Abstract
Context:
Incidental benign adrenocortical adenomas, adrenal incidentalomas are found in 4.5% of abdominal computed tomography scans, with the incidence increasing to 10% in patients older than 70 years of age. These incidentalomas frequently show evidence of excess cortisol secretion but without overt Cushing's syndrome. The mortality rate is increased in Cushing's syndrome.
Objective:
This study sought to investigate whether patients with adrenal incidentalomas have an increased mortality.
Design:
This was a retrospective, longitudinal cohort study.
Setting:
The study was carried out in an Endocrine Investigation Unit in a University Teaching Hospital.
Patients:
Two hundred seventy-two consecutive patients with an incidental adrenal mass underwent a dedicated diagnostic protocol, which included dexamethasone testing for hypercortisolism between 2005 and 2013. Overall survival was assessed in 206 patients with a benign, adrenocortical adenoma.
Main Outcome Measures:
Survival analysis was carried out by using Kaplan-Meier curves and the effect of dexamethasone cortisol estimated by Cox-regression analysis. Cause-specific mortality was ascertained from death certificates and compared with local and national data.
Results:
Eighteen of 206 patients died and the mean time (SD) from diagnosis to death was 3.2 (1.7) years. Seventeen of 18 patients who died had a post dexamethasone cortisol >1.8 μg/dL and there was a significant decrease in survival rate with increasing dexamethasone cortisol levels (P = .001). Compared with the <1.8 μg/dL group, the hazard ratio (95% confidence interval) for the 1.8–5μg/dL group was 12.0 (1.6–92.6) whereas that of the >5 μg/dL group was 22.0 (2.6–188.3). Fifty percent and 33% of deaths were secondary to circulatory or respiratory/infective causes, respectively.
Conclusions:
Patients with adrenal incidentalomas and a post-dexamethasone serum cortisol >1.8 μg/dL have increased mortality, mainly related to cardiovascular disease and infection.
Benign adrenocortical adenomas are common, being found in 4.5% of all computed tomography (CT) scans as incidental findings—“adrenal incidentaloma.” In the aging population, adrenal incidentalomas are more common, present in 10% of those aged 70 years (1). These tumors are identified at cross-sectional imaging of the abdomen in patients across a wide variety of medical and surgical specialties. On routine clinical testing, up to half of all patients exhibit biochemical evidence of excess cortisol secretion (2–4), but without associated overt clinical features of Cushing's syndrome (5), and this has been called “subclinical Cushing's syndrome” or “subclinical hypercortisolism” (2, 6). The true prevalence of subclinical hypercortisolism is debated becasuse diagnostic tests may have false-positive rates, and whether associated with diabetes and hypertension arguable, becasuse both are highly prevalent in the general population, making causal links less than straightforward (2, 7). Nevertheless, a number of studies have shown an increased risk of cardiovascular disease in patients with adrenal incidentalomas (8, 9), and a recent study has suggested increased mortality from cardiovascular causes (10). Therefore, it is essential to understand the significance of cortisol measurements in these patients.
Cortisol plays a major role in glucose, fat, and protein metabolism, and is a crucial regulator of the immune system. Disturbances in cortisol physiology interrupt homeostatic equilibrium with pathological consequences, as evident in patients with Cushing's syndrome (5). Overt endogenous Cushing's syndrome and use of excess exogenous glucocorticoids are associated with increases in cardiovascular events and mortality from cardiovascular disease or infection (11–14). Low-grade excess cortisol secretion in adrenal incidentalomas has also been associated with an increased prevalence of insulin resistance, hypertension, and visceral obesity (15), and we hypothesized, therefore, that patients with benign adrenocortical incidentalomas might also have excess mortality. To test this hypothesis, we assessed rates and cause of death, and compared these to local and national mortality data in patients with benign adrenocortical incidentalomas and studied the relationship with their serum cortisol taken after a dexamethasone-suppression test.
Materials and Methods
Setting and participants
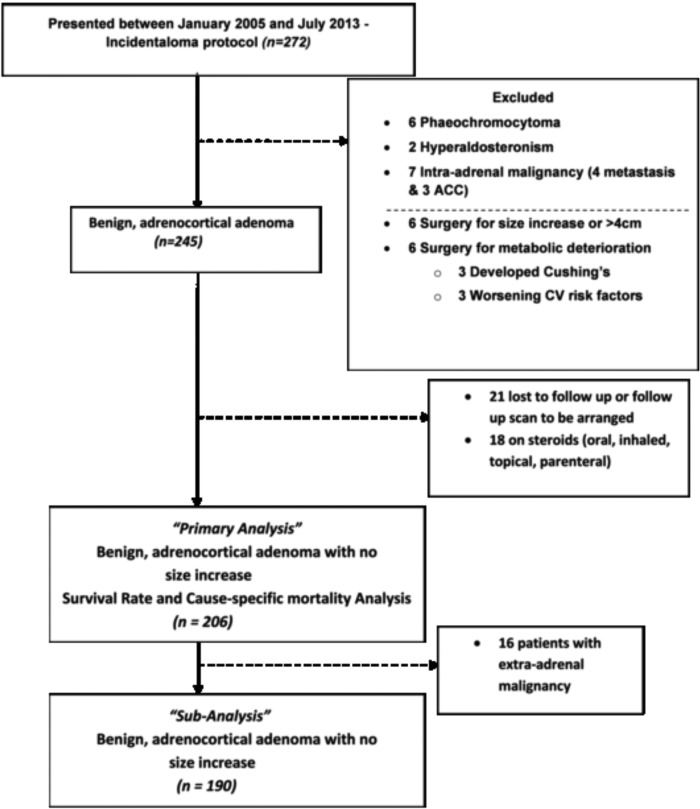
A retrospective, longitudinal, cohort study was performed on a consecutive series of 272 patients diagnosed with adrenal incidentalomas between January 2005 and July 2013, and referred to the Endocrine Investigation Unit, Royal Hallamshire Hospital, Sheffield Teaching Hospitals National Health Service Foundation Trust, Sheffield, United Kingdom, a University Hospital. Each patient underwent a clinically indicated specific diagnostic protocol, which included a full medical history and clinical examination, routine laboratory investigations, review of computed tomography (CT) scans, and an endocrine workup. Patients were referred from a wide range of specialties once an adrenal incidentaloma had been identified on CT performed for the valuation of unrelated disease approximately 3–6 months prior to referral. A Siemens Volume Zoom 4 slice scanner and a GE XT 64 slice scanner (GE Healthcare Hatfield) were used to image patients and all scans were assessed by a radiologist with specialist expertise in adrenal disease. Patients underwent a follow-up CT or magnetic resonance imaging (Siemens 1.5 Tesla magnetic resonance imaging) scan 6 months after the initial scan that identified the incidentaloma in accordance with current guidelines to exclude an increase in size of the adrenal mass (16). Our final analysis included only patients who had undergone a repeat abdominal CT or magnetic resonance imaging scan approximatly 6 months after the initial scan that had demonstrated the adrenal incidentaloma (Figure 1).
Figure 1.
Recruitment Strategy Flow chart. Patients attending for incidentaloma protocol at an Endocrine Investigation Unit between January 2005 and July 2013.
From the total of 272 we limited our incidentaloma cohort to lipid-rich benign adrenocortical adenoma with precontrast density less than 10 Hounsfield units or increased contrast washout if necessary, excluding patients with pheochromocytoma and primary hyperaldosteronism detected on our incidentaloma endocrine protocol and those with a suspicion of adrenal malignancy or, suspicion of adrenal malignancy. We excluded patients who we referred for adrenalectomy on the basis of suspicion of malignancy: incidentaloma greater than 4 cm in diameter or demonstrated increase in size at 6-month follow-up, patients who had worsening metabolic features (worsening blood pressure control or worsening control of diabetes), or patients with Cushing's syndrome phenotype. This left a group of 245 patients. We then excluded patients on chronic inhaled, oral, topical, or parenteral glucocorticoid treatments (18 patients), given that these could directly influence the results of the dexamethasone suppression tests and effect on morbidity and mortality. No patients were on estrogens. In addition, 21 patients were lost to follow-up. The final survival analysis was carried out on 206 patients (primary analysis). We also performed a subanalysis on the same group of patients but excluding patients with known extra-adrenal malignancy at referral (n = 190).
The study was approved and registered as an institutional case-notes review at Sheffield Teaching Hospitals National Health Service Foundation Trust (Registration No. 5133). The database was registered with the Department of Information Governance, Sheffield Teaching Hospitals. The study is reported as recommended by the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines, that recommend parameters to be included in a complete and accurate report of an observational study (17).
Study procedures
Deaths were identified from hospital databases that are updated in real time with death data received from the National Health Service Spine network, part of the critical national infrastructure that provides health care and supports the delivery of health services in the United Kingdom and is updated at least on a weekly basis. After data extraction, patient records were anonymized. Survival was calculated as the time from the date of radiological diagnosis to 1) the date of death for those who died, or 2) July 2013 for those who survived. The primary cause of death was ascertained from death certificates obtained from the General Register Office in the United Kingdom so that all-cause and cause-specific mortality could be determined. The General Register Office maintains the national archive of all deaths, births, and marriages dating back to 1837. Death certificates were coded using the 10th revision of the International Classification of Disease (ICD-10). The cause-specific mortality in the adrenal incidentaloma patients was compared with local incidence figures in 2010 obtained from the Sheffield Director of Public Health Report, 2011 (18). Approximately 550 000 people make up the population of Sheffield. The data were also compared with national United Kingdom incidence data in 2010 obtained from the Office for National Statistics, 2011 (19). Deaths were classified into four general categories: circulatory diseases, including ischemic heart disease, hypertensive heart disease, cerebrovascular disease, congestive heart failure, and atherosclerotic vascular disease; respiratory disease and infection; malignancy; and other causes. The age of death of adrenal incidentaloma patients was also compared with the general population life expectancy at birth data (18).
All patients were assessed by a standard incidentaloma protocol. Two 24-hour urine collections for catecholamines and metanephrines (to screen for pheochromocytoma) and in hypertensive patients, a plasma aldosterone:renin ratio were collected. The latter was obtained after the patient had been out of bed for at least 2 hours and then seated comfortably for 5–15 minutes. Screening for hypercortisolism was carried out by an out-patient 1-mg overnight dexamethasone suppression test, in which a 1-mg dexamethasone tablet is administered at 2300 h and serum cortisol level is measured at 0900 h the following morning. We classified patients with a post-dexamethasone cortisol >1.8 μg/dL (>50 nmol/L) into two groups. Group 1, post-dexamethasone cortisol 1.8–5 μg/dL (50–137 nmol/L); and group 2, post-dexamethasone cortisol >5 μg/dL (>138 nmol/L) (10, 16). A post-dexamethasone cortisol >1.8 μg/dL is in keeping with a diagnosis of cortisol excess as recommended by Endocrine Society guidelines (20). Other recommended cutoffs in the literature include 3 μg/dL (>83 nmol/L) by European centers (2) and 5 μg/dL by the National Institutes of Health (21) and the Italian Association of Clinical Endocrinologists (16). Only a baseline overnight dexamethasone suppression test is carried out in our unit (although we do acknowledge that in some cases post-dexamethasone cortisol levels may worsen (10) or normalize (22) on followup). Blood tests also included hematological and biochemical profiles taken after an overnight fast at 0900 h.
Assays
Between January 2005 and January 2011 total serum cortisol was measured in a Siemens Advia Centaur Cortisol assay (Siemens): analytical range, 0.2–75 μg/dL (5.5–2069 nmol/L); interassay coefficient of variation, 9% at 0.1 μg/dL (3.5 nmol/L), 5% at 2.3 μg/dL (62.9 nmol/L), and 6.2% at 4.9 μg/dL (134 nmol/L). From February 2011 to July 2013 the assay was changed to the Roche e602 serum cortisol assay, which showed negligible bias at 1.8 μg/dL and 7% bias at 21.7 μg/dL (600 nmol/L) on external quality assessment for comparison.
Statistical analysis
The incidentaloma database was managed using Microsoft Excel 2010 and all statistical analyses were performed using SPSS Version 19.0 (SPSS) or Stata 12.1 (StataCorp). Rates and percentages were calculated for categorical data. Continuous variables were summarized as means and SDs unless asymmetrically distributed, in which case medians and interquartile ranges (IQR) are presented. The relationships between dexamethasone cortisol and patient characteristics was assessed by independent sample t test for categorical characteristics and Pearson correlation for continuous characteristics, unless asymmetric, in which case nonparametric alternatives (specifically, Mann-Whitney test and Spearman correlation, respectively) were used. A significant result was taken as P < .05 and two-tailed tests were used.
Kaplan-Meier survival curves were plotted to demonstrate survival according to post-dexamethasone serum cortisol cutoffs described in the literature: 1.8 μg/dL [Endocrine Society guidelines (20)] and 5 μg/dL [National Institutes of Health 2002 (21)]: group 1, post-dexamethasone cortisol 1.8–5 μg/dL; group 2, post-dexamethasone cortisol >5 μg/dL. The log-rank test for trend was performed to assess significance and hazard ratios, and 95% confidence intervals were estimated by Cox regression. Proportional hazards were assessed using Schoenfeld residuals and the related test proposed by Grambsch and Therneau (23).
Results
From a total of 272 patients, 66 subjects were excluded so that 206 patients with a benign, adrenocortical adenoma were assessed for survival (Figure 1). The baseline characteristics for this group are shown in Table 1 and presented using a post-dexamethasone serum cortisol cutoff of 1.8 μg/dL. The mean (SD) length of followup of these patients was 4.2 (2.3) years, and between January 2005 and July 2013 there were 18 deaths. The median (IQR) age of death was 73 (69–79) years (men, 74 years; women, 72 years), and the mean (SD) time from diagnosis to death was 3.2 (1.7) years. The average overall life expectancy for the local and national population during this time period was approximately 78 years for men and 82 years for women (18). The death rates were similar: women 12/137 (8.8%) vs men 6/69 (8.7%).
Table 1.
Baseline Demographic and Endocrine Characteristics
| Characteristic | Dexamethasone Cortisol <1.8 μg/dL (n = 95) | Dexamethasone Cortisol >1.8 μg/dL (n = 111) | P Value |
|---|---|---|---|
| Sex, female/male, No. | 65/30 | 72/39 | |
| Age at diagnosis, ya | 63 (54–70) | 69 (61–75) | <.001 |
| Dexamethasone cortisol, μg/dLa | 1.2 (1.0–1.4) | 2.9 (2.2–4.5) | <.001 |
| Length of follow up, yb | 4.1 (2.4) | 4.3 (2.3) | .66 |
| Side of adenoma, right/left/bilateral | 23/66/6 | 39/52/20 | |
| Size of adenoma, cma | 2.0 (1.4–2.5) | 2.5 (2.0–3.0) | <.001 |
| Weight, kga | 86.2 (63.0–103.5) | 78.5 (62.3–101.1) | .36 |
| On hypertensive treatment, % | 58 | 64 | |
| No of HT drugs | |||
| n = 0, % | 42 | 36 | .67 |
| n = 1, % | 13 | 19 | |
| n = 2, % | 15 | 19 | |
| n = 3, % | 8 | 6 | |
| n > 3, % | 22 | 20 | |
| On diabetes treatment, % | 21 | 24 | .58 |
| Fasting glucose, mmol/La | 5.4 (4.8 − 6.7) | 5.5 (4.8–7.3) | .65 |
| On statins, % | 46 | 47 | .94 |
| Neutrophil/lymphocyte ratioa | 2.26 (1.78–3.08) | 2.63 (2.02–3.78) | .014 |
Abbreviation: HT, hypertension.
Median (IQR).
Mean (SD).
Post-dexamethasone serum cortisol was correlated with size of adenoma (Spearman's ρ = 0.35, P < .001) and age (Spearman's ρ = 0.32; P < .001). Patients with bilateral disease also had a higher post-dexamethasone serum cortisol level, although this relationship was less pronounced (median, 2.25 μg/dL [62 nmol/L] bilateral vs 1.8 μg/dL [51 nmol/L] unilateral; P = .019). There was no significant difference in post-dexamethasone serum cortisol between sexes (median 1.88 μg/dL [52 nmol/L] women vs 1.92 μg/dL [53 nmol/L] men; P = .72). The neutrophil to lymphocyte ratio was significantly higher in patients with a serum cortisol >1.8 μg/dL post dexamethasone (P = .014).
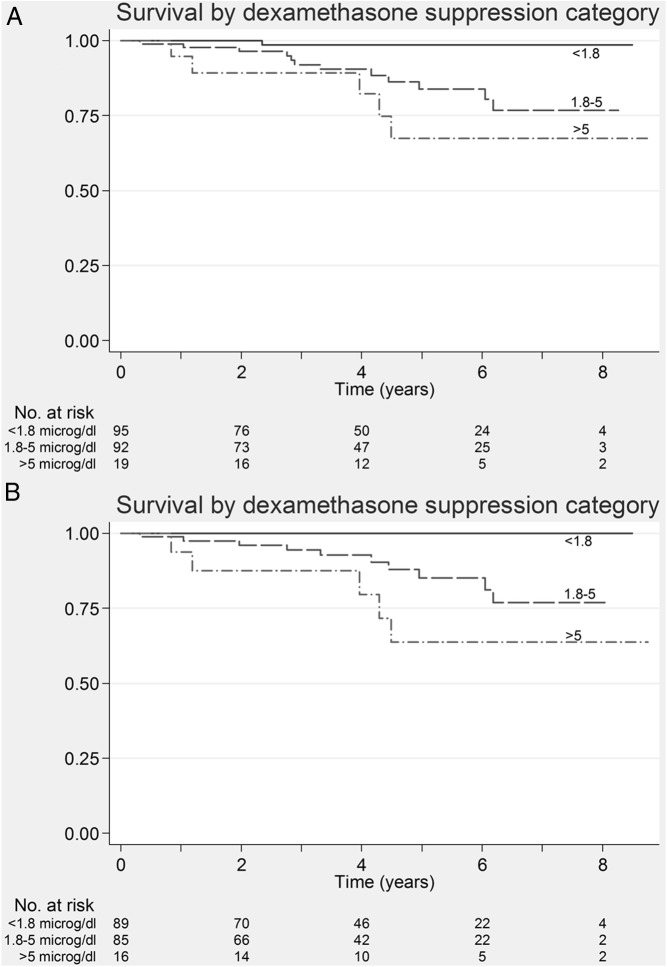
Kaplan-Meier survival curves were plotted for the different dexamethasone serum cortisol categories and are shown in Figure 2A. These show a significant decrease in survival rate that worsens with increasing post-dexamethasone cortisol cutoffs (log-rank trend test, P = .001). Twelve of 92 (13%) and 5/19 (26%) patients in the 1.8–5 μg/dL and >5 μg/dL groups died, respectively. There was 1/95 (1%) death in the group with post-dexamethasone serum cortisol <1.8 μg/dL. The mean (95% confidence interval) survival for the post-dexamethasone groups were as follows: cortisol <1.8, 1.8–5, and >5 μg/dL was 8.4 (8.2–8.6), 7.3 (6.8–7.8), and 6.9 (5.6–8.3) years, respectively. The hazard ratio (95% confidence interval) for the 1.8–5-μg/dL group was 12.0 (1.6–92.6) whereas that of the >5 μg/dL group was 22.0 (2.6–188.3) using the <1.8-μg/dL group as reference. When excluding patients on drugs that induce CYP3A4 (n = 5) and inhibit CYP3A4 (n = 8) results were similar. There was no relationship between the presence of hypertension (P = .8), dyslipidemia (P = .5), nor diabetes (P = .6) and mortality. Fasting glucose levels were similar in patients who survived or died.
Figure 2.
A, Kaplan-Meier survival curve in patients categorized according to post dexamethasone cortisol levels. This shows worsening survival with increasing post dexamethasone serum cortisol levels (log rank P = .001) categorized using standard cut-offs from literature (<1.8 μg/dL, 1.8–5 μg/dL, >5 μg/dL) (Primary analysis). B, Kaplan-Meier survival curve in patients categorized according to post-dexamethasone cortisol levels. This shows worsening survival with increasing post dexamethasone serum cortisol levels (log-rank P < .001) after excluding patients with benign adrenocortical adenomas and extra-adrenal malignancy. Hazard ratios could not be calculated because no patients from the <1.8-μg/dL group died (subanalysis).
Kaplan-Meier curves were also plotted after excluding patients with extra-adrenal malignancy (n = 190) (Figure 2B) in a subanalysis. Findings were similar with significantly decreasing survival rates associated with higher dexamethasone cortisol cutoffs (log-rank trend test, P < .001). There were 15 deaths in total: none with serum cortisol 1.8 μg/dL; 10 with serum cortisol 1.8–5 μg/dL (10/85[12%]); and five with a serum cortisol >5 μg/dL (5/16[31%]). Hazard ratios in relation to the <1.8-μg/dL group could not be calculated because no deaths were observed in this group.
Attempts to fit post-dexamethasone serum cortisol as a continuous covariate via regression models were limited by the lack of events for patients with low levels, meaning the hazard function could not be reliably estimated; hence, the three-level categorization was retained. Furthermore, multivariable modeling was limited by the small number of events (24), which would yield unstable models and imprecise coefficients. Nonetheless, tentative models that incorporate the post-dexamethasone cortisol category along with one other potentially confounding covariate (age, sex, presence of bilateral disease, and log-transformed size) confirmed the univariate findings. The model assumption (proportional hazards) was not contradicted by the assessment of residuals, although again the formal tests are limited by the low death rate.
The cause of death was ascertained from patient death certificates. The results are tabulated in Table 2. In our cohort there were 50% circulatory and 33% respiratory/infective causes of death, which were higher than the 31% circulatory and the 14% respiratory/infective causes recorded locally in Sheffield, and nationally in the United Kingdom. All our respiratory cases were pneumonias and one of our circulatory cases was a pulmonary embolism. Conversely, 6% of our patients died directly resulting from malignancy, a percentage much lower than the local and national incidence of 29% (Figure 3). Similar results were observed in the cohort of patients excluding those with extra-adrenal malignancy in whom there were 53, 27, and 7% deaths from circulatory, respiratory/infection, and malignant causes, respectively.
Table 2.
Cause of Mortality in Relation to Dexamethasone Cortisol Levels
| Pt No | Sex | Post-Dexamethasone Cortisol μg/dL (nmol/L) | Age at Diagnosis, y | Age at Death, y | Dys | HT | DM | Cardiovascular Diseasea | Primary Cause of Deathb |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 3.4 (93) | 71 | 75 | No | No | No | Yes | Circulatory |
| 2 | M | 6.1 (169) | 68 | 69 | No | No | No | No | Circulatory |
| 3 | M | 2.2 (60) | 67 | 73 | No | Yes | No | No | Circulatory |
| 4 | F | 3.5 (97) | 77 | 81 | Yes | Yes | Yes | Yes | Peritonitis from bowel ischemia |
| 5 | M | 3.2 (88) | 74 | 79 | No | Yes | No | Yes | Malignancy |
| 6 | F | 0.3 (8) | 58 | 60 | No | Yes | No | No | Sepsis from cellulitis |
| 7 | F | 9.5 (262) | 89 | 93 | Yes | Yes | Yes | Yes | Pneumonia |
| 8 | F | 4.6 (126) | 71 | 72 | Yes | No | Yes | No | Circulatory |
| 9 | M | 11.7 (322) | 69 | 70 | Yes | Yes | No | No | Pneumonia |
| 10 | F | 6.3 (175) | 57 | 61 | Yes | No | No | No | Pneumonia |
| 11 | F | 1.8 (51) | 66 | 69 | Yes | Yes | Yes | No | Sepsis from gallbladder empyema |
| 12 | F | 2.6 (71) | 69 | 71 | No | No | No | Yes | Circulatory |
| 13 | M | 2.9 (81) | 82 | 85 | Yes | Yes | Yes | No | Circulatory |
| 14 | F | 2.2 (60) | 63 | 69 | Yes | No | No | No | Circulatory |
| 15 | F | 3.9 (107) | 73 | 75 | Yes | Yes | No | Yes | Circulatory |
| 16 | M | 6.1 (167) | 70 | 74 | Yes | Yes | No | Yes | Other |
| 17 | F | 3.6 (99) | 59 | 59 | No | No | Yes | No | Other |
| 18 | F | 4.7 (131) | 77 | 80 | No | No | No | No | Circulatory |
Abbreviations: DM, diabetes mellitus Dys, dyslipidemia; HT, hypertension; Pt No, patient number.
History of cardiovascular disease: classified as ischemic heart disease, hypertensive heart disease, cerebrovascular disease, and atherosclerotic vascular disease.
The disease or condition immediately causing death.
Figure 3.
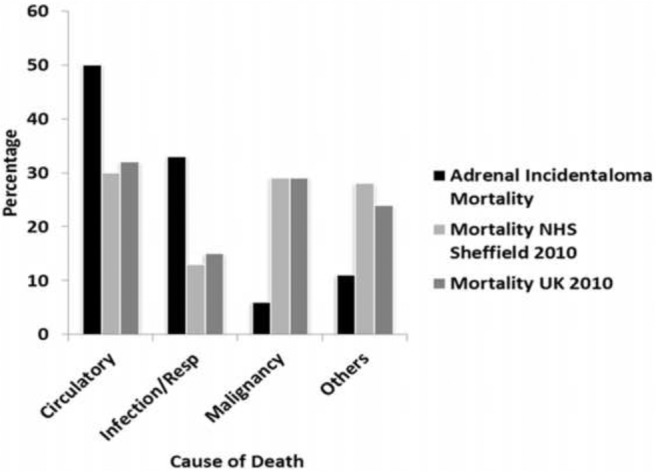
Causes of mortality. Mortality rate in adrenal incidentalomas compared with local incidence mortality (Sheffield) and national incidence mortality (United Kingdom). Graphs show a higher rate of mortality from circulatory and infective/respiratory (resp) causes in the adrenal incidentaloma group of patients between January 2005 and July 2013.
Discussion
Adrenal incidentalomas secreting excess cortisol are associated with multiple complications including diabetes, hypertension, visceral obesity, low bone mineral density, vertebral fractures, a predisposition to thrombosis (2, 15, 25, 26), and cardiovascular disease (8, 15). Here, we show that patients with adrenal incidentalomas who fail to suppress serum cortisol on dexamethasone testing have greater mortality, which increases the higher the post-dexamethasone serum cortisol level. The main causes of death were atherosclerotic cardiovascular disease and infections, mainly pneumonias. Our findings are supported by recent data indicating increased cardiovascular mortality in 198 patients with adrenal incidentalomas followed up for approximately 7.5 years (10). However, the rate of infection-related mortality was higher in our cohort and the rate of malignancy lower. The difference between the two studies could relate to different exclusion strategies, and the fact that our data for mortality come directly from death certificates. In our subanalysis we excluded patients both with intra- and extra-adrenal malignancy: many patients with malignancy have CT scans carried out to exclude recurrence or spread, such as adrenal metastasis. Nevertheless, our two independent studies come to the same broad conclusions, increasing confidence in the observed relationships. Di Dalmazi et al (10) studied mortality of patients with adrenal incientaloma in Italy but the mean time to death was not reported. In our study, however, the mean time to death was 3.2 years. In addition, the age of death for patients with adrenal incidentalomas was lower than the life expectancy at birth of the general population (men/women, 74/72 vs 78/82 y) (18). When adjusting for age the relationship between postdexamethasone cortisol category and mortality remained identical.
Patients with overt Cushing's syndrome have increased mortality due mainly to cardiovascular disease and infection (27). The mortality rate improves considerably in Cushing's syndrome patients in remission post treatment, and when due to adrenal disease returns to that of the background population following successful intervention (28, 29). In most highly selected surgical studies patients with adrenal incidentalomas secreting excess cortisol have experienced improvement in their cardiovascular risk profile when compared with patients treated conservatively (30, 31), but this finding is not universal (32). Our data, on a consecutive series of patients, extend these observations. The small number of events hampers the extent of modeling, which can be undertaken and the precision of the estimates, but nonetheless the data strongly suggest that hypercortisolemia in the context of an adrenal incidentaloma is detrimental. To establish causality, an appropriately powered prospective randomized formal intervention study would be needed, but this has never been performed.
Several lines of evidence from other disease states suggest that excess low-grade cortisol exposure over long periods, as may occur in patients with adrenal incidentaloma, can have a detrimental effect on cardiovascular risk. For example, nonsuppression on dexamethasone testing and higher baseline serum cortisol levels has been associated with cardiovascular disease death in patients with mood disorders (33). Cross-sectional data show that increased total cortisol exposure, measured in salivary cortisol samples, a marker of free cortisol, is associated with the development of atherosclerosis in the elderly, independent of the presence of other cardiovascular risk factors (34). Furthermore, higher 24-hour urine cortisol levels predicted cardiovascular mortality during a six year follow up in an elderly population with or without baseline cardiovascular disease, suggesting a direct effect of cortisol on the cardiovascular system (35). Hypercortisolism and increased sensitivity to glucocorticoids in patients with glucocorticoid receptor polymorphisms have also been associated with severe atherosclerosis and coronary artery disease (36). This effect may also be explained by the binding of glucocorticoids to glucocorticoid and mineralocorticoid receptors in cardiovascular tissues such as the heart and vascular walls, enhancing contractility of vascular smooth muscle by increasing sensitivity to noradrenaline and decreasing endothelium-dependent vasodilatation (37). Patients with Cushing's syndrome have significantly greater levels of coronary artery calcification and noncalcified plaques, increasing the risk for cardiovascular mortality (38), whereas similarly, patients with rheumatoid arthritis on exogenous glucocorticoids also have more carotid plaque formation and lower-limb arterial noncompressibility, independent of cardiovascular risk factors and inflammatory disease manifestations (39).
A high prevalence of infection-related mortality in our population could be related to loss of the normal physiological interaction between cortisol and the immune system. Under normal circumstances in the young there is a circadian change to the total number of lymphocytes and some lymphocyte subpopulations such as CD3 (total T cells), CD4 (T-helper), and CD20 (total B-cells) with higher levels at night when cortisol levels are lowest, whereas CD8 (T-suppressor and cytotoxic) and CD16 (natural killer) cells are higher at 1200 h (40). Changes in cortisol exposure may distort lymphocyte rhythms resulting in impaired immune function. An important effect of glucocorticoids is to reduce Th-1 responses needed to counter bacterial infection, and reduce the Th-1:Th-2 cell ratio (41), and lower cellular immunity (42). Glucocorticoids also stimulate apoptosis of eosinophils (43) and impair neutrophil apoptosis (44). A high prevalence of infection-related mortality in our population could be related to loss of physiological interaction between cortisol and the immune system. A higher neutrophil/lymphocyte ratio in our patients who failed to suppress serum cortisol after dexamethasone might be an indicator of such a relationship.
Limiting factors in our study include a small number of deaths and the retrospective nature of the analysis that precludes causative extrapolation. We did, however, carefully ascertain our subjects to define the benign adrenocortical adenoma and assess the significance of the presence of this and excess cortisol as evidenced by dexamethasone testing. Locally, all patients diagnosed with adrenal incidentalomas, after radiological investigation, were referred for investigation to the endocrinology unit irrespective of underlying diagnosis and health status. Dexamethasone levels were not measured, but recent data suggest that measuring dexamethasone levels does not improve the diagnostic performance of the 1-mg overnight dexamethasone-suppression test (45). Therefore, it would be highly unlikely that measurement of dexamethasone levels would have affected our final study outcome, although in clinical practice these may be helpful to rule out false-positive cases on an individual basis. We also acknowledge that hypercortisolism in a patient with a benign adenoma could be secondary to other common conditions, such as, alcoholism, depression, and psychotic disease that result in excess cortisol, but our aim was to investigate the relationship between excess cortisol and death in a nonbiased sample that truly represents adrenal incidentalomas in the general population. Importantly, there were no deaths from suicide or from alcohol-related issues in our study. It is important to note that our subjects only had an initial dexamethasone suppression test, and this is a limitation of the study. In the United Kingdom it is recommended for all patients age 65 years and over to receive the flu and pneumococcal vaccines but we did not have any records of this and cannot comment on whether this may have influenced infection-related mortality.
Our data show an increased mortality both from cardiovascular disease and infections in patients with adrenal incidentaloma and low-grade excess cortisol. Despite death being due to events that are plausibly related to excess cortisol, it is not possible to know whether the patients died because of their hypercortisolism or with their hypercortisolism, and this can only be assessed by prospective studies aimed at reducing the effects of such hypercortisolism. Therefore, this study supports the need for prospective interventional studies to assess whether medical or surgical interventions can be tailored to the individual and improve outcomes in this common condition.
Acknowledgments
We thank all members of the Department of Endocrinology, especially the Endocrine Unit nurse specialists Kaye Dunkley, Vicky Ibbotson, and Betty Roberts, and MDT Coordinator, Helen Sutcliffe.
Author Contributions: M.D., M.Br., R.J.R., and J.N.-P. conceived the project; M.D., M.Br., M.Bu., B.H., R.J.R., and J.N.-P. contributed to data acquisition, analysis, and interpretation; M.D., M.Br., and J.N.-P. drafted initial and additional versions of the manuscript, and M.Bu., B.H., and R.J.R. critically revised drafts for important intellectual content.; All authors approved the final version of the manuscript.
Part of the costs for this study came from a National Institute for Health Research (NIHR) Fellowship Grant DH_BFR-2011-005 (to M.D.).
This paper presents independent research partly funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CT
- computed tomography.
References
- 1. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: Update in diagnosis and management. Endocr Rev. 2004;25:309–340. [DOI] [PubMed] [Google Scholar]
- 2. Chiodini I. Clinical review: Diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011;96:1223–1236. [DOI] [PubMed] [Google Scholar]
- 3. Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637–644. [DOI] [PubMed] [Google Scholar]
- 4. Terzolo M, Pia A, Reimondo G. Subclinical Cushing's syndrome: Definition and management. Clin Endocrinol (Oxf). 2012;76:12–18. [DOI] [PubMed] [Google Scholar]
- 5. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006;367:1605–1617. [DOI] [PubMed] [Google Scholar]
- 6. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–285. [DOI] [PubMed] [Google Scholar]
- 7. Stewart PM. Is subclinical Cushing's syndrome an entity or a statistical fallout from diagnostic testing? Consensus surrounding the diagnosis is required before optimal treatment can be defined. J Clin Endocrinol Metab. 2010;95:2618–2620. [DOI] [PubMed] [Google Scholar]
- 8. Di Dalmazi G, Vicennati V, Rinaldi E, et al. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: A large cross-sectional study. Eur J Endocrinol. 2012;166:669–677. [DOI] [PubMed] [Google Scholar]
- 9. Morelli V, Reimondo G, Giordano R, et al. Long-term follow-up in adrenal incidentalomas: An Italian multicenter study. J Clin Endocrinol Metab. 2014:jc20133527. [DOI] [PubMed] [Google Scholar]
- 10. Di Dalmazi G, Vicennati V, Garelli S, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing's syndrome: A 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2:396–405. [DOI] [PubMed] [Google Scholar]
- 11. Fardet L, Petersen I, Nazareth I. Risk of cardiovascular events in people prescribed glucocorticoids with iatrogenic Cushing's syndrome: Cohort study. BMJ. 2012;345:e4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klein NC, Go CH, Cunha BA. Infections associated with steroid use. Infect Dis Clin North Am. 2001;15:423–432, viii [DOI] [PubMed] [Google Scholar]
- 13. Ntali G, Asimakopoulou A, Siamatras T, et al. Mortality in Cushing's syndrome: Systematic analysis of a large series with prolonged follow-up. Eur J Endocrinol. 2013;169:715–723. [DOI] [PubMed] [Google Scholar]
- 14. Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, Johannsson G. Premature mortality in patients with Addison's disease: A population-based study. J Clin Endocrinol Metab. 2006;91:4849–4853. [DOI] [PubMed] [Google Scholar]
- 15. Tauchmanovà L, Rossi R, Biondi B, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87:4872–4878. [DOI] [PubMed] [Google Scholar]
- 16. Terzolo M, Stigliano A, Chiodini I, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164:851–870. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 18. 2011. Sheffield Director of Public Health Report. http://wwwpublichealthsheffield2011nhsuk/the-story-so-far/cause-of-death/.
- 19. 2011. Office for National Statistics. http://wwwonsgovuk/ons/rel/vsob1/mortality-statistics–deaths-registered-in-england-and-wales–series-dr-/2010/stb-deaths-by-cause-2010html.
- 20. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consens State Sci Statements. 2002;19:1–25. [PubMed] [Google Scholar]
- 22. Bernini GP, Moretti A, Oriandini C, Bardini M, Taurino C, Salvetti A. Long-term morphological and hormonal follow-up in a single unit on 115 patients with adrenal incidentalomas. Br J Cancer. 2005;92:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Therneau TM, Grambsch PM. 2000. Modeling survival data: Extending the Cox model. New York: Springer; 350. [Google Scholar]
- 24. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. [DOI] [PubMed] [Google Scholar]
- 25. Chiodini I, Mascia ML, Muscarella S, et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med. 2007;147:541–548. [DOI] [PubMed] [Google Scholar]
- 26. Debono M, Prema A, Hughes TJ, Bull M, Ross RJ, Newell-Price J. Visceral fat accumulation and postdexamethasone serum cortisol levels in patients with adrenal incidentaloma. J Clin Endocrinol Metab. 2013;98:2383–2391. [DOI] [PubMed] [Google Scholar]
- 27. Dekkers OM, Horváth-Puhó E, Jørgensen JO, et al. Multisystem morbidity and mortality in Cushing's syndrome: A cohort study. J Clin Endocrinol Metab. 2013;98:2277–2284. [DOI] [PubMed] [Google Scholar]
- 28. Lindholm J, Juul S, Jørgensen JO, et al. Incidence and late prognosis of cushing's syndrome: A population-based study. J Clin Endocrinol Metab 2001;86:117–123. [DOI] [PubMed] [Google Scholar]
- 29. Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing's syndrome in New Zealand. Clin Endocrinol (Oxf). 2011;75:436–442. [DOI] [PubMed] [Google Scholar]
- 30. Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E. Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: A prospective randomized study. Ann Surg. 2009;249:388–391. [DOI] [PubMed] [Google Scholar]
- 31. Tsuiki M, Tanabe A, Takagi S, Naruse M, Takano K. Cardiovascular risks and their long-term clinical outcome in patients with subclinical Cushing's syndrome. Endocr J. 2008;55:737–745. [DOI] [PubMed] [Google Scholar]
- 32. Sereg M, Szappanos A, Toke J, et al. Atherosclerotic risk factors and complications in patients with non-functioning adrenal adenomas treated with or without adrenalectomy: A long-term follow-up study. Eur J Endocrinol. 2009;160:647–655. [DOI] [PubMed] [Google Scholar]
- 33. Jokinen J, Nordström P. HPA axis hyperactivity and cardiovascular mortality in mood disorder inpatients. J Affect Disord. 2009;116:88–92. [DOI] [PubMed] [Google Scholar]
- 34. Dekker MJ, Koper JW, van Aken MO, et al. Salivary cortisol is related to atherosclerosis of carotid arteries. J Clin Endocrinol Metab. 2008;93:3741–3747. [DOI] [PubMed] [Google Scholar]
- 35. Vogelzangs N, Beekman AT, Milaneschi Y, Bandinelli S, Ferrucci L, Penninx BW. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J Clin Endocrinol Metab. 2010;95:4959–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alevizaki M, Cimponeriu A, Lekakis J, Papamichael C, Chrousos GP. High anticipatory stress plasma cortisol levels and sensitivity to glucocorticoids predict severity of coronary artery disease in subjects undergoing coronary angiography. Metabolism. 2007;56:222–226. [DOI] [PubMed] [Google Scholar]
- 37. Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol 2007;157:545–559. [DOI] [PubMed] [Google Scholar]
- 38. Neary NM, Booker OJ, Abel BS, et al. Hypercortisolism is associated with increased coronary arterial atherosclerosis: Analysis of noninvasive coronary angiography using multidetector computerized tomography. J Clin Endocrinol Metab. 2013;98:2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. del Rincón I, O'Leary DH, Haas RW, Escalante A. Effect of glucocorticoids on the arteries in rheumatoid arthritis. Arthritis Rheum. 2004;50:3813–3822. [DOI] [PubMed] [Google Scholar]
- 40. Mazzoccoli G, De Cata A, Greco A, et al. Aging related changes of circadian rhythmicity of cytotoxic lymphocyte subpopulations. J Circadian Rhythms. 2010;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–5595. [PubMed] [Google Scholar]
- 42. Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999;10:359–368. [DOI] [PubMed] [Google Scholar]
- 43. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. [DOI] [PubMed] [Google Scholar]
- 44. Saffar AS, Ashdown H, Gounni AS. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Curr Drug Targets. 2011;12:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sharma ST, Yanovski J, Abraham S, Nieman L. Utility of measurement of dexamethasone levels in diagnostic testing for Cushing's syndrome. Program of the 96th Annual Meeting of The Endocrine Society, Chicago, IL, 2014, (Abstract ORO2-1). [Google Scholar]