Abstract
Context:
Mutations in the MCT8 (SLC16A2) gene, encoding a specific thyroid hormone transporter, cause an X-linked disease with profound psychomotor retardation, neurological impairment, and abnormal serum thyroid hormone levels. The nature of the central nervous system damage is unknown.
Objective:
The objective of the study was to define the neuropathology of the syndrome by analyzing brain tissue sections from MCT8-deficient subjects.
Design:
We analyzed brain sections from a 30th gestational week male fetus and an 11-year-old boy and as controls, brain tissue from a 30th and 28th gestational week male and female fetuses, respectively, and a 10-year-old girl and a 12-year-old boy.
Methods:
Staining with hematoxylin-eosin and immunostaining for myelin basic protein, 70-kDa neurofilament, parvalbumin, calbindin-D28k, and synaptophysin were performed. Thyroid hormone determinations and quantitative PCR for deiodinases were also performed.
Results:
The MCT8-deficient fetus showed a delay in cortical and cerebellar development and myelination, loss of parvalbumin expression, abnormal calbindin-D28k content, impaired axonal maturation, and diminished biochemical differentiation of Purkinje cells. The 11-year-old boy showed altered cerebellar structure, deficient myelination, deficient synaptophysin and parvalbumin expression, and abnormal calbindin-D28k expression. The MCT8-deficient fetal cerebral cortex showed 50% reduction of thyroid hormones and increased type 2 deiodinase and decreased type 3 deiodinase mRNAs.
Conclusions:
The following conclusions were reached: 1) brain damage in MCT8 deficiency is diffuse, without evidence of focal lesions, and present from fetal stages despite apparent normality at birth; 2) deficient hypomyelination persists up to 11 years of age; and 3) the findings are compatible with the deficient action of thyroid hormones in the developing brain caused by impaired transport to the target neural cells.
Mutations of the monocarboxylate transporter 8 (MCT8) or SLC16A2 gene cause an X-linked syndrome of psychomotor retardation and altered thyroid hormone (TH) levels. Patients show global developmental delay, severe intellectual disability (intelligence quotient < 30), lack of speech, and severe neuromotor impairment with central hypotonia, spastic quadriplegia, and dystonic movements. This condition was described in 1944 by Allan et al (1). In 2004 patients with similar clinical pictures were shown to have elevated serum T3, low T4, and rT3, with normal or slightly elevated TSH, suggesting a defect in TH metabolism. The X-chromosome-linked mode of inheritance led to the identification of mutations of the MCT8 gene, encoding a specific TH transporter (2, 3). MCT8 mutations were subsequently found in affected members of the original families with Allan-Herndon-Dudley syndrome (4).
MCT8 is an integral plasma membrane protein specific for T4 and T3 transport (5). It is expressed in several tissues including brain, in which it is present in the blood-brain barrier, choroid plexus, tanycytes, and neurons (6–8). Given the role of THs in brain development (9), it is reasonable to surmise that the neurological impairment in MCT8 defect is due to impaired TH availability to neural cells. This hypothesis has been impossible to prove experimentally because Mct8 knockout mice do not present neurological impairment (10, 11), and the causal relationship between TH transport and neurological damage is uncertain.
Here we describe, for the first time, the brain histopathology of MCT8 defects by analyzing a 30th gestational week male fetus and an 11-year-old boy with MCT8 mutations. The results support the notion that impaired supply of TH to the brain, starting at the prenatal stages, is the cause of brain damage.
Materials and Methods
Procedures were approved by our institution's Ethics Committee (Consejo Superior de Ivestigaciones Cientifícas, permit SAF2011-25608). Tissues were obtained with written informed consent from the parents of all subjects, in agreement with the Declaration of Helsinki. Personal data were treated anonymously.
Subject 1
A 30th gestational week male fetus with MCT8 mutation (L494P) was identified by amniocentesis. The mutation was also present in a brother with neurological and thyroid function abnormalities typical of MCT8 deficiency and in a heterozygous mother. Pregnancy was terminated after an officially designated committee approved the parental request based on the genetic prenatal diagnosis (Wolfson Medical Center, Holon, Israel, and the Sackler School of Medicine, Tel Aviv, Israel). Body length was 45 cm, body weight 1500 g, head circumference 28 cm, and chest circumference 26 cm. Paraffin blocks were provided by the Wolfson Medical Center. Fetal ages estimated by menstrual history and by ultrasonography were concordant. The control was a 30th gestational week male fetus aborted due to placental abruption. Paraffin blocks were provided by the IdiPAZ Biobank (Spanish Biobank Network, www.redbiobancos.es). A 28th gestational week female fetus lacking the corpus callosum was used as control for biochemical analysis. Gestational age was estimated from clinical and pathology data.
Subject 2
Subject 2 was an 11-year-old boy with severe psychomotor impairment; seizures requiring antiepileptics, high serum T3, low rT3, and T4; mildly elevated TSH; and an MCT8 mutation (Q96X). At 9 months he had mildly delayed myelination on magnetic resonance imaging (MRI) but no abnormalities at 6.5 years except mild dilatation of the lateral ventricles frontal horns. Death was from respiratory failure secondary to aspiration pneumonia. Paraffin blocks were provided by the Sydney Children's and Prince of Wales Hospitals, Randwick, Australia. At autopsy, body weight was 29.9 kg, length 151 cm, brain weight 1285.5 g, cerebral hemispheres 1100 g, cerebellum 127 g, and brainstem 25 g. Controls were a 10-year-old girl who died from acute pulmonary edema during preparation for a bone marrow transplant and a 12-year-old boy who died from diffuse lymphocytic myocarditis. Paraffin blocks were provided, respectively, by the IdiPAZ Biobank, and the Biobanc per a la Investigació de l'Hospital Infantil Sant Joan de Déu, Barcelona (National Network Biobanks of the Instituto de Salud Carlos III, Madrid, Spain).
Tissue processing and histological techniques are described in the Supplemental Methods.
Results
MCT8 deficiency was associated with generalized signs of delayed brain maturation, hypomyelination, altered neuronal differentiation, and deficient synaptogenesis. No focal lesions were observed.
Delayed maturation of the cerebral cortex and cerebellum
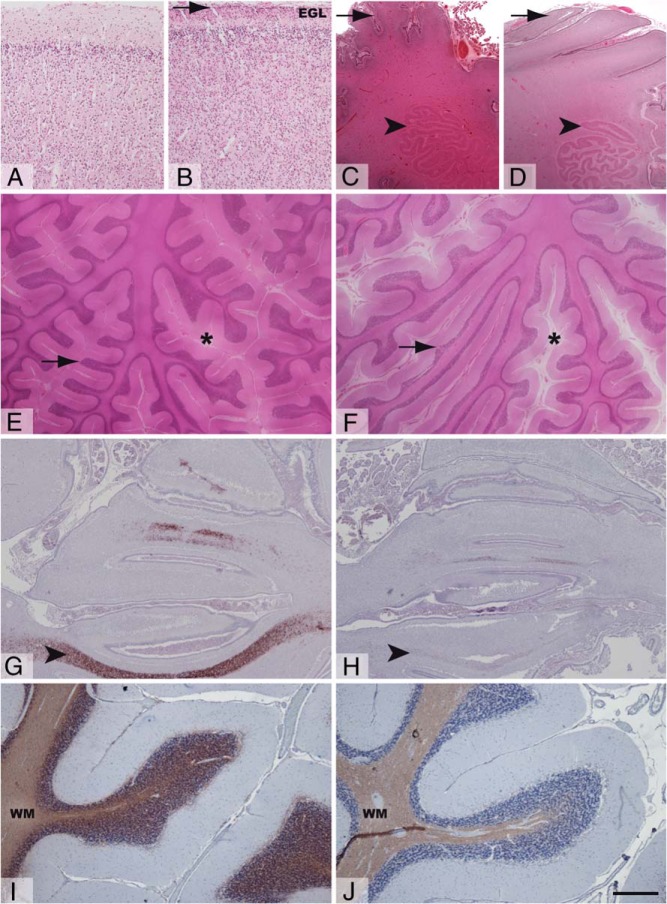
In comparison with the control (Figure 1A), the MCT8-deficient fetal cerebral cortex showed poorly defined lamination, reduced thickness, higher cellularity, and abnormal persistence of the external granular layer (Figure 1B). The MCT8-deficient fetal cerebellum showed poor folial development, a delayed maturation of Purkinje cells, and a less folded dentate nucleus than the control (Figure 1, C and D, and Supplemental Figure 1). The cerebellum of the MCT8-deficient child (Figure 1F) presented a wider subarachnoid space and thinner and sparser cerebellar folia compared with the control (Figure 1E). The morphology of the granular and Purkinje cell layers (PCLs) appeared normal (Supplemental Figure 1).
Figure 1.
Structure and myelination of the fetal and juvenile cerebral cortex and cerebellum. Representative images showing hematoxylin-eosin staining (A–F) and MBP immunostaining (G–J) of tissue sections from the frontal cerebral cortex (A and B), cerebellar hemispheres (C and D), and cerebellar vermis (E–J) from control fetus (A, C, and G), MCT8-deficient fetus (B, D, and H), control child (E and I), and MCT8-deficient child (F and J). Arrowhead in panel B points to the EGL in frontal cortex of the MCT8-deficient fetus. Arrows and arrowheads in panels C and D indicate the folial development and the folding of the dentate nucleus, respectively, to illustrate the immature cerebellar histology in the MCT8-deficient fetus. Asterisks in E and F indicate the subarachnoid space in the cerebellum; note that it is wider in the MCT8-deficient subject (F). Arrows in panels E and F point to cerebellar folia; note their thinner size in the cerebellum of the MCT8-deficient boy (F). Arrowheads indicate immunopositive axons in panels G and H. Note the lower proportion of immunopositive axons in the vermis from the MCT8-deficient fetus (H). Note the lower intensity of MBP immunostaining in the cerebellum of the MCT8-deficient boy (J) in comparison with the control (I). Control samples for the MCT8-deficient boy correspond to the 10-year-old girl. EGL, external granular layer; WM, white matter. Scale bars represent 538 μm (panels A, B); low magnification (panels C–F); and 1 μm (panels G and H) and 444 μm (panels I and J).
Hypomyelination
In the control fetal cerebellum, myelin basic protein (MBP) was present in the vermal white matter (Figure 1G), the hilum of the dentate nucleus, and the deep white matter (not shown). In the MCT8-deficient fetal cerebellum, MBP was very low or absent in all these locations (Figure 1H). Signs of deficient myelination were also present in the MCT8-deficient child's cerebellum, with paler MBP staining (Figure 1J) than the control subject (Figure 1I). Expression of other myelin proteins gave similar results (not shown).
Altered neuronal differentiation
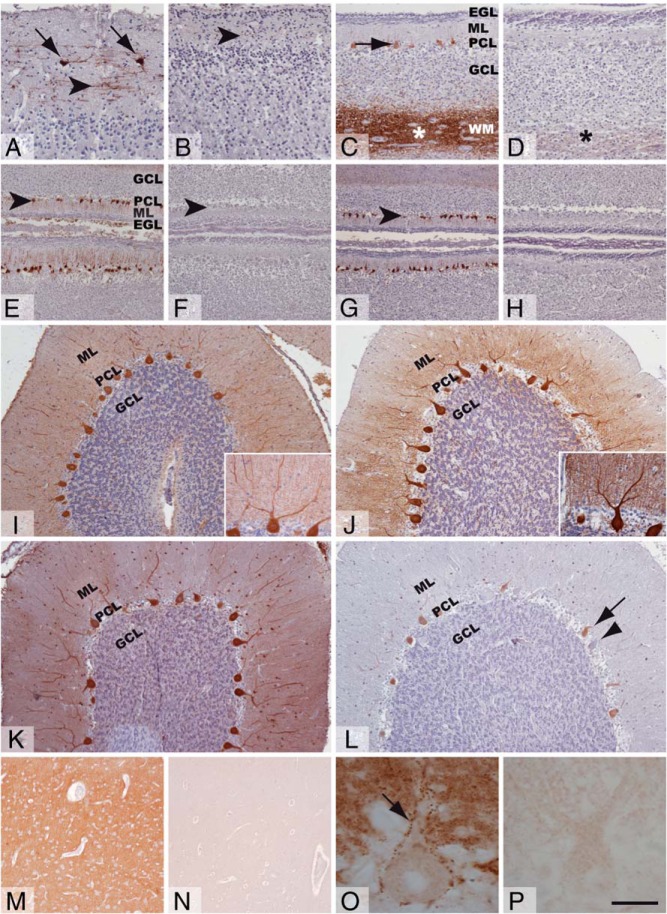
The light 70-kDa neurofilament subunit (NEFL) and the Ca2+-binding proteins parvalbumin (PVALB) and calbindin-D28k (CALB) were expressed abnormally.
The control fetus showed NEFL expression in tangential axons of cerebral cortex layer 1, interneurons, and Cajal-Retzius cells (Figure 2A). There was little or no staining in the MCT8-deficient cortex (Figure 2B). In the control cerebellum, NEFL was present in the axons of the vermal white matter and in some vermal Purkinje cells (Figure 2C), the hilum, and dentate nucleus neurons (Supplemental Figure 1). The MCT8-deficient fetus stained sparsely only in some axons of the vermal white matter (Figure 2D).
Figure 2.
Neuronal differentiation in the fetal and juvenile brain. Representative images showing 70-kDa neurofilament (A–D), calbindin-D28k (E, F, I, and J), parvalbumin (G, H, K, and L), and synaptophysin expression (M–P) in immunostained tissue sections of the frontal cortex (A, B, M, and N) and cerebellar vermis (C-L, O, and P) from control fetus (A, C, E, and G), MCT8-deficient fetus (B, D, F, and H), control child (I, K, M, and O), and MCT8-deficient child (J, L, N, and P). The arrowheads indicate NEFL-immunopositive tangential axons in the ML of the frontal cortex (A and B) and Purkinje cells in panels E, F, G and L. The Arrows point to the immunopositive Cajal Retzius cells in the frontal cortex (A), Purkinje cells in the vermis (C and L), and the puncta pattern of SYP expression in the cytoplasmic membrane of Purkinje cells (O). Asterisks indicate immunopositive axons in the white matter vermal folia (C and D). Note the lower immunopositive signal in the vermal white matter in the MCT8-deficient fetus (D). Note the low expression of CALB (F) and absence of expression of PVALB (H) in the MCT8-deficient fetus. Note the higher CALB expression in J; insets in panels I and J are to illustrate the similar morphology of Purkinje cells in the cerebellum of the control and MCT8-deficient children. Note the variable PVALB expression in Purkinje cells of the cerebellum of the MCT8-deficient child, from low (arrow) to absent (arrowhead) in panel L. Note the much lower SYP expression in the frontal cortex and cerebellum of the MCT8-deficient child (N and P). Control samples for the MCT8-deficient boy correspond to the 10-year-old girl for cerebellum. EGL, external granular layer; GCL, granular cell layer. Scale bars represent 90 μm (panels A and B); 125 μm (panels C and D); 108 μm (panels E–H); 157 μm (panels I–L); 44 μm (inset in panels I and J); and 212 μm (panels M and N).
The cerebellar molecular layer (ML) and PCL of the control fetus expressed CALB (Figure 2E) and PVALB (Figure 2G). CALB was almost absent and PVALB completely absent from the MCT8-deficient cerebellum (Figure 2, F and H). In the control child, CALB was expressed throughout the brain, and in the cerebellum, CALB was present in the Purkinje cells (Figure 2I). Interestingly, in the affected cerebellum, CALB expression followed the same pattern but with stronger intensity (Figure 2J).
In the control child, the PVALB was expressed in cortical interneurons (Supplemental Figure 1), reaching a density in the sensory cortex of approximately 25 ± 4 cells/mm2. The PVALB was also present in the ML and PCL of the cerebellar cortex (Figure 2K) and in the folial and the deep white matter (data not shown). In contrast, in the MCT8-deficient child, the PVALB was not detected in the cerebral cortex (Supplemental Figure 1) and was of variable intensity from low to nonexistent in many of the Purkinje cells (Figure 2L). Thus, in the presence of normal Purkinje cells' morphology (Figure 2, I and J insets), CALB expression was increased and PVALB expression was decreased in the MCT8-deficient cerebellum (Figure 2, I and J vs K and L, respectively).
Altered synaptogenesis
To evaluate synaptogenesis, we used synaptophysin (SYP) immunohistochemistry. There were no differences in the fetuses (not shown). In contrast, big differences were found in the children sections. The most striking finding was the virtual lack of SYP expression in the MCT8-deficient frontal cortex (Figure 2N). In contrast, the control subject showed high expression in the neuropil (Figure 2M). In the MCT8-deficient cerebellum, SYP expression was very low (Figure 2P and Supplemental Figure 1), whereas in the control, robust SYP expression was found with the typical punctate pattern decorating the Purkinje cell membranes and the primary dendrites (Figure 2O).
Tissue-specific alteration of iodothyronines and their deiodinases
T4, T3, and rT3 concentrations were reduced by 50% in the cerebral cortex of the MCT8-deficient fetus, but T3 and T4 were normal in the liver. The hormonal deficiency in brain produced the expected increase in type 2 deiodinase and decrease in type 3 deiodinase mRNA expression (Supplemental Table 1).
Discussion
This is the first description of brain pathology in subjects with MCT8 gene mutations. Our study is consistent with a major role of the altered TH transport as a primary cause of the neurological syndrome. The MCT8-deficient brains showed no focal lesions but histological signs of immaturity, deficient myelination, and altered expression of TH-dependent neuronal proteins, all reminiscent of alterations produced by severe developmental hypothyroidism in rodents. The conclusion agrees with the data in the fetal brain showing low T4, T3, and rT3 concentrations and increased type 2 and decreased type 3 deiodinases mRNAs, with normal T4 and T3 in the liver. This tissue-selective hormonal deprivation agrees with observations in Mct8-deficient mice (10, 11).
TH deprivation is likely the cause of the hypomyelination observed in MCT8-deficient patients as a direct consequence of defective action of TH on oligodendrocyte differentiation and myelin gene expression (12). Our data show that altered myelination is a persistent feature; even if by MRI, it may no longer show such evidence in patients beyond 5–6 years of age (13, 14). In our case the MRI did not detect abnormalities at age 6.5 years, but hypomyelination was present by histopathology at 11 years.
Hypomyelination can also be a consequence of neuronal immaturity. This was demonstrated by the altered expression of NEFL, and Ca2+-binding proteins. It was remarkable that PVALB expression was completely absent in the fetus and in the child's frontal cerebral cortex. Neurofilaments and Ca2+-binding proteins are under the control of TH and its receptors. Hypothyroidism in rodents decreases Nefm, Nefh, and Pvalb and increases Calb1 expression (15). The altered SYP expression is also compatible with the action of TH in the formation and maintenance of synapses and in the control of the expression of synaptic proteins (16).
The histopathology may be correlated to some clinical findings. Alterations in axonal maturation are compatible with spasticity, hyperreflexia, motor retardation, and muscular weakness. Abnormal expression of the Ca2+-binding proteins could be related to the development of seizures (1, 17). The observed alterations in cortex cytoarchitecture, the content of calcium binding proteins, and the hypomyelination of cortical connections could result in dysfunction of intracortical and corticosubcortical circuits that may lead to the severe mental retardation and the later development of seizures. The intense structural alterations in cerebellum could underlie, at least in part, features such as mental retardation, nystagmus, trunk hypotonia, poor head control, and speech alterations.
The pathological findings described here cannot be compared with those reported in neurological cretinism (18) or more recently in the endemic regions of China (19). Endemic cretinism has many clinical forms, depending on the geographical setting (20). The TH deficiency affects the whole body, whereas in MCT8 deficiency, it is selective to the brain and may affect differentially the brain areas in relation to the expression of other TH transporters. In congenital hypothyroidism TH deficiency starts around birth due to the protection afforded by the maternal TH, whereas the insult to the brain in MCT8-deficiency occurs before the 30th gestational week.
Acknowledgments
We are grateful to the subjects' parents who consented the use of brain tissues for this investigation and to the clinicians involved in their care including Dr Ian Andrews at Sydney Children's Hospital, Sydney, Australia, and Dr Tally Lerman-Sagie at Wolfson Medical Center, Holon, Israel. We are indebted to the following institutions for the provision of brain samples: the IdiPAZ Biobank integrated in the Spanish Biobank Network (www.redbiobancos.es), for the generous gifts of clinical samples used in this work. The IdiPAZ Biobank is supported by the Instituto de Salud Carlos III, Spanish Health Ministry (Red Temática de Investigación Cooperativa en Salud RD09/0076/00073), and Farmaindustria, through the Cooperation Program in Clinical and Translational Research of the Community of Madrid; the Biobanc de l'Hospital Infantil Sant Joan de Déu per a la Investigació integrated in the National Network Biobanks of Instituto de Salud Carlos III, Barcelona, Spain, for the sample and data procurement; the Prince of Wales Hospital, Randwick, Australia, and the Wolfson Medical Center, Holon, Israel, and the Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel. We acknowledge Dr Isabel Lastres Becker for translating a reference (18) from German to English and David Gómez-Andrés and Irene Pulido-Valdeolivas for helpful comments.
Supplemental data are available online.
This study was supported by Grant SAF2011-25608 from the Plan Nacional de I+D+i and the Center for Research on Rare Diseases (Centro de Investigación Biomédica en Red Enfermedades Raras), Instituto de Salud Carlos III, Madrid, Spain; Grant DK15070 from the National Institutes of Health, Bethesda, Maryland; and a grant from the Sherman Family. D.L.-E. is recipient of a fellowship from the “Fellowship Training Program for Advanced Human Capital, Becas Chile” from the National Commission for Scientific and Technological Research, Gobierno de Chile.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CALB
- calbindin-D28k
- MBP
- myelin basic protein
- MCT8
- monocarboxylate transporter 8
- ML
- molecular layer
- MRI
- magnetic resonance imaging
- NEFL
- neurofilament subunit
- PCL
- Purkinje cell layer
- PVALB
- parvalbumin
- SYP
- synaptophysin
- TH
- thyroid hormone.
References
- 1. Allan W, Herndon CN, Dudley FC. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic. 1944;48:325–334. [Google Scholar]
- 2. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74(1):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friesema EC, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364(9443):1435–1437. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz CE, May MM, Carpenter NJ, et al. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet. 2005;77(1):41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278(41):40128–40135. [DOI] [PubMed] [Google Scholar]
- 6. Alkemade A, Friesema EC, Unmehopa UA, et al. Neuroanatomical pathways for thyroid hormone feedback in the human hypothalamus. J Clin Endocrinol Metab. 2005;90(7):4322–4334. [DOI] [PubMed] [Google Scholar]
- 7. Roberts LM, Woodford K, Zhou M, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149(12):6251–6261. [DOI] [PubMed] [Google Scholar]
- 8. Chan SY, Martin-Santos A, Loubiere LS, et al. The expression of thyroid hormone transporters in the human fetal cerebral cortex during early development and in N-Tera-2 neurodifferentiation. J Physiol. 2011;589(Pt 11):2827–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernal J, Guadaño-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003;13(11):1005–1012. [DOI] [PubMed] [Google Scholar]
- 10. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147(9):4036–4043. [DOI] [PubMed] [Google Scholar]
- 11. Trajkovic M, Visser TJ, Mittag J, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117(3):627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodríguez-Peña A, Ibarrola N, Iñiguez MA, Muñoz A, Bernal J. Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J Clin Invest. 1993;91(3):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gika AD, Siddiqui A, Hulse AJ, et al. White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev Med Child Neurol. 2010;52(5):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaurs-Barrière C1, Deville M, Sarret C, et al. Pelizaeus-Merzbacher-Like disease presentation of MCT8 mutated male subjects. Ann Neurol. 2009;65(1):114–118. [DOI] [PubMed] [Google Scholar]
- 15. Gil-Ibañez P, Morte B, Bernal J. Role of thyroid hormone receptor subtypes α and β on gene expression in the cerebral cortex and striatum of postnatal mice. Endocrinology. 2013;154(5):1940–1947. [DOI] [PubMed] [Google Scholar]
- 16. Gong J, Dong J, Wang Y, et al. Developmental iodine deficiency and hypothyroidism impair neural development, up-regulate caveolin-1 and down-regulate synaptophysin in rat hippocampus. J Neuroendocrinol. 2010;22(2):129–139. [DOI] [PubMed] [Google Scholar]
- 17. Holden KR, Zuniga OF, May MM, et al. X-linked MCT8 gene mutations: characterization of the pediatric neurologic phenotype. J Child Neurol. 2005;20(10):852–857. [DOI] [PubMed] [Google Scholar]
- 18. Wegelin C. Pathologische anatomie und histologie. In: F. De Quervain CW, ed. Der Endemische Kretinismus. Berlin und Wien: Verlag Von Julius Springer; 1936:110–118. [Google Scholar]
- 19. Yuquin Y, Chunxiang G, Lili L, Jianqun L. Quantitative histology study on brain nervous cells of neurological endemic cretins. In: Delong G, Robbins J, Condliffe P, eds. Iodine and the Brain. New York: Plenum Press; 1989:359. [Google Scholar]
- 20. DeLong GR. Effects of nutrition on brain development in humans. Am J Clin Nutr. 1993;57(suppl 2):286S–290S. [DOI] [PubMed] [Google Scholar]