Abstract
Context:
Large cell calcifying Sertoli cell tumors (LCCSCT) present in isolation or, especially in children, in association with Carney Complex (CNC) or Peutz-Jeghers Syndrome (PJS). These tumors overexpress aromatase (CYP19A1), which leads to increased conversion of delta-4-androstenedione to estrone and testosterone to estradiol. Prepubertal boys may present with growth acceleration, advanced bone age, and gynecomastia.
Objective:
To investigate the outcomes of aromatase inhibitor therapy (AIT) in prepubertal boys with LCCSCTs.
Design:
Case series of a very rare tumor and chart review of cases treated at other institutions.
Setting:
Tertiary care and referral center.
Patients:
Six boys, five with PJS and one with CNC, were referred to the National Institutes of Health for treatment of LCCSCT. All patients had gynecomastia, testicular enlargement, and advanced bone ages, and were being treated by their referring physicians with AIT.
Interventions:
Patients were treated for a total of 6–60 months on AIT.
Main Outcome Measures:
Height, breast tissue mass, and testicular size were all followed; physical examination, scrotal ultrasounds, and bone ages were obtained, and hormonal concentrations and tumor markers were measured.
Results:
Tumor markers were negative. All patients had decreases in breast tissue while on therapy. Height percentiles declined, and predicted adult height moved closer to midparental height as bone age advancement slowed. Testicular enlargement stabilized until entry into central puberty. Only one patient required unilateral orchiectomy.
Conclusions:
Patients with LCCSCT benefit from AIT with reduction and/or elimination of gynecomastia and slowing of linear growth and bone age advancement. Further study of long-term outcomes and safety monitoring are needed but these preliminary data suggest that mammoplasty and/or orchiectomy may be foregone in light of the availability of medical therapy.
Large cell calcifying Sertoli cell tumors (LCCSCT) account for 0.4%–1.5% of testicular tumors (1). These tumors consist of cords, sheets, ribbons, trabeculae, or irregular nests of polygonal cells with frequent islands of calcifications and sometimes necrosis (1, 2). Although macrocalcifications may lead to an irregular shape and coarse texture of the testis, microcalcifications are more frequent and often hard to detect by physical examination (2). Testicular ultrasound (US) may detect the microcalcifications, and blood flow is typically increased around the tumor (3).
The neoplastic Sertoli cells overexpress p450 aromatase (CYP19A1) (4), which is normally found in only low concentrations in prepubertal Leydig cells. Aromatase allows for increased conversion of δ-4-androstenedione (the major source of androgens from the adrenal gland in prepubertal males with LCCSCTs) to estrone. Testosterone is also converted to estradiol, but this is less of an issue in prepubertal boys with LCCSCTs (4). Although these hormone levels may still remain below the detection limit of standard assays, the sensitivity of the growth plates and breast tissue to estrogens may lead to growth acceleration, advanced bone age, and gynecomastia in prepubertal boys. Such presentation is similar to that observed in cases of aromatase excess syndrome due to rearrangements in the CYP19A1 gene.
LCCSCTs are found in isolation in over 60% of cases, typically in adults, but one-third are found in association with syndromes (1), when they typically present bilaterally as increased testicular volume at a younger age. Such syndromes include Carney Complex (CNC), where the prevalence of LCCSCT is approximately a third to a half of all male patients (5, 6) and Peutz-Jeghers Syndrome (PJS), which presents with LCCSCT in a much smaller percentage of cases. Both of these syndromes are inherited in an autosomal-dominant fashion with variable penetrance. CNC is typically caused by a mutation of the PRKAR1A gene, which regulates protein kinase A (PKA), although other genes are also involved, and thus, the diagnosis is made mostly clinically (5, 6). Increased cAMP signaling in LCCSCTs most likely is responsible for increased aromatase expression in these tumors, although the exact mechanism remains unknown (7). Features of CNC can include cutaneous, mucosal, cardiac, and breast myxomas, primary pigmented nodular adrenocortical disease, growth-hormone secreting adenomas, thyroid carcinoma or nodules, psammomatous melanotic schwannomas, blue nevi, breast ductal adenomas, and osteochondromyxomas (5). PJS is usually caused by a mutation in STK11 (also known as LKB1), a tumor suppressor gene on chromosome 19p13.3 with effects on metabolic and other signaling (8). Mutations in LKB1/STK11 lead to increased aromatase expression (9). PJS is mainly characterized by multiple hamartomatous polyps in the gastrointestinal (GI) tract. There is increased risk of bowel, breast, gastric, and pancreatic cancer. Both CNC and PJS present with mucocutaneous hyperpigmented macules on the vermillion border of the lips and around the eyes, as well as on the vaginal and penile mucosa in CNC and on the bowel mucosa, axilla, palms, and soles in PJS.
Although LCCSCTs are more frequent in CNC than in PJS, these tumors are far more frequently aggressive and aromatizing intensely in the context of PJS. Malignancy is found in approximately 17% of patients with LCCSCT but is rare in young patients with bilateral tumors or in association with a genetic syndrome (1, 10). Features that are more concerning for malignancy include older age, unifocal, and unilateral disease (11). Given this low risk, it has been suggested that treatment of LCCSCT may be generally conservative (12, 13). Aromatase inhibitors have been used as therapy in individual cases of patients with LCCSCT (14–17) but no concerted effort to treat a larger number of patients has been reported.
We present a series of six cases of LCCSCT seen at our institution over the last 15 years all treated for gynecomastia in their referring institutions. Aromatase inhibitors, originally anastrazole and later letrozole, were used in each of these patients. Medical treatment led to avoidance of surgery (bilateral orchiectomy had been offered in all these patients elsewhere) and limitation of bone age advancement, slower growth velocity, and successful reversal of gynecomastia; the latter also led to avoidance of surgical breast reduction.
Materials and Methods
Six pediatric patients were referred to the National Institutes of Health (NIH). All molecular and genetic studies were conducted under clinical protocol 95-CH-0059 that was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Informed consent from the patients' parents (and assent from older children) was obtained for all patients. An endocrinologist performed a medical history and a physical examination. Midparental height (MPH) was calculated as the average of the mother's height plus 13 cm (cm) and the father's height. The patient's height was measured three times to the nearest millimeter by a stadiometer, and weight was measured to the nearest 0.1 kg with a calibrated digital scale. Testicular volume (mL) was measured by using a set of orchidometer beads as standards according to Prader (18), and breast tissue was palpated and measured using a rigid ruler in the horizontal and vertical directions along the ventral surface. Total breast area was calculated by measuring height and width.
Serum was collected for estradiol, estrone, total testosterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH), and insulin-like growth factor −1 (IGF-1). Tumor markers β-human chorionic gonadotropin (hcg), AFP, CA-125, and CEA were also sent. Estrone and total testosterone were measured with LC-MS/MS. LH, FSH, CA-125, and CEA were measured by solid-phase, two-site chemiluminescent immunometric assay on Siemens Immulite 2500; βHCG was measured with automated two-site chemiluminescent assay done by Mayo Medical labs. Skeletal age was determined by a left-hand roentgenogram and assessed by an endocrinologist according to the technique of Greulich and Pyle (19). Predicted adult height (PAH) was calculated using the Bailey-Pinneau method (20). Scrotal ultrasound (US) was performed to assess testicular volume, calculated as 0.71 × width × length × depth. Patients were followed in conjunction with the referring physicians. Each patient was treated with an aromatase inhibitor at the referring institution, either anastrozole or letrozole. Patients were followed serially with exams, skeletal age roentgenograms, and scrotal ultrasounds, as per protocol 95-CH-0059.
Results
Five patients were found to have a deletion or substitution in the STK11/LKB1 gene leading to PJS, and one patient was enrolled with a substitution in the PRKARIA gene leading to CNC (Table 1). Table 1 also describes their presenting features, past medical history, and family history. Figure 1 displays photographs of some of the clinical findings at presentation to the NIH. Of note, patient 1 initially presented to pediatric endocrinology at 2.75 years of age and underwent a computed tomography (CT) scan of the abdomen, pelvis, and adrenal glands. Evaluation continued at 3.5 years of age with normal magnetic resonance imaging (MRI) scans. Although patient 2 was diagnosed within 6 months of presentation, a second opinion and genetics evaluation were sought. Subsequent evaluation included a leuprolide stimulation test with prepubertal results, a normal pituitary MRI and US of the abdomen, and a 46,XY karyotype. Patient 3 was only noted to have lip freckling at age 2, so no pediatric endocrine evaluation was sought until age 7 when unilateral gynecomastia was discovered. Testicular calcifications were found, but the patient was only monitored clinically for the following year. Clinical features of PJS were not observed with patient 4 until age 7.5 years; autistic features and attention deficit problems were also identified. Patient 5 was always noted to have breast tissue, which became more prominent around 8 years. Despite a diagnosis of PJS in his father, he was not referred to a pediatric endocrinologist until almost age 10, when his gynecomastia was in a Tanner III distribution. Despite an enlarged testis at presentation and a family history of a cardiac myxoma in the mother of patient 6, symptoms of CNC were not noted in this patient until his seventh birthday. Evaluation of the testis and referral of both the patient and his mother to the NIH were then rapidly pursued.
Table 1.
Patient Characteristics
| Pt | Dx | Genetic Mutation | Age at Initial Symptoms | PMHx | FamHx |
|---|---|---|---|---|---|
| 1 | PJS | STK11/LKB1 p.D237E (c.711C>G) | Age 2 gynecomastia, testicular enlargement, lip freckling | · Undescended left testis | · MPH 176.5 cm |
| · Orchiopexy at 14 months of age | · Father, Ewing sarcoma in spine | ||||
| · Maternal aunt, Crohns | |||||
| 2 | PJS | STK11/LKB1 gross deletion of full gene | Age 4 gynecomastia and increased growth velocity | · Mild speech delay | · MPH 178.5 cm |
| 3 | PJS | STK11/LKB1 p.D194N (c.580G>A) | Age 3 lip freckling | · Conceived by in vitro fertilization | · MPH 177.8 cm |
| Age 7 gynecomastia | · Food allergies and eczema | · Mother hypothyroidism, cervical CA, hypertension | |||
| · Maternal GF, colonic polyps | |||||
| · Paternal GF, colon CA | |||||
| 4 | PJS | STK11/LKB1 gene microdeletion | Age 7.5 gynecomastia, testicular enlargement, lip freckling | · Bilateral hydroceles | · MPH 170.2 |
| · Autism and attention problems | · Mother, irritable bowel syndrome | ||||
| · Multiple ear infections and ear tubes | · Great GF, pancreatic CA | ||||
| · Multiple hamartomas on colonoscopy | |||||
| 5 | PJS | Age 8 gynecomastia | · Intussusception of small intestine | · Father, PJS | |
| 6 | CNC | PRKAR1A p.Glu297Glu (c.891G>A) | Age 7 left sided testicular enlargement, gynecomastia, lip freckling | · Previously healthy | · MPH 177.8 cm |
| · Mother, cardiac myxoma leading to embolic stroke; also found to have CNC | |||||
| · Father, co-arctation of the aorta |
MPH, midparental height; cm, centimeters; Mat, maternal; Pat, paternal; GF, grandfather; CA, cancer.
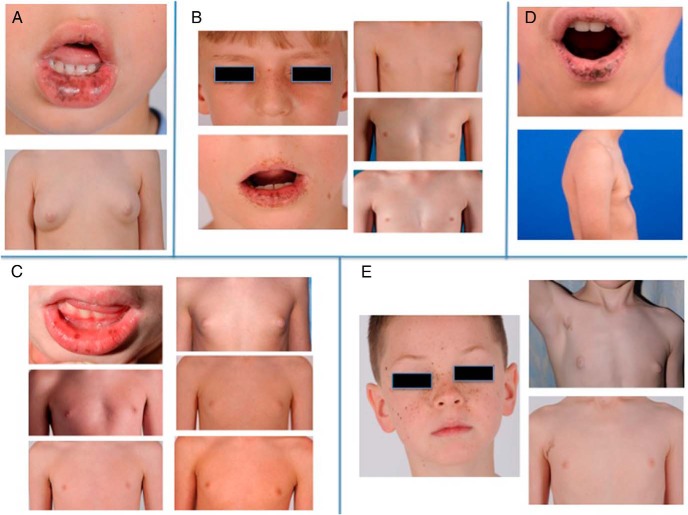
Figure 1.
(A) Patient 1. Top: Multiple dark pigmented freckles on the lower lip. Bottom: Baseline gynecomastia prior to aromatase therapy. (B) patient 3. Left: Facial freckling. Right Top: Baseline gynecomastia prior to AIT. Right Middle: Gynecomastia following one year of AIT. Right Bottom: Full recession of gynecomastia. (C) patient 4. Top Left: Freckling of lips. Top Right: Baseline gynecomastia prior to AIT. Continuing clockwise, gynecomastia after 5 months, 3.75 years, and 4.75 years on treatment, and 2 years after cessation of treatment. (D) Patient 5. Top: Freckling on lips. Bottom: Baseline gynecomastia prior to AIT. (E) Patient 6. Left: Facial freckling. Right Top: Axillary freckling and baseline gynecomastia prior to AIT. Right Bottom: Recession of gynecomastia after 6 months of therapy.
All six patients were initially evaluated at outside institutions. Table 2 describes the clinical features and radiographic findings at the time of referral to the NIH. All patients had areas of hyperpigmentation, gynecomastia, and bilateral testicular enlargement, although pubic hair was only present for patient 6, the only patient with CNC. Patient 5 refused a genital examination at the time of presentation to the NIH. Bone age advancement by 1.5–5.5 years was noted in all patients. Predicted adult height was lower than MPH in all but two patients (patients 3 and 5).
Table 2.
Patient Exam Findings and Radiology Studies at Presentation to the NIH
| Age at Presentation to the NIH | Height | Skin Pigmentation | Gynecomastia | Testes/Pubic Hair (PH) | Scrotal US | Bone age (BA) and Predicted Adult Height (PAH) | |
|---|---|---|---|---|---|---|---|
| 1 | 4.6 y | 99.9th percentile (+3.3 sd) | · Freckling on lower lip | · Right 8 × 9 cm | · Right 12 mL | · Right, micro-calcifications and a hydrocele | · BA 10 y |
| · Brown papule left thigh | · Left 10 × 10 cm | · Left 10 mL | · Left, several irregular echogenic foci, measuring up to 2 mm but most less than 1 mm | · PAH 161.4 cm | |||
| · CAL left abdomen | · No PH | ||||||
| 2 | 5.2 y | 99.9th percentile (+3.1 sd) | · Two CAL, 13 × 15 cm and 2 × 2 cm, on back | · 10 × 9 cm b/liter | · Right 6 mL | · Right 1.6 × 1.2 × 3 cm | · BA 9 y (1 month prior) |
| · CAL 30 × 17 cm on chest | · Left 5 mL | · Left 2.8 × 1.1 × 1.7 cm | · PAH 174.2 cm | ||||
| · No PH | |||||||
| 3 | 8.6 y | 99.5th percentile (+2.6 sd) | · Dark pigmented lines, freckling on lower lip | · Right 4 × 5 cm | · 10 mL b/liter | · Right – 2.9 × 1.4 × 2 cm | · BA 10 y (3 months prior to presentation) |
| · 0.5 cm skin tag on neck | · Left 7 × 7 cm | · No PH | · Left – 2.7 × 1.3 × 2 cm | · PAH 194.2 cm | |||
| · Lentigenes on fingertips | · Microcalcifications b/liter | ||||||
| 4 | 8.7 y | 96.6th percentile (+1.8 sd) | · Freckling on lower lip | · Tanner IV sized b/liter | · Right 12 mL | · Right 1.5 × 1.7 × 3.3 cm | · BA 10.5 y |
| · Left 10 mL | · Left 3.2 × 2 × 1.5 cm | · PAH 163.8 cm | |||||
| · Hydroceles b/liter | · Several echogenic lesions b/liter | ||||||
| · No PH | |||||||
| 5 | 10.1 y | 99.5th percentile (+2.6 sd) | · Freckling on lips | · 5 × 6 cm b/liter | · 10 mL b/litera | · Right 2.5 × 1.4 × 2.5 cm | · BA 13.5 y |
| · Left 4.6 × 2.5 × 2.1 cm | · PAH 182 cm | ||||||
| · Many punctate densities | |||||||
| 6 | 7.5 y | 87.3rd percentile (+1.1 sd) | · 40–50 brown to black ink macules on central face, 2–4 mm | · Tanner II sized b/liter | · Right 8 mL | · Right 2.5 × 1.6 × 2.8 cm | · BA 10.5 y |
| · 2 mm light brown macule on lower lip | · Left >25 mL (lemon sized) | · Left 3.6 × 3.9 × unmeasurable cm, 1 × 0.5 cm fluid collection without vascularity, ring shape calcifications, central necrosis | · PAH 172.8 | ||||
| · 3 × 3.5 cm macule right axilla | · Tanner II PH |
sd, standard deviation; CAL, café' au lait; cm, centimeters; mL, milliliters; b/liter, bilaterally.
Patient 5 refused genital exam at time of presentation to the NIH. Testicular volume based on prior exam with referring endocrinologist.
Initial biochemical evaluation revealed undetectable estradiol in patients 3, 5, and 6, although patient 3 had previously had a measurable level of 17 pg/mL (62 pmol/L). Estradiol levels at presentation to the NIH measured 19 pg/mL (70 pmol/L) in patient 1, 13 pg/mL (48 pmol/L) in patient 2, and 20 pg/mL (73 pmol/L) in patient 4. Estrone was measured in patient 1 as 21 pg/mL (78 pmol/L), and was undetectable in patient 6. Testosterone was undetectable in patients 1, 2, and 5, and measured 8 ng/dL (0.28 nmol/L) in patient 3, 14 ng/dL (0.49 nmol/L) in patient 4, and 114 ng/dL (3.96 pmol/L) in patient 6. Prepubertal gonadotropins were noted in patients 1, 2, 4, and 6. Patient 5 had an LH of 1.8 U/L and FSH of 0.2 U/L. No data are available for patient 3. Tumor markers (AFP, hcg, CA-125, and CEA) were negative for all patients, although patient 4 did not have CEA measured, and patient 5 did not have an AFP measured. Mean IGF-1 levels were 175 ng/mL (range 101–284), and IGF-1 levels were within the normal range for age.
AIT was started as treatment to reduce gynecomastia and slow bone age advancement in all six patients by the referring physician. Patients were followed for 6–60 months in consultation with the referring clinical centers. In three out of six cases, AIT was started after their first visit to the NIH. Patients 2, 3, and 5 began therapy just prior to the first NIH visit (2 months, 3 months, and 1 month, respectively). Patient 5 was switched to letrozole after his visit to the NIH. Patient 3 was initially continued on anastrozole, but the dose was increased after 4 months of therapy when his gynecomastia demonstrated only minimal decrease in size, although there was mild slowing of the growth velocity with a height percentile at 99.3 (+2.5 SD). On re-evaluation after 14 months of therapy, his bone age continued to advance to 12.25 years, although the growth velocity had slowed with a height percentile of 98.1 (+2.1 SD). Although the gynecomastia was improving with the right breast measuring 3 × 3 cm and the left measuring 4.5 × 5 cm, the lack of full resolution led to a substitution of anastrozole for letrozole, which was started at 2.5 mg daily. All other patients were placed on letrozole at the start of therapy.
Results of change in height percentile, bone age, breast size, and testicular size are displayed in Table 3. Figure 1 displays photographs of changes in gynecomastia after therapy. All patients demonstrated a decline in height percentile. Bone age advancement was slowed in all but patient 1 (who was only monitored at NIH for 6 months and had an equivalent increase in bone age over that time, but his referring endocrinologist reported a stable bone age of 10–11 years after a year of therapy) and patient 5. PAH increased for the four patients whose initial PAH was lower than MPH, and decreased for the two patients whose initial PAH was higher than MPH. Full resolution of gynecomastia was observed in three patients, with all patients showing a decrease in the amount of breast tissue while on therapy.
Table 3.
Therapy and Outcomes for Each Patient With LCCSCT Treated With Aromatase Inhibitors
| Therapy | Age at Start of Therapy | Total Time on Therapy | Change in Ht Percentile (Change in Ht sd) | Change in Bone Age (BA) and Predicted Adult Height (PAH)a | Change in Breast Size | Change in Testicular Sizeb |
||
|---|---|---|---|---|---|---|---|---|
| By Exam | By US | |||||||
| 1 | Ltzl 2.5 mg | 4.6 y | 6 mo | −0.1 (−0.4 sd) | BA: +0.5 y | R − 42 cm2, L − 52 cm2 | R − 2 mL, L + 2 mL | R + 1.9 mL, L + 0.4 mL |
| PAH: +0.8 cm | ||||||||
| 2 | Ltzl 2.5 mg | 5.1 y | 17 mo | −0.9 (−0.8 sd) | BA: None | −48 cm2 bilaterally | R no change, L + 1 mL | R − 0.1 mL, L + 2 mL |
| PAH: +5.9 cm | ||||||||
| 3 | Anzl 1 mg × 4 mo, | 8.5 y | 60 mo | −4.8 (−1 sd) | BA: +2.75 y | Full resolution | R − 2 mL, L − 2 mL | R − 1.4 mL, L − 0.9 mL |
| Anzl 1.5 mg × 10 mo, | OnA: −1.4 (−0.5 sd) | —OnA: +2.25 y | OnA: R − 11 cm2, L − 26.5 cm2 | OnA: R − 1 mL, L − 2 mL | ||||
| Ltzl 2.5 mg × 46 mo | OnL: −3.4 (−0.5 sd) | —OnL: +0.5 y | OnL: R − 9 cm2, L − 22.5 cm2 | OnL: R − 1 mL, L no change | ||||
| PAH: −10.8 cm | ||||||||
| 4 | Ltzl 2–3 mg | 8.7 y | 54 mo | −7.5 (−0.8 sd) | BA: +3 y | Full resolution (almost all within first year of treatment) | R − 2 mL, L no change | R + 9.2 mL, L + 0.1 mL |
| PAH: +23.3 cm | ||||||||
| 5 | Anzl 1 mg × 1 mo, | 10 y | 13 mo | −0.4 (0.2 sd) | BA: +1.5 y | Full resolution | Refused exam | R + 10.9 mL, L + 16.1 mL |
| Ltzl 2.5 mg × 12 mo | PAH: −13.6 cm | |||||||
| 6 | Ltzl 1.25 mg | 7.5 y | 6 mo | −1.8 (−0.4 sd) | BA: None | Small degree of decrease | R + 2–4 mL, (L removed) | R + 2.8 mL |
| PAH: +3.7 cm | ||||||||
Ltzl, Letrozole; Anzl, Anastrozole; mg, milligram; mo, months; y, years; Ht, height; sd, standard deviation; OnA, during time on anastrozole; OnL, during time on letrozole; R, right side; L, left side; cm, centimeters; mL, milliliters; US, ultrasound.
For patient 1, bone age data collected after 11 months of follow up; no data available from 17 month follow up visit.
For patients 3 and 4, change in testicular size is only evaluated from start of therapy until the last visit before onset of central puberty (36 months on treatment for patient 3 and 17 months for patient 4). Following onset of central puberty, both patients progressed to 25 mL testes bilaterally by physical exam.
Testicular size changed minimally until entry into central puberty. Data in Table 3 reflects testicular size prior to entry into puberty, which occurred at age 12.5 years for patient 3 with elevated gonadotropins and testicular volume of 12 mL. By 13.5 years, testicular volume was 25 mL. Estradiol and estrone remained undetectable.
The testicular volume remained relatively stable for patient 4 for the first year of treatment; however, he entered central puberty shortly thereafter. By the next visit at age 12.3 years, his testicular volume had increased to 25 mL, his gonadotropins were well in the pubertal range, and his testosterone rose to 1690 ng/dL (58.64 nmol/L). Despite these pubertal advancements, bone age progression remained slow, with his chronological age almost equaling his bone age at 12.3 years and surpassing his bone age by the age of 13.3 years. His growth velocity declined with a fall of height percentiles to 87.1 (+1.1 SD) at the age of 12.3 years; however, with the onset of puberty, he grew more rapidly and height increased to the 90.5 percentile (+1.3 SD) by age 13.3 years. Gynecomastia continued to recede with no detectable breast tissue by 13.3 years; thus, he was weaned off letrozole at that time, after 4.5 years of therapy. A follow-up visit 2 years after the discontinuation of letrozole revealed persistent resolution of the gynecomastia. Height velocity had continued to rise with a height percentile of 95.4 (+1.7 SD). Testosterone had fallen to 844 ng/dL (29.29 nmol/L) with normal gonadotropins.
Given the lack of viable left-sided testicular tissue based on ultrasound examination, the presence of a sizeable tumor, and the risk for torsion, patient 6 underwent a left orchidectomy during his initial visit to the NIH. In addition to letrozole, the patient was also started on leuprolide injections given his elevated testosterone, by the referring endocrinologist. Follow-up 6 months later revealed a fall in testosterone concentrations to 35 ng/dL (1.21 nmol/L), although LH rose to 0.3 U/L. No further follow up at the NIH has occurred.
Discussion
We report six boys with PJS or CNC who were treated by their physicians with AIT for their hormone-producing LCCSCTs. All of the patients presented with gynecomastia, testicular enlargement, and abnormal skin pigmentation; there was no apparent genotype-phenotype correlation. Furthermore, patients with deletions vs point mutations in the STK11/LKB1 gene were not distinguishable clinically in response to AIT.
Breast development significantly decreased or fully regressed in all six patients, even for those patients with Tanner IV or V gynecomastia. Treatment with aromatase inhibitors also affected linear growth. All patients were tall at initial evaluation, with five patients above the 95th percentile and four patients above the 99th percentile. All six boys experienced slowing in growth velocity as evidenced by declines in height percentiles. PAH moved closer to MPH, although for two of these patients PAH was initially above MPH (but decreased while on therapy). Despite initial enlargement in testicular size at the time of diagnosis, testicular growth did not continue to any significant degree until entry into central puberty.
Patients in our study were started on AIT 6 months to 2.5 years after gynecomastia was first reported. It is possible that if AIT is begun earlier while breast tissue is in a more proliferative stage, a better effect may be observed. Earlier initiation of therapy may prove beneficial, but a larger randomized controlled trial (RCT) is needed to test this hypothesis. In our experience, we have observed regression of breast tissue in patients with PJS even after initiation of therapy at later Tanner stages. Although gynecomastia may have presented earlier and been missed in some of these cases, there seems to be no correlation with the length of time these patients were untreated and the extent of gynecomastia at time of treatment initiation. Furthermore, given that all breast tissue regressed significantly, there seems to be no correlation to pretreatment estrogen exposure and potential for resolution of gynecomastia. Similarly, testicular enlargement and change in testicular size after treatment seemed unrelated to duration of disease prior to treatment.
Letrozole was chosen as the aromatase inhibitor for recently diagnosed patients. In two, anastrozole was started prior to the first evaluation at the NIH. In one patient, anastrozole was continued for 14 months, but gynecomastia persisted so the patient was switched to letrozole. While the literature on comparison of the potency of different aromatase inhibitors is predominately related to breast cancer, it appears that letrozole should be used as the more potent aromatase inhibitor for treatment of LCCSCT related gynecomastia (21).
Following completion of central pubertal development, AIT can be discontinued in patients with LCCSCT. The increased aromatization of estrogens has little impact in fully virilized males with higher androgen levels. Testicular ultrasound should still be followed routinely for their LCCSCTs (11).
Patients with LCCSCT classically demonstrate microcalcifications on testicular ultrasound as seen in our patients. Tumor markers were consistently negative in all six patients in this study. The risk of metastasis with LCCSCT in the PJS and CNC population is thought to be very low, so conservative management is acceptable. Orchiectomy is not recommended unless there is lack of viable healthy tissue and/or a risk for testicular torsion. We performed unilateral orchiectomy only once to prevent this complication.
Our results are similar to prior studies of aromatase inhibitors in patients with LCCSCT. Previous reports have been limited to individual or sibling pair case reports (14–17). The older case reports described the use of testolactone, a first generation aromatase inhibitor, while the more recent studies utilized anastrozole. The six boys described in these studies all had PJS, and five of them had gynecomastia. Aromatase inhibitors were successful in reducing gynecomastia in all of these cases. Of note, we have observed that gynecomastia is more common in males with PJS than in males with CNC. We followed 21 male children with CNC and a mean age at their first visit to NIH of 6.8 years ± 4.3. Over a mean length of follow up of 4.3 ± 5.3 years, 12 out of the 21 children (57%) were found to have testicular calcifications, but only 2 had a history of gynecomastia (9.5%). In contrast, 15 male children with PJS were seen, who presented at the NIH at a mean age of 8.8 ± 4.2 years. Over a mean length of follow up of 2.4 ± 2.6 years, 9 out of 11 (81%) of these males developed testicular calcifications, and 7 out of 11 had gynecomastia reported (64%). The molecular basis for this discrepancy is not known.
This study describes six patients more systematically and allows for comparison across cases. However, it is limited by the lack of placebo controls as well as detailed retrospective data on growth velocity and testicular volume prior to the onset of treatment.
In conclusion, aromatase inhibitors, particularly letrozole, provide a benefit to patients with LCCSCT due to PJS or CNC. Long-term data on safety and outcomes are still needed.
Acknowledgments
We would like to thank the patients and their families. In addition, we would like to thank the referring physicians, including Michelle Klein, MD, Mt. Sinai Hospital New York, NY, David Cooke, MD and Ines Guttmann-Bauman MD, Johns Hopkins University School of Medicine, Baltimore, MD, Daniel Hale, MD, University of Texas Health Science Center at San Antonio, TX, Patrick Lynch, MD, University of Texas MD Anderson Cancer Center, Houston, TX, and Ann Marie Straight MD, San Antonio, TX.
This work was supported by The Intramural Research Programs of The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health Clinical Center.
Disclosure Summary: Authors have nothing to disclose.
Footnotes
- AIT
- aromatase inhibitor therapy
- CNC
- Carney Complex
- FSH
- follicle stimulating hormone
- hcg
- human chorionic gonadotropin
- IGF-1
- insulin-like growth factor −1
- LCCSCT
- large cell calcifying Sertoli cell tumors
- LH
- luteinizing hormone
- MPH
- midparental height
- PAH
- predicted adult height
- PJS
- Peutz-Jeghers Syndrome
- PKA
- protein kinase A.
References
- 1. Giglio M, Medica M, De Rose AF, Germinale F, Ravetti JL, Carmignani G. Testicular sertoli cell tumours and relative sub-types. Analysis of clinical and prognostic features. Urologia Internationalis. 2003;70:205–210. [DOI] [PubMed] [Google Scholar]
- 2. Young RH. Sex cord-stromal tumors of the ovary and testis: their similarities and differences with consideration of selected problems. Mod Pathol. 2005;18 Suppl 2:S81–S98. [DOI] [PubMed] [Google Scholar]
- 3. Gierke CL, King BF, Bostwick DG, Choyke PL, Hattery RR. Large-cell calcifying Sertoli cell tumor of the testis: appearance at sonography. AJR. 994;163:373–375. [DOI] [PubMed] [Google Scholar]
- 4. Brodie A, Inkster S, Yue W. Aromatase expression in the human male. Molecular and Cellular Endocrinology. 2001;178:23–28. [DOI] [PubMed] [Google Scholar]
- 5. Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clinical Endocrinology and Metabolism. 2001;86:4041–4046. [DOI] [PubMed] [Google Scholar]
- 6. Libé R, Horvath A, Vezzosi D, et al. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clinical Endocrinology and Metabolism. 2011;96:E208–E214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forlino A, Vetro A, Garavelli L, et al. PRKACB and Carney complex. N Engl J Med. 2014; 370:1065–1067. [DOI] [PubMed] [Google Scholar]
- 8. Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. [DOI] [PubMed] [Google Scholar]
- 9. Ham S, Meachem SJ, Choong CS, et al. Overexpression of aromatase associated with loss of heterozygosity of the STK11 gene accounts for prepubertal gynecomastia in boys with Peutz-Jeghers syndrome. J Clinical Endocrinology and Metabolism. 2013;98:E1979–E1987. [DOI] [PubMed] [Google Scholar]
- 10. Kratzer SS, Ulbright TM, Talerman A, et al. Large cell calcifying Sertoli cell tumor of the testis: contrasting features of six malignant and six benign tumors and a review of the literature. Am J Surgical Pathology. 1997;21:1271–1280. [DOI] [PubMed] [Google Scholar]
- 11. Gourgari E, Saloustros E, Stratakis CA. Large-cell calcifying Sertoli cell tumors of the testes in pediatrics. Current Opinion in Pediatrics. 2012;24:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nonomura K, Koyama T, Kakizaki H, Murakumo M, Shinohara N, Koyanagi T. Testicular-sparing surgery for the prepubertal testicular tumor. Experience of two cases with large cell calcifying Sertoli cell tumors. European Urology. 2001;40:699–704. [DOI] [PubMed] [Google Scholar]
- 13. Kaluzny A, Matuszewski M, Wojtylak S, et al. Organ-sparing surgery of the bilateral testicular large cell calcifying sertoli cell tumor in patient with atypical Peutz-Jeghers syndrome. Int Urology and Nephrology. 2012;44:1045–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grandone A, del Giudice EM, Cirillo G, Santarpia M, Coppola F, Perrone L. Prepubertal gynecomastia in two monozygotic twins with Peutz-Jeghers syndrome: two years' treatment with anastrozole and genetic study. Hormone Research in Paediatrics. 2011;75:374–379. [DOI] [PubMed] [Google Scholar]
- 15. Lefevre H, Bouvattier C, Lahlou N, Adamsbaum C, Bougnères P, Carel JC. Prepubertal gynecomastia in Peutz-Jeghers syndrome: incomplete penetrance in a familial case and management with an aromatase inhibitor. Eur J Endocrinology / European Federation of Endocrine Societies. 2006;154:221–227. [DOI] [PubMed] [Google Scholar]
- 16. Alikasifoglu A, Gonc EN, Akcoren Z, et al. Feminizing Sertoli cell tumor associated with Peutz-Jeghers syndrome. J Pediatric Endocrinology, Metabolism: JPEM. 2002;15:449–452. [DOI] [PubMed] [Google Scholar]
- 17. Kara C, Kutlu AO, Tosun MS, Apaydin S, Senel F. Sertoli cell tumor causing prepubertal gynecomastia in a boy with peutz-jeghers syndrome: the outcome of 1-year treatment with the aromatase inhibitor testolactone. Hormone Research. 2005;63:252–256. [DOI] [PubMed] [Google Scholar]
- 18. Zachmann M, Prader A, Kind HP, Häfliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatrica Acta. 1974;29:61–72. [PubMed] [Google Scholar]
- 19. Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 20. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatrics. 1952;40:423–441. [DOI] [PubMed] [Google Scholar]
- 21. Lønning PE. The potency and clinical efficacy of aromatase inhibitors across the breast cancer continuum. Ann Oncol. 2011;22:503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]