Abstract
Context:
The hypothalamic pituitary-adrenal axis is thought to play a role in Type 2 Diabetes (T2D). However, the evidence for an association between diurnal cortisol patterns and T2D is equivocal.
Objective:
The aim was to examine the association of cortisol patterns throughout the day with T2D status in a community-dwelling population.
Design:
This was a cross-sectional study of T2D status and salivary cortisol from phase 7 (2002–2004) of the Whitehall II study, United Kingdom.
Setting:
The occupational cohort was originally recruited in 1985–1988.
Participants:
Three-thousand, five-hundred eight white men and women including 238 participants with T2D aged 50–74 years with complete information on cortisol secretion participated.
Outcome Measures:
We measured diurnal cortisol (nmol/L) patterns from six saliva samples obtained over the course of a normal day: at waking, +30 min, +2.5, +8, +12 hours, and bedtime. The cortisol awakening response and slope in diurnal secretion were calculated.
Results:
T2D status was associated with a flatter slope in cortisol decline across the day (b = 0.004; confidence interval [CI], 0.001–0.007; P = .014) and greater bedtime cortisol (b = 0.063; CI, 0.010–0.117; P = 0.020) independent of a wide range of covariates measured at the time of cortisol assessment. There was no association between morning cortisol, the cortisol awakening response, and T2D (P > .05).
Conclusions:
In this nonclinical population, T2D was associated with a flatter slope in cortisol levels across the day and raised bedtime cortisol values.
Type 2 diabetes (T2D) is a chronic metabolic and endocrine disorder characterized by impaired insulin resistance and pancreatic β-cell dysfunction (1). The hypothalamic pituitary adrenal (HPA) axis is thought to play a role in T2D (2). Pathological (3) and experimental (4) exposure to excessive cortisol (a product of the HPA system) is related to metabolic disturbances such as hypertension, hyperlipidemia, and central obesity, all of which are risk factors for T2D. Elevated cortisol concentrations, assessed from single plasma samples (5) and 24-hour urinary free samples (6) have been associated with raised plasma glucose (5) and insulin resistance (5, 6) in healthy participants. T2D is also a recognized complication of long-term cortisol excess as seen in Cushing's syndrome (7) and in glucocorticoid-treated patients (8).
Increasingly, the marked diurnal patterning in the release of cortisol has been the focus of large-scale HPA axis research (9). The diurnal cortisol pattern is typically characterized by high cortisol levels on waking, followed by an increase that reaches a peak 30 minutes after waking (termed the cortisol awakening response [CAR]) and subsequent decline across day (9). Flatter slope in cortisol over the day has been associated with diabetes-related outcomes such as central adiposity (10) and an increased risk of cardiovascular disease (11). However, the reported associations between diurnal cortisol patterns and T2D are equivocal.
In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, participants with T2D were found to have a significantly lower CAR than those without T2D (12). Bruehl et al (13), similarly observed a blunted CAR in participants with T2D, but found no association of T2D with slope in cortisol across the day. In contrast, Lederbogen et al (14), observed a flattened slope in diurnal cortisol secretion among those with diabetes. Whereas, Vreeburg et al (15), observed no association between diabetes status and the CAR or diurnal cortisol slope.
The reasons for these divergent findings are unknown. It is possible that differences in participant characteristics or in the number and timing of cortisol samples between studies may be involved. We therefore sought to examine the association of diurnal cortisol secretion with diabetes status in sample of 3508 community-dwelling men and women of the Whitehall II study. We predicted that individuals with T2D would have a greater CAR and flatter slope in cortisol across the day.
Methods and Measures
Participants
We used data from phase 7 (2002–2004) of the British Whitehall II study. The cohort of 10 308 participants was initially recruited between 1985 and 1988 from 20 London-based civil service departments. The total number of participants at phase 7 was 6967, and of these, 6484 had a clinical assessment. Saliva collection for the assessment of cortisol was instigated partway through phase 7 and of those participants that were asked to collect saliva samples, 90.1% (n = 4608) returned samples. This group had fewer participants in the lowest civil service employment grades compared with phase 1 of the study: however, this difference was small. We restricted our analysis to those with complete information on time of waking, cortisol measures, and diabetes status, giving a final sample of 3508 participants (see Figure 1). Ethical approval for the Whitehall II study was obtained from the University College London Medical School committee on the ethics of human research. Informed consent for involvement in the study was obtained from all participants.
Figure 1.
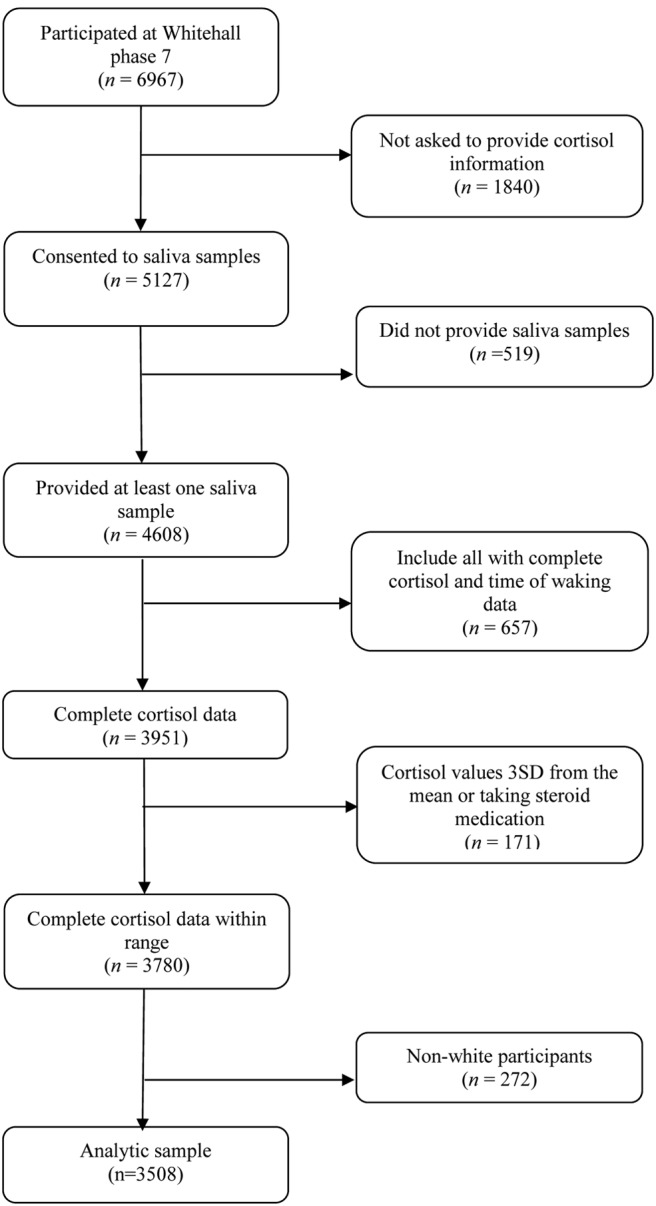
Flow diagram of participants included and excluded from the analyses.
Cortisol collection and analysis
The collection of cortisol from phase 7 of the study has been described previously (16). Participants were provided with a set of Salivettes and asked to take six samples over the course of a normal weekday (Monday–Friday) at waking, after 30 minutes, 2.5 hours, 8 hours, and 12 hours, and at bedtime. They were instructed not brush teeth or consume any food or beverages for 15 minutes prior to sample collection. An instruction booklet was used to record information on the day of sampling including wake time, time each sample was taken, and stressful events. The salivettes and booklet were returned by post. Salivettes were centrifuged at 3000× g for 5 minutes, resulting in a clear supernatant of low-viscosity. Salivary cortisol levels were measured using a commercial chemiluminescence immunoassay (IBL). The lower concentration limit of the assay was 0.44 nmol/L and the intra- and interassay coefficients of variance were less than 8%. Any sample over 50 nmol/L was reanalyzed.
T2D
T2D was defined as a fasting glucose at least 7.0 mmol/l or a 2-hour post-load glucose at least 11.1 mmol/l during the oral glucose tolerance test (OGTT) performed at the Whitehall clinical assessment or by reported doctor diagnosed diabetes, or the use of diabetes medication (17). For the purposes of the OGTT, participants provided a venous blood sample 8 hours after fasting and at 2 hours post administration of a 75-g glucose solution. Blood glucose was measured using the glucose oxidase method (18) on a YSI 2300 STAT PLUS Analyzer (YSI Corporation; mean coefficient of variation, 1.4–3.1%) (19). Of the 238 participants with prevalent diabetes in our sample, 126 had known diabetes (confirmed by report of doctor diagnosis and diabetic medication) at the beginning of phase 7. An additional 112 participants with diabetes were identified by the OGTT carried out at the Whitehall clinical assessment.
Covariates
We used standard protocols to assess characteristics of the participants at the time diurnal cortisol patterns were measured (phase 7; 2002–2004). Age, sex, and current or most recent civil service employment grade, a measure of social position, were assessed by questionnaire. Smoking status was defined as current smokers vs noncurrent smokers (16). Waking up time was assessed by participants' records on the day of the collection of saliva. Time difference between waking and taking first sample was categorized into 5-minute intervals. Body mass index (BMI) was assessed by measurement of height and weight at the clinical assessment. Height was assessed using a stadiometer with the head in the Frankfort plane, and weight was assessed using a portable digital scale (Tanita, Yiewsley). BMI was calculated as kg/m2. For presentational purposes, BMI is categorized as obese (≥30 kg/m2) or nonobese (<29.9 kg/m2). We have previously reported an association between fatigue and alterations in diurnal cortisol secretion (20), and fatigue is common in individuals with T2D (21). Therefore, we included fatigue as a covariate in the study. Fatigue was assessed using the vitality subscale of the Short Form-36 (22). At the clinical assessment it was recorded whether participants had a history of coronary heart disease (CHD). Participants also provided details of current medication use and these were subsequently coded using the British National Formulary (23). Cardiovascular medication usage was defined as the use of β-blockers, antihypertensives, lipid-lowering drugs, nitrates, or antiplatelet medications.
Statistical analysis
Participants with cortisol values outside three SD from the mean and those taking steroid medications were removed from the analyses (n = 171). Despite this, cortisol data were skewed and were therefore logged for analysis. The CAR was calculated by subtracting cortisol measured at time 1 (waking) from cortisol measured at time 2 (+30 minutes). Conventionally, analyses are restricted to samples that are collected within 10 minutes of waking (sample 1 taken >10 min; n = 646) because of a reduced CAR in those with longer delays (24). We did not see a difference in sample delays by diabetes status so all participants were retained. Most participants (n = 3395, 96.8%) took cortisol sample 2 on time. We not see a difference in late sample 2 collection by diabetes status so this was not included as a covariate in the analyses. The method used to calculate the diurnal slope in cortisol secretion has been previously described (10, 11, 20). In brief, the slope of the decline in cortisol levels over the day was calculated by regressing cortisol values on time after waking for samples 1 (waking), 3 (+2.5 hours), 4 (+8 hours), 5 (+12 hours), and 6 (bedtime). Because it is suggested that the CAR and slope in cortisol secretion are under different neurobiological control systems (9), sample 2 was not included to ensure that the CAR did not obscure the slope calculation. Lower (more negative) slopes suggest a more rapid decline in cortisol levels, whereas slope values closer to zero reflect flatter diurnal rhythms. Descriptive and clinical characteristics of the sample were compared using t tests for continuous variables and χ2 tests for categorical variables. Associations between prevalent diabetes and the cortisol measures were analyzed using linear regression. Multivariable linear regressions using waking cortisol, CAR, slope, and bedtime cortisol as outcome variables were performed to analyze associations with prevalent diabetes. Age, sex, grade of employment, smoking, waking time, late saliva collection, fatigue, BMI, cardiovascular medication, and history of CHD were included as covariates in all analyses. Participants with missing covariate information were excluded from the analyses. Previous research has shown sex differences in the relationship between cortisol and diabetes status (12). Therefore, we investigated whether diabetes status interacted with sex, but found no significant associations with cortisol measures, so interaction terms were not included in the final models. We have previously shown a nonlinear relationship between BMI and slope (10). We investigated whether the pattern of results changed including BMI as a quadratic term. Because the results were robust to controlling for the nonlinear effects of BMI, only BMI as a continuous variable was included in the final models. Of the Whitehall II participants who provided cortisol data 92.8% (n = 3508) were of white ethnicity. We investigated whether prevalent diabetes interacted with ethnicity in the current sample. This interaction term was significant for slope (P < .001) and bedtime cortisol (P = .015). Therefore, we limited the present analysis to individuals of white ethnicity. Results are presented as unstandardized regression coefficients (b) with 95% confidence intervals (CI). The slope estimates were generated using 1 MLWin version 2.10 beta 6, all other analyses were conducted using SPSS version 21 (SPSS).
Results
We restricted our analysis to those with complete information on time of waking, cortisol measures, and diabetes status. This resulted in 3508 participants. The characteristics of participants included and excluded from the analysis are displayed in Table 1. The groups significantly differed in diabetic prevalence. However, this effect did not remain when nonwhite participants were removed from the excluded group (P = .380, data not shown). The group with complete cortisol data were younger, more likely to be male, and had fewer participants in the lowest civil service employment grades. They were less likely to take cardiovascular medication and have a history of CHD than the phase-7 group members who did not provide saliva samples.
Table 1.
Characteristics of Participants at Phase 7 (2002–2004) of Whitehall II Study
| Participants Included in the Cortisol Analyses (n = 3508) | Participants Excluded from the Cortisol Analyses (n = 3459) | P Value | |
|---|---|---|---|
| Sex, % men | 2636 (75.1%) | 2257 (65.3%) | <.001 |
| Mean age, y, SD | 61.04 (5.94) | 61.44 (6.06) | .005 |
| Current smoker, % yes | 230 (6.6%) | 274 (8.0%) | .056 |
| Employment grade, % lowest | 271 (7.7%) | 489 (14.6%) | <.001 |
| Mean BMI, SD | 26.68 (4.29) | 26.84 (4.49) | .131 |
| Fatigued, % yes | 682 (19.5%) | 700 (21.3%) | .068 |
| Cardiovascular medication, % yes | 1005 (28.6%) | 1135 (33.1%) | <.001 |
| History of CHD, % yes | 467 (13.7%) | 570 (17.2%) | <.001 |
| Type 2 Diabetes, % yes | 238 (6.78%) | 309 (8.9%) | <.001 |
Data are presented as means ± SDs or N (%).
The characteristics of the participants who provided cortisol samples are displayed in Table 2. Two hundred and thirty eight participants (6.78%) had prevalent diabetes at the time of saliva collection. The group with diabetes were older on average and more likely to be in the lowest civil service employment grades. They were more likely to be obese, have a history of CHD, and take cardiovascular medicine than those without diabetes. Cortisol collection measures such as waking time on day of sampling did not differ by diabetes status.
Table 2.
Characteristics of Participants at Time of Cortisol Assessment by Diabetes Status
| N of Participants | No Diabetes (n = 3270) | Prevalent Diabetes (n = 238) | P Value | |
|---|---|---|---|---|
| Mean age, y, SD | 3508 | 60.85 (5.89) | 63.64 (5.99) | <.001 |
| Sex, % men | 3508 | 2455 (75.1%) | 181 (76.1%) | .816 |
| Current smoker, % yes | 3506 | 212 (6.5%) | 18 (7.6%) | .522 |
| Employment grade, % lowest | 3498 | 237 (7.3%) | 34 (14.3%) | <.001 |
| Obese, % yes | 3494 | 567 (17.4%) | 80 (33.8%) | <.001 |
| Fatigued, % yes | 3491 | 625 (19.2%) | 57 (24.1%) | .075 |
| Late saliva collection, % yes | 3508 | 597 (18.3%) | 49 (20.6%) | .386 |
| Cardiovascular medication, % yes | 3508 | 861 (26.3%) | 144 (60.5%) | <.001 |
| History of CHD, % yes | 3398 | 407 (12.8%) | 60 (26.2%) | <.001 |
Data are presented as means ± SDs or N (%).
The average CAR in the sample was 7.33 (SD = 11.575). As shown in Table 3, the CAR did not differ by diabetes status (b = 0.002; CI, −0.036–0.039; P = .923). The average diurnal slope estimated from the hierarchical linear model was −0.1290 nmol/l per h (SD = 0.023). Participants with diabetes had a flatter slope in cortisol across the day than those without diabetes (b = 0.004; CI, 0.001–0.007; P = .014). This association was robust to adjustment for age, sex, grade of employment, smoking, waking time, late saliva collection, fatigue, BMI, cardiovascular medication, and history of CHD. A flatter slope in cortisol patterns across the day can be due to low waking values or high evening values of cortisol. We examined the association of these cortisol measures with diabetes status. Although participants with diabetes had higher waking levels on average compared with those without diabetes, this difference was not significantly different (b = 0.014; CI, −0.018–0.046; P = .383). In contrast, cortisol measures at bedtime differed significantly between the groups. Participants with diabetes had significantly greater bedtime cortisol values than those without controlling for covariates (b = 0.063; CI, 0.010–0.117; P = .020). This suggests that raised evening cortisol levels accounted for the difference in slope between the two groups.
Table 3.
Mean Scores of Measures of Cortisol by Diabetes Status at Phase 7
| Prevalent Diabetes (n = 238) | No Diabetes (n = 3270) | P Value | Adjusted P Value | |
|---|---|---|---|---|
| Waking cortisol, nmol/L | 16.32 (7.74) | 15.82 (7.18) | .383 | |
| CAR, nmol/L | 7.35 (10.64) | 7.54 (10.96) | .923 | |
| Slope across the day, nmol/L/h | −0.125 (0.022) | −0.129 (0.023) | .002 | .014 |
| Bedtime cortisol, nmol/L | 2.59 (2.57) | 2.34 (2.95) | .002 | .020 |
Data are presented as means ± SDs.
Adjusted for age, sex, smoking, grade of employment, waking time, fatigue, late saliva collection, BMI, CVD medication, and history of CHD.
Discussion
This study investigated the cross-sectional association between components of the diurnal cortisol profile and diabetes status in a large population of community-dwelling adults. We found that the slope in cortisol across the day was flatter in those with compared with those without T2D. Our data suggest that the flat slope in cortisol in individuals with T2D is due to raised late evening cortisol levels rather than depressed morning levels. These findings were robust to adjustment for a range of covariates. No association emerged for the CAR.
Previous reports of the association between cortisol secretion and diabetes status are mixed (12–15). In the present study, we observed a flattened diurnal cortisol slope in participants with T2D. This corroborates the results of Lederbogen et al (14), who found an association between diabetes status and flatter daily cortisol profiles in 979 individuals from a community cohort. Similar to our analysis, individuals with T2D were observed to have raised evening cortisol concentrations compared with nondiabetic controls. Elevated late-night cortisol levels have been suggested as a diagnostic criterion for Cushing's syndrome (25). We removed participants with very high cortisol concentrations from our analysis, which would serve to exclude individuals with Cushing's syndrome. Our findings were also independent of obesity, which is strongly associated with the disorder. Indeed, raised late-night salivary cortisol levels have been previously been described in individuals with diabetes but without Cushing's syndrome (26).
In contrast with our findings, Vreeburg et al (15), found no association between T2D and diurnal cortisol slope in 491 individuals without psychopathology from The Netherlands Study of Depression and Anxiety (NESDA) cohort. Participants in the study provided four saliva samples within an hour of waking and two late-evening samples. The additional samples collected in the late morning and afternoon in the present investigation may account for the diverging findings, as we were better able to define the shape of the diurnal cortisol curve. It is possible that the lack of information on late morning and afternoon cortisol levels reduced the ability of Vreeburg et al to examine the curvilinear nature of the decline in cortisol across the day (9).
We failed to find an association between diabetes status and the CAR. This result is in contrast with the findings of Bruehl et al (13) and Champaneri et al (12), who observed a blunted CAR in T2D individuals relative to controls. The reasons for the inconsistent results are unclear. However, our study is considerably larger than previous studies and consisted of a well-defined group of white, community-dwelling individuals. In contrast, the study by Bruehl et al (13) was limited by low participant numbers and a lack of adjustment for potential confounding factors. Champaneri et al (12) investigated the association between diabetes status and cortisol secretion in a cohort of over a 1000 individuals. However, the sample used was ethnically diverse and greater than 60% of the participants were of Hispanic origin. The present study was unpowered to detect the potential ethnic differences in the association between cortisol secretion and T2D and this may account for the differing findings between the studies.
Waking cortisol was not related to diabetes status in the current analysis. We have previously reported a relationship between fatigue and lower cortisol on waking (20) and fatigue is a common complaint among individuals with diabetes (21). Fatigue was independently associated with the diurnal cortisol slope and bedtime cortisol in the present study (data not shown). However, the association between T2D and these cortisol measures was robust to adjustment for this factor.
The causes of a flattened slope in diurnal cortisol are unknown and the mechanisms by which T2D is related to the HPA axis also remain to be elucidated. Cortisol plays a pivotal role in many physiological processes relevant to diabetes. It directly reduces insulin sensitivity and decreases insulin secretion by acting through glucocorticoid receptors, which are expressed on pancreatic β-cells. It triggers hepatic gluconeogenesis, promotes lipolysis and the release of fatty free acids into the circulation and the accumulation of triglycerides in adipose tissue (2). Obesity is common in T2D and visceral adipose tissue expresses high levels of glucocorticoid receptors (27). It has been hypothesized that adipocytes are a source of cortisol. Research has shown that transgenic mice overexpressing 11β-hydroxysteroid dehydrogenase type 1, the enzyme activating the inactive form of glucocorticoids, have increased adipose levels of corticosterone (28). Increased 11β-hydroxysteroid dehydrogenase type 1 activity in human visceral adipose tissue has been associated with symptoms of the metabolic syndrome (29). Thus, obesity offers one possible mechanism through which T2D might be associated with alterations in cortisol secretion.
Participants with diabetes in our sample were significantly more likely to be obese and obesity has been associated with a flattened slope in diurnal cortisol secretion (10). As previously reported (10), BMI as a continuous measure was not associated with the slope in diurnal cortisol secretion. However, obesity was independently associated with the diurnal cortisol slope and bedtime cortisol (data not shown). Despite this the relationship between diabetes status and these cortisol measures was robust to adjustment for obesity.
Inflammation is another pathway through which T2D might be related to alterations in HPA axis function. Inflammatory cytokines are involved in the pathogenesis of T2D. Circulating cytokine levels are elevated in diabetic individuals (30) and heightened concentrations are predictive of T2D development in initially healthy samples (31). Cortisol is involved in the regulation of inflammation (2) and circadian rhythms are regulated at the hypothalamic level by the suprachiasmatic nuclei. It has been suggested that circadian control is an important aspect of hypothalamic-immune communication, and that glucocorticoids may dysregulate the immune response via circadian-immune communication (32). It is also possible that disturbances in circadian rhythms may act on T2D through the alteration of glucose metabolism. Recent experimental work suggests that circadian disruption increases both fasting and postprandial plasma glucose concentrations through inadequate pancreatic insulin secretion (33). Additional research is needed to examine whether changes in inflammation and alterations in glucose metabolism may underlie the association between flatter slopes in diurnal cortisol section and T2D.
Another mechanism that may explain the relationship between diurnal cortisol slope and T2D is psychosocial stress. Results from meta-analyses and longitudinal studies suggest that psychosocial factors increase the risk of developing T2D (34) and contribute to disease progression in diabetic individuals (35). Cortisol levels are elevated by exposure to stress (36) and the flattening of the diurnal cortisol slope has been associated with both acute and chronic stress factors (37). Acute stress assessed by stressful events on the day of saliva sampling was not associated with the slope in cortisol or evening cortisol in the current analysis and our findings remained independent of acute stress (data not shown). However, it is possible that the findings could be attributed to long-term changes in circadian regulation as a result of chronic stress in people with T2D. Additional research is needed to examine whether chronic stress factors may underlie the association of flattened diurnal cortisol slopes with T2D.
In the present study we assessed cortisol across the day in a large community-based sample. The participants with T2D were well-characterized and we were able to use data from the larger cohort study to adjust for a number of potentially confounding factors in our analysis. However, our findings should be interpreted in light of some limitations. The Whitehall II study is an occupational cohort of civil servants and as such, our sample is not representative of the general population. The larger cohort study is predominately of white ethnicity, and due to ethnic differences in the pathogenesis of T2D we restricted the current analysis to white individuals. Therefore, the present results may not generalize to other populations. T2D was assessed by self-report of doctor diagnosis, use of diabetic medications, or OGTT rather than clinical diagnosis. We also lacked data on the duration of T2D, which may be related to neuroendocrine function. The associations observed in the analyses were small. However, these patterns are thought to be representative of chronic differences that are present on an everyday basis. Under these circumstances, even modest effects may contribute to substantial accumulated differences in cortisol output over time. Studies specifically designed to test the association between T2D and neuroendocrine function would provide richer data. The cross-sectional design makes it impossible to draw causal conclusions about the temporal relationship between aberrant cortisol output and diabetes. Cortisol was assessed over a single day and it has been suggested that this may obscure the CAR to situational rather than chronic correlates (38). Furthermore, the night release of cortisol was not assessed and therefore it was not possible to evaluate total 24-hour circadian cortisol exposure. We only measured free cortisol levels in the study and did not assess glucocorticoid receptor function. It is possible that the findings reflect reduced episodic cortisol release, which has been reported to modify the regulation of glucocorticoid sensitive genes (39). We relied on self report for the timing of sample collection. Our prevalence of “late” reporting was similar to previously reported rates (24) and evidence suggests that participants are generally accurate in their recording of this information (40).
Despite these considerations, our findings indicate that flat slopes in salivary cortisol, particularly raised evening levels of cortisol, are associated with T2D in nonclinical population of middle-aged men and women. The mechanisms by which these associations occur remain to be determined. It is possible that neuroendocrine dysfunction may be related to the pathophysiology of T2D. However, longitudinal studies are required to assess the prognostic properties of cortisol secretion for T2D.
Acknowledgments
R.A.H. and A.S. are funded by the British Heart Foundation (BHF). M.K. is partially supported by the Economic and Social Research Council International Centre for Life Course Studies in Society and Health (RES-596-28-0001). The Whitehall II study is supported by the BHF; Medical Research Council; National Heart, Lung and Blood Institute (HL36310); and National Institute on Aging (AG13196). The funding sources had no role in the design, conduct, or reporting of this study.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CAR
- cortisol awakening response
- CHD
- coronary heart disease
- CI
- confidence interval
- HPA
- hypothalamic pituitary-adrenal
- MESA
- Multi-Ethnic Study of Atherosclerosis
- NESDA
- Netherlands Study of Depression and Anxiety
- OGTT
- oral glucose tolerance test
- T2D
- type 2 diabetes.
References
- 1. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Dalmazi G, Pagotto U, Pasquali R, Vicennati V. Glucocorticoids and Type 2 Diabetes: From Physiology to Pathology. J Nutr Metab. 2012;525093:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing's disease over 50 years in Stoke-on-Trent, UK: Audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011;96(3):632–642. [DOI] [PubMed] [Google Scholar]
- 4. Connell JM, Whitworth JA, Davies DL, Lever AF, Richards AM, Fraser R. Effects of ACTH and cortisol administration on blood pressure, electrolyte metabolism, atrial natriuretic peptide and renal function in normal man. J Hypertens. 1987;5(4):425–433. [PubMed] [Google Scholar]
- 5. Phillips DI, Barker DJ, Fall CH, et al. Elevated plasma cortisol concentrations: A link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83(3):757–760. [DOI] [PubMed] [Google Scholar]
- 6. Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A. Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab. 2008;295(2):E385–E392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006;367(9522):1605–1617. [DOI] [PubMed] [Google Scholar]
- 8. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469–474. [DOI] [PubMed] [Google Scholar]
- 9. Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436. [DOI] [PubMed] [Google Scholar]
- 10. Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2010;95(9):4415–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: Findings from the Whitehall II Study. J Clin Endocrinol Metab. 2011;96(5):1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Diez Roux AD, Golden SH. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis. Metabolism. 2012;61(7):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009;34(6):815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lederbogen F, Hummel J, Fademrecht C, et al. Flattened circadian cortisol rhythm in Type 2 Diabetes. Exp Clin Endocrinol Amp Diabetes. 2011;119(09):573–575. [DOI] [PubMed] [Google Scholar]
- 15. Vreeburg SA, Kruijtzer BP, van Pelt J, et al. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology. 2009;34(8):1109–1120. [DOI] [PubMed] [Google Scholar]
- 16. Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92(3):819–824. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Supplement 1):S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cooper GR. Methods for determining the amount of glucose in blood. CRC Crit Rev Clin Lab Sci. 1973;4(2):101–145. [DOI] [PubMed] [Google Scholar]
- 19. Astles JR, Sedor FA, Toffaletti JG. Evaluation of the YSI 2300 glucose analyzer: Algorithm-corrected results are accurate and specific. Clin Biochem. 1996;29(1):27–31. [DOI] [PubMed] [Google Scholar]
- 20. Kumari M, Badrick E, Chandola T, et al. Cortisol secretion and fatigue: Associations in a community based cohort. Psychoneuroendocrinology. 2009;34(10):1476–1485. [DOI] [PubMed] [Google Scholar]
- 21. Fritschi C, Quinn L. Fatigue in patients with diabetes: A review. J Psychosom Res. 2010;69(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 23. Joint Formulary Committee. British National Formulary. Pharmaceutical Press; 2013;84–482. [Google Scholar]
- 24. Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65(2):313–319. [DOI] [PubMed] [Google Scholar]
- 25. Carroll T, Raff H, Findling J. Late-night salivary cortisol for the diagnosis of Cushing Syndrome: A meta-analysis. Endocr Pract. 2009;15(4):335–342. [DOI] [PubMed] [Google Scholar]
- 26. Liu H, Bravata DM, Cabaccan J, Raff H, Ryzen E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf). 2005;63(6):642–649. [DOI] [PubMed] [Google Scholar]
- 27. Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation. 2007;116(11):1234–1241. [DOI] [PubMed] [Google Scholar]
- 28. Masuzaki H, Paterson J, Shinyama H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294(5549):2166–2170. [DOI] [PubMed] [Google Scholar]
- 29. Walker BR, Andrew R. Tissue production of cortisol by 11β-hydroxysteroid dehydrogenase Type 1 and metabolic disease. Ann N Y Acad Sci. 2006;1083(1):165–184. [DOI] [PubMed] [Google Scholar]
- 30. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–823. [DOI] [PubMed] [Google Scholar]
- 31. Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arjona A, Sarkar DK. Are circadian rhythms the code of hypothalamic-immune communication? Insights from natural killer cells. Neurochem Res. 2008;33(4):708–718. [DOI] [PubMed] [Google Scholar]
- 33. Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43–129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pouwer F, Kupper N, Adriaanse MC. Does emotional stress cause type 2 diabetes mellitus? A review from the European Depression in Diabetes (EDID) Research Consortium. Discov Med. 2010;9(45):112–118. [PubMed] [Google Scholar]
- 35. Chida Y, Hamer M. An association of adverse psychosocial factors with diabetes mellitus: A meta-analytic review of longitudinal cohort studies. Diabetologia. 2008;51(12):2168–2178. [DOI] [PubMed] [Google Scholar]
- 36. Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. [DOI] [PubMed] [Google Scholar]
- 37. Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci. 2006;103(45):17058–17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32(1):80–86. [DOI] [PubMed] [Google Scholar]
- 39. Stavreva DA, Wiench M, John S, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11(9):1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33(1):77–82. [DOI] [PubMed] [Google Scholar]


