Abstract
Context:
Diabetes in neonates nearly always has a monogenic etiology. Earlier sulfonylurea therapy can improve glycemic control and potential neurodevelopmental outcomes in children with KCNJ11 or ABCC8 mutations, the most common gene causes.
Objective:
Assess the risks and benefits of initiating sulfonylurea therapy before genetic testing results become available.
Design, Setting, and Patients:
Observational retrospective study of subjects with neonatal diabetes within the University of Chicago Monogenic Diabetes Registry.
Main Outcome Measures:
Response to sulfonylurea (determined by whether insulin could be discontinued) and treatment side effects in those treated empirically.
Results:
A total of 154 subjects were diagnosed with diabetes before 6 months of age. A genetic diagnosis had been determined in 118 (77%), with 73 (47%) having a mutation in KCNJ11 or ABCC8. The median time from clinical diagnosis to genetic diagnosis was 10.4 weeks (range, 1.6 to 58.2 wk). In nine probands, an empiric sulfonylurea trial was initiated within 28 days of diabetes diagnosis. A genetic cause was subsequently found in eight cases, and insulin was discontinued within 14 days of sulfonylurea initiation in all of these cases.
Conclusions:
Sulfonylurea therapy appears to be safe and often successful in neonatal diabetes patients before genetic testing results are available; however, larger numbers of cases must be studied. Given the potential beneficial effect on neurodevelopmental outcome, glycemic control, and the current barriers to expeditious acquisition of genetic testing, an empiric inpatient trial of sulfonylurea can be considered. However, obtaining a genetic diagnosis remains imperative to inform long-term management and prognosis.
Persistent hyperglycemia occurring at a very early age has long been termed neonatal diabetes mellitus (NDM), although it is often diagnosed after the first few weeks of life. Infants diagnosed within the first 6 months of life are especially likely to have an underlying monogenic cause, but a small fraction of those diagnosed at later ages may also be found to have similar single gene mutations (1). Studies of European populations provide incidence estimates of 1 in 90 000 to 160 000 live births, representing approximately 0.2% of pediatric diabetes cases (1–3). Approximately half of the cases will have permanent NDM, whereas the remainder will be transient (4). Autoimmune type 1 diabetes is unusual in infancy, so whereas NDM is a rare condition, the yield from targeted genetic testing is high (5). Clinicians are faced with an expanding list of over 20 identified genetic causes of NDM, but many of these are syndromic, with clinical clues that can direct genetic testing (6). Although NDM was historically treated with insulin, studies over the last decade have in many cases clarified the underlying molecular mechanism and pointed to disease-specific therapy.
Heterozygous mutations of the genes KCNJ11 and ABCC8 encoding the two subunits of the ATP-sensitive potassium (KATP) channel account for approximately 50% of cases of permanent NDM (7–9). Because KATP channel closure is a key step in insulin secretion, diabetes in these patients results from inappropriate activation of the channels that fail to close in response to the usual signal of rising plasma glucose and the resulting rise in intracellular ATP (10, 11). In most patients with KATP-related diabetes, oral sulfonylurea therapy permits insulin secretion through ATP-independent closure of overly active mutated channels (7, 9, 12, 13). A few other relatively common heterozygous causes include mutations in the insulin gene (INS) itself and GATA6 mutations causing pancreatic agenesis (14, 15). Insulin remains the mainstay of treatment for these causes as well as the many other rare recessive causes, although in a few cases sulfonylureas have been used with limited success (16).
Even accounting for the high cost of clinical genetic testing, transitioning NDM patients with KATP channel mutations to sulfonylurea therapy results in significant cost savings due to improved glycemic control, quality of life, and decreased complications (17). Furthermore, our recent data have suggested that early sulfonylurea treatment could ameliorate the neurodevelopmental disability experienced by many of these patients (18). Clinicians treating a baby recently diagnosed with neonatal diabetes are thus faced with the question of whether to attempt treatment immediately with sulfonylurea or to await approval for and completion of genetic testing. Utilizing data from the University of Chicago Monogenic Diabetes Registry (http://monogenicdiabetes.uchicago.edu/registry/), we consider the potential benefits and risks of a trial of sulfonylurea therapy before genetic testing results are available.
Patients and Methods
Subjects with diabetes diagnosed before 1 year of age were consented for participation through the University of Chicago Monogenic Diabetes Registry, through which longitudinal information regarding the diagnosis and treatment of diabetes, other medical problems or complications, family history, and genetic testing results is collected through surveys and medical records (19). For all available time points, key data gathered include age, weight, HbA1c, and medication information. All subjects were consented for participation through protocols approved by the Institutional Review Board at the University of Chicago.
Genetic testing was completed commercially by the referring clinicians or was performed on a research basis at the University of Chicago. DNA used for research-based testing was isolated from blood or saliva samples. Standard Sanger methodology was used to sequence the protein-coding regions of KCNJ11, ABCC8, INS, and other genes causing NDM. Testing for 6q24-related transient neonatal diabetes (6q24-TND) was performed either commercially or through a research collaboration by the Wessex regional genetics laboratory (20).
Results
A total of 154 subjects within the Monogenic Diabetes Registry were diagnosed with diabetes by 6 months of age; 118 (77%) of those studied have had an underlying monogenic cause identified. Ninety-five percent of NDM subjects with KCNJ11 or ABCC8 mutations were found to have successfully achieved insulin independence through sulfonylurea use. Since July 2006, the median time from clinical diagnosis of NDM to a genetic test diagnosis (either research or clinical) was 10.4 weeks (range, 1.6 to 58.2 wk).
We identified nine probands within the Monogenic Diabetes Registry who were diagnosed with diabetes by 6 months of age and were given an empiric trial of sulfonylurea (glyburide/glibenclamide in all cases) before genetic testing results were available (Table 1). These attempts at treatment were performed in an inpatient setting using published protocols while awaiting either commercial or research genetic testing results (7). Six patients were successfully transitioned off insulin therapy within 6 days of initiation of oral sulfonylurea, whereas two cases continued to require supplemental insulin for 14 days and 11 days after glyburide was started (UC0212 and UC0425, respectively). One case (UC0224) was given increasing doses of glyburide for 5 days, up to 1.0 mg/kg/d, at which point he continued to require a replacement dose of insulin, and his C-peptide levels (as a measure of endogenous insulin secretion) remained undetectable, so sulfonylurea treatment was discontinued. Five patients who successfully switched to sulfonylurea were subsequently found to have a mutation in KCNJ11 (p.Gly53Asp, p.Arg50Pro, p.Arg201His, and p.Arg201Cys) or ABCC8 (p.Val1523Met). Three patients were found to have chromosome 6q24-TND, and all demonstrated the expected remission of diabetes within weeks of diabetes diagnosis. Interestingly, they exhibited a variable response to sulfonylurea: one case was able to stop insulin injections within 1 day of glyburide initiation and continued on glyburide monotherapy for 79 days until diabetes remission, whereas the other two cases continued to require insulin for 14 days and 11 days after the glyburide was started, and diabetes remission occurred within 5 days and 10 days of insulin cessation. The only proband for whom a genetic cause has yet to be revealed tested negative for known gene causes and remains on exclusive insulin therapy. No significant adverse effects of sulfonylurea therapy were reported in any of the cases. Glyburide doses in those with 6q24-TND were gradually reduced and were discontinued by the supervising clinicians in response to mild hypoglycemia.
Table 1.
NDM Patients Given an Empiric Trial of Sulfonylurea Before Genetic Testing Results Were Available
| Subject ID | Age of DM Dx | Time From DM Dx to SU Trial, d | Time From DM Dx to Genetic Dx, d | Time From SU Trial to Insulin Discontinuation, d | Mutation |
|---|---|---|---|---|---|
| UC0193 | Birth | 3 | 60 | 6 | KCNJ11 p.Gly53Asp |
| UC0212 | 14 d | 6 | 75 | 14 | 6q24-TND |
| UC0224 | Birth | 28 | N/A | N/A | N/Aa |
| UC0403 | Birth | 6 | 213 | 0 | ABCC8 p.Val1523Met |
| UC0425 | 4 d | 1 | 43 | 11 | 6q24-TND |
| UC0439 | Birth | 3 | 28 | 4 | KCNJ11 p.Arg50Pro |
| UC0610 | 4 d | 5 | 77 | 3 | KCNJ11 p.Arg201Cys |
| UC0693 | 4 d | 2 | 252 | 0 | KCNJ11 p.Arg201His |
| UC0781 | 1 d | 14 | 121 | 1 | 6q24-TND |
Abbreviations: N/A, not applicable; DM, diabetes mellitus; Dx, diagnosis; SU, sulfonylurea. For KCNJ11, the nucleotide sequence is based on NCBI reference sequence NM_000525.3, and for ABCC8, the nucleotide sequence is based on NCBI reference sequence NM_000352.3.
Genetic testing failed to identify cause of neonatal diabetes, and SU trial was unsuccessful.
Discussion
Genetic testing is mandatory in any case of neonatal diabetes because it informs medical decision-making, familial recurrence risk, information on possible associated clinical features, and long-term prognosis. Commercial testing is available for the most common gene causes of permanent neonatal diabetes and some causes of transient neonatal diabetes. However, barriers to implementation of universal genetic testing—at least in the United States—include the high cost that leads to frequent denial of medical insurance coverage for genetic testing, as well as the lack of provider knowledge regarding the importance of testing and which genes to test. We hypothesized that such factors might lead to the delay of sulfonylurea treatment in patients with potentially sulfonylurea-responsive NDM. Indeed, the current study demonstrates that although the recent genetic discoveries in neonatal diabetes revealed the importance of genetic testing, there is a median delay of 10.4 weeks from the time of diabetes diagnosis to genetic diagnosis, with a range from 1.6 to 58.2 weeks. The inordinate delay seen in many cases supports a consideration of attempting sulfonylurea therapy in newly diagnosed neonatal diabetes cases. The current study reports on the outcome of nine cases for whom clinicians implemented a clinical trial of glyburide before genetic testing results were available. Eight of the nine cases had a favorable response to treatment; importantly, the case without a response appeared to suffer no ill effects from the treatment.
Sulfonylurea treatment in this setting is not without potential known or unknown risks, and the pros and cons should be weighed before considering an empiric trial (Table 2). Sulfonylureas, like many other medicines, are not US Food and Drug Administration (FDA) approved for use in young patients (particularly neonates) or at the higher doses typically required by those with KATP channel mutations (21). Despite this, the data to date suggest that sulfonylurea use is well tolerated with few adverse events (22, 23) and is highly efficacious in that glycemic control improves dramatically after the switch to sulfonylureas in responsive cases of NDM (7). To our knowledge, no severe adverse events have been reported in the use of sulfonylureas in NDM. Gastrointestinal side effects are commonly reported in older patients (24). These symptoms were not reported in the current series, perhaps because they are less apparent in neonates. Dental discoloration related to high-dose glyburide use has been described in older patients and appears to be manageable by dental cleanings (23). The most common major side effect associated with sulfonylureas in type 2 diabetes is hypoglycemia. However, there have been very few reported severe hypoglycemic episodes in KATP channel mutation patients successfully treated with sulfonylurea monotherapy, suggesting that the risk of hypoglycemia is decreased compared to insulin therapy and may be less than in those using sulfonylureas for type 2 diabetes (12, 25). Caregivers should be cautioned that NDM may be transient and that iatrogenic hypoglycemia may be caused by a trial of sulfonylurea therapy. Hypoglycemia in neonates can be difficult to recognize and treat. We advocate continued glucose monitoring in neonates that have successfully transitioned, particularly during a time of illness or if the child is irritable, listless, feeding poorly, tremulous, unresponsive, or has a seizure. A molecular genetic diagnosis should be established as early as possible because this may give an indication as to whether the hyperglycemia is likely to be transient.
Table 2.
Pros and Cons of Empiric Sulfonylurea Trial Before Genetic Testing Results Are Available
| Pro | Con |
|---|---|
| May ameliorate neurodevelopmental disability in some cases | Inappropriately high family expectations leading to disappointment (and possibility of increased cost) if attempt fails |
| Could shorten inpatient stay | Molecular diagnosis may guide treatment based on reported sulfonylurea response in patients with identical mutation |
| If successful, will reduce overall treatment costs | Risk of hypoglycemia during transition and in those with transient forms of NDM |
| Much easier to give pills than insulin | Sulfonylurea is not approved by FDA for use in infants (also the case for many forms of insulin) |
| Safety profile of sulfonylurea use in children remains excellent to date | Unknown long-term risks of sulfonylurea use |
| Genetic testing approval, results, and/or interpretation could cause delay | Second attempt needed if initial trial fails but genetic testing reveals a KCNJ11 or ABCC8 mutation |
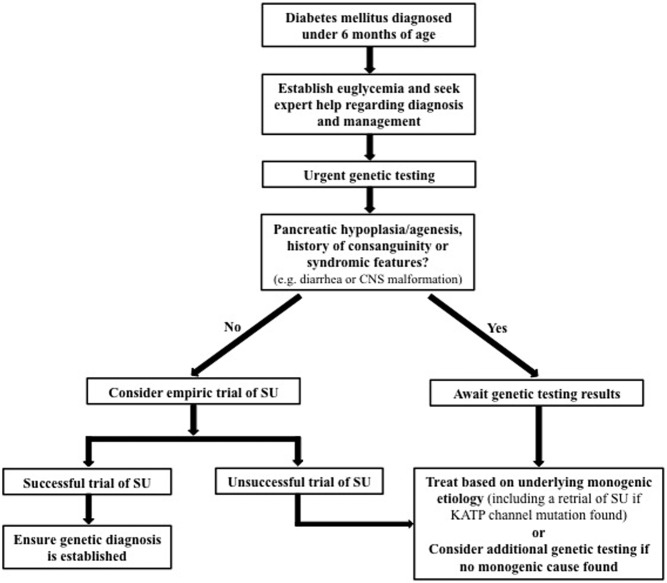
Careful monitoring is required to avoid severe hyperglycemia and ketosis during the transition, particularly in those who do not have an adequate response to sulfonylurea. Several forms of monogenic diabetes are caused by mechanisms that would not be amenable to sulfonylurea treatment, such as mutations in the gene encoding insulin, and genes associated with pancreatic agenesis or β-cell development, most of which are rare autosomal recessive causes (6). It would thus be prudent to first assess for the presence of the pancreas (usually by ultrasound) and elicit any history of potential consanguinity. We would recommend not proceeding with a trial if there is a clear history of consanguinity or pancreatic hypoplasia/agenesis. Early additional features that suggest rare causes not expected to respond to sulfonylurea treatment include unremitting diarrhea, cerebellar hypoplasia/agenesis, microcephaly, and gastrointestinal or cardiac malformations (Figure 1). It is important to emphasize the use of a protocol that maintains adequate glucose control so as not to compromise patient safety. If a sufficient dose is reached (most KATP mutations would be expected to show a response to 1 mg/kg/d of glyburide) and no response is apparent (by glucose values or measurable increment in C-peptide levels), then the trial should be abandoned. An initial twice-daily dose of 0.1 mg/kg can typically be increased each day so as to achieve a dose of 1 mg/kg/d within 5–6 days. If a responsive mutation is later found by genetic testing, then a repeat attempt can be arranged. Of note, some non-KATP forms of NDM may also respond at least partially to sulfonylureas, such as the cases of 6q24-TND reported here. However, in some of these cases the diabetes itself may remit during treatment, making the true effectiveness of the treatment uncertain.
Figure 1.
Decision algorithm for an empiric trial of sulfonylurea. CNS, central nervous system; SU, sulfonylurea. International monogenic diabetes registries can be contacted (http://monogenicdiabetes.uchicago.edu and http://www.diabetesgenes.org) if local expert help is unavailable.
An important possible negative consequence of an empiric sulfonylurea trial is that family expectations for success may be unrealistic, and treatment failure could result in great disappointment. However, families may appreciate that the treatment was attempted even if it does not work. This highlights the importance of including the family in the decision-making about whether a trial should be considered. It also emphasizes the need for genetic testing to provide further explanation for treatment failure.
Insulin management of diabetes is complicated, especially in infants, and requires extensive training that often requires days of education during the initial inpatient admission before the patient is safe for discharge. The transition protocol calls for appropriate glucose control with continued insulin injections while the sulfonylurea dose is increased. We would suggest that training of family members regarding insulin administration proceed during this time in case the transition is not successful. However, sulfonylurea therapy would be predicted to be effective in nearly half of cases and would provide great relief to families facing the prospect of taking home a baby with diabetes, especially for those who may have limited education, resources, and support.
Transition from insulin to sulfonylurea therapy has been done safely in hundreds of cases worldwide using an established inpatient protocol (7). The protocol allows for an increase to a sufficiently high dose within 5 days, although the transition could take place in as little as 2 days, given that younger patients often require a lower dose. Because glyburide is an inexpensive generic medication, transition to oral therapy also reduces medical costs compared to insulin injections, even accounting for an elective 5-day inpatient admission at the time of transition (17). If the transition took place during the initial admission when diabetes was diagnosed, it is possible that such a policy would reduce the overall costs associated with length of stay; however, additional data would be needed to demonstrate whether this is the case.
It is possible that our results are most applicable to the United States, where barriers remain to obtaining genetic testing with appropriate cost reimbursement. Regardless of where they live, we encourage clinicians and families to contact the established national/international monogenic diabetes registries for consultation on treatment and to ensure longitudinal follow-up of these rare forms of diabetes. We echo the concerns of others that a successful trial of sulfonylurea does not obviate the need for genetic testing, which should be pursued without delay in all patients with NDM (26). There are few instances in medicine where genetic testing has such a dramatic and immediate impact on both treatment and clinical outcome. We urge policy makers within the United States and other countries to improve access to genetic testing for all individuals with NDM.
Treatment with sulfonylureas at later ages appears to be associated with increased sulfonylurea dose requirements and reduced chance of achieving independence from exogenous insulin (7, 9, 27). But perhaps the most compelling reason to ensure that sulfonylurea treatment is initiated as early as possible is the potential beneficial effect on neurodevelopmental outcome. A sizeable proportion of KATP channel mutations are associated with a spectrum of neurodevelopmental impairment, likely due to expression of mutated channels in the brain, where their function is poorly understood (28). Developmental delay, epilepsy, and neonatal diabetes (DEND) and intermediate DEND are frequently described in some children with KATP channel mutations (4, 18). Milder developmental coordination problems and attention deficits can also be found in those without DEND or intermediate DEND (29). Just as sulfonylureas restore channel function in β-cells, case reports have also suggested improvement in neurodevelopmental effects after treatment. Given that brain development continues through infancy and beyond, any possible beneficial consequence of sulfonylurea treatment may be most pronounced at the earliest ages (30, 31). Indeed, we previously showed that visuomotor performance on standardized testing in KCNJ11 subjects later in life was associated with initiation of sulfonylurea therapy before 1 year of age (18). Larger studies are needed to determine whether early sulfonylurea intervention and higher sulfonylurea dosing will have implications for the more subtle developmental problems seen in individuals with KATP channel mutations.
Conclusion
We have shown that genetic testing results in newly diagnosed NDM are often delayed. We report the outcome of nine patients who underwent empiric treatment with sulfonylureas before genetic testing results were available. Such treatment was successful in most cases, and none of the cases were associated with any adverse events, even when treatment failed. Although genetic testing remains mandatory in all cases of NDM, sulfonylurea treatment before such results are available can be considered due to several potential benefits, including improved neurodevelopmental outcome.
Acknowledgments
We acknowledge the continued support of Dr D. Mackay (Division of Human Genetics, University of Southampton School of Medicine, UK) for performing diagnostic support for 6q24-TND. We thank Dr Paul Kaplowitz, Dr Stuart Chalew, and all the clinicians providing care for patients within the Monogenic Diabetes Registry. We are most grateful to all of the wonderful patients and families who participated in these studies.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health through grants supporting the University of Chicago Diabetes Research and Training Center (DK020595), and S.A.W.G.'s K23 Award (DK094866), as well as by a grant from the American Diabetes Association (1-11-CT-41), and gifts from the Kovler Family Foundation and the Lewis-Sebring Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DEND
- developmental delay, epilepsy, and neonatal diabetes
- KATP
- ATP-sensitive potassium
- NDM
- neonatal diabetes mellitus
- 6q24-TND
- 6q24-related transient neonatal diabetes.
References
- 1. Greeley SA, Tucker SE, Naylor RN, Bell GI, Philipson LH. Neonatal diabetes mellitus: a model for personalized medicine. Trends Endocrinol Metab. 2010;21:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiedemann B, Schober E, Waldhoer T, et al. Incidence of neonatal diabetes in Austria-calculation based on the Austrian Diabetes Register. Pediatr Diabetes. 2010;11:18–23. [DOI] [PubMed] [Google Scholar]
- 3. Iafusco D, Massa O, Pasquino B, et al. Minimal incidence of neonatal/infancy onset diabetes in Italy is 1:90,000 live births. Acta Diabetol. 2012;49:405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49:1190–1197. [DOI] [PubMed] [Google Scholar]
- 5. Edghill EL, Dix RJ, Flanagan SE, et al. HLA genotyping supports a nonautoimmune etiology in patients diagnosed with diabetes under the age of 6 months. Diabetes. 2006;55:1895–1898. [DOI] [PubMed] [Google Scholar]
- 6. Greeley SA, Naylor RN, Philipson LH, Bell GI. Neonatal diabetes: an expanding list of genes allows for improved diagnosis and treatment. Curr Diab Rep. 2011;11:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pearson ER, Flechtner I, Njølstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. [DOI] [PubMed] [Google Scholar]
- 8. Babenko AP, Polak M, Cavé H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456–466. [DOI] [PubMed] [Google Scholar]
- 9. Støy J, Greeley SA, Paz VP, et al. Diagnosis and treatment of neonatal diabetes: a United States experience. Pediatr Diabetes. 2008;9:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. [DOI] [PubMed] [Google Scholar]
- 11. Koster JC, Remedi MS, Dao C, Nichols CG. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 2005;54:2645–2654. [DOI] [PubMed] [Google Scholar]
- 12. Rafiq M, Flanagan SE, Patch AM, Shields BM, Ellard S, Hattersley AT, Neonatal Diabetes International Collaborative Group. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iafusco D, Bizzarri C, Cadario F, et al. No β cell desensitisation after a median of 68 months on glibenclamide therapy in patients with KCNJ11-associated permanent neonatal diabetes. Diabetologia. 2011; 54:2736–2738. [DOI] [PubMed] [Google Scholar]
- 14. Støy J, Edghill EL, Flanagan SE, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci USA. 2007;104:15040–15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lango Allen H, Flanagan SE, Shaw-Smith C, et al. GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat Genet. 2012;44:20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turkkahraman D, Bircan I, Tribble ND, Akçurin S, Ellard S, Gloyn AL. Permanent neonatal diabetes mellitus caused by a novel homozygous (T168A) glucokinase (GCK) mutation: initial response to oral sulphonylurea therapy. J Pediatr. 2008;153:122–126. [DOI] [PubMed] [Google Scholar]
- 17. Greeley SA, John PM, Winn AN, et al. The cost-effectiveness of personalized genetic medicine: the case of genetic testing in neonatal diabetes. Diabetes Care. 2011;34:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah RP, Spruyt K, Kragie BC, Greeley SA, Msall ME. Visuomotor performance in KCNJ11-rElated neonatal diabetes is impaired in children with DEND-associated mutations and may be improved by early treatment with sulfonylureas. Diabetes Care. 2012;35:2086–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greeley SA, Naylor RN, Cook LS, Tucker SE, Lipton RB, Philipson LH. Creation of the Web-based University of Chicago Monogenic Diabetes Registry: using technology to facilitate longitudinal study of rare subtypes of diabetes. J Diabetes Sci Technol. 2011;5:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mackay DJ, Temple IK, Shield JP, Robinson DO. Bisulphite sequencing of the transient neonatal diabetes mellitus DMR facilitates a novel diagnostic test but reveals no methylation anomalies in patients of unknown aetiology. Hum Genet. 2005;116:255–261. [DOI] [PubMed] [Google Scholar]
- 21. Laughon MM, Avant D, Tripathi N, et al. Drug labeling and exposure in neonates. JAMA Pediatr. 2014;168:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Codner E, Flanagan S, Ellard S, García H, Hattersley AT. High-dose glibenclamide can replace insulin therapy despite transitory diarrhea in early-onset diabetes caused by a novel R201L Kir6.2 mutation. Diabetes Care. 2005;28:758–759. [DOI] [PubMed] [Google Scholar]
- 23. Kumaraguru J, Flanagan SE, Greeley SA, et al. Tooth discoloration in patients with neonatal diabetes after transfer onto glibenclamide: a previously unreported side effect. Diabetes Care. 2009;32:1428–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. U.K. Prospective Diabetes Study Group. Diabetes Care. 1998;21:87–92. [DOI] [PubMed] [Google Scholar]
- 25. Codner E, Flanagan SE, Ugarte F, et al. Sulfonylurea treatment in young children with neonatal diabetes: dealing with hyperglycemia, hypoglycemia, and sick days. Diabetes Care. 2007;30:e28–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chakera AJ, Flanagan SE, Ellard S, Hattersley AT. Comment on: Khurana et al . The diagnosis of neonatal diabetes in a mother at 25 years of age. Diabetes Care 2012;35:e59 Diabetes Care. 2013;36:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wambach JA, Marshall BA, Koster JC, White NH, Nichols CG. Successful sulfonylurea treatment of an insulin-naïve neonate with diabetes mellitus due to a KCNJ11 mutation. Pediatr Diabetes. 2010;11:286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. [DOI] [PubMed] [Google Scholar]
- 29. Busiah K, Drunat S, Vaivre-Douret L, et al. Neuropsychological dysfunction and developmental defects associated with genetic changes in infants with neonatal diabetes mellitus: a prospective cohort study [corrected]. Lancet Diabetes Endocrinol. 2013;1:199–207. [DOI] [PubMed] [Google Scholar]
- 30. Slingerland AS, Hurkx W, Noordam K, et al. Sulphonylurea therapy improves cognition in a patient with the V59M KCNJ11 mutation. Diabet Med. 2008;25:277–281. [DOI] [PubMed] [Google Scholar]
- 31. Koster JC, Cadario F, Peruzzi C, Colombo C, Nichols CG, Barbetti F. The G53D mutation in Kir6.2 (KCNJ11) is associated with neonatal diabetes and motor dysfunction in adulthood that is improved with sulfonylurea therapy. J Clin Endocrinol Metab. 2008;93:1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]