Abstract
Context:
The inability to accurately localize the parathyroid glands during parathyroidectomy and thyroidectomy procedures can prevent patients from achieving postoperative normocalcemia. There is a critical need for an improved intraoperative method for real-time parathyroid identification.
Objective:
The objective of the study was to test the accuracy of a real-time, label-free technique that uses near-infrared (NIR) autofluorescence imaging to localize the parathyroid.
Setting:
The study was conducted at the Vanderbilt University endocrine surgery center.
Subjects and Methods:
Patients undergoing parathyroidectomy and/or thyroidectomy were included in this study. To validate the intrinsic fluorescence signal in parathyroid, point measurements from 110 patients were collected using NIR fluorescence spectroscopy. Fluorescence imaging was performed on 6 patients. Imaging contrast is based on a previously unreported intrinsic NIR fluorophore in the parathyroid gland. The accuracy of fluorescence imaging was analyzed in comparison with visual assessment and histological findings.
Main Outcome Measure:
The detection rate of parathyroid glands was measured.
Results:
The parathyroid glands in 100% of patients measured with fluorescence imaging were successfully detected in real time. Fluorescence images consistently showed 2.4 to 8.5 times higher emission intensity from the parathyroid than surrounding tissue. Histological validation confirmed that the high intrinsic fluorescence signal in the parathyroid gland can be used to localize the parathyroid gland regardless of disease state.
Conclusion:
NIR fluorescence imaging represents a highly sensitive, real-time, label-free tool for parathyroid localization during surgery. The elegance and effectiveness of NIR autofluorescence imaging of the parathyroid gland makes it highly attractive for clinical application in endocrine surgery.
One of the most common problems in surgical intervention of patients with parathyroid and thyroid disease is the inability to localize the parathyroid glands (1–3). Intraoperative parathyroid identification is difficult due to their small size, inconspicuous coloring, and variable location (4).
The challenge of parathyroid localization is heightened in patients undergoing reoperation or presenting with ectopic and/or supernumerary abnormal glands (5). Accidental removal of healthy parathyroid glands during thyroidectomy results in postoperative hypocalcemia in 20% to 30% of thyroidectomy cases (6). Conversely, insufficient removal of diseased parathyroid glands during parathyroidectomy can require reoperation due to persistent hypercalcemia (7).
High complication rates during cervical endocrine procedures have spurred efforts to image parathyroid location (8–10). Currently, the most commonly employed image guidance techniques for parathyroid localization in hyperparathyroidism cases are sestamibi scintigraphy, ultrasound, computed tomography, and magnetic resonance imaging (11). However, these methods are limited by their inability to reliably localize healthy glands or provide real-time intraoperative information. In the case of sestamibi scintigraphy, the administration of radiotracers requires an additional step and can suffer from nonselective tissue uptake (11, 12). Current intraoperative methods for parathyroid detection include frozen section analysis and fine-needle aspiration with intact PTH assay. Frozen section analysis may require partial excision of a gland, which risks damage to its blood supply (13). Fine-needle aspiration with intact PTH assay suffers from false-negative rates and is usually employed ex vivo once the parathyroid has been excised (14). There is currently no intraoperative method for identifying the parathyroid gland in real time regardless of disease state. Surgeons must therefore rely on visual inspection, which is highly subjective and dependent on experience (15–17).
Optical imaging methods can improve the accuracy of surgical resection by producing higher-resolution images in real time (18). Near-infrared (NIR) fluorescence imaging is particularly attractive because it takes advantage of an optical window between 700 and 900 nm where light can penetrate deep into tissue with relatively little scattering or absorption (19). Because reports of endogenous NIR fluorophores are rare, clinical use of NIR fluorescence has mostly involved exogenous contrast agents (20, 21). A method that relies on intrinsic NIR fluorescence could avoid the challenges associated with the use of contrast agents such as potential toxicity, photobleaching, and localization. Such a method could provide real-time intraoperative information while taking advantage of the longer NIR wavelength.
A promising target for parathyroid identification and localization is a novel, intrinsic, NIR fluorophore that has been discovered in high concentrations in the parathyroid gland regardless of disease state (22, 23). In this study, the nature of this fluorophore is investigated over a large patient population using NIR fluorescence spectroscopy. To improve visualization, an imaging system was designed for intraoperative anatomical guidance to the parathyroid gland via this fluorescence signal. In this pilot study, we report the results of the first real-time intraoperative intrinsic fluorescence imaging of the parathyroid gland to aid cervical endocrine surgery in human patients.
Patients and Methods
The central goal of this study was to evaluate the feasibility of NIR autofluorescence imaging to localize the parathyroid gland intraoperatively. We began by measuring the spectral properties of the fluorescence signal from neck tissues in humans (n = 110) during parathyroidectomy and thyroidectomy. We then showed proof-of concept using a custom intraoperative NIR fluorescence imaging system to image the parathyroid glands (n = 6). We then used histology to validate the high fluorescence signals imaged in healthy parathyroid glands of canine subjects (n = 6).
Patient recruitment
Patients undergoing parathyroidectomy and/or thyroidectomy were recruited for this study. Approval was obtained from the Investigational Review Board at Vanderbilt University. Study inclusion criteria included 1) adult patients between the ages of 18 and 99 years and 2) presenting with primary thyroid or parathyroid disease. The attending surgeon at the Vanderbilt Clinic conducted a preoperative evaluation to ensure each subject was a safe and acceptable candidate for the study. Informed written consent was obtained from each patient enrolled. All optical procedures were performed such that patient care was not compromised.
Clinical fluorescence spectroscopy
An NIR fluorescence spectroscopy system was assembled using a 785-nm diode laser (Innovative Photonic Solutions; I0785SL0050PA) connected to a fluorescence spectrometer (Ocean Optics; S2000-FL) through a fiber optic probe. The probe consists of 7-around-1 fibers with inline filtering to attenuate the signals generated in the delivery and collection fibers. The parathyroidectomy or thyroidectomy procedure was performed per routine. When the parathyroid was exposed, fluorescence spectra were acquired from parathyroid, thyroid, muscle, and fat. The visual assessment and level of confidence of the surgeon on the type of tissue and pathology were recorded. Six measurements were collected at each tissue site by the surgeon at an integration time of 300 milliseconds with the overhead operating room lights off. The data were corrected for the spectral response of the system using a calibrated tungsten white light source. Each spectrum was normalized to peak thyroid fluorescence to account for day to day variations in the source and system response. Spectra were background subtracted, and the 6 recordings for each site were averaged together.
Intraoperative fluorescence imaging
The NIR fluorescence imaging system was developed at Vanderbilt by customizing a clinical endoscope camera typically used for visible fluorescence applications (Supplemental Figure 1). It is composed of a 3-chip color camera with an integrated parfocal zoom lens (Karl Storz, PDD Camera) driven by a camera control unit (Karl Storz, Tricam SL). A long-pass 808-nm filter was placed in front of the detector to block excitation light from the 785-nm diode laser while enabling white light and NIR image capture. The system has a field-of-view of 25 cm2 with a spatial resolution of 170 μm from a 15-cm working distance.
Images were collected after the surgeon had identified and exposed parathyroid tissue on the surface of the surgical field. The camera was held stationary 15 cm above the tissue, and white light images were collected. NIR fluorescence images were captured with the operating room lights off and the laser evenly irradiating the surgical field at 1 W/cm2. Intraoperative images were displayed on a screen that was not visible to the operating surgeon so that the research study would not dictate surgical decisions. Histopathology of all tissues removed during surgery served as the gold standard to compare fluorescence measurements. Normal parathyroid diagnosis was based on visual inspection only. Raw images were processed after the operation, and parathyroid contrast was calculated as the ratio of fluorescence intensity of the parathyroid compared with fluorescence of all other surrounding tissues.
Ex vivo tissue measurements
Because histological validation of the fluorescence signal cannot be performed on healthy human parathyroid tissue, canine tissues were used due to their similarity in structure and function. Six healthy mongrel canines of either sex were euthanized, and parathyroid and thyroid tissue was excised. Excised tissues were cut in two, and half was snap-frozen and maintained at −80°C for further analysis. The other half was immediately fixed in 10% neutral buffered formalin and processed for histological analysis. For measurement, samples were thawed to room temperature in PBS, and fluorescence spectroscopy and imaging were performed as in humans.
Statistical analysis
Statistical analysis was performed with a Student's t test to detect significant differences in the fluorescence signal from parathyroid and surrounding neck tissue. The sample size of 6 patients for the imaging study was chosen as the minimum sample size needed to provide at least 80% power to detect the mean difference between parathyroid fluorescence and background as seen in the clinical spectroscopy study. Statistical significance was assigned for P values ≤.05.
Results
Validation of parathyroid autofluorescence in a large patient population
The presence of the NIR fluorescence signal in parathyroid tissue was confirmed during surgery using single-point spectroscopy. Measurements were collected from various neck tissues of 110 patients undergoing parathyroidectomy and thyroidectomy. Results show that the parathyroid glands in all patients exhibited a significantly higher fluorescence signal than thyroid, muscle, and fat. Fluorescence detection of the parathyroid showed 100% sensitivity when measured with spectroscopy, with the parathyroid signal ranging from 1.2 to 25 times higher than thyroid, and all surrounding tissues emitted no signal (Supplemental Figure 2). Using a 2-sample t test at a 99.9% significance level, the parathyroid fluorescence intensity was found to be significantly higher than thyroid fluorescence with a P value of 3.54 × 10−11.
High NIR fluorescence signals from parathyroid tissue were confirmed with histology when available. Histological validation was available for the diseased parathyroid in 47 patients, for the diseased thyroid in 81 patients, and for both diseased tissue types in 14 patients. Histology confirmed the accuracy of NIR fluorescence spectroscopy in detecting the parathyroid in all cases of excised parathyroid glands. In some instances, NIR fluorescence improved upon the visual assessment of the surgeon (Supplemental Table 1). The efficacy of using this technique to localize the parathyroid was assessed using a receiver operating characteristic (ROC) curve (Figure 1). Results showed the area under the ROC curve, a measure of predictive power, was 0.9945. For a tissue to be identified as parathyroid, the optimal cutoff value of the fluorescence signal when normalized to the thyroid was determined to be 1.29 based on the appropriate tradeoff between sensitivity and specificity from the ROC curve.
Figure 1.

ROC curve of the NIR parathyroid fluorescence signal normalized to thyroid fluorescence. The area under the curve is 0.9945.
Intraoperative imaging of the parathyroid
The patients recruited for the study included 3 patients diagnosed with primary hyperparathyroidism, 2 patients with nontoxic nodular thyroid goiters, and 1 patient with papillary thyroid cancer (Table 1). The time for fluorescence image capture during surgery ranged from 4 to 9 minutes with a mean time of 6 minutes. Measurements did not interfere with the flow of the surgical procedure. Because the source of contrast is intrinsic, no preoperative preparation was needed for intraoperative imaging.
Table 1.
Individual Demographics and Intraoperative Fluorescence Imaging Results for 6 Patients Undergoing Parathyroidectomy or Thyroidectomy
| Postoperative Diagnosis | Age (Year) | Sex | Race | Surgeon Assessment (by Visual Observation) | Confidence Level of Surgeon in Visual Assessment | Parathyroid Contrast Using Fluorescence Imaging | Histopathological Confirmation of Parathyroid? |
|---|---|---|---|---|---|---|---|
| Parathyroid disease | |||||||
| Primary hyperparathyroidism | 65 | Male | White | Parathyroid | High | 2.53 | Yes |
| Primary hyperparathyroidism | 67 | Male | White | Parathyroid | High | 4.17 | Yes |
| Primary hyperparathyroidism | 53 | Female | Black | Parathyroid | High | 3.71 | Yes |
| Thyroid disease | |||||||
| Nontoxic multinodular goiter | 40 | Female | White | Parathyroid | High | 8.48 | No |
| Nontoxic uninodular goiter | 29 | Female | White | Parathyroid | High | 5.9 | No |
| Papillary thyroid cancer | 75 | Male | White | Parathyroid | High | 2.91 | No |
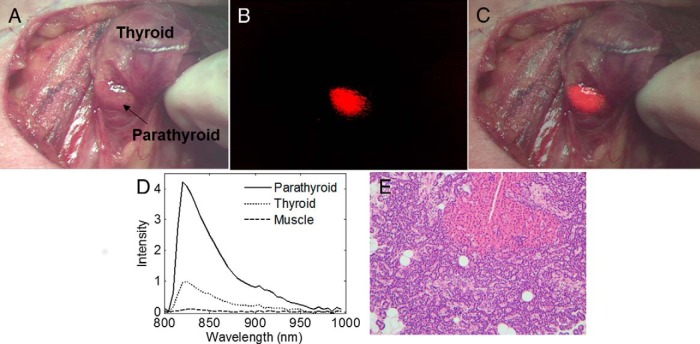
Intrinsic parathyroid fluorescence was detected in all 6 patients regardless of disease state. In a patient with a nontoxic multinodular goiter, parathyroid fluorescence (shown in red) overlaid on a bright light color image of the surgical field clearly delineated the parathyroid gland from surrounding tissue (Figure 2, A–C). The parathyroid signal intensity exceeded the fluorescence from all surrounding tissue such as fat, muscle, thyroid, nerve, and trachea in each patient measured. Although in some cases a low level signal from the thyroid was imaged, the detector was optimized to image parathyroid fluorescence with maximum contrast. Point measurements with fluorescence spectroscopy collected after imaging confirm the presence of a high fluorescence signal in the parathyroid (Figure 2D). Imaging results show parathyroid glands from thyroidectomy patients on average emit stronger fluorescence signal than that of parathyroidectomy patients. The average parathyroid contrast (fluorescence intensity of parathyroid to that of surrounding tissue) for the parathyroid glands measured across 6 patients was 5.76 for patients with primary thyroid disease and 3.47 for patients with primary parathyroid disease. These results show normal parathyroids in patients undergoing thyroidectomy had a statistically significantly higher signal than affected/hypercellular parathyroids in patients undergoing parathyroidectomy (P = .021), a finding that is also observed in the 110 patients measured with probe-based spectroscopy (Figure 3).
Figure 2.
Intraoperative fluorescence images of the parathyroid gland. A, Bright-field image of parathyroid and thyroid from patient undergoing thyroidectomy. B, NIR fluorescence image shows parathyroid in red. C, Bright-field and fluorescence images overlaid show parathyroid fluorescence. D, Fluorescence spectra of tissues show in A–C confirm fluorescence signal. E, Hematoxylin and eosin-stained slide of parathyroid gland from patient undergoing parathyroidectomy confirms that intraoperative fluorescence signal successfully identified parathyroid tissue.
Figure 3.
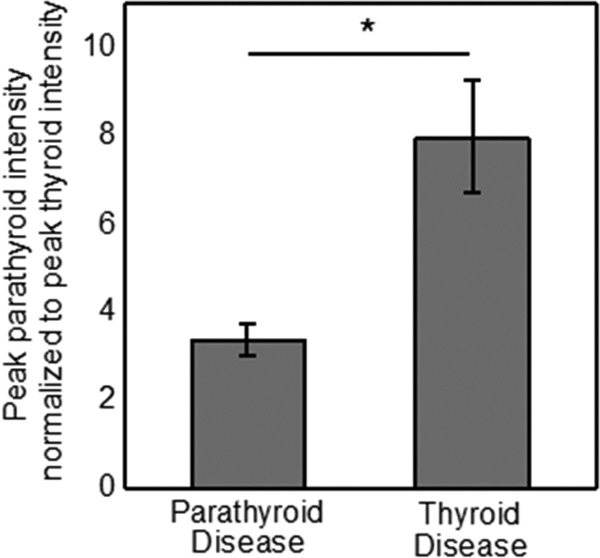
Average normalized parathyroid signal from patients with primary thyroid disease show significantly higher fluorescence intensity than patients with primary parathyroid disease.
Accuracy of parathyroid identification using fluorescence imaging was compared with the gold standard of histopathology in cases where diseased parathyroid tissue was removed. A representative example of histopathological analysis is depicted in Figure 2E from an excised parathyroid gland that exhibited a parathyroid contrast of 3.71 with intraoperative fluorescence imaging. If parathyroid glands were not excised, the identity of the parathyroid was confirmed by visual inspection by experienced surgeons who rated their confidence as high, medium, or low. Surgeons were highly confident in their visual identification of all fluorescent parathyroid tissues imaged (Table 1).
Ex vivo fluorescence imaging of healthy canine parathyroid tissue
Imaging permitted real-time identification of ex vivo healthy canine parathyroid gland by detecting higher fluorescence than thyroid tissue and background (Figure 4A). The fluorescence signal of healthy tissue was measured with fluorescence spectroscopy (Figure 4B). Histological analysis of the same sample measured in Figure 4, A and B, confirmed that healthy parathyroid tissue exhibits a high NIR fluorescence signal (Figure 4C).
Figure 4.
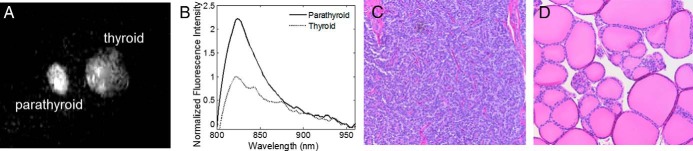
Healthy parathyroid and thyroid fluorescence in canine subjects. A, Representative NIR fluorescence image of parathyroid (left) and thyroid (right) shows a higher fluorescence intensity from parathyroid in vitro. B, NIR fluorescence spectra collected from the same samples confirm high level of fluorescence in parathyroid with peak emission at 822 nm. C and D, Microphotographs of hematoxylin and eosin-stained slide of healthy canine parathyroid (C) and thyroid tissue (D) (×20 magnification) provide histological validation that healthy tissues exhibit NIR fluorescence.
Discussion
Insufficient parathyroid identification during cervical endocrine surgery can irreversibly damage the body's calcium regulation, which has led it to become one of the principal causes of surgical malpractice litigation (24). In this study, we investigate the utility of NIR autofluorescence imaging of the parathyroid using a previously undiscovered source of biological contrast. Intraoperative detection of the parathyroid glands aims at improving the rates of postoperative normocalcemia.
The robust and consistent NIR autofluorescence signal in the parathyroid and thyroid glands of 110 patients indicate a novel NIR fluorophore that is excited at 785 nm and emits at 822 nm. The higher-intensity signal in the parathyroid than the thyroid suggests the fluorophore is present at a greater concentration in the parathyroid gland. The high fluorescence of the parathyroid gland is present both in patients with primary thyroid and parathyroid disease, indicating this surgical navigation technique is valid regardless of disease state. This study shows that NIR fluorescence can improve upon the visual inspection of the surgeon by identifying the parathyroid gland with greater accuracy. Although fluorescence spectroscopy can convey shape and intensity of the fluorescence, it is inherently restricted because it can provide measurements only at single points. NIR fluorescence imaging provides a more useful surgical guidance tool by capturing spatial information on parathyroid fluorescence intraoperatively.
Here we present for the very first time in vivo, real-time, intraoperative surgical guidance of endocrine surgery using NIR fluorescence imaging to provide specific and sensitive localization of the parathyroid gland. It has been shown that the complication rate in thyroid and parathyroid surgery is inversely proportional to the experience of the operating surgeon (25, 26), indicating that a tool for intraoperative parathyroid localization has the greatest potential for impact in the hands of less experienced surgeons. However, even the meticulous dissection of highly experienced surgeons can result in incidental parathyroid excision during thyroidectomy. Highly experienced parathyroid surgeons may question the identity of the parathyroid in cases when the anatomy is distorted (adenopathy, thyroiditis, large multinodular goiters, radiation, vascular Graves' glands). An intraoperative parathyroid localization tool would be useful to surgeons of all experience levels to anatomically identify the parathyroid during thyroidectomy for the sake of preserving its vasculature and avoiding trauma. In this study, the parathyroid glands of all 6 patients imaged showed higher fluorescence than surrounding tissues, but patients with thyroid disease showed significantly greater parathyroid contrast than patients with parathyroid disease. The utility of this imaging technique is therefore emphasized as normal parathyroid glands that must be preserved during surgery are the most difficult to visually identify yet emit the greatest fluorescence contrast. This method is potentially advantageous over intraoperative methods because the system is associated with a one-time cost that is approximately one-third the cost of a year's worth of fine-needle aspirations in an expert center.
In addition to a robust, real-time solution to intraoperative parathyroid detection, we postulate the discovery of a unique NIR fluorophore with potentially widespread applications. The discovery of an intrinsic NIR fluorophore is transformational in the fields of both surgical imaging and biomedical photonics because endogenous NIR fluorophores with emission greater than 800 nm have not been reported. Currently, the molecular basis of the parathyroid autofluorescence is unknown. Because peak fluorescence emission from the parathyroid and thyroid occurs at the same wavelength, it is hypothesized that the same fluorophore is responsible in both tissues. The relative fluorescence signals from the parathyroid and thyroid suggest that potential candidates are likely to be present in high concentrations in the parathyroid, lower concentrations in the thyroid, and not present in surrounding tissues.
A primary fluorophore candidate is the extracellular calcium-sensing receptor, which is a G-protein coupled receptor present at highest concentrations in the chief cells of the parathyroid gland; lower concentrations in the parafollicular cells of the thyroid, colon, kidney; and not present in other neck tissues (27–29). Calcium-sensing receptors are known to be downregulated in patients with hyperparathyroidism (30), which is consistent with the lower fluorescence intensity measured in the parathyroid glands of patients with hyperparathyroidism. To assess the validity of this hypothesis, bulk human kidney and colon were measured for NIR fluorescence due to their high expression of the calcium-sensing receptor. The signal emitted from colon and kidney possessed the same peak fluorescence at 822 nm when excited with 785 nm as parathyroid and thyroid tissue, and the intensity of this fluorescence was the same order of magnitude as the parathyroid signal (results not shown). Calcium-sensing receptors constitute our leading fluorophore hypothesis at this time based on preliminary findings, but because it is yet to be validated, other potential fluorophores should not be overlooked. Other possible fluorophore candidates are secretory granules because of their high concentration in parathyroid glands, porphyrin derivatives because they are the longest emitting fluorophore currently identified, or precursors or byproducts of these molecules.
One limitation of the reported imaging system is that it requires all operating room lights be off during image capture. Although this step created only minor inconvenience during the study, a system that is unaffected by ambient light would be ideal for widespread use. Future iterations of the imaging system will contain optical filters to allow image collection under operating room lights. Additionally, this technique has not been used to test the ability to detect parathyroid glands located deep within tissue. Although NIR wavelengths are considered the optical window due to their deep penetration in biological tissue (∼2–3 mm), the intensity of the fluorescence signal will decrease as the distance from the parathyroid to the exposed surface increases. The excitation and collection geometry of future iterations of the system can be altered such that spatial offset methods are employed to detect light at specific depths within tissue (31).
In summary, our study is the first to report real-time intraoperative imaging of the parathyroid during surgery using intrinsic biological fluorescence. Our results indicate a novel NIR biological fluorophore that has not previously been reported in the literature. The high levels of this fluorophore in the parathyroid gland offers the unique opportunity for tissue-selective imaging without administered contrast agents. With future knowledge of the fluorophore identity, NIR fluorescence imaging can be expanded beyond parathyroid detection to other surgical guidance applications involving tissues expressing this NIR fluorophore.
Acknowledgments
We would also like to thank Phil Williams at the Vanderbilt division of surgical sciences for supplying canine tissue. We would also like to thank the nurses at the Vanderbilt Endocrine Surgery center for their assistance in data collection.
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 0909667 and the National Institute of Health under Grant No. NIHR41 EB015291.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- NIR
- near-infrared
- ROC
- receiver operating characteristic.
References
- 1. Gourgiotis S, Moustafellos P, Dimopoulos N, Papaxoinis G, Baratsis S, Hadjiyannakis E. Inadvertent parathyroidectomy during thyroid surgery: the incidence of a complication of thyroidectomy. Langenbeck's Archives of Surgery. 2006;391(6):557–560. [DOI] [PubMed] [Google Scholar]
- 2. Sakorafas GH, Stafyla V, Bramis C, Kotsifopoulos N, Kolettis T, Kassaras G. Incidental parathyroidectomy during thyroid surgery: an underappreciated complication of thyroidectomy. World J Surg. 2005;29(12):1539–1543. [DOI] [PubMed] [Google Scholar]
- 3. Gonçalves Filho J, Kowalski LP. Surgical complications after thyroid surgery performed in a cancer hospital. Otolaryngol Head Neck Surg. 2005;132(3):490–494. [DOI] [PubMed] [Google Scholar]
- 4. Mariani G, Gulec SA, Rubello D, et al. Preoperative localization and radioguided parathyroid surgery. J Nucl Med. 2003;44(9):1443–1458. [PubMed] [Google Scholar]
- 5. Sebag F, Shen W, Brunaud L, Kebebew E, Duh QY, Clark OH. Intraoperative parathyroid hormone assay and parathyroid reoperations. Surgery. 2003;134(6):1049–1055, discussion 1056. [DOI] [PubMed] [Google Scholar]
- 6. Christou N, Mathonnet M. Complications after total thyroidectomy. J Visc Surg. 2013;150(4):249–256. [DOI] [PubMed] [Google Scholar]
- 7. Caron NR, Sturgeon C, Clark OH. Persistent and recurrent hyperparathyroidism. Curr Treat Options Oncol. 2004;5(4):335–345. [DOI] [PubMed] [Google Scholar]
- 8. O'Doherty MJ, Kettle AG, Wells P, Collins RE, Coakley AJ. Parathyroid imaging with technetium-99m-sestamibi: preoperative localization and tissue uptake studies. J Nucl Med. 1992;33(3):313–318. [PubMed] [Google Scholar]
- 9. Majithia A, Stearns MP. Methylene blue toxicity following infusion to localize parathyroid adenoma. J Laryngol Otol. 2006;120(2):138–140. [DOI] [PubMed] [Google Scholar]
- 10. Prosst RL, Weiss J, Hupp L, Willeke F, Post S. Fluorescence-guided minimally invasive parathyroidectomy: clinical experience with a novel intraoperative detection technique for parathyroid glands. World J Surg. 2010;34(9):2217–2222. [DOI] [PubMed] [Google Scholar]
- 11. Mohebati A, Shaha AR. Imaging techniques in parathyroid surgery for primary hyperparathyroidism. Am J Otolaryngol. 2012;33:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taillefer R, Boucher Y, Potvin C, Lambert R. Detection and localization of parathyroid adenomas in patients with hyperparathyroidism using a single radionuclide imaging procedure with technetium-99m-sestamibi (double-phase study). J Nucl Med. 1992;33(10):1801–1807. [PubMed] [Google Scholar]
- 13. Perrier ND, Ituarte P, Kikuchi S, et al. Intraoperative parathyroid aspiration and parathyroid hormone assay as an alternative to frozen section for tissue identification. World J Surg. 2000;24(11):1319–1322. [DOI] [PubMed] [Google Scholar]
- 14. Bancos I, Grant CS, Nadeem S, et al. Risks and benefits of parathyroid fine-needle aspiration with parathyroid hormone washout. Endocr Pract. 2012;18(4):441–449. [DOI] [PubMed] [Google Scholar]
- 15. Zarebczan B, Chen H. Influence of surgical volume on operative failures for hyperparathyroidism. Adv Surg. 2011;45:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erbil Y, Barbaros U, Ozbey N, Aral F, Ozarmağan S. Risk factors of incidental parathyroidectomy after thyroidectomy for benign thyroid disorders. Int J Surg. 2009;7(1):58–61. [DOI] [PubMed] [Google Scholar]
- 17. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228(3):320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kherlopian AR, Song T, Duan Q, et al. A review of imaging techniques for systems biology. BMC Syst Biol. 2008;2(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hadjipanayis CG, Jiang H, Roberts DW, Yang L. Current and future clinical applications for optical imaging of cancer: from intraoperative surgical guidance to cancer screening. Semin Oncol. 2011;38:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsui A, Tanaka E, Choi HS, et al. Real-time, near-infrared, fluorescence-guided identification of the ureters using methylene blue. Surgery. 2010;148(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirche C, Murawa D, Mohr Z, Kneif S, Hünerbein M. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010;121:373–378. [DOI] [PubMed] [Google Scholar]
- 22. Paras C, Keller M, White LM, Phay JE, Mahadevan-Jansen A. Near-infrared auto-fluorescence for the detection of parathyroid glands. J Biomed Opt. 2011;16(6):067012 [DOI] [PubMed] [Google Scholar]
- 23. McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT. A novel optical approach to intraoperative detection of parathyroid glands. Surgery. 2013;154(6):1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dralle H, Lorenz K, Machens A. Verdicts on malpractice claims after thyroid surgery: Emerging trends and future directions. Head Neck. 2012;34(11):1591–1596. [DOI] [PubMed] [Google Scholar]
- 25. Stavrakis AI, Ituarte PH, Ko CY, Yeh MW. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery. 2007;142(6):887–899. [DOI] [PubMed] [Google Scholar]
- 26. Lin DT, Patel SG, Shaha AR, Singh B, Shah JP. Incidence of inadvertent parathyroid removal during thyroidectomy. Laryngoscope. 2002;112(4):608–611. [DOI] [PubMed] [Google Scholar]
- 27. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81(1):239–297. [DOI] [PubMed] [Google Scholar]
- 28. Chattopadhyay N, Cheng I, Rogers K, et al. Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am J Physiol. 1998;274(1 Pt 1):G122–G130. [DOI] [PubMed] [Google Scholar]
- 29. Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol. 1998;274(3):F611–F622. [DOI] [PubMed] [Google Scholar]
- 30. Gogusev J, Duchambon P, Hory B, et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51(1):328–336. [DOI] [PubMed] [Google Scholar]
- 31. Vaithianathan T, Tullis ID, Everdell N, et al. Design of a portable near infrared system for topographic imaging of the brain in babies. Rev Sci Instrum. 2004;75(10):3276–3283. [Google Scholar]