Abstract
Context:
The distinctive presentation of primary hyperparathyroidism (PHPT) in adults and youths suggest that PHPT is a fundamentally different disease in these two groups.
Objective:
To understand the difference in PHPT between adults and youths we compared the biochemistry of PHPT in these two groups.
Design:
This study is a systematic review and meta-analysis of retrospective studies published 1966–2014 on PHPT.
Data Sources:
All studies were obtained through Medline (1966–2014).
Study Selection and Data Extraction:
Only studies that included post-surgical subjects and that explicitly described biochemical results from more than one decade were included. Data were extracted from each article to generate the mean and SE for multiple biochemical parameters.
Data Synthesis:
We analyzed 16 studies describing 268 unique youths and 2405 adults with PHPT. Youths with PHPT had significantly (P < .05) greater serum and urinary calcium than adults with PHPT (3.2 ± 0.1 mmol/L vs 2.8 ± 0.0 mmol/L for serum calcium, and 9.95 ± 1.26 mmol/d vs 7.15 ± 0.56 mmol/d for urine calcium, [mean ± SEM]). There were no significant differences in serum intact PTH, phosphorus, or alkaline phosphatase.
Conclusions:
Juvenile PHPT has greater hypercalcemia and hypercalciuria than adult PHPT at similar concentrations of serum intact PTH. These observations suggest that there are differences in the pathophysiology of PHPT between juvenile and adult patients who reflect an apparent decrease in the sensitivity of the parathyroid adenoma to negative feedback by calcium and increased sensitivity of target tissues to the effects of PTH.
Primary hyperparathyroidism (PHPT) is characterized by hypercalcemia and elevated or inappropriately normal serum concentrations of intact PTH. PHPT primarily affects older adults, yet it also occurs in a small number of children and adolescents. In adults, PHPT is usually an asymptomatic disorder that presents as incidentally discovered hypercalcemia (1, 2). In the juvenile population (age 0–25 y), by contrast, PHPT is uncommon and clinically symptomatic (3). One potential explanation for this difference is ascertainment bias, because routine biochemical screening is common in adults but not in youths. Hence, PHPT is identified in younger patients only when they become symptomatic. Alternatively, it is also conceivable that juvenile PHPT is a different disease, and specifically, a more aggressive disease, than adult PHPT. This understanding, though widely held, remains incompletely examined (4–6). Previous analyses of juvenile PHPT have disclosed qualitative differences between adult PHPT and juvenile PHPT but lacked power to determine biochemical differences. Here we report a systematic review of the biochemical features of pediatric and adult PHPT through meta-analysis of postsurgical studies published over similar time periods (4–25).
Materials and Methods
Selection criteria and search strategy
Our selection approach is described below and summarized in Figure 1 (PRISMA flow chart). Articles describing PHPT in pediatrics were identified through Medline via PubMed search using the terms “pediatric” and “primary hyperparathyroidism.” We selected articles from this group for this systematic analysis using the following criteria: publication in the English language, limitation to patients under the age of 25 years (or separation of patients < 25 y), and greater than four patients in the study. In addition, the citation lists of recent reviews were used to identify studies not identified by this approach. For our meta-analysis neonatal severe hyperparathyroidism was excluded. To select articles for comparison, we attempted to find articles published in the last 30 years describing more than one decade of adult subjects with PHPT, by searching for 1) “adult,” “primary hyperparathyroidism,” and “surgery” or for 2) “adult,” “primary hyperparathyroidism,” and “biochemistry.” From this group we selected articles using the following criteria: use of surgical record for ascertainment, publication in the English language, greater than four patients in the study, and explicit statistical description of more than a single decade. By using postsurgical patients for both groups we attempted to decrease ascertainment bias in the juvenile group; in adult studies on PHPT performed using population screening (rather than chart review), only 20% of those identified by biochemical criteria end up having surgery (26) over a follow-up period of at least one decade. All juvenile studies reviewed here covered several decades; we attempted to decrease any bias due to changing measurement methods by using adult studies covering more than a single decade (eg, to account for the evolution of measurement methods from the 1960s to 2010s).
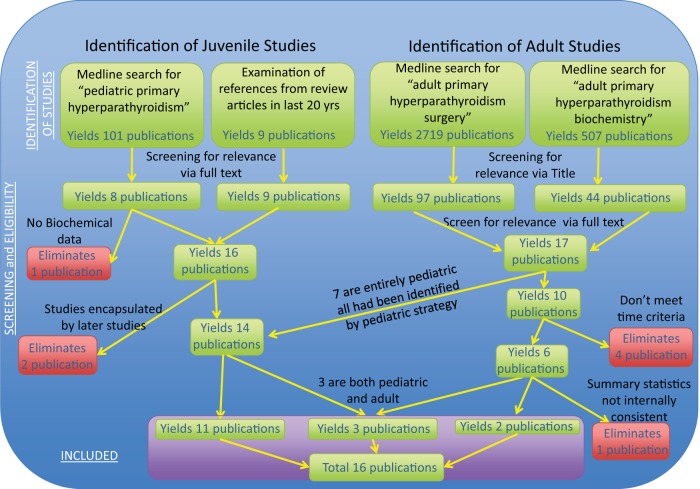
Figure 1.
PRISMA Flow Chart. All articles describing PHPT in pediatrics were identified through Medline via pubmed search using the terms “pediatric” and “primary hyperparathyroidism.” From this group of articles on pediatric PHPT, we selected articles for this systematic analysis using the following criteria: publication in the English language, limitation to patients under the age of 25 y (or separation of patients < 25 y), and > 4 patients in the study. In addition, the citation lists of recent reviews were used to identify any other studies not identified by this approach. For this article, neonatal severe hyperparathyroidism was excluded. To select articles for comparison, we attempted to find articles published in the last 30 years describing more than one decade of adult subjects with PHPT (eg, sampling adults for a period > 10 y), by searching for 1) “adult,” “primary hyperparathyroidism,” and “surgery” as well as 2) “adult,” “primary hyperparathyroidism,” and “biochemistry.” From this group we selected articles using the following criteria: use of surgical record for ascertainment, publication in the English language, > 4 patients in the study, and explicit statistical description of more than a single decade by the study. By using postsurgical patients for both groups we attempted to decrease ascertainment bias in the pediatric group.
All of the studies selected by the method above reported descriptive statistics regarding the mean calcium concentration, but other important analytes (eg, PTH, urine calcium, phosphorus, and alkaline phosphatase) were variably described. In addition, for a specific analyte, some articles provide normal ranges and summary statistics as well as individual values (9, 13, 20, 27), some articles provide summary statistics, whereas other articles omit a normal range. Finally, some articles provide a normal range (and note that the mean was different from the normal range) but provide no summary statistics or individual statistics (7).
All of the studies in this analysis reported histology (adenoma vs hyperplasia) and several studies did not report genetic pathology (ie, MEN-1, MEN-2a, or familial PHPT). Several studies attempted to exclude those with familial syndromes causing PHPT (6, 28), and the remaining adult studies did not report any cases of PHPT due to MEN-1, MEN2a, or familial PHPT. Overall, the adult studies reported PHPT occurring in 1959 subjects with adenomas, 281 subjects with hyperplasia, and nine subjects with carcinoma; and the juvenile studies reported PHPT occurring in 230 subjects with adenomas, 30 subjects with hyperplasia, and four subjects with carcinoma. In the single juvenile study to compare subgroups by either histology or genetic cause (14), there were not significant differences or trends toward differences between subgroups. The vast majority of studies did not report a direct measure of renal function, or the criteria by which familial hypocalciuric hypercalcemia (FHH) was excluded, but the predominance of adenomatous disease in these series is reassuring that diseases aside from PHPT (ie, renal failure or FHH), which can masquerade as PHPT, were generally excluded from the studies included here. A single study (6) divided PHPT into three groups: those below 25 years of age, those between 25–50 years of age, and those over 50 years of age. We contacted the authors for this study and they were unable to provide separate data for those under 18 years. Because we were unable to separate those below 18 years from the group below 25 years in this study, our analysis was performed using 25 years as the cutoff point for the juvenile age range. For all outcomes where a comparison was possible, there were no differences in the overall significance of our analysis (eg, significant results remained significant and nonsignificant results remained nonsignificant) when omitting this study and only comparing those under 18 years to adults.
Statistical analyses
Data were extracted from each article to generate standard statistical descriptors (mean and SD) for the following analytes when they were reported: total serum calcium, intact PTH, serum phosphorus, serum alkaline phosphatase, urine calcium, and gland weight. When required, we used a method described by Hozo, et al (29) for estimation of mean and SD to determine the mean and SD from the median and range. We used OpenMetaAnalyst (version 09.19.13) and input the mean, the SD, and used a continuous-random effects model (DerSimonian-Laird method) to generate estimated 95% confidence intervals (CIs) for each study and the two subgroups of juvenile or adult populations. Statistical significance of the cumulative mean was confirmed using a t test with Welch's correction for unequal variances in GraphPad Prism (version 6). Doing the same calculation using equal variances generated results with equal or increased statistical significance in every case but did not alter the significance level of any result. Frequency-weighted regression was performed on calcium vs PTH using calcium as the dependent variable using R for MacOS (www.r-project.org, version 3.0.2). For any study reporting individual values or reporting subsets of values (ie, subgroup analyses), we tested consistency between individual values or subgroup values. One study was discarded because sets of subgroup summary statistics within the article were inconsistent with overall summary statistics (22). The population from each of several studies (8, 10) was subsumed within subsequent studies (6, 30), and so for those studies, data from the later and larger study were used. For intact PTH, values reported in pg/mL were converted to pmol/L. We used standard pediatric reference ranges (for ages 8–12 y) and adult reference ranges for the analyses described for phosphorus, alkaline phosphatase. These ranges were similar to what was reported in the studies examined in our meta-analysis. For PTH, the reference range is similar across age groups (1–5.2 mmol/L). A single study (31) reported phosphorus and a normal range for phosphorus in a range dramatically increased relative to other reported normal ranges and so these phosphorus values were not used in our analysis.
Results
Using the selection criteria above, we identified 11 studies of only pediatric PHPT (n = 213), three studies comparing juvenile and adult PHPT (n = 1976; 1921 adults and 55 juvenile patients), and two studies of only adult PHPT (n = 541) that were published over the time period 1966–2012. These reports yielded a total of 268 juvenile patients and 2405 adult patients. Summed results for all analytes are in Table 1.
Table 1.
Biochemical Comparison of Juvenile and Adult Primary Hyperparathyroidism
| Analyte | Juvenile |
Adult |
||||
|---|---|---|---|---|---|---|
| Mean | sem | n | Mean | sem | n | |
| Serum calcium, mmol/L | 3.2 | 0.1 | 268 | 2.9a | 0.0 | 2405 |
| PTH, pmol/L | 35.1 | 11.6 | 246 | 18.5 | 3.0 | 2385 |
| Phosphorus, mmol/L | 0.90 | 0.05 | 224 | 0.96 | 0.03 | 155 |
| Alkaline phosphatase, U/L | 596 | 234 | 122 | 154 | 304 | 396 |
| Urinary calcium, mmol/d | 9.95 | 1.26 | 43 | 7.15a | 0.56 | 221 |
Different from juvenile mean with P < .05 (paired t test with unequal variances).
Serum total calcium
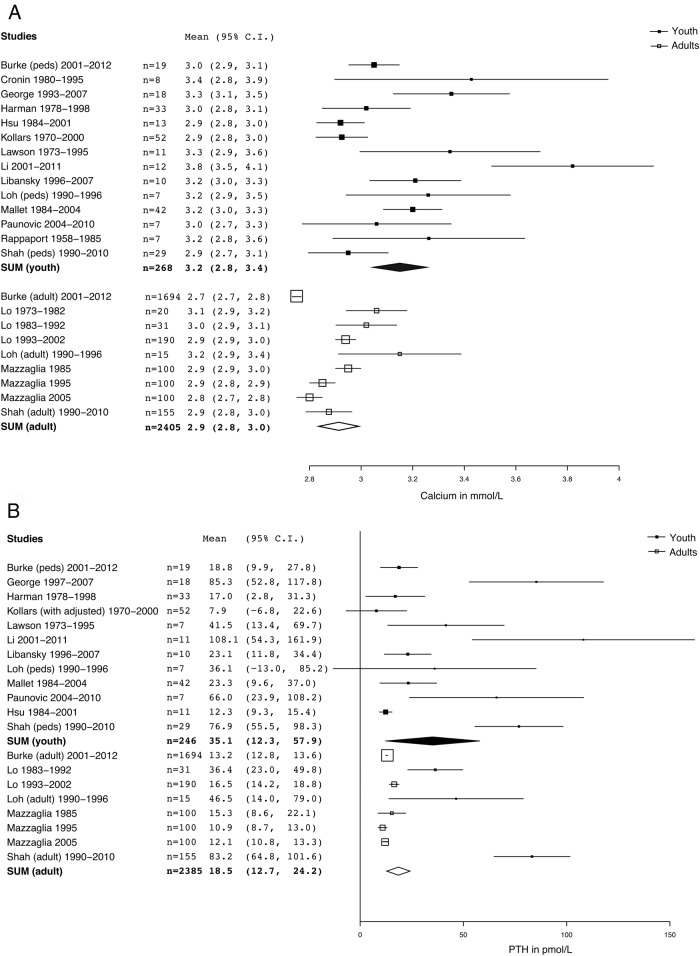
The total serum calcium concentration, unadjusted for serum protein concentration, was the most consistently reported analyte in these studies. Serum calcium was significantly greater in juvenile PHPT (mean, 3.2 mmol/L; 95% CI, 2.8–3.4; SEM, 0.1) than adult PHPT (mean, 2.8 mmol/L; 95% CI, 2.7–2.9; SEM, 0.0) in analyses using either all studies (Table 1 and Figure 2A: P < .05, paired t test with Welch's correction for unequal variances) as well as in analyses only including studies with mean parathyroid gland mass less than 1188 mg (Supplemental Figure 1B).
Figure 2.
A. Forest plot of comparison of serum total calcium between juvenile PHPT and adult PHPT. Serum calcium in pediatric PHPT is significantly increased relative to Adult PHPT, P < .05 (paired t test with Welch's correction for unequal variances), pediatric mean, 3.2 mmol/L (95% CI, 2.8–3.4) vs 2.9 mmol/L (95% CI, 2.8–3.0). Summary or individual statistics for calcium were reported for all of the subjects in this study (n = 2673). Studies separating out multiple decades [Lo et al (24) and Mazzaglia et al (25)] are graphically represented by a square for each group. Square size is proportional to n and SD. B. Forest plot of comparison of serum PTH between juvenile PHPT and adult PHPT. Differences between groups are not statistically significant, P = .17 (paired t test with Welch's correction for unequal variances); pediatric mean, 35.1 pmol/L (95% CI, 12.2–58.0) vs 18.5 pmol/L (95% CI, 12.7–24). Summary or individual statistics for PTH were reported for 2388 subjects. Studies separating out multiple decades [Lo et al (24) and Mazzaglia et al (25)] are graphically represented by a square for each group. Square size is proportional to n and SD.
PTH
Serum intact PTH was not significantly different between adult PHPT (mean, 18.5 pmol/L; 95% CI, 12.7–24.3; SEM, 3.0) and juvenile PHPT (mean, 35.1 pmol/L; 95% CI, 12.2–58.0; SEM, 11.6) in analyses using either all studies (Table 1 and Figure 1B: P = .17 [paired t test with Welch's correction for unequal variances]), as well as in analyses only including studies with mean gland mass less than 1188 mg (Supplemental Figure 1C).
Phosphorus
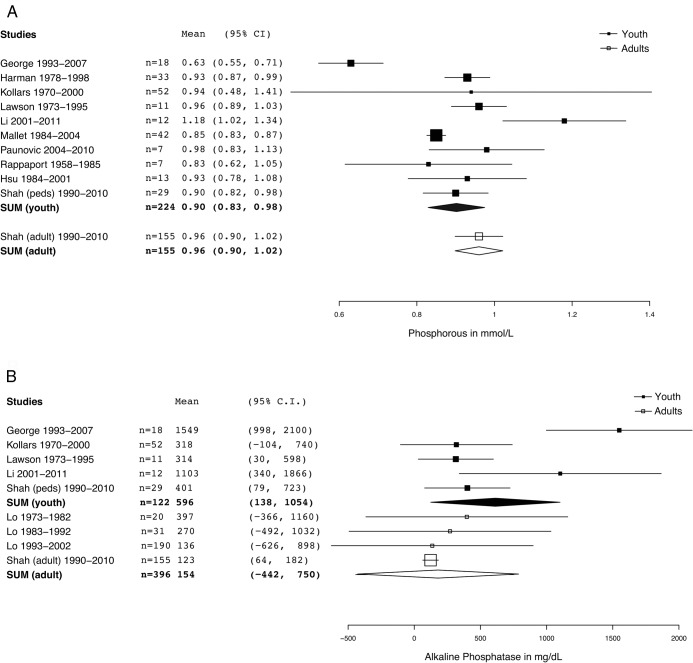
Phosphorus was measured in a minority of studies and was not reported in any adult studies aside from one where the mean parathyroid gland weight was greater than 1188 mg. Therefore, we did not perform any comparison between adult and juvenile studies eliminating studies describing PHPT with PTH glands larger than 1188 mg. Table 1 and Figure 3A show that serum phosphorus was not significantly different (P = .37, paired t test with Welch's correction for unequal variances) between juvenile PHPT (mean, 0.90 mmol/L; 95% CI, 0.83–0.98; SEM, 0.05) and adult PHPT (mean, 0.96 mmol/L; 95% CI, 0.90–1.02; SEM, 0.03). Reanalysis of the data by normalizing serum phosphorus levels to the appropriate reference range [juvenile, 1.13–1.71 mmol/L; adult, 0.87–1.45 mmol/L) reveals a difference in the deviation from the reference range between juvenile studies and adults studies; juvenile mean, −3.57 SDs from mean, with SEM/(normal range SD) 0.36; adult mean −1.41 SDs from mean, with SEM/(normal range SD) 0.21]. However, given that we are estimating the SD in these values as the top of the range minus the mean of the range divided by two, it is difficult to determine in a statistically rigorous fashion whether this difference is significant.
Figure 3.
A. Forest plot of comparison of phosphorus between juvenile PHPT and adult PHPT. Differences between groups are not statistically significant, P = .37 (paired t test with Welch's correction for unequal variances); pediatric mean, 0.90 mmol/L (95% CI, 0.83–0.98) vs 0.96 (95% CI, 0.90–1.02). Summary or individual statistics for serum phosphorous were reported for 379 subjects. B. Forest plot of comparison of alkaline phosphatase between juvenile PHPT and adult PHPT. Differences between groups are not statistically significant, P = .25 (paired t test with Welch's correction for unequal variances); pediatric mean, 596 mg/dL (95% CI, 133–1059) vs 154 mg/dL (95% CI, 443–752). Summary or individual statistics for Alkaline phosphatase were reported for 363 subjects.
Alkaline phosphatase
Alkaline phosphatase was measured in very few studies and was reported in only one adult study in which the mean parathyroid adenoma weight was greater than 1188 mg (6). Therefore, we did not perform any comparison between adult and juvenile studies eliminating studies describing PHPT with PTH glands larger than 1188 mg. Alkaline phosphatase activity (Table 1 and Figure 3B) was not significantly different between juvenile PHPT (mean, 596 U/L; 95% CI, 133–1059; SEM, 234) and adult PHPT (mean, 154 U/L; 95% CI, 443–752; SEM, 304; P = .25; paired t test with Welch's correction for unequal variances). Normalizing values to the appropriate reference range also did not reveal any significant differences between the juvenile and adult values even when we determined the difference of the mean from the middle of the normal range (juvenile mean, 3.67 SDs from mean, with SEM/(normal range SD) 2.67; adult mean, 2.26 SDs from mean, with SEM/(normal range SD) 12.67.
Urinary calcium
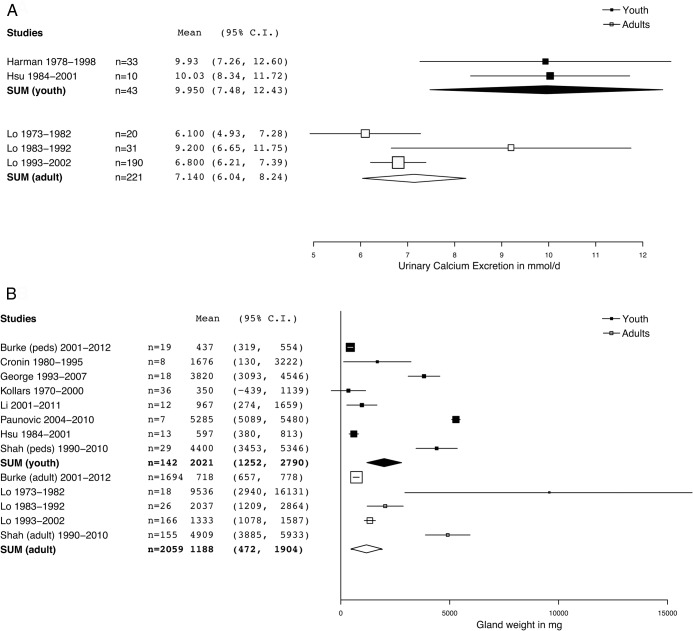
Urinary calcium was reported in only three adult or juvenile studies that met our criteria for inclusion in this study, and was significantly greater (Table 1 and Figure 4A: P < .05, paired t test with Welch's correction for unequal variances) in juvenile PHPT (mean, 9.95 mmol/d; 95% CI, 7.4–12.7; SEM, 1.26 mmol/d) than in adult PHPT (mean, 7.15 mmol/d; 95% CI, 6.03–8.24; SEM, 0.56). Because these numbers do not account for the lesser body weight of juvenile patients, it is conceivable that expression of urinary calcium excretion on the basis of body weight would further increase the differences we noted in urinary calcium excretion. Unfortunately, only one of the three studies reporting urinary calcium reported it as a function of weight (13). Increased urinary calcium might reflect increased renal calcium load; however, in the studies reporting urinary and serum calcium there was no correlation between serum calcium and urinary calcium.
Figure 4.
A. Forest plot of comparison of urine calcium between juvenile PHPT and adult PHPT. Urine calcium in pediatric PHPT is significantly increased relative to adult PHPT, P < .05 (paired t test with Welch's correction for unequal variances); pediatric mean, 9.95 mmol/d (95% CI, 7.4–12.7) vs 7.15 mmol/d (95% CI, 6.03–8.24). Summary or individual statistics for urinary calcium were reported for 264 subjects. B. Forest plot of comparison of gland size between juvenile PHPT and adult PHPT. Differences between groups are not statistically significant, P = .12, (paired t test with Welch's correction for unequal variances); mean mass for pediatric gland, 2021 mg (95% CI, 1246–2797), vs mean mass for adult gland, 1188 mg (95% CI, 472–1905).
Parathyroid gland weight
Gland weight was reported in the vast majority of patients in these studies and is surprisingly heterogeneous between studies (Figure 4B). When all values are included in the meta-analysis, we found a nonsignificant (P = .12, paired t test with Welch's correction for unequal variances) trend for greater weight of juvenile parathyroid adenomas (2021 mg; 95% CI, 1246–2797 mg) than adult parathyroid adenomas (1188 mg; 95% CI, 472–1905 mg). This trend was reinforced by several smaller studies that reported very large adenoma weights (> 1188 mg), however. Because of the correlation between gland weight and severity of disease at extreme values (32) we performed our analysis with all studies as well as limiting to studies reporting parathyroid adenoma weights below the adult weighted mean parathyroid gland weight of 1188 mg (eg, dividing the glands into small and large glands and limiting the analysis to small glands [Supplemental Figure 1A]). This approach significantly decreased heterogeneity within each group, but did not change the level of statistical significance for any single parameter.
Discussion
The notion that juvenile PHPT differs from adult PHPT has been based principally on studies that emphasized the greater proportion of youths with PHPT who presented with symptomatic hypercalcemia and clinically significant bone or renal stone disease. Because youths, unlike adults, do not undergo routine biochemical screening, it is conceivable that a cohort of youths with asymptomatic PHPT also exists. However, the observation that PHPT typically presents as recent-onset hypercalcemia in adults in the fifth and sixth decades argues against this concept. Hence, it is likely, but unproven, that there are distinct biological factors accounting for the bimodal development of PHPT in youths and adults.
The percentage of adults presenting with symptomatic PHPT has steadily declined during the past 75 years, as documented in extensive case studies: 100% of 14 individuals in 1934 (33); 95% of 380 individuals in 1961 (34); 77% of 57 individuals in 1974; 53% of 100 individuals in 1978 (23); to currently approximately 20% or even fewer (26). The decrease in symptomatic presentation has been brought about by the introduction in the late 1970s of routine biochemical screening that used panels including serum calcium. However, the reduced number of adult patients with symptomatic PHPT does not represent a secular trend, but rather a statistical artifact, as symptomatic patients now represent a smaller proportion of a dramatically larger number of ascertained cases [eg, a 20-fold increase in annual PHPT diagnosis at the Mayo clinic between 1943–1961 (34) and a 7-fold increase in annual PHPT diagnosis in Hong Kong (24)]. The biological basis for the dichotomy between symptomatic and asymptomatic PHPT in adults remains unresolved as well.
In this analysis, we sought to compare the biochemical and histological characteristics between youths and adults with PHPT undergoing surgery. By selecting for adults undergoing surgery we attempted to decrease the ascertainment bias due to differences in the biochemical screening in these populations. These two groups shared significant similarity, with no significant differences between the two groups in alkaline phosphatase activity or weight of resected parathyroid adenomas. In contrast, concentrations of serum and urine calcium were significantly greater in young patients with PHPT compared with adults with PHPT, and when adjusted for age-appropriate reference ranges, serum phosphorus was lower in juvenile patients than adult patients. The studies we reviewed did not consistently report individual values for adenoma weights, and as noted above, there was significant heterogeneity between the average or median adenoma weights reported.
One of the most interesting findings of our comprehensive analysis was the unexpected determination that the size and prevalence of parathyroid adenomas were similar in adults and juveniles. In this context, the rates of reported hyperplasia for juvenile PHPT (11% of 264) and adult PHPT (12% of 2249) were also similar. These results are in conflict with the widely held notion, as yet unproven, that juvenile PHPT is more likely to be multiglandular. We believe this reflects two important considerations, one biological and the other descriptive: firstly, expression of PHPT in MEN1 occurs earlier than sporadic parathyroid adenoma, but rarely before age 21 years; and second, elimination of patients with FHH, which is a more common cause of hypercalcemia than PHPT in young individuals with an elevated or nonsuppressed PTH level, from several of the reported series.
Because the studies we reviewed did not consistently report serum levels of 25(OH)D, we were unable to determine whether a greater prevalence of vitamin D insufficiency in the adult PHPT patients could account for lower serum and urinary calcium levels relative to those in young patients with PHPT. In the single study included in our analysis comparing serum vitamin D concentrations between the juvenile population and the adult population there was no statistically significant difference in serum 25(OH)D between age groups (6). In addition, based on a growing number of studies that show similar rates of vitamin D insufficiency and deficiency in the healthy young and adult populations, it is unlikely that the differences we found in hypercalcemia and hypercalciuria are due to an increased prevalence of vitamin D deficiency or insufficiency in adults with PHPT compared with younger patients with PHPT. To ensure that our results were not due to country of origin effects, we repeated our analysis limiting studies to those performed in the United States. Because the adult postsurgical studies did not report other analytes, this limitation meant that we could only compare three parameters: gland size, serum total calcium, and intact PTH. The results of the comparison of three parameters limited to studies in the United States were similar to the overall results: with statistically different serum calcium concentrations (juvenile mean ± SEM, 3.0 ± 0.1 vs 2.8 ± 0.0 for adults; two-tailed t test corrected for unequal variances, 0.03), and no statistical difference between gland size or intact PTH between groups (gland size, juvenile mean ± SEM, 422 ± 300 mg vs adults, 718 ± 31 mg; two-tailed t test corrected for unequal variances, P = .33; PTH, juvenile mean ± SEM, 14.1 ± 8.8 vs adults, 13.4 ± 1.7; two-tailed t test corrected for unequal variances, P = .93).
As illustrated in Figure 5 and Supplemental Figure 2, our analyses reveal 1) greater levels of serum and urinary calcium in youths with PHPT than adults, suggesting enhanced sensitivity of target organs to PTH in youths with PHPT; and 2) an apparent decrease in sensitivity of the parathyroid adenoma to calcium-mediated inhibition of PTH secretion. The apparently greater autonomy of parathyroid adenomas in young patients with PHPT is consistent with a presumed difference in the fundamental biology of parathyroid adenomas in juvenile and adult patients with PHPT. At present there are no molecular studies comparing the mRNA profiles of juvenile and adult PHPT that can provide insights into these potential differences. Germline mutation is the cause for PHPT in inverse proportion to the age of PHPT diagnosis; reportedly occurring in fewer than 5% of all adult cases (1), in 23.5% of those with PHPT less than 45 years of age and (35), and in 29% of patients less than 18 years who were tested (16). Thus, it might be argued that biochemical differences between juvenile and adult PHPT are due to variable genetic differences between the two groups. However, in the only study that discriminated juvenile PHPT by the presence or absence of a causative germline mutation, those with the PHPT-germline mutation syndromes had milder biochemical alterations from the norm of those with sporadic parathyroid adenomas (14).
Figure 5.
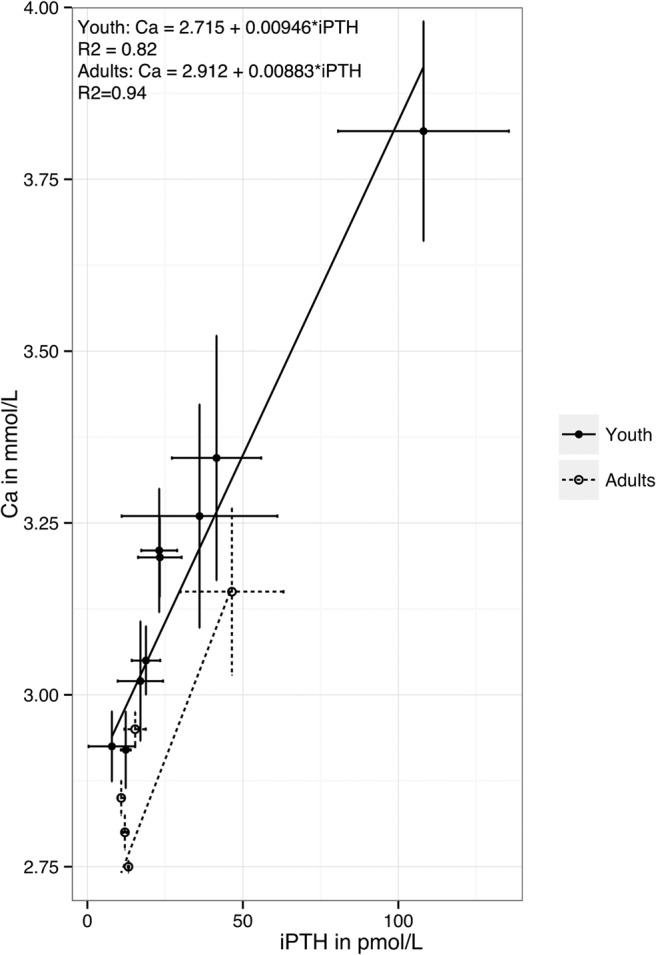
Comparison of frequency-weighted regression of calcium vs PTH curve in pediatric PHPT and adult PHPT in studies with mean gland size < 1188 mg. For juveniles the linear regression gives: calcium = 2.715 + 0.00946×iPTH (R2 = 0.82), for adults: calcium = 2.912 + 0.00883*iPTH (R2 = 0.94). These lines provide an estimation of the relationship between calcium and PTH. Thus, differences between the lines imply that the regulation of calcium by PTH is different between these two groups.
The greater severity of hypercalcemia in juvenile patients with PHPT likely reflects the increased sensitivity of young, growing bone to both PTH and 1,25(OH)2D. This apparent difference may be reflected in a recent study revealing different inflection points in the correlation between iPTH and calcium in the juvenile population from those previously reported in adults (36). It is also well known that PTH-dependent renal production of 1,25(OH)2D decreases with age (37), suggesting that concentrations of serum 1,25(OH)2D are likely to be greater in young compared with older patients with PHPT. Too few juvenile studies on PHPT have reported either bone densitometry or serum levels of 1,25(OH)2D for us to confirm this hypothesis. The greater degree of hypercalciuria in juvenile patients with PHPT compared with adults likely reflects the increased filter load of calcium presented to the kidney by the more severe hypercalcemia, with no difference in circulating levels of PTH.
Our study has several important strengths. First, we analyzed data sets representing a wide variety of countries and eras. The large relative volume of this analysis gave us unprecedented power to detect heretofore-unreported differences between juvenile and adult PHPT. Second, we used rigorous exclusionary and inclusionary requirements to optimize comparisons. Our study also has several notable limitations. First, not all studies that we reviewed reported all the data we desired. For example, the juvenile studies, and many adult studies, did not report data for bone markers, bone densitometry, or serum vitamin D metabolites. Second, the number of juvenile patients is small, limiting some analyses. Nevertheless, this systematic study comparing juvenile PHPT and adult PHPT reveals decreased parathyroid adenoma sensitivity and increased target tissue responsiveness to PTH to be the important biological difference between PHPT in young and older patients. These results provide the rationale for a larger, multicentered prospective study of childhood PHPT that can inform our understanding of the biology of this disease.
Acknowledgments
This study was performed on publically available anonymized data and therefore did not require Internal Review Board approval.
This work was supported by the National Institutes of Health (NIH) through NIH T32-HD043021, NIH R01DK079970 as well as the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- FHH
- familial hypocalciuric hypercalcemia
- PHPT
- primary hyperparathyroidism.
References
- 1. Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. N. Engl. J. Med. 2011;365:2389–2397. [DOI] [PubMed] [Google Scholar]
- 2. Silverberg SJ, Walker MD, Bilezikian JP. Asymptomatic primary hyperparathyroidism. J Clin Densitom. 2013;16:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roizen J, Levine MA. Primary hyperparathyroidism in children and adolescents. J Chin Med Assoc. 2012;75:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke JF, Jacobson K, Gosain A, Sippel RS, Chen H. Radioguided parathyroidectomy effective in pediatric patients. J Surg Res. 2013;184:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loh KC, Duh QY, Shoback D, Gee L, Siperstein A, Clark OH. Clinical profile of primary hyperparathyroidism in adolescents and young adults. Clin Endocrinol (Oxf). 1998;48:435–443. [DOI] [PubMed] [Google Scholar]
- 6. Shah VN, Bhadada SK, Bhansali A, Behera A, Mittal BR, Bhavin V. Influence of age and gender on presentation of symptomatic primary hyperparathyroidism. J Postgrad Med. 2012;58:107–111. [DOI] [PubMed] [Google Scholar]
- 7. Allo M, Thompson NW, Harness JK, Nishiyama RH. Primary hyperparathyroidism in children, adolescents, and young adults. World J Surg. 1982;6:771–776. [DOI] [PubMed] [Google Scholar]
- 8. Bhadada SK, Bhansali A, Dutta P, Behera A, Chanukya GV, Mittal BR. Characteristics of primary hyperparathyroidism in adolescents. J Pediatr Endocrinol Metab. 2008;21:1147–1153. [DOI] [PubMed] [Google Scholar]
- 9. Cronin CS, Reeve TS, Robinson B, Clifton-Bligh P, Guinea A, Delbridge L. Primary hyperparathyroidism in childhood and adolescence. J Paediatr Child Health. 1996;32:397–399. [DOI] [PubMed] [Google Scholar]
- 10. Durkin ET, Nichol PE, Lund DP, Chen H, Sippel RS. What is the optimal treatment for children with primary hyperparathyroidism? J Pediatr Surg. 2010;45:1142–1146. [DOI] [PubMed] [Google Scholar]
- 11. George J, Acharya SV, Bandgar TR, Menon PS, Shah NS. Primary hyperparathyroidism in children and adolescents. Indian J Pediatr. 2010;77:175–178. [DOI] [PubMed] [Google Scholar]
- 12. Girard RM, Belanger A, Hazel B. Primary hyperparathyroidism in children. Can J Surg. 1982;25:11–13, 32. [PubMed] [Google Scholar]
- 13. Hsu SC, Levine MA. Primary hyperparathyroidism in children and adolescents: The Johns Hopkins Children's Center experience 1984–2001. J Bone Miner Res. (17 Suppl) 2002;2:N44–50. [PubMed] [Google Scholar]
- 14. Kollars J, Zarroug AE, van Heerden J, et al. Primary hyperparathyroidism in pediatric patients. Pediatrics. 2005;115:974–980. [DOI] [PubMed] [Google Scholar]
- 15. Lawson ML, Miller SF, Ellis G, Filler RM, Kooh SW. Primary hyperparathyroidism in a paediatric hospital. QJM. 1996;89:921–932. [DOI] [PubMed] [Google Scholar]
- 16. Li CC, Yang C, Wang S, Zhang J, Kong XR, Ouyang J. A 10-year retrospective study of primary hyperparathyroidism in children. Exp Clin Endocrinol Diabetes. 2012;120:229–233. [DOI] [PubMed] [Google Scholar]
- 17. Libánský P, Astl J, Adámek S, et al. Surgical treatment of primary hyperparathyroidism in children: Report of 10 cases. Int J Pediatr Otorhinolaryngol. 2008;72:1177–1182. [DOI] [PubMed] [Google Scholar]
- 18. Mallet E. Primary hyperparathyroidism in neonates and childhood. The French experience (1984–2004). Horm Res. 2008;69:180–188. [DOI] [PubMed] [Google Scholar]
- 19. Norwood S, Andrassy RJ. Primary hyperparathyroidism in children: A review. Mil Med. 1983;148:812–814. [PubMed] [Google Scholar]
- 20. Paunovic I, Zivaljevic V, Stojanic R, Kalezic N, Kazic M, Diklic A. Primary hyperparathyroidism in children and young adults:–a single institution experience. Acta Chir Belg. 2013;113:35–39. [DOI] [PubMed] [Google Scholar]
- 21. Rapaport D, Ziv Y, Rubin M, Huminer D, Dintsman M. Primary hyperparathyroidism in children. J Pediatr Surg. 1986;21:395–397. [DOI] [PubMed] [Google Scholar]
- 22. Almquist M, Bergenfelz A, Mårtensson H, Thier M, Nordenström E. Changing biochemical presentation of primary hyperparathyroidism. Langenbecks Arch Surg. 2010;395:925–928. [DOI] [PubMed] [Google Scholar]
- 23. Lafferty FW. Primary hyperparathyroidism. Changing clinical spectrum, prevalence of hypertension, and discriminant analysis of laboratory tests. Arch Intern Med. 1981;141:1761–1766. [DOI] [PubMed] [Google Scholar]
- 24. Lo CY, Chan WF, Kung AW, Lam KY, Tam SC, Lam KS. Surgical treatment for primary hyperparathyroidism in Hong Kong: Changes in clinical pattern over 3 decades. Arch Surg. 2004;139:77–82. [DOI] [PubMed] [Google Scholar]
- 25. Mazzaglia PJ, Berber E, Kovach A, Milas M, Esselstyn C, Siperstein AE. The changing presentation of hyperparathyroidism over 3 decades. Arch Surg. 2008;143:260–266. [DOI] [PubMed] [Google Scholar]
- 26. Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, Grant CS, Melton LJ., 3rd Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993–2001: An update on the changing epidemiology of the disease. J Bone Miner Res. 1993;21:171–177. [DOI] [PubMed] [Google Scholar]
- 27. Rapaport D, Ziv Y, Rubin M, Huminer D, Dintsman M. Primary hyperparathyroidism in children. J Pediatr Surg. 1986;21:395–397. [DOI] [PubMed] [Google Scholar]
- 28. Harman CR, van Heerden JA, Farley DR, Grant CS, Thompson GB, Curlee K. Sporadic primary hyperparathyroidism in young patients: A separate disease entity? Arch Surg. 1999;134:651–655; discussion 655–656. [DOI] [PubMed] [Google Scholar]
- 29. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burke JF, Naraharisetty K, Schneider DF, Sippel RS, Chen H. Early-phase technetium-99m sestamibi scintigraphy can improve preoperative localization in primary hyperparathyroidism. Am J Surg. 2013;205:269–273; discussion 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bilezikian JP, Silverberg SJ. Clinical practice. Asymptomatic primary hyperparathyroidism. N Engl J Med. 2004;350:1746–1751. [DOI] [PubMed] [Google Scholar]
- 32. Mózes G, Curlee KJ, Rowland CM, et al. The predictive value of laboratory findings in patients with primary hyperparathyroidism. J Am Coll Surg. 2002;194:126–130. [DOI] [PubMed] [Google Scholar]
- 33. Albright F, Aub JC, Bauer W. Hyperparathyroidism: A common and polymorphic condition as illustrated by seventeen proved cases from one clinic. J Am Med Assoc. 1934; 102(16):1276–1287. [Google Scholar]
- 34. Keating FR., Jr. 1961 Diagnosis of primary hyperparathyroidism. Clinical and laboratory aspects. J Am Med Assoc. 178:547–555. [DOI] [PubMed] [Google Scholar]
- 35. Starker LF, Akerström T, Long WD, Delgado-Verdugo A, Donovan P, Udelsman R, Lifton RP, Carling T. Frequent germ-line mutations of the MEN1, CASR, and HRPT2/CDC73 genes in young patients with clinically non-familial primary hyperparathyroidism. Horm Cancer. 2012;3:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atapattu N, Shaw N, Högler W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr Res. 2013;74:552–556. [DOI] [PubMed] [Google Scholar]
- 37. Armbrecht HJ, Boltz MA, Forte LR. Effect of age on parathyroid hormone and forskolin stimulated adenylate cyclase and protein kinase activity in the renal cortex. Exp Gerontol. 1986;21:515–522. [DOI] [PubMed] [Google Scholar]