Abstract
Background:
Low total T is associated with an increased risk of atherosclerotic complications. However, the magnitude of this association in middle-aged patients with type 2 diabetes (T2D) has not been determined.
Materials and Methods:
This cross-sectional study evaluated atherosclerotic disease markers in T2D patients with normal and low plasma total T. A total of 115 male patients, aged younger than 70 years, without a history of cardiovascular events, and with normal [≥3.5 ng/mL (≥12.1 nmol/L), n = 79] or low [< 3.5 ng/mL (≤12.1 nmol/L), n = 36] total T underwent the measurement of highly sensitive C-reactive protein, carotid artery carotid intima-media thickness (IMT), and atherosclerotic plaque by high-resolution B-mode ultrasound and to asses endothelial function by brachial artery flow-mediated dilation.
Results:
Carotid IMT was negatively correlated with total T concentration (r = −0.39, P < .0001). Compared with subjects with normal T, a higher proportion of patients with low total T had carotid IMT of 0.1 cm or greater [80% vs 39%, odds ratio (OR) 6.41; 95% CI 2.5–16.4, P < .0001], atherosclerotic plaques (68.5% vs 44.8%, OR 2.60, 95% CI 1.12–6.03, P < .0001); endothelial dysfunction (80.5% vs 42.3%, OR 5.77, 95% CI 2.77–14.77, P < .003), and higher highly sensitive C-reactive protein levels (2.74 ± 5.82 vs 0.89 ± 0.88 mg/L, P < .0001). Similar results were found when free T was considered. Multiple logistic regression analyses adjusted for age, diabetes mellitus duration, hemoglobin A1c, lipids, treatment effect, and body mass index reported that a low total T level was independently associated with greater IMT [OR 8.43 (95% CI 2.5–25.8)] and endothelial dysfunction [OR 5.21 (95% CI 1.73–15.66)] but not with the presence of atherosclerotic plaques (OR 1.77, 95% CI 0.66–4.74).
Conclusions:
Low T is associated with more advanced atherosclerotic disease markers in middle-aged patients with T2D.
Cardiovascular events are the main cause of death in patients with type 2 diabetes (T2D). Patients with diabetes have twice the risk of coronary heart disease and ischemic strokes compared with subjects without diabetes (1). Longitudinal epidemiological studies have shown that a low T level at baseline is associated with increased all-cause mortality (2), as well as with higher prevalence of coronary artery disease, and cardiovascular mortality (3). This association can be explained in part by the adverse effects of low T on key cardiovascular risk factors, including central obesity, hyperglycemia, dyslipidemia, hypertension, and insulin resistance (4). Low plasma total T levels have been associated with a proinflammatory state and oxidative stress, which improve with T replacement (5, 6). T replacement has also been associated with improved insulin sensitivity and visceral adiposity (7, 8), higher skeletal muscle mass, and lower abdominal obesity (9). Additionally, T replacement modulates a number of cellular mechanisms intimate to the atherosclerotic process (10, 11).
Increasing evidence indicates a high prevalence of hypogonadism and low total, free, and bioavailable T in men with T2D and obesity (5, 12, 13). Dhindsa et al (14) reported that up to one-third of patients with T2D have low serum T concentrations with clinical evidence of hypogonadism. In addition, diabetic and nondiabetic subjects with low T have a higher prevalence of upper abdominal obesity, hyperinsulinism, and lower insulin sensitivity, which are established risk factors for the development of T2D and cardiovascular disease (15). Nevertheless, the cause effect of hypogonadism on risk markers of atherosclerotic and vascular disease is unknown.
Carotid carotid intima-media thickness (IMT), endothelial dysfunction, and highly sensitive C-reactive protein (Hs-CRP) are widely accepted markers of atherosclerotic disease risk (16, 17). Furthermore, increased carotid IMT and the presence of carotid plaques are important risk factors for acute myocardial infarction and cardiovascular mortality in patients with and without T2D (18). Few studies have examined the association between these atherosclerotic disease markers and low total T in patients with T2D. Our hypothesis was that male T2D patients with low total T levels have worse profile on the risk markers for vascular disease accordingly in this study; we compared the presence of altered atherosclerotic markers in middle-aged T2D subjects with normal and low total T.
Research Design and Methods
This cross-sectional prospective study was conducted in a single referral center in Buenos Aires, Argentina, between September 2010 and November 2012. We screened 148 male patients with a known history of T2D; 115 of the patients fulfilled the following inclusion criteria: male gender, age less than 70 years, and no history of cardiovascular disease. Exclusion criteria comprised the presence of structural cardiomyopathy, arrhythmia, valvular heart disease, previous cardiovascular events, endocrine disorders that could affect the hypothalamic-pituitary-gonadal axis and previous androgen replacement treatment. The Institutional Review Board and Ethics Committee at Sanatorio Guemes (Buenos Aires) approved the study. Written and signed informed consent was obtained from all patients and participants prior to the study.
To assess the association between T concentration and atherosclerotic disease, we divided the study population into 2 groups: subjects with low total T (<12.1 nmol/L) and subjects with normal total T (≥12.1 nmol/L). We analyzed atherosclerotic disease on patients with free T below 70 pg/mL (250 pmol/L). The rationale for using these cutoff values was the consensus by the International Society of Andrology regarding no substitution at levels total T of 3.5 ng/mL or greater or 12.1 nmol/L or greater (19). Each patient was screened with a questionnaire detailing his medical history, family history for cardiovascular disease, diabetic complications, and use of pharmacological agents for the treatment of hypogonadism (androgen replacement), diabetes, lipids, and hypertension.
Carotid artery IMT with the presence of carotid atherosclerotic plaques was assessed by high-resolution B-mode ultrasound of the common carotid artery using the standardized evaluation as recommended by the American Society of Echocardiography, 34th Report of the Bethesda Conference Task Force number 3 Noninvasive Atherosclerosis Evaluation (20). We considered an IMT of 0.1 cm or greater to be a significant value of end-organ damage, as recommended by European guidelines (21, 22). Patients were examined in the supine position, and each carotid wall and segment were examined to identify IMT. The IMT was defined as the distance between the echogenic line representing the intima-blood interface and the outer echogenic line representing the adventitia junction.
Endothelial function was assessed by brachial artery flow-mediated dilation (23). Ultrasound images of the brachial artery were obtained at baseline under standardized conditions and 60 seconds after the induction of reactive hyperemia by cuff occlusion of the forearm for 5 minutes. Image landmarks and surface markers were used to ensure anatomical consistency between serial imaging studies. The ultrasound was performed by a physician experienced in vascular studies and certified in cardiovascular imaging by the Argentinean Society of Cardiology. The physician was blinded to the patient's clinical data. The flow-mediated dilatation was reported as the percentage increase in diameter from the baseline.
Laboratory methods
Plasma glucose and lipid levels were measured on a chemistry analyzer (Beckman Diagnostics) using reagents and calibrators from Beckman Diagnostics. Blood samples for T assays were drawn between 7:00 and 10:00 am and analyzed by a chemiluminescence assay (Elecsys 2010; normal range 2.50–1500 ng/dL or 0.087–52.0 nmol/L). Free T levels were calculated from total T and SHBG levels according to the Vermeulens equation (24) and chemiluminescence assay. SHBG was measured by electrochemiluminescence, and ultrasensitive C-reactive protein (Hs-CRP) was measured by immune turbidimetric quantitative assay. Prolactin, LH, and FSH were measured in each subject to rule out the presence of abnormalities of the hypothalamic-pituitary-gonadal axis.
Statistical analysis
Data were analyzed using the STATA 12 package (Stata Corp). Values are expressed as a percentage of each group or as mean ± SE. Data were obtained by a simple calculation. The Mann-Whitney U test was used to compare demographics and continuous variables between the low and normal T groups. The correlation between total T and IMT was calculated by the Spearman correlation coefficient. Simple and multiple logistic regressions were used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) between the T levels and IMT greater than 0.1 cm, plaque presence, and the presence of endothelial dysfunction. The Shapiro-Wilk test and nonparametric test were used to compare data nonnormally distributed. A two-tailed α < .05 was considered statistically significant.
Results
Patient characteristics are reported in Table 1. Of the 115 patients, 36 (31%) had low serum T levels (<12.1 nmol/L), whereas 79 (69%) had normal serum T levels (≥12.1 nmol/L). Forty-eight patients (41.7%) had free T below 70 pg/mL. There were no differences in the presence of a family history for cardiovascular disease, age, weight, body mass index (BMI), glucose, plasma triglycerides, low-density lipoprotein (LDL) cholesterol, or duration of diabetes between the patients with low and normal T levels. High-density lipoprotein (HDL) cholesterol was higher in the normal T group (P < .002). As expected, the mean total T, free T, and SHBG levels were significantly lower in the low total T group than in the normal total T group.
Table 1.
Comparison Between Patients With Low T (≤12.1 nmol/L) and Normal T (≥12.1 nmol/L)
| Group | Low Total T (<12.1 nmol/L) | Normal Total T (≥12.1 nmol/L) | P Value |
|---|---|---|---|
| Number of patients, n | 36 | 79 | |
| Age, y | 59 ± 5.8 | 58 ± 7.01 | .78 |
| Diabetes duration, y | 7.9 ± 3.06 | 6.0 ± 3.19 | .06 |
| Weight, kg | 90 ± 9.8 | 88 ± 13.8 | .44 |
| BMI, kg/m2 | 29.8 ± 2.9 | 29.1 ± 3.9 | .27 |
| Triglycerides, mg/dL | 124 ± 27.9 | 130 ± 74.0 | .63 |
| LDL cholesterol, mg/dL | 127 ± 23.1 | 118 ± 24.5 | .17 |
| HDL cholesterol, mg/dL | 37 ± 6.8 | 41 ± 8.3 | .002 |
| HbA1c, % | 7.05 ± 0.58 | 6.9 ± 1.04 | .10 |
| Glucose, mg/dL | 133 ± 14.5 | 132 ± 28.3 | .92 |
| SHBG, nmol/L | 34.79 ± 10.4 | 42.76 ± 22.4 | <.001 |
| Total T, nmol/L (ng/L) | 10.03 ± 1.86 (2.9 ± 0.54) | 17.64 ± 4.53 (5.1 ± 1.31) | |
| Free T, pmol/L (pg/mL) | 202.3 ± 82 (58.3 ± 23.8) | 308 ± 176 (89.0 ± 51.0) | <.005 |
| Carotid IMT, cm | 0.107 ± 0.013 | 0.083 ± 0.019 | <.0001 |
| Endothelial dysfunction, n (% of patients) | 29/36 (80.5%) | 33/78. (42.3%) | <.003 |
| Plaque, n (% of patients) | 24/ 35 (68.5) | 35/78 (44,8%) | <.02 |
| C-reactive protein, mg/L | 2.74 ± 1.37 | 0.89 ± 0.93 | <.0001 |
Data are expressed as mean ± SE.
The whole study population had a mean age of 56.6 years, T2D duration of 6 years, HbA1c. High-density lipoprotein (HDL) levels of 6.9%, a BMI of 30.1 kg/m2, LDL cholesterol of 119 mg/dL, and a mean HDL of 39.9 mg/dL. The T2D and other pharmacological treatments were similar between the men with low and normal T. Metformin was the most common treatment (88%), followed by insulin secretagogues (∼30%), DPP-4 inhibitors (10%), and insulin (5.5%). Angiotensin-converting enzyme inhibitors were used in 55 of 115 in the low T group (48.6%) and 61 of 115 in the normal T group (53%) (P = NS). Statins were used in 45% of both groups.
Atherosclerotic disease markers
Intima-media thickness
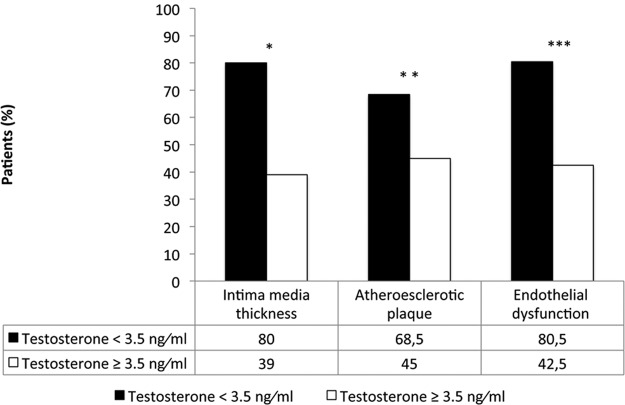
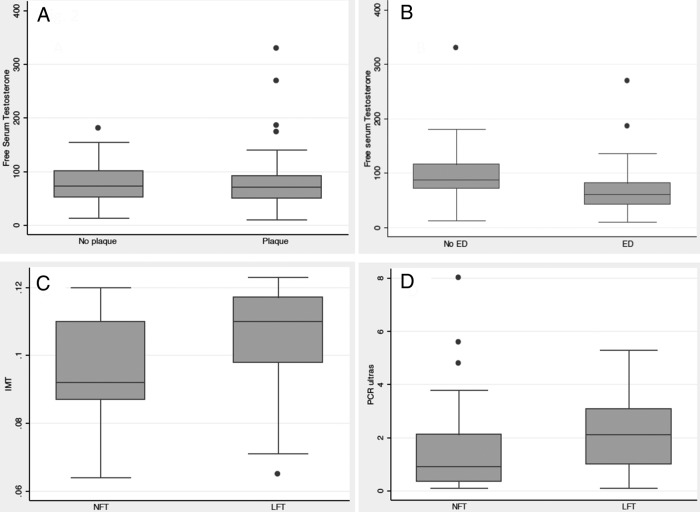
The IMT was significantly higher in patients with low total T compared with those with normal total T (0.107 ± 0.01 cm and 0.083 ± 0.01 cm, P < .0001) (Table 1). Higher IMT was found in the group of patients with low free T with respect to the group with normal free T (0.105 ± 0.01cm and 0.095 ± 0.01 P < .0001). IMT of 0.1 cm or greater was present in 80% of the patients with low T and 39% with normal T. In addition, 68.5% of the patients with low T had atherosclerotic plaques compared with 44.8% of the subjects with normal T (P < .02) (Figure 1). Plaque presence was not different between patients with normal or low free T (P < .65) No difference on serum free T was observed in the patients with or without plaque presence (Figure 2). IMT was negatively correlated with total T levels (r = −0.39, P = .0001) (Figure 3).
Figure 1.
Percentage of patients with low and normal T concentration and IMT (carotid) greater than 0.1 cm, atherosclerotic plaque presence, and endothelial dysfunction. *, P < .02; **, P < .001; ***, P < .003.
Figure 2.
Atherosclerotic risk markers and free serum T. A, Plaque presence and serum free T levels (picograms per milliliter) (P = NS). B, Endothelial function and serum free T levels (picograms per milliliter) (P < .001). C, IMT less than 0.0001, D, Serum C-reactive protein levels (milligrams per liter) (P < .001). ED, endothelial dysfunction; LFT, patients with low free testosterone; NFT, patients with normal free T; No ED, no endothelial dysfunction.
Figure 3.
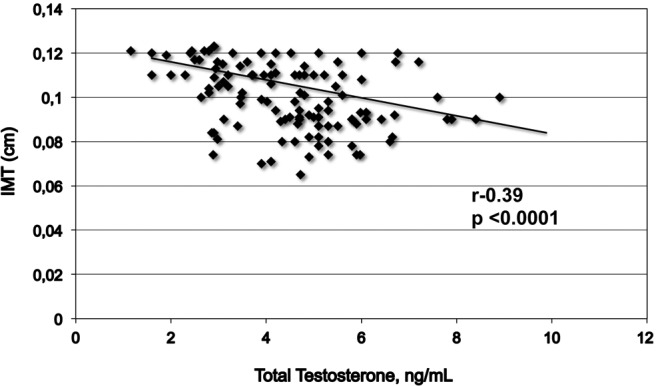
Correlation between total T and IMT.
Endothelial function
All 115 patients underwent endothelial function assessment. Patients with positive tests had 13.52 ± 4.74 nmol/L and 96.5 ± 48.6 pg/mL serum total and free T, respectively, and patients without endothelial dysfunction had serum total T of 18 ± 4.74 nmol/L and free T 66.1 ± 40.1 pg/mL (P < .001). In the low total T group, 80.5% of the patients had positive tests compared with the normal total T group, in which 42.3% had normal endothelial function (P < .003) (Table 1). Similar results were found on the low free T group with respect to the normal free T group (P < .00001)
The Hs-CRP concentration was significantly higher in the low total T group than in the normal T group (2.74 ± 1.37 and 0.89 ± 0.93 mg/L, respectively, P < .0001). Similar results were found considering the low and normal free T groups (2.07 ± 1.20 mg/L and 1.43 ± 1.32 mg/L, respectively P < .0017).
When the presence of these three major atherosclerotic markers were analyzed together for each individual patient (IMT ≥ 0.1 cm, plaque presence, and endothelial dysfunction), we observed that 54% of the patients with low total T and 10% of subjects with normal T evidenced a higher risk for vascular disease.
Multiple logistic regression analysis showed that low total T independently predicted the markers of atherosclerotic disease, IMT greater than 0.1 cm (OR 6.41; CI 2.50–16.43, P < .0001), plaque presence (OR 2.6; CI 1.12–6.03, P < .001), and endothelial dysfunction (OR 5.77; CI 2.77–14.77, P < .001). After adjustments for age, BMI, lipids, T2D duration, and type of diabetes treatment, HbA1c, IMT (OR 8.43; CI 2.5–25.8, P < .001), and endothelial dysfunction remained significant (OR 8.43; 95% CI 2.5–25.8, P < .001, and OR 5.21; CI 1.73–15.63, P < .003, respectively). However, plaque presence was not significantly associated with total T levels (OR 1.77; CI 0.66–4.74, P = .26).
Discussion
This cross-sectional study aimed at evaluating the association between atherosclerotic markers and total T in male patients with T2D. We found that one-third of adult patients with T2D have low total serum T. A low T level in patients with diabetes was found to be negatively correlated with the presence of high values on the risk markers for atherosclerosis, including higher IMT and Hs-CRP concentration and more endothelial dysfunction.
To our knowledge, this is the first cross-sectional study evaluating the association between major atherosclerotic disease markers and low serum T levels in middle-aged men with T2D. Our results indicate that, in contrast to T2D subjects with normal serum T levels, subjects with T2D and low total and free T levels evidence increased atherosclerosis as supported by the presence of endothelial dysfunction, higher IMT, and Hs-CRP. Nearly half of our patients with low total T had a measured IMT greater than 0.1 cm, with atherosclerotic plaques and endothelial dysfunction. Our results indicate that diabetic patients with low total T had a 6-fold higher risk for increased carotid IMT and endothelial dysfunction compared with subjects with normal total T. In multiple regression models, a low total T level less than 12.1 nmol/L was found to be an independent marker for increased IMT and endothelial dysfunction. This association was independent of age, BMI, diabetes duration, lipid levels, and treatment of hypertension. These findings are in agreement with previous studies that reported increased IMT in nondiabetic patients with low T levels, indicating accelerated atherosclerosis (25).
Extensive evidence from observational studies has indicated a strong association between low serum T and the presence of metabolic syndrome and increased cardiovascular risk factors (26). Patients with low T levels, with and without diabetes, have higher BMI, abdominal obesity, hypertension, and insulin resistance as well as higher HbA1c, LDL cholesterol, and triglyceride concentrations compared with those with normal serum T (16, 27). In addition, several cross-sectional studies have reported an association between low serum T, increased all-cause mortality, and higher cardiovascular events in elderly men with and without diabetes (28).
T can mediate vascular vasodilation and influence arterial stiffness and atherosclerosis progression (29, 30). Circulating T levels are negatively correlated with factors involved in atherogenesis, including fibrinogen, plasminogen activator inhibitor-1, and insulin concentrations (31). These levels are positively correlated with HDL cholesterol (32). It is not clear, however, whether low T has a direct role in atherogenesis or is merely a marker of more advanced atherosclerotic disease (12, 33). Previous studies of T replacement have reported increased muscle mass, healthier fat distribution, improved insulin sensitivity, and some improvement in the components of metabolic syndrome (34, 35). However, T replacement in older men with hypogonadism does not reduce cardiovascular events and, conversely, has been associated with an increased risk of cardiovascular events (36).
There are several limitations in this cross-sectional study, including the lack of longitudinal and long-term follow-up to determine the progression of atherosclerosis in diabetic subjects with low and normal total T and lack of information about smoking history. In addition, we did not assess the role of T replacement in cardiovascular markers among the subjects with low T. However, we did show that this association is present in middle-aged men. Prospective randomized studies are needed to assess the clinical significance of our findings and the clinical impact of T replacement on cardiovascular risk factors in diabetic patients with low total T.
In summary, middle-aged men with low T, less than 12.1 nmol/L, have evidence of more advanced atherosclerotic disease, including a higher presence of carotid atherosclerotic plaques and greater IMT. These patients also show evidence of endothelial dysfunction and higher Hs-CRP concentrations. This study's results confirm previous reports from observational studies on the association between increased cardiovascular events and hypogonadism in patients with diabetes.
Acknowledgments
Authors' contributions include the following: J.M.F. is the guarantor of this work. As such, J.M.F. had full access to all study data and takes responsibility for the integrity and accuracy of the data analysis. J.M.F. and M.T. wrote the initial research proposal and manuscript. M.T. conducted the cardiological evaluations. M.K. reviewed the research proposal and conducted the statistical analyses. G.E.U. reviewed and edited the research proposal and manuscript and contributed to the discussion.
G.E.U. is supported in part by research grants from the American Diabetes Association (Grant 1–14-LLY-36), Public Health Service Grant UL1 RR025008 from the Clinical Translational Science Award Program (Grant M01 RR-00039), National Institutes of Health, and National Center for Research Resources.
Disclosure Summary: J.M.F., M.T., and M.K. have nothing to disclose. GEU has also received unrestricted research support for inpatient studies (to Emory University) from Sanofi, Merck, Novo Nordisk, Boehringer Ingelheim, Eli Lilly, and Endo Barrier and has received consulting fees and/or honoraria for membership in advisory boards from Sanofi, Merck, and Boehringer Ingelheim.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- HDL
- high-density lipoprotein
- Hs-CRP
- highly sensitive C-reactive protein
- HbA1c
- hemoglobin A1c
- IMT
- intima-media thickness
- LDL
- low-density lipoprotein
- OR
- odds ratio
- T2D
- type 2 diabetes.
References
- 1. The Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375(9733):2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haring R, Volzke H, Steveling A. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J. 2010;31:1494–1501. [DOI] [PubMed] [Google Scholar]
- 3. English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000;11:890–894. [DOI] [PubMed] [Google Scholar]
- 4. Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab. 2010;21:496–503. [DOI] [PubMed] [Google Scholar]
- 5. Kapoor D, Hazei A, Clark S, Jones H. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes. Diabetes Care. 2007;30:911–917. [DOI] [PubMed] [Google Scholar]
- 6. Nettleship JE, Pugh PJ, Channer KS, Jones T, Jones RD. Inverse relationship between serum levels of interleukin-1b and testosterone in men with stable coronary artery disease. Horm Metab Res. 2007;39:366–371. [DOI] [PubMed] [Google Scholar]
- 7. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. [DOI] [PubMed] [Google Scholar]
- 8. Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 Study). Diabetes Care. 2011;34:828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mårin P, Krotkiewski M, Björntorp P. Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissues. Eur J Med. 1992;6:329–336. [PubMed] [Google Scholar]
- 10. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217:R25–R45. [DOI] [PubMed] [Google Scholar]
- 11. Khaw KT, Dowsett M, Folkerd E. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–2701. [DOI] [PubMed] [Google Scholar]
- 12. Vikan T, Schirmer H, Njølstad I. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromsø Study. Eur J Endocrinol. 2009;161:435–442. [DOI] [PubMed] [Google Scholar]
- 13. Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34:1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–5468. [DOI] [PubMed] [Google Scholar]
- 15. Chandel A, Dhindsa S, Topiwala S. Testosterone concentration in young patients with diabetes. Diabetes Care. 2008;31(10):2013–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grossman M, Merlin CT, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–1840. [DOI] [PubMed] [Google Scholar]
- 17. Iglesias del Sol A, Bots ML, Grobbee DE, Hofman A, Witteman JC. Carotid intima-media thickness at different sites: relation to incident myocardial infarction: the Rotterdam Study. Eur Heart J. 2002;23:934–940. [DOI] [PubMed] [Google Scholar]
- 18. Johnsen SH, Mathiesen EB, Joakimsen O. Carotid atherosclerosis is a stronger predictor of myocardial infarction in study women than in men: a 6-year follow-up study of 6226 persons: the Tromso. Stroke. 2007;38:2873–2880. [DOI] [PubMed] [Google Scholar]
- 19. Wang C, Nieschlag E, Wu FCW. Investigation, treatment and monitoring of late-onset hypogonadism in males. Eur J Endocrinol. 2008;159:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizzo M, Corrado E, Novo S. Prediction of cardio- and cerebrovascular events in patients with subclinical carotid atherosclerosis and low HDL cholesterol. Atherosclerosis. 2008;200:389–395. [DOI] [PubMed] [Google Scholar]
- 21. Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111; quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 22. Brunner H, Cockcroft JR, Deanfield J, et al. Part II: Association with cardiovascular risk factors and diseases: a statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–246. [DOI] [PubMed] [Google Scholar]
- 23. Vogel RA. Measurement of endothelial function by brachial arterial flow-mediated vasodilation. Am J Cardiol. 2001;88(2A):31E–34E. [DOI] [PubMed] [Google Scholar]
- 24. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- 25. Ruige JB, Mahmoud AM, De Bacquer D. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97:870–875. [DOI] [PubMed] [Google Scholar]
- 26. Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. [DOI] [PubMed] [Google Scholar]
- 27. Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. [DOI] [PubMed] [Google Scholar]
- 28. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. [DOI] [PubMed] [Google Scholar]
- 29. Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109(17):2074–2079. [DOI] [PubMed] [Google Scholar]
- 30. Lu Y, Fu Y, Ge Y, Juncos LA, Reckelhoff JF, Liu R. The vasodilatory effect of testosterone on renal afferent arterioles. Gend Med. 2012;9(2):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saldanha PA, Cairrão E, Maia CJ, Verde I. Long- and short-term effects of androgens in human umbilical artery smooth muscle. Clin Exp Pharmacol Physiol. 2013;40(3):181–189. [DOI] [PubMed] [Google Scholar]
- 32. De Pergola G, De Mitrio V, Sciaraffia M, et al. Lower androgenicity is associated with higher plasma levels of prothrombotic factors irrespective of age, obesity, body fat distribution, and related metabolic parameters in men. Metabolism. 1997;46(11):1287–1293. [DOI] [PubMed] [Google Scholar]
- 33. Phillips GB, Pinkernell BH, Jing T-Y. The Association of Hypotestosteronemia with coronary artery disease in Men. Arterioscler Thromb Vasc Biol. 1994;14:701–706. [DOI] [PubMed] [Google Scholar]
- 34. Rubinow KB, Vaisar T, Tang C, Matsumoto AM, Heinecke JW, Page ST. Testosterone replacement in hypogonadal men alters the HDL proteome but not HDL cholesterol efflux capacity. J Lipid Res. 2012;53(7):1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol. 2014;2014:527470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]