt solution (Sigma-Aldrich) and transported to the laboratory for isolation of HESCs and long-term culture as described below and as published previously (40).
Ectopic endometrial and matched eutopic endometrial samples were obtained from 17 reproductive age women with endometriosis, who were undergoing laparoscopy. All women in this study group had advanced stages of endometriosis (stage III–IV) according to the criteria described in the revised American Fertility Society classification system (41). None of the patients had undergone hormonal treatment within the 3 months preceding their laparoscopy. The presence of endometriosis was confirmed by histopathologic assessment of the implants. Ectopic and eutopic endometrial samples from women with endometriosis were grouped based on menstrual cycle phase and determined by menstrual history and endometrial histology (39) of the eutopic endometrial sample obtained by endometrial biopsy at the time of laparoscopy: EP [days 1–5], n = 3; MP [days 6–10], n = 3; LP [days 11–14], n = 2; ES [days 15–18], n = 3; MS [days 19–23], n = 3; and LS [days 24–28], n = 3. For immunohistochemical analysis, samples were fixed in 10% buffered formalin and processed for paraffin embedding.
Immunohistochemistry
Immunohistochemical analysis was performed as described previously (40), with modifications. In brief, 5-μm sections of paraffin-embedded endometrial and endometriotic tissue were deparaffinized in xylene, rehydrated in graded series of alcohol, and immersed in distilled water. To block endogenous peroxidase activity, samples were treated with 3% hydrogen peroxide in distilled water with 50% methanol at room temperature for 20 minutes. After rinses in PBS, slides were incubated with 10% blocking horse serum (Vector Laboratories) in PBS in a humidified chamber for 30 minutes at room temperature. Then excess serum was drained, and sections were incubated with a polyclonal goat anti-human TIA-1 antibody (dilution 1:100; Santa Cruz Biotechnology, Inc) at 37°C for 1 hour in a humidified chamber. An equivalent concentration of goat IgG (Vector Laboratories) was used for negative controls. Slides were then rinsed in Tris-buffered saline (TBS) and incubated with biotinylated horse anti-goat antibody (1.5 mg/mL; Vector Laboratories) at 1:500 dilution for 30 minutes at room temperature. An avidin-biotin-peroxidase kit (LabVision) was used to detect the antigen-antibody complex, and diaminobenzidine (3,3-diaminobenzidine tetrahydrochloride dihydrate; LabVision) was used as the chromogen. Finally, sections were counterstained with hematoxylin and eosin.
TIA-1 immunostaining intensity was evaluated semiquantitatively using a scale from 0 (no staining) to 3 (the most intense staining) as described previously (40). A histological score (HSCORE) value was derived for each slide by summing the percentages of stained cells at each intensity multiplied by the score of intensity [HSCORE = ΣPi(i + 1)], where i is the intensity score and Pi is the corresponding percentage of the cells. Ten different areas were evaluated under the microscope for each slide, and the percentage of the cells for each intensity within each of these areas was determined at different times by 3 investigators blinded to the source of the tissues. The average of the 3 independent scores was used. Epithelial and stromal cells were separately evaluated and scored.
Isolation and culture of HESCs
HESCs were isolated and cultured as described previously (40). In brief, endometrial tissues were minced and digested in Hanks' balanced salt solution (Sigma-Aldrich) containing collagenase B (1 mg/mL [15 U/mg]; Roche), DNase I (0.1 mg/mL [1500 U/mg]; Roche), penicillin (200 U/mL), and streptomycin (200 mg/mL) at 37°C for 45 to 60 minutes with agitation. Dispersed HESCs were separated by filtration through a wire sieve (73-μm-diameter pore; Sigma-Aldrich) and cultured in 75-cm2 flasks (Falcon; BD Biosciences) incubated in a standard 95% air-5% CO2 incubator at 37°C in DMEM/Ham's F-12 (1:1, v/v; Sigma-Aldrich) containing penicillin (200 U/mL), streptomycin (200 mg/mL), and fetal bovine serum (10%, v/v; Gibco/BRL). When they reached confluence, cells were trypsinized (0.25%, Sigma-Aldrich), plated in 12-well plates at 5 × 104 cells/well concentration, and cultured to preconfluence. For each experiment, cells were cultured in serum-free, phenol red–free medium (Sigma-Aldrich) for 24 hours before treatment with vehicle, E2 (E1, 100 pM; E2, 1 nM; E3, 10 nM), progesterone (P1, 10 nM; P2, 100 nM; P3, 1 μM), TNF-α (10 ng/mL), IL-6 (100 or 500 ng/mL), or lipopolysaccharide (LPS) (1000 ng/mL) for 24 hours. After treatments, plates were rinsed in PBS and stored at −80°C until analysis. For experiments involving ELISAs, cell culture supernatants were collected and stored at −80°C until analyzed. All experiments were repeated 3 times using endometrial tissue from different women.
Western blot analysis
Protein extraction was performed using T-PER protein extraction reagent (Pierce) supplemented with a protease inhibitor cocktail (1 mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 mM phenylmethylsulfonylfluoride (Calbiochem). Protein quantification was performed using the Bradford protein assay (Bio-Rad Laboratories) according to the manufacturer's instructions. Western blot analysis was performed as described previously (40). In brief, for each sample, 10 μg of protein was fractionated by SDS-PAGE using 10% Tris-HCl Ready Gels (Bio-Rad Laboratories) and electroblotted onto a nitrocellulose membrane (Bio-Rad Laboratories). Blocking was performed using 5% nonfat dry milk in TBS-T buffer (0.1% Tween 20 in TBS) for 1 hour at room temperature, followed by incubation with the primary antibody (polyclonal goat anti-human TIA-1 antibody [dilution 1:500; Santa Cruz Biotechnology, Inc] or monoclonal rabbit antibody against nuclear factor κ-light chain enhancer of activated B cells [NF-κB, dilution 1:1000; Cell Signaling Technology]) overnight at 4°C. Membranes were then washed 3 times with TBS-T for 20 minutes each and incubated with peroxidase-labeled secondary antibody for 1 hour at room temperature (horse anti-goat IgG for TIA-1 [1:10,000 dilution; Vector Laboratories] or peroxidase-labeled goat anti-rabbit IgG for NF-κB [1:10 000 dilution; Vector Laboratories]). After 3 washes with TBS-T for 20 minutes each, expression levels were detected using chemiluminescent detecting reagents (PerkinElmer Life Sciences) and BioMax film (Kodak).
Subsequently, membranes were treated with stripping solution (Pierce) and reprobed with polyclonal rabbit anti-human β-actin antibody (Cell Signaling Technology). Quantification of immunoblot bands was performed using a laser densitometer. Each TIA-1 and NF-κB band was normalized to the value obtained from the corresponding β-actin band.
Lentiviral production
Lentiviral transduction vectors targeting human TIA-1 transcripts for repression/knockdown via intracellular production of short hairpin RNAs (shRNAs) were obtained from Santa Cruz Biotechnology (TIA-1 shRNA, catalog no. sc-29504-V; control shRNA, catalog no. sc-108080). These vectors, along with a vector containing the TIA-1 open reading sequence (PCR amplified from human kidney cDNA) cloned into an pCDH-CMV-puro vector (System Biosciences) were used to produce lentiviral particles as described previously (42). In brief, the target vector pCDH-TIA-1 or pLK0.1-TIA-1-shRNA was cotransfected into HEK293T cells (at 70% confluence in a 150-mm dish) with the delta 8.9 plasmid (expressing gag, pol, and rev), and the vesicular stomatitis virus G expression plasmid (expressing env) to produce replication-incompetent but highly infective viral particles. The packaged, unconcentrated virus–containing supernatant was collected over a period of 5 days posttransfection, filtered, concentrated by ultracentrifugation, and resuspended in sterile PBS. To determine the viral titers, HEK293T cells were infected with serial dilutions (from 10−2 to 10−5) of the concentrated viruses, and the transduced cell population was enriched after selection with puromycin (10 μg/mL). After a period of 10 to 14 days, cells were stained with crystal violet solution (catalog no. HT90132; Sigma-Aldrich), and stained colonies were counted. Lentiviral particles were used at 9 × 107 colony-transducing units/mL titer.
Lentiviral transduction of endometrial stromal cells
To achieve lentiviral transduction of HESCs, approximately 2.5 × 104 cells/well were plated in 12-well plates, and lentiviral particles were added to the cells in medium containing 8 μg/mL hexadimethrine bromide (catalog no. H9268; Sigma-Aldrich). The next day the medium was changed to medium without hexadimethrine bromide. Starting from 48 hours posttransduction, medium containing puromycin (2 μg/mL) was added to the cells.
ELISAs
IL-6 and TNF-α protein in HESC culture supernatants were quantified by ELISAs (R&D Systems). Each experiment was performed using 3 replicates for each condition, and supernatants from each experiment were tested in duplicate in the same ELISA. Each experiment was, in turn, performed independently on 3 occasions (3 biological repeats). IL-6 and TNF-α levels were normalized to total protein content in each respective well as determined by a Bio-Rad protein assay (catalog no. 500-0006).
Statistical analyses
The data from the immunohistochemistry HSCOREs, ELISAs, and Western blot analyses were normally distributed (determined by the Kolmogorov-Smirnov test). Statistical analyses were performed using the Student t test or one-way ANOVA followed by post hoc Holm-Sidak testing where appropriate. SigmaStat for Windows, version 3.0 (Jandel Scientific Corp) was used for statistical analyses. Statistical significance was defined as a value of P < .05 for the Student t test or one-way ANOVA and P < .005 for post hoc analyses.
Results
TIA-1 protein expression in human endometrium is temporally regulated during the menstrual cycle
We first assessed expression of TIA-1 in endometrial tissue samples obtained from women without known endometrial pathologic conditions or endometriosis. Specimens collected at different phases of the menstrual cycle were assessed by immunohistochemistry. The menstrual cycle distributions for the specimens were as follows: EP (days 1–5), n = 4; MP (days 6–10); n = 4, LP (days 11–14) n = 4; ES (days 15–18); n = 5, MS (days 19–23) n = 6; and LS (days 24–28) n = 7.
TIA-1 immunoreactivity was detected in the nucleus and cytoplasm, with higher intensity in the nucleus in both glands and stroma (Figure 1A). TIA-1 expression in both stromal cells (Figure 1B) and glandular cells (Figure 1C) increased at the LS phase and the highest expression was detected in the EP phase, whereas the lowest expression was detected at midcycle (P < .05) (Figure 1, B and C). Negative control staining (nonspecific IgG) on tissue obtained during the MS stage of the menstrual cycle is shown in Supplemental Figure 1, A and B.
Figure 1.
A, TIA-1 protein expression in representative samples of eutopic endometrial tissue from the EP, MP, LP, ES, MS, and LS phases of the menstrual cycle. TIA-1 immunostaining is nuclear and cytoplasmic, with a higher intensity in the nucleus. B, TIA-1 protein expression in normal eutopic endometrial stromal cells according to the menstrual cycle phase. HSCORE analysis was performed to quantitate endometrial stromal expression of TIA-1 from biopsy samples. TIA-1 protein expression is significantly higher in the EP, MP, LP, and LS phases, with the highest expression during the EP phase. P < .01: *, EP vs LP, ES, MS, and LS; **, MP vs LP, ES, and MS; ***, LP vs ES; ****, LS vs ES and MS. C, TIA-1 protein expression in eutopic endometrial glandular cells according to the menstrual cycle phase. HSCORE analysis was performed to quantitate the endometrial glandular expression of TIA-1 from biopsy samples. TIA-1 protein expression is significantly higher in the LS and EP phases, with the highest expression during the EP phase. P < .01: *, EP vs LP, ES, and MS; **, LS vs ES.
TIA-1 protein expression in HESCs is not directly regulated by estrogen or progesterone
Having observed that endometrial TIA-1 expression is regulated throughout the menstrual cycle in vivo, we asked whether the sex steroids that regulate cyclic endometrial growth and differentiation regulate the expression of TIA-1 in isolated HESCs in vitro. HESCs in culture were treated with vehicle, estrogen (100 pM, 1 nM, and 10 nM) or progesterone (10 nM, 100 nM, or 1 μM) for 24 hours. All experiments were repeated 3 times using endometrial cells isolated from different women.
TIA-1 protein expression was determined by Western blot analysis and normalized to β-actin. Estrogen or progesterone treatments, even when titrated to physiologic doses, did not result in a change in TIA-1 expression in cultured HESCs compared with the result for treatment with vehicle alone (Figure 2). We previously confirmed that primary HESCs express estrogen receptor α and progesterone receptor protein and respond to hormone treatment (40).
Figure 2.

TIA-1 protein expression in normal primary HESCs treated with E2 and progesterone. Cultured HESCs were treated with vehicle, 3 different doses of estrogen (E1, 100 pM; E2, 1 nM; E3, 10 nM) or 3 different doses of progesterone (P1, 10 nM; P2, 100 nM; P3, 1 μΜ) for 24 hours. Total protein was extracted, and TIA-1 protein expression was analyzed by Western blot. A band corresponding to TIA-1 was observed at ∼45 kDa. Reprobing the blot with an antibody against β-actin provided the internal loading control. Bars represent means + SD. P = nonsignificant for control vs E1, E2, and E3 or P1, P2, and P3.
Immune factors enhance the expression of TIA-1 in HESCs
We next asked whether inflammatory factors might regulate TIA-1 expression in endometrium. Primary HESCs were treated with either vehicle, TNF-α (10 ng/mL), LPS (1000 ng/mL), or IL-6 (100 or 500 ng/mL) for 24 hours. All experiments were repeated 3 times using endometrial tissue from different women.
Western blot analysis revealed that TIA-1 expression is increased in response to TNF-α or LPS treatments of HESCs (Figure 3). Expression of NF-κB was also enhanced in these cells in response to TNF-α and LPS treatments, confirming the responsiveness of these cells to inflammatory stimuli (Figure 3). However, there was no change in TIA-1 expression in response to treatment with IL-6 (Supplemental Figure 2). The specificity of the TIA-1 antibody is demonstrated by a single band of the predicted molecular weight on Western blot analysis (Supplemental Figure 3).
Figure 3.
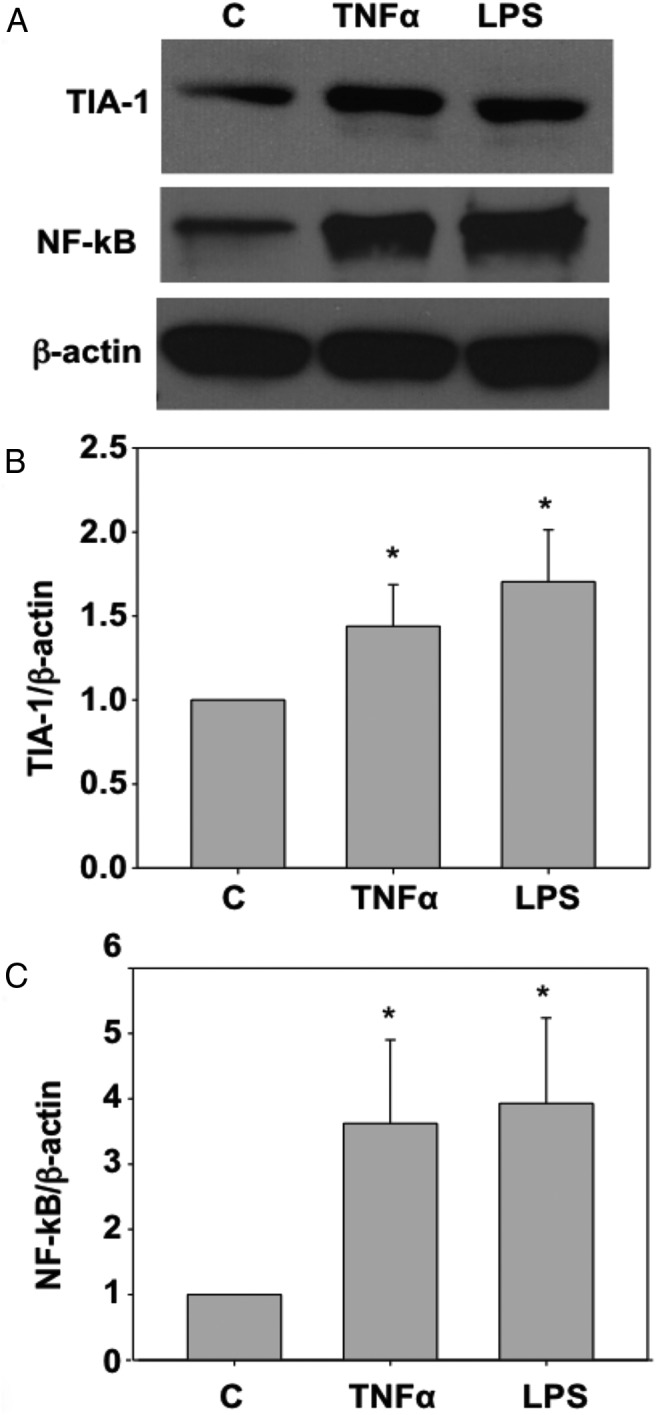
A, TIA-1 and NF-κB protein expression levels in primary HESCs treated with TNF-α or LPS. Cultured HESCs were treated with vehicle, TNF-α (10 ng/mL), or LPS (1000 ng/mL) for 24 hours each. Total protein was extracted and TIA-1 protein was analyzed by Western blot. A band corresponding to TIA-1 was observed at ∼45 kDa. Reprobing the blot with an antibody against NF-κB and then β-actin provided the positive control for immune stimulation and the internal loading control, respectively. Bars represent means + SD. B and C, TIA-1 (B) and NF-κB (C) expression in HESCs increased significantly in response to TNF-α or LPS treatment. *, P < .05 for TNF-α or LPS vs control.
In women with endometriosis, TIA-1 protein expression in eutopic and ectopic endometrium is decreased compared with that in normal control endometrium
Ectopic and eutopic endometrial samples obtained from 17 women with endometriosis were evaluated by immunohistochemistry. The distribution of samples according to menstrual cycle phase was as follows: EP (days 1–5), n = 3; MP (days 6–10), n = 3; LP (days 11–14), n = 2; ES (days 15–18), n = 3; MS (days 19–23), n = 3; and LS (days 24–28), n = 3.
TIA-1 expression was nuclear and cytoplasmic, with a higher intensity in the nucleus in all ectopic endometrial tissues (Figure 4A). The mean TIA-1 immunoreactivity in stromal and glandular cells of ectopic endometrial tissues was compared with that of eutopic endometrial samples from the same women and also compared with phase-matched endometrial expression in normal control women. The TIA-1 expression in eutopic and ectopic endometrium of women with endometriosis was significantly reduced compared with the expression in normal endometrium in EP, MP, and LS phases in stromal cells and in EP and LS phases in glandular cells (P < .005) (Figure 4, B and C).
Figure 4.
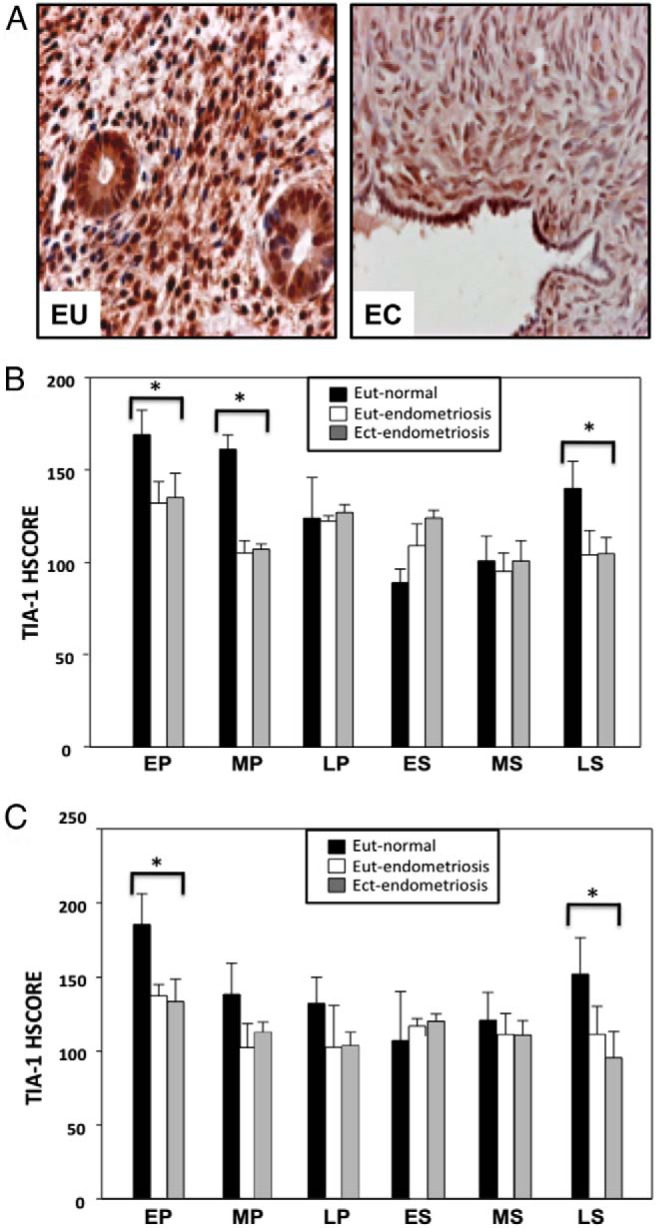
A, Representative micrographs of TIA-1 protein immunostaining in normal eutopic endometrium (EU) from a woman without endometriosis and in ectopic endometrium from a woman with endometriosis (EC) in the EP phase. TIA-1 expression is cytoplasmic and nuclear in both the glandular and stromal components of normal endometrium and ectopic endometrium. B, TIA-1 protein expression in stromal cells of eutopic endometrium from women without endometriosis (■; Eut-normal) and eutopic (□; Eut-endometriosis) and ectopic (▩; Ect-endometriosis) endometrium from women with endometriosis. HSCORE analysis was performed to quantitate endometrial stromal cell expression of TIA-1 from biopsy samples. *, P < .005, normal endometrium vs eutopic or ectopic endometrium from women with endometriosis in the same menstrual cycle phase. C, TIA-1 protein expression in glandular cells of eutopic endometrium from women without endometriosis (■; Eut-normal) and eutopic (□; Eut-endometriosis) and ectopic (▩; Ect-endometriosis) endometrium from women with endometriosis. HSCORE analysis was performed to quantitate endometrial glandular expression of TIA-1 from biopsy samples. *, P < .005; normal endometrium vs eutopic or ectopic endometrium from women with endometriosis in the same menstrual cycle phase.
TIA-1 suppresses IL-6 and TNF-α expression in HESCs
To determine whether TIA-1 regulates expression of proinflammatory cytokines in the endometrium, we investigated the effects of altered expression of TIA-1 on IL-6 and TNF-α secretion in HESCs. First, we confirmed that lentiviral expression systems can be used to overexpress and suppress TIA-1 in primary HESCs in culture (Figure 5A). We then tested whether overexpression or suppression of TIA-1 in HESCs altered expression of IL-6 or TNF-α protein. Cells were placed in serum-free culture medium for 24 hours before treatment with vehicle control or TNF-α (10 ng/mL) or LPS (1000 ng/mL). Culture medium was then tested by ELISA, normalized to the total protein in each well. Overexpression of TIA-1 resulted in 4- to 5-fold reduced expression of IL-6 in basal and TNF-α-stimulated cells, whereas suppression of TIA-1 expression led to 2- to 3-fold elevated IL-6 expression in cultured HESCs under basal and TNF-α–stimulated conditions (Figure 5B). The effect of TIA-1 overexpression and down-regulation on TNF-α expression was similarly evaluated. As observed for IL-6, overexpression of TIA-1 caused a 2- to 3-fold decrease in TNF-α expression in both basal and LPS-stimulated cells, whereas suppression of TIA-1 expression resulted in approximately 2-fold enhancement of TNF-α expression (Figure 5C).
Figure 5.
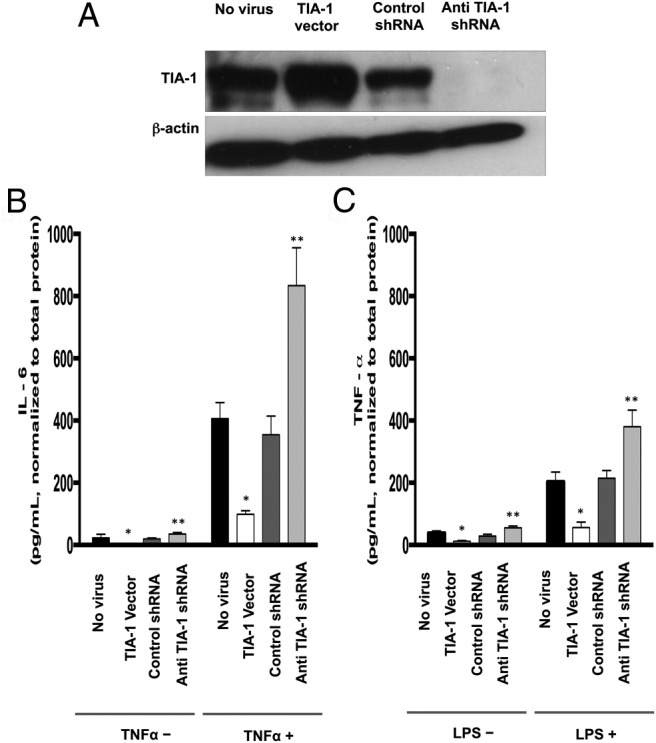
A, TIA-1 expression in HESCs after lentiviral-mediated overexpression and knockdown. Cells were transduced with vehicle (No virus), TIA-1–expressing lentiviral particles (TIA-1 vector), control shRNA-expressing lentiviral particles (Control shRNA) or anti-TIA-1 shRNA-expressing lentiviral particles (Anti TIA-1 shRNA). Total protein was extracted, and TIA-1 protein expression was analyzed by Western blot. A band corresponding to TIA-1 was observed at ∼45 kDa. Reprobing the blot with an antibody against β-actin provided the internal loading control. B, IL-6 expression by HESCs after lentiviral-mediated overexpression and knockdown of TIA-1 with or without TNF-α pretreatment. Cells were transduced with vehicle (No virus), TIA-1–expressing lentiviral particles (TIA-1 Vector), control shRNA–expressing lentiviral particles (Control shRNA), or anti-TIA-1 shRNA-expressing lentiviral particles (Anti TIA-1 shRNA). Cells were grown to preconfluence and then treated with vehicle or TNF-α (10 ng/mL) in serum-free medium for 24 hours. Culture medium was collected, and IL-6 levels were detected in the supernatants of cells by ELISAs. *, P < .05, TIA-1 vector vs no virus or control shRNA; **, P < .05, anti TIA-1 shRNA vs no virus or control shRNA. C, TNF-α expression by HESCs after lentiviral-mediated overexpression and knockdown of TIA-1 with or without LPS pretreatment. Cells were transduced with vehicle (No virus), TIA-1–expressing lentiviral particles (TIA-1 Vector), control shRNA-expressing lentiviral particles (Control shRNA), or anti-TIA-1 shRNA-expressing lentiviral particles (Anti TIA-1 shRNA). Cells were grown to preconfluence and then were treated with vehicle or LPS (1000 ng/mL) in serum-free medium for 24 hours. Culture medium was collected, and TNF-α levels were detected in the supernatants of cells by ELISAs. *, P < .05, TIA-1 vector vs no virus or control shRNA; **, P < .05; anti TIA-1 shRNA vs no virus or control shRNA
Discussion
TIA-1 is an ARE-BP that functions as a translational silencer for a multitude of proinflammatory genes including TNF-α, IL-1β, IL-6, and COX-2 (34, 35). In this study, we showed that TIA-1 is expressed in normal human endometrium and that its expression is regulated throughout the menstrual cycle. We also demonstrated that endometrial TIA-1 expression is regulated by immune mediators but not by sex steroids. Further, TIA-1 regulates expression of the inflammatory cytokine IL-6 and TNF-α in HESCs. Importantly, we found that TIA-1 expression is suppressed in ectopic and eutopic endometrium of women with endometriosis. Previous studies have indicated a role for immune factors in regulating normal endometrial functions as well as in contributing to the pathogenesis of endometriosis. Our findings suggest that TIA-1 may play a role in normal endometrial physiology and in the development or maintenance of ectopic endometrial implants in women with endometriosis.
During the endometrial cycle, cytokines and growth factors play critical roles in regulating leukocyte migration and function. Moreover, these factors are involved in the proliferation, differentiation, and perimenstrual apoptotic loss of cells in the endometrium. A significant number of cytokines and growth factors are expressed by endometrial cells, collectively helping to attract immune cells including T lymphocytes, monocytes, basophils, and eosinophils to the endometrium in a highly regulated spatiotemporal program (43). Many of these cytokines and growth factors, including TNF-α (44, 45), IL-1β (46), IL-6 (47, 48), IL-8 (49, 50), IL-11 (51), monocyte chemoattractant protein-1 (50), TGF-β (52), and COX-2 (50), are most highly expressed in the endometrium during the perimenstrual period of the menstrual cycle coincident with the need for leukocyte infiltration. We found that TIA-1 protein expression is significantly higher in the LS and EP phases, consistent with a role for TIA-1 in modulating cytokine expression in these phases. Low TIA-1 expression was similarly associated with menstrual cycle phases that demonstrate low inflammatory cytokine expression in the endometrium (midcycle). Whether increased TIA-1 expression during the EP phase is a necessary compensatory mechanism that buffers the expression of inflammatory cytokines in the endometrium remains to be tested.
We recently characterized the expression of another ARE-BP, HuR/ELAVL1, in human endometrium throughout the menstrual cycle (40). HuR promotes the mRNA stability of a large number of ARE-bearing transcripts encoding inflammatory and immune factors, including TNF-α (53), IL-1β (54), IL-3 (55), IL-8 (56), granulocyte macrophage–colony-stimulating factor (55), COX-2 (57), and vascular endothelial growth factor (55). We found that HuR expression is significantly lower in the LP and EP phases (40), when TIA-1 levels are at their highest. Our data support a complementary role for HuR (stabilizing) and TIA-1 (destabilizing) ARE-BPs in menstrual cycle–specific regulation of important inflammatory cytokines and growth factors.
Because TIA-1 expression in human endometrium was regulated during the menstrual cycle, we tested whether steroid hormones play a role in controlling TIA-1 expression in HESCs. We found no direct effect of estrogen or progesterone on TIA-1 expression in isolated HESCs (Figure 2). These data do not rule out indirect or paracrine effects of steroid hormone exposure on TIA-1 expression in vivo, as has been described for the expression of the cytokine regulated upon activation, normal T cell expressed and secreted (58). Although we were unable to identify a direct effect of steroid hormones on the regulation of TIA-1 expression in HESCs, our in vitro findings indicate that immune factors play a role in regulating TIA-1 expression in endometrium (Figure 3). Our data demonstrating LPS- and TNF-α–mediated enhancement of TIA-1 expression, along with TIA-1–mediated suppression of IL-6 and TNF-α secretion in HESCs, are consistent with a compensatory response of TIA-1 to inflammatory stimuli in endometrial cells.
Based on our in vitro findings demonstrating up-regulation of TIA-1 in HESCs in response to immune mediators and because of the elevated levels of cytokines and growth factors in the peritoneal fluid of women with endometriosis (1, 5), we anticipated a compensatory elevation in TIA-1 expression in endometriotic implants. Likewise, we observed that expression of HuR, a stabilizer of cytokine transcripts, is decreased in ectopic endometrial implants, consistent with a compensatory reduction of HuR expression in the setting of high cytokine concentrations (40). Surprisingly, we observed that eutopic and ectopic endometrium from women with endometriosis express decreased levels of TIA-1 during the LS and EP phases (perimenstrual timeframe). We propose that the decreased TIA-1 expression in these patients contributes to the inflammatory environment in the peritoneum and intrauterine cavity, contributing to the pathogenesis of endometriosis and exacerbating the pain and infertility often associated with the disease.
In eukaryotic cells, several pathways of mRNA decay converge to influence the stability and translation of target mRNAs. Many signaling pathways and proteins participate in these processes, some of which promote and others of which suppress mRNA expression. ARE-mediated mRNA degradation is one pathway of mRNA translational silencing or decay. TIA-1 promotes translational suppression (34, 35) or decay (59) of a multitude of ARE-containing transcripts including those encoding for cytokines/chemokines (34, 35). Importantly, the mRNA targets of TIA-1 depend upon the cell type and cell milieu (60), and identifying the mRNA targets of TIA-1 in endometrial cells will be important in elucidating a role for TIA-1 in endometrial function and in the pathogenesis of endometriosis.
Abnormalities in the posttranscriptional control of ARE-containing mRNAs have been implicated in disease states involving chronic inflammation and cancer (33). An enhanced understanding of the function of ARE-binding proteins governing the stability and/or translation of ARE-containing mRNAs is likely to lead to the development of new therapeutic tools for the treatment of a multitude of inflammatory disorders. Our findings suggest that TIA-1 gene expression plays a central role in endometrial physiology and possibly in the pathogenesis of endometriosis. Whether the suppressed TIA-1 expression observed in women with endometriosis contributes to the development and growth of endometriotic implants remains to be tested. Whether modulation of TIA-1 expression in these cells would constitute a useful therapy for the treatment of endometriosis-related infertility and/or pain also remains to be determined.
Acknowledgments
E.S. is supported by the National Institutes of Health (NIH) (Grant R01 HD059909). C.B.K. is supported by the NIH (Grant R01 DK091841). The content of the manuscript is solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARE
- adenosine- and uridine-rich element
- ARE-BP
- adenosine- and uridine-rich element–binding protein
- COX-2
- cyclooxygenase 2
- E2
- estradiol
- HSCORE
- histological score
- HESC
- human endometrial stromal cell
- LPS
- lipopolysaccharide
- NF-κB
- nuclear factor κ-light chain enhancer of activated B cells
- P
- progesterone
- shRNA
- short hairpin RNA
- TBS
- Tris-buffered saline
- TIA-1
- T-cell intracellular antigen.
References
- 1. Seli E, Berkkanoglu M, Arici A. Pathogenesis of endometriosis. Obstet Gynecol Clin North Am. 2003;30:41–61. [DOI] [PubMed] [Google Scholar]
- 2. Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41–46. [PubMed] [Google Scholar]
- 3. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86:1561–1572. [DOI] [PubMed] [Google Scholar]
- 4. Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:394–404. [DOI] [PubMed] [Google Scholar]
- 5. Seli E, Arici A. Endometriosis: Interaction of immune and endocrine systems. Semin Reprod Med. 2003;21:135–144. [DOI] [PubMed] [Google Scholar]
- 6. Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue in peritoneal cavity. Am J Obstet Gynecol. 1927;15:422. [Google Scholar]
- 7. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- 8. Liu DT, Hitchcock A. Endometriosis: its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br J Obstet Gynaecol. 1986;93:859–862. [DOI] [PubMed] [Google Scholar]
- 9. Eisermann J, Gast MJ, Pineda J, Odem RR, Collins JL. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertil Steril. 1988;50:573–579. [DOI] [PubMed] [Google Scholar]
- 10. Mori H, Sawairi M, Nakagawa M, Itoh N, Wada K, Tamaya T. Peritoneal fluid interleukin-1 beta and tumor necrosis factor in patients with benign gynecologic disease. Am J Reprod Immunol. 1991;26:62–67. [DOI] [PubMed] [Google Scholar]
- 11. Taketani Y, Kou TM, Mizuno M. Comparison of cytokine levels and embryo toxicity in peritoneal fluid in infertile women with untreated or treated endometriosis. Am J Obstet Gynecol. 1992;167:265–270. [DOI] [PubMed] [Google Scholar]
- 12. Mier-Cabrera J, Jiménez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernández-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118:6–16. [DOI] [PubMed] [Google Scholar]
- 13. Fakih H, Baggett B, Holtz G, Tsang KY, Lee JC, Williamson HO. Interleukin-1: a possible role in the infertility associated with endometriosis. Fertil Steril. 1987;47:213–217. [PubMed] [Google Scholar]
- 14. Hill JA, Anderson DJ. Lymphocyte activity in the presence of peritoneal fluid from fertile women and infertile women with and without endometriosis. Am J Obstet Gynecol. 1989;161:861–864. [DOI] [PubMed] [Google Scholar]
- 15. Keenan JA, Chen TT, Chadwell NL, Torry DS, Caudle MR. Interferon-γ (IFN-γ) and interleukin-6 (IL-6) in peritoneal fluid and macrophage-conditioned media of women with endometriosis. Am J Reprod Immunol. 1994;32:180–183. [DOI] [PubMed] [Google Scholar]
- 16. Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol. 1996;174:1522–1526. [DOI] [PubMed] [Google Scholar]
- 17. Schröder W, Gaetje R, Baumann R. Interleukin-6 and soluble interleukin-6 receptor in peritoneal fluid and serum of patients with endometriosis. Clin Exp Obstet Gynecol. 1996;23:10–14. [PubMed] [Google Scholar]
- 18. Harada T, Yoshioka H, Yoshida S, et al. Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol. 1997;176:593–597. [DOI] [PubMed] [Google Scholar]
- 19. Mahnke JL, Dawood MY, Huang JC. Vascular endothelial growth factor and interleukin-6 in peritoneal fluid of women with endometriosis. Fertil Steril. 2000;73:166–170. [DOI] [PubMed] [Google Scholar]
- 20. Kalu E, Sumar N, Giannopoulos T, et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33:490–495. [DOI] [PubMed] [Google Scholar]
- 21. Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN. Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril. 1995;63:929–932. [PubMed] [Google Scholar]
- 22. Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL. Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod. 1996;2:40–45. [DOI] [PubMed] [Google Scholar]
- 23. Pizzo A, Salmieri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest. 2002;54:82–87. [DOI] [PubMed] [Google Scholar]
- 24. Calhaz-Jorge C, Costa AP, Santos MC, Palma-Carlos ML. Peritoneal fluid concentrations of interleukin-8 in patients with endometriosis depend on the severity of the disorder and are higher in the luteal phase. Hum Reprod. 2003;18:593–597. [DOI] [PubMed] [Google Scholar]
- 25. Ho HN, Wu MY, Chao KH, Chen CD, Chen SU, Yang YS. Peritoneal interleukin-10 increases with decrease in activated CD4+ T lymphocytes in women with endometriosis. Hum Reprod. 1997;12:2528–2533. [DOI] [PubMed] [Google Scholar]
- 26. Tabibzadeh S, Becker JL, Parsons AK. Endometriosis is associated with alterations in the relative abundance of proteins and IL-10 in the peritoneal fluid. Front Biosci. 2003;8:a70–a78. [DOI] [PubMed] [Google Scholar]
- 27. Arici A, Oral E, Attar E, Tazuke SI, Olive DL. Monocyte chemotactic protein-1 concentration in peritoneal fluid of women with endometriosis and its modulation of expression in mesothelial cells. Fertil Steril. 1997;67:1065–1072. [DOI] [PubMed] [Google Scholar]
- 28. Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol. 1993;169:1545–1549. [DOI] [PubMed] [Google Scholar]
- 29. McLaren J, Prentice A, Charnock-Jones DS, Smith SK. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11:220–223. [DOI] [PubMed] [Google Scholar]
- 30. Anderson P, Phillips K, Stoecklin G, Kedersha N. Post-transcriptional regulation of proinflammatory proteins. J Leukoc Biol. 2004;76:42–47. [DOI] [PubMed] [Google Scholar]
- 31. Wilusz CJ, Wang W, Peltz SW. Curbing the nonsense: the activation and regulation of mRNA surveillance. Genes Dev. 2001;15:2781–2785. [DOI] [PubMed] [Google Scholar]
- 32. Garneau NL, Wilusz J, Wilusz CJ. the highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. [DOI] [PubMed] [Google Scholar]
- 33. Espel E. The role of the AU-rich elements of mRNAs in controlling translation. Semin Cell Dev Biol. 2005;16:59–67. [DOI] [PubMed] [Google Scholar]
- 34. Piecyk M, Wax S, Beck AR, et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-α. EMBO J. 2000;19:4154–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dixon DA, Balch GC, Kedersha N, Anderson P, Zimmerman GA, Beauchamp RD, Prescott SM. Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. J Exp Med. 2003;198:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Subramaniam K, Ooi LL, Hui KM. Transcriptional down-regulation of IGFBP-3 in human hepatocellular carcinoma cells is mediated by the binding of TIA-1 to its AT-rich element in the 3′-untranslated region. Cancer Lett. 2010;297:259–268. [DOI] [PubMed] [Google Scholar]
- 37. Bossowski A, Czarnocka B, Bardadin K, et al. Identification of chosen apoptotic (TIAR and TIA-1) markers expression in thyroid tissues from adolescents with immune and non-immune thyroid diseases. Folia Histochem Cytobiol. 2010;48:178–184. [DOI] [PubMed] [Google Scholar]
- 38. Phillips K, Kedersha N, Shen L, Blackshear PJ, Anderson P. Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis. Proc Natl Acad Sci USA. 2004;101:2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. [DOI] [PubMed] [Google Scholar]
- 40. Karipcin FS, Ensari TA, Kayisli UA, Guzel E, Kallen CB, Seli E. The mRNA-binding protein HuR is regulated in the menstrual cycle and repressed in ectopic endometrium. Reprod Sci. 2011;18:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. American Fertility Society. Revised American Fertility Society classification of endometriosis:1985. Fertil Steril. 1985;43:351–352. [DOI] [PubMed] [Google Scholar]
- 42. Zhang H, Taylor WR, Joseph G, et al. mRNA-binding protein ZFP36 is expressed in atherosclerotic lesions and reduces inflammation in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. [DOI] [PubMed] [Google Scholar]
- 44. Hunt JS, Chen HL, Hu XL, Tabibzadeh S. Tumor necrosis factor-α messenger ribonucleic acid and protein in human endometrium. Biol Reprod. 1992;47:141–147. [DOI] [PubMed] [Google Scholar]
- 45. Philippeaux MM, Piguet PF. Expression of tumor necrosis factor-α and its mRNA in the endometrial mucosa during the menstrual cycle. Am J Pathol. 1993;143:480–486. [PMC free article] [PubMed] [Google Scholar]
- 46. Simón C, Piquette GN, Frances A, Polan ML. Localization of interleukin-1 type I receptor and interleukin-1β in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab. 1993;77:549–555. [DOI] [PubMed] [Google Scholar]
- 47. Tabibzadeh S, Kong QF, Babaknia A, May LT. Progressive rise in the expression of interleukin-6 in human endometrium during menstrual cycle is initiated during the implantation window. Hum Reprod. 1995;10:2793–2799. [DOI] [PubMed] [Google Scholar]
- 48. von Wolff M, Thaler CJ, Zepf C, Becker V, Beier HM, Strowitzki T. Endometrial expression and secretion of interleukin-6 throughout the menstrual cycle. Gynecol Endocrinol. 2002;16:121–129. [PubMed] [Google Scholar]
- 49. Arici A, Seli E, Senturk LM, Gutierrez LS, Oral E, Taylor HS. Interleukin-8 in the human endometrium. J Clin Endocrinol Metab. 1998;83:1783–1787. [DOI] [PubMed] [Google Scholar]
- 50. Jones RL, Hannan NJ, Kaitu'u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155–6167. [DOI] [PubMed] [Google Scholar]
- 51. Dimitriadis E, Slamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod. 2000;6:907–914. [DOI] [PubMed] [Google Scholar]
- 52. Casslén B, Sandberg T, Gustavsson B, Willén R, Nilbert M. Transforming growth factor β1 in the human endometrium. Cyclic variation, increased expression by estradiol and progesterone, and regulation of plasminogen activators and plasminogen activator inhibitor-1. Biol Reprod. 1998;58:1343–1350. [DOI] [PubMed] [Google Scholar]
- 53. Atasoy U, Curry SL, López de Silanes I, et al. Regulation of eotaxin gene expression by TNF-α and IL-4 through mRNA stabilization: involvement of the RNA-binding protein HuR. J Immunol. 2003;171:4369–4378. [DOI] [PubMed] [Google Scholar]
- 54. Suswam EA, Nabors LB, Huang Y, Yang X, King PH. IL-1β induces stabilization of IL-8 mRNA in malignant breast cancer cells via the 3′ untranslated region: Involvement of divergent RNA-binding factors HuR, KSRP and TIAR. Int J Cancer. 2005;113:911–919. [DOI] [PubMed] [Google Scholar]
- 55. Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palanisamy V, Park NJ, Wang J, Wong DT. AUF1 and HuR proteins stabilize interleukin-8 mRNA in human saliva. J Dent Res. 2008;87:772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dixon DA, Tolley ND, King PH, et al. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J Clin Invest. 2001;108:1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Akoum A, Lemay A, Maheux R. Estradiol and interleukin-1β exert a synergistic stimulatory effect on the expression of the chemokine regulated upon activation, normal T cell expressed, and secreted in endometriotic cells. J Clin Endocrinol Metab. 2002;87:5785–5792. [DOI] [PubMed] [Google Scholar]
- 59. Yamasaki S, Stoecklin G, Kedersha N, Simarro M, Anderson P. T-cell intracellular antigen-1 (TIA-1)-induced translational silencing promotes the decay of selected mRNAs. J Biol Chem. 2007;282:30070–30077. [DOI] [PubMed] [Google Scholar]
- 60. Saito K, Chen S, Piecyk M, Anderson P. TIA-1 regulates the production of tumor necrosis factor alpha in macrophages, but not in lymphocytes. Arthritis Rheum. 2001;44:2879–2887. [DOI] [PubMed] [Google Scholar]



