Abstract
Context:
Medullary thyroid cancer (MTC) is a rare form of thyroid cancer comprising approximately 4% of all thyroid cancers. The majority of patients have a relatively good prognosis; however, a subgroup of patients will require systemic therapy. Large phase III randomized trials led to the approval of two drugs—vandetanib and cabozantinib—for progressive or symptomatic MTC. The decision regarding which drug to initiate first is not entirely clear and is a common concern amongst treating physicians.
Evidence Acquisition and Synthesis:
A review of the literature in English was conducted, and data were summarized and integrated into a decision matrix.
Conclusions:
The decision regarding which drug to initiate first for progressive MTC should be based on a careful review of the medical history, physical examination findings, medication list, electrocardiogram, laboratory results, and tumor characteristics. It is necessary to consider the relative contraindications when choosing which drug to initiate first.
Clinical Case
A 61-year-old man with a history of coronary artery disease complicated with cardiac arrest presented with a right neck mass. Sonogram of the neck revealed a 1-cm right thyroid nodule and bulky lymphadenopathy. Fine-needle aspiration confirmed a medullary thyroid cancer (MTC). Germline RET testing was negative. Calcitonin was 930 pg/mL (normal, <5 pg/mL), and abdominal computed tomography scan was normal. The patient underwent a total thyroidectomy with lymph node dissection. Sonogram of the neck 6 months after surgery was normal and calcitonin was 247 pg/mL. One year later, the patient presented with recurrent right neck lymphadenopathy and multiple liver metastases. The patient complained of diarrhea (10–13 bowel movements daily). Calcitonin was 1835 pg/mL. The patient had hypokalemia, 3.3 mEq/L (normal range, 3.5–5.0), and hypomagnesemia, 1.5 mg/dL (normal range, 1.8–2.9). Electrocardiogram (EKG) was normal. The patient started potassium and magnesium supplementation. The patient was considered a candidate for systemic therapy due to progressive disease.
This article reviews the elements in a patient's disease and personal medical history that play a significant role in the decision regarding which agent to initiate. A personalized treatment strategy is required in patients with progressive MTC.
Background
MTC accounts for approximately 4% of thyroid cancers. It is derived from the neuroendocrine “C” cells. These tumors secrete calcitonin and carcinoembryonic antigen (CEA), which are sensitive biomarkers for the disease. Patients present with a thyroid nodule with or without cervical lymphadenopathy, and frequently with distant metastases to the liver, lungs, and/or bone. Diarrhea and/or flushing are present in approximately 30% of cases.
Most patients with MTC have a relatively good prognosis. Stage at diagnosis is highly predictive of overall survival. The 10-year survival rate is 96% among patients with localized disease (tumor confined to the thyroid gland), compared to 76% in patients with regional disease (extension beyond the thyroid directly into surrounding tissues or regional lymph nodes) (1). Distant metastases are evident at presentation in 7–23% of patients; the median overall survival of these patients is about 3 years (2). A substantial number of patients with distant metastases may have indolent disease that remains quiescent or slow growing over years of routine observation.
Postoperative calcitonin and CEA doubling times (DTs) are predictors of aggressive tumor behavior. Patients who have a calcitonin DT of more than 1 year have a 95% 10-year survival rate and a 73% 5-year recurrence-free survival. In contrast, patients whose calcitonin DT is less than or equal to 1 year have 10-year survival rates and 5-year recurrence-free survival rates of 18 and 20%, respectively (3). Calculation of DT is helpful and is recommended for identifying high-risk patients who should be monitored more frequently for tumor progression (4).
Initial Treatment and Follow-up
Currently, the only curative treatment for MTC is surgery. However, when cervical lymph node metastases are present at the time of initial surgery, the cure rate is low, and 90% of patients will demonstrate residual disease, either radiologically or biochemically (5). Patients who have persistent neck disease can be observed or managed with repeat surgery if progression is proven over time. Many patients with distant metastases have indolent disease that may not require systemic treatment for many years. Localized therapy with external beam radiation may be considered to palliate painful bone metastases or to prevent other skeletal-related events (eg, spinal cord compression, fracture). We and others have observed that tyrosine kinase inhibitors (TKIs) may offer limited efficacy in thyroid cancer patients with bony metastases (6, 7). Therefore, progressive or symptomatic bone disease treatments, such as radiation therapy and/or an antiresorptive (intravenous bisphosphonate or RANK-ligand inhibitor), need to be considered if feasible. Embolization or cryoablation of metastatic disease in the liver or bone may be beneficial in some cases as a means of decreasing tumor burden, alleviating pain, or treating refractory diarrhea (8).
Systemic Therapy
Only a select population of patients with metastatic MTC should be considered for systemic therapy. The criteria for initiating systemic therapy are clinically significant disease progression (9) (within 12–14 mo), symptomatic tumor burden that cannot be managed with localized therapy, or tumor involvement that threatens organ function or vital structures that cannot be managed with localized therapy (10). It should be emphasized that rising tumor markers alone are not sufficient evidence of progression to warrant initiation of systemic therapy. In patients with severe, intractable diarrhea due to MTC, systemic therapy may decrease tumor burden and improve symptoms. However, in some instances, these therapies also may worsen diarrhea. Therefore, a decision to start chemotherapy based solely on diarrhea should be made by physicians who are experienced in managing MTC-related diarrhea.
Until 2011, there were no effective agents approved for advanced MTC. Vandetanib and cabozantinib are antiangiogenic, multikinase inhibitors recently approved by the Food and Drug Administration (FDA) for symptomatic or progressive MTC. The choice of drug may be dictated by certain findings on the history, examination, laboratory, and EKG findings and the behavior of the tumor, which are explained further below. It should be pointed out that if a patient is not a good candidate for vandetanib or cabozantinib, a clinical trial or other commercially available TKIs may be considered (10).
Vandetanib
Vandetanib is an oral multikinase inhibitor of RET, VEGFR2 and -3, and EGFR, approved in 2011 for symptomatic and/or progressive MTC in patients with unresectable or metastatic disease. The recommended starting dosage is 300 mg by mouth once daily, and the drug can be taken with or without food.
Vandetanib's approval was based on a phase III, randomized, double-blinded, placebo-controlled trial that demonstrated a progression-free survival (PFS) advantage in MTC patients treated with vandetanib (PFS estimated at 30 months) when compared with placebo (PFS = 19 months; P < .0001) (11). The overall response rate to vandetanib was 44%. Progression before enrollment was not an entry criterion, which may have contributed to the long PFS found in the placebo group. Crossover from placebo to the treatment arm due to tumor progression was permitted.
Vandetanib can be prescribed only by physicians who have completed the Vandetanib Risk Evaluation and Mitigation Strategy (REMS) program because of a black box warning for prolongation of the QT interval, torsades de pointes, and sudden death in clinical trials. Routine EKGs, review of concomitant medications that may prolong the QT interval, and correction of electrolyte abnormalities (hypocalcemia, hypokalemia, hypomagnesemia) are required during treatment. Patients with a history of long QT syndrome, baseline rate-corrected QT (using the Fridericia formula; QTcF) of > 450 milliseconds, or uncorrectable electrolyte disturbances should not be treated with this drug.
Cabozantinib
Cabozantinib is a multikinase inhibitor of VEGFR2, RET, and c-MET, approved in 2012 for the treatment of patients with progressive, metastatic MTC. The recommended starting dosage is 140 mg by mouth once a day. Our recommendation is to start one (100 mg) or two (60 mg) dose levels lower than the recommended starting dose because the full dose is associated with a greater degree of toxicity and can be difficult to tolerate. The dose can then be titrated up sequentially to the full dose, if a response is not achieved and the patient tolerates a dosing level well. Conversely, cabozantinib can be decreased further to 40 mg if patients experience grade 2 or intolerable toxicities that cannot be managed medically. Our clinical experience has been that responses are seen even at dose levels below 40 mg. Patients should be instructed to take the drug on an empty stomach. The label carries a black box warning for perforations, fistulas, and hemorrhage; therefore, this drug should be used with caution or avoided in patients with a history of neck/mediastinal irradiation or injury to the gastrointestinal (GI) mucosa.
In contrast to clinical trials of vandetanib, trials evaluating cabozantinib in MTC required patients to demonstrate radiological progression before enrollment. The drug's approval was based on the phase III randomized, double-blind, placebo-controlled trial that demonstrated a PFS advantage among MTC patients treated with cabozantinib (PFS, 11.2 mo) when compared with placebo (PFS, 4 mo; P < .0001) (12). Overall response rate was 28%.
Effectiveness of vandetanib and cabozantinib and patient's mutational status
In the phase III vandetanib trial, patients with sporadic MTC harboring a somatic RET M918T mutation had a higher response rate to vandetanib (54.5%; 55 of 101) than patients without this mutation (32%; 33 of 103) (11). In the phase III cabozantinib trial, patients with somatic/germline RET mutations treated with cabozantinib exhibited longer PFS when compared with those treated with placebo (60 vs 20 wk; hazard ratio, 0.23; 95% confidence interval, 0.14, 0.38). PFS was longer in patients with somatic/germline M918T mutation than in patients with other RET mutations treated with cabozantinib (61 vs 36 wk). Of interest, patients with RAS mutations treated with cabozantinib exhibit longer PFS when compared with those treated with placebo (47 vs 8 wk; hazard ratio, 0.15; 95% confidence interval, 0.02, 1.10) (13). Although these results are suggestive, we cannot recommend the use of these drugs based on RET status. In fact, some patients without RET mutations have benefited from vandetanib and cabozantinib.
In vitro data showed that RET V804M and V804L mutations confer resistance to vandetanib (14). Although cabozantinib could be considered a better therapeutic option for these patients, clinical confirmation is required; thus, vandetanib could still be offered to patients with MTC with RET 804 mutations.
Adverse events (AEs) associated with vandetanib and cabozantinib
Although TKIs generally are better tolerated than cytotoxic chemotherapy, many patients develop side effects. These drugs are continued until the patient exhibits radiological progression or develops intolerable AEs associated with the drug. Tables 1 and 2 show the most common AEs associated with vandetanib and cabozantinib.
Table 1.
| Vandetanib | Cabozantinib |
|---|---|
| Diarrhea, 57% | Diarrhea, 63% |
| Rash, 53% | Stomatitis, 51% |
| Dermatitis acneiform/acne, 35% | Hand-foot skin reaction,50% |
| Nausea, 33% | Weight loss, 48% |
| Hypertension, 33% | Decreased appetite, 46% |
| Headache, 26% | Nausea, 43% |
| Fatigue, 24% | Fatigue, 41% |
| Decreased appetite, 21% | Dysgeusia, 34% |
| Abdominal pain, 21% | Hair color changes/graying, 34% |
| Dry skin, 15% | Hypertension, 33% |
| Vomiting, 15% | Constipation, 27% |
| QT prolongation, 14%a | Abdominal pain, 27% |
| Photosensitivity reaction, 13% | Vomiting, 24% |
| Dysphonia, 20% | |
| Pruritus, 11% | Headache, 18% |
| Dyspepsia, 11% | Dysphagia, 13% |
| Proteinuria, 10% | Dyspepsia, 11% |
| Depression, 10% |
Sixty-nine percent had QTc prolongation > 450 ms and 7% had QTc > 500 ms using the Fridericia correction.
Table 2.
Lab Abnormalities Associated with Vandetanib and Cabozantinib in Order of Frequency
| Vandetanib | Cabozantinib |
|---|---|
| Calcium decreased, 57% | AST increased, 86% |
| ALT increased, 51% | ALT increased, 86% |
| Glucose decreased, 24% | Alkaline phosphatase increased, 52% |
| Creatinine increased, 16% | Calcium decreased, 52% |
| Bilirubin increased, 13% | Phosphorus decreased, 28% |
| Magnesium decreased, 7% | Bilirubin increased, 25% |
| Potassium decreased, 6% | Magnesium decreased, 19% |
| Potassium increased, 6% | Potassium decreased, 18% |
| Neutropenia, 10% | Sodium decreased, 10% |
| Thrombocytopenia, 9% | Neutropenia, 35% |
| Thrombocytopenia, 35% | |
| TSH increased, 57% |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The most common AEs associated with vandetanib are diarrhea, rash, acne, fatigue, nausea, hypertension, headache, vomiting, QT prolongation, and photosensitivity. The most common laboratory abnormalities are hypocalcemia, liver enzyme elevations, hypoglycemia, hypothyroidism, and increase in creatinine. Rare but serious AEs include Stevens-Johnson syndrome, interstitial lung disease, ischemic cerebrovascular events, hemorrhage, heart failure, and reversible posterior leukoencephalopathy syndrome. Only 2.5% of patients treated with vandetanib on the phase III clinical trial were reported to have a fatal AE (15).
The most common AEs associated with cabozantinib are diarrhea, stomatitis, hand-foot skin reaction, weight loss, decreased appetite, nausea, fatigue, dysgeusia, graying of the hair, hypertension, constipation, abdominal pain, vomiting, dysphonia, and headaches. Common laboratory abnormalities include elevation of liver enzymes, hypocalcemia, hypophosphatemia, elevation in alkaline phosphatase, hyperbilirubinemia, neutropenia, thrombocytopenia, hypothyroidism, and low magnesium, potassium, and sodium. Rare but serious AEs include perforation and fistula, hemorrhage, thromboembolic events, poor wound healing, osteonecrosis of the jaw, and reversible posterior leukoencephalopathy syndrome. Six percent of patients treated with cabozantinib on the phase III clinical trial experienced a fatal adverse reaction (16). A complete list of AEs for both drugs can be found in the package inserts (15, 16).
Because neither drug is curative, long-term use is required and can lead to diminished quality of life. Aggressive, conscientious management of AEs is required to maintain patient compliance, optimize therapy, and avoid potentially life-threatening consequences.
Choice of TKI
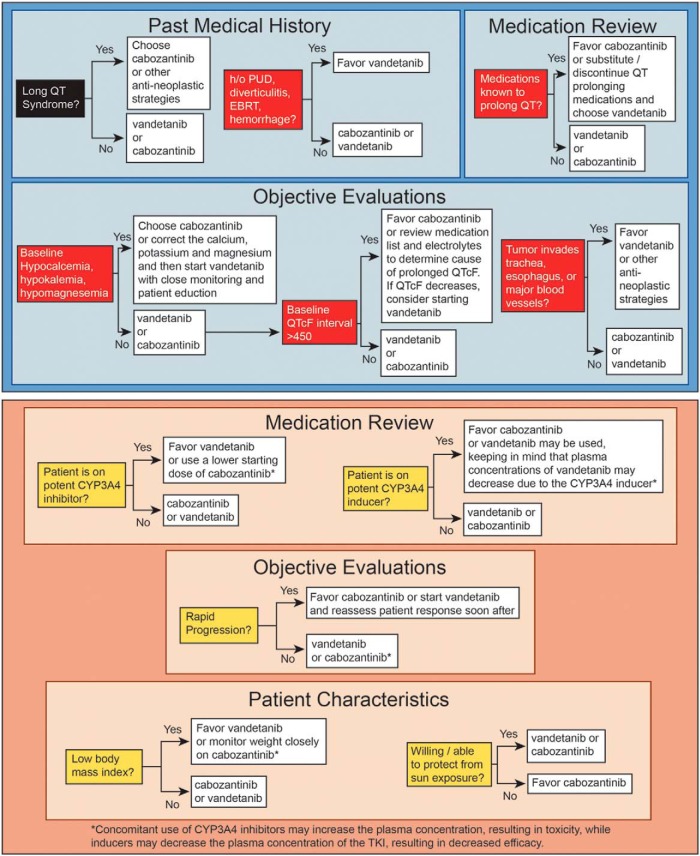
Oftentimes the choice of an approved agent to initiate is unclear. However, a patient-centered approach should be taken with careful consideration of the patient's medical history, physical examination, and baseline tests—with safety being the top priority. Figure 1 illustrates the primary and secondary considerations that should be taken into account before deciding which drug to start first.
Figure 1.
Top panel, Relative contraindications to consider when choosing vandetanib or cabozantinib for progressive or symptomatic MTC. A careful review of the medical history, patient medications, EKG, and tumor characteristics should be performed to determine which drug to start in patients who qualify for systemic therapy for MTC. Vandetanib should be avoided in patients who are at risk of prolonged QT interval as a result of personal cardiac history, difficulty in managing hypocalcemia, or use of certain medications that cannot be substituted. Cabozantinib should be avoided in patients with a history of peptic ulcer disease, diverticulitis, or a tumor invading the trachea, esophagus, or major vessels due to the risk of perforation or fistula. In these patients, vandetanib should be used with caution. Bottom panel, Factors that may be taken into consideration when a patient has no relative contraindications to either vandetanib or cabozantinib (see top panel). A careful review of the medication list, tumor characteristics, and patient characteristics is important when selecting which drug to initiate in patients with MTC, especially if the patient has no contraindications to either drug. Concomitant use of CYP3A4 inhibitor drugs may increase the plasma concentration of cabozantinib, whereas CYP3A4 inducers may decrease the plasma concentration of vandetanib. A list of CYP3A4 inhibitors and inducers can be found at www.medicine.iupui.edu/clinpharm/ddis/. Cabozantinib is the only drug proven to be effective in patients with progressive MTC; therefore, the rate of progression may be important when selecting which drug to initiate. Low body mass index and a patient's willingness to protect him- or herself from sun exposure are additional factors to be considered. Cabozantinib causes weight loss, whereas vandetanib may cause weight gain. Photosensitivity is an adverse effect of vandetanib. h/o, history of; PUD, peptic ulcer disease; QTcF, rate-corrected QT interval using the Fridericia formula; EBRT, external beam radiation therapy.
Vandetanib carries a black box warning because of QT interval prolongation, torsade de pointes, and sudden death, which have been observed in clinical trials. In the phase III vandetanib trial, QT prolongation was reported in 14% of patients randomized to vandetanib and in 1% of patients randomized to placebo. Vandetanib should not be given to patients with a history of torsades de pointes, congenital long QT syndrome, bradyarrhythmias, or uncompensated heart failure. Vandetanib should not be started in patients whose corrected QT interval (QTcF, Fridericia formula) is greater than 450 milliseconds and should be used with caution in patients taking antiarrhythmic drugs or drugs that cause QT prolongation. Patients should be made aware of all drugs that must be avoided while taking vandetanib.
One purpose of the REMS program for vandetanib is to ensure that patients are monitored for QT prolongation. EKGs should be obtained on patients at baseline, at 2–4 weeks, at 8–12 weeks, and then every 3 months during treatment with vandetanib. Electrolytes should be monitored closely because hypokalemia, hypocalcemia, and hypomagnesemia may cause QT prolongation. These electrolyte abnormalities are common in thyroid cancer patients who may have hypoparathyroidism as a complication of neck surgery, diarrhea attributable to MTC, and/or diarrhea due to drug toxicity. Drugs that prolong the QT interval should be avoided. These include commonly used drugs such as some antibiotics, antiemetics (ie, 5-HT3 antagonists and dopamine D2 antagonists), and antidepressants. A complete list can be found at www.crediblemeds.org. In the rare patients who develop ectopic Cushing's syndrome due to MTC, the use of vandetanib should be undertaken cautiously due to the associated electrolyte and cardiac disturbances. Consideration should be given to referring such a patient to a center with extensive experience in caring for these patients. Cushing's patients exhibit hypokalemia that places them at risk for a prolonged QT and torsades de pointes. A few reports have described successful control of ectopic Cushing's syndrome with vandetanib (17, 18); nevertheless, some patients may not respond to it (authors' experience), and it is potentially dangerous to give this drug to a patient at risk of hypokalemia. Because it is currently not possible to predict which MTC patients would respond to vandetanib, it is important to stabilize the Cushing's syndrome and/or correct it in a definitive manner (bilateral adrenalectomy) before initiating vandetanib treatment. If this is not possible, cabozantinib may be a better option.
Cabozantinib carries a black box warning for perforations, fistulas, and hemorrhage. The medical history should include questions regarding any history of hemoptysis, diverticulitis, chronic inflammatory GI disease (Crohn's disease or ulcerative colitis), active peptic ulcer disease, or radiation to the neck or mediastinum because these patients may have a higher risk of GI perforation, hemorrhage, and tracheoesophageal fistula.
Invasion of the tumor into the trachea, bronchus, or esophagus may increase the risk of fistula formation. Tumors that encase major vessels or invade the GI mucosa, cholecystitis, cholangitis, and appendicitis are associated with a high risk of bleeding or GI perforation. All patients should be aware of these potentially life-threatening risks. Consideration of vandetanib is appropriate among patients at high risk of developing these complications. Cabozantinib has no specific monitoring guidelines; however, guidance on how to monitor patients on TKIs can be found elsewhere (19).
If a patient has no contraindications for vandetanib and cabozantinib, other clinical factors may be considered (Figure 1, bottom panel). For example, patients with rapidly progressive disease may be considered for cabozantinib because the clinical trial that led to its FDA approval required progression, with one-third of enrolled patients having rapid progression (within 6 months before study entry) (12), whereas the vandetanib trial did not. Photosensitivity is a common side effect of vandetanib, and patients who are unable or unwilling to avoid unprotected sun exposure may have less risk of phototoxicity on cabozantinib. Hand-foot skin reaction is a common side effect of cabozantinib. For patients whose jobs mostly comprise the use of their hands (eg, musicians), vandetanib may be preferred. In addition, cabozantinib is associated with weight loss, whereas vandetanib is associated with weight gain (20). Therefore, with a patient who is losing weight as a result of cancer, vandetanib may be considered as a first-line agent over cabozantinib. A review of the medication list should be conducted to determine whether the patient is taking a CYP3A4 inhibitor or inducer. Concomitant use of CYP3A4 inhibitors may increase toxicity to cabozantinib by increasing plasma concentrations of the drug. CYP3A4 inducers can decrease the plasma concentration of vandetanib, making the drug less efficacious. A complete and frequently updated list can be found at www.medicine.iupui.edu/clinpharm/ddis/.
Clinical Case Continued
Because of the electrolyte disturbances and history of cardiac arrest in our patient, cabozantinib was chosen. After 2 months of therapy, diarrhea improved (two or three bowel movements daily), and a 28% tumor size reduction was noted. Calcitonin was decreased to 53 pg/mL. Due to fatigue, the cabozantinib dose was reduced from 140 to 100 mg/d. The authors wish to emphasize that the selection of cabozantinib was based on careful consideration of the patient's overall clinical picture. As described in this article, the final selection of the agent to initiate in patients with progressive MTC is a patient-centered approach that must weigh potential risks and benefits of the treatment selected.
Conclusions
In summary, a significant number of patients with residual or recurrent MTC have indolent disease, characterized by stable or slowly rising calcitonin and/or CEA levels, and can be actively surveyed for progression at appropriate intervals. Given the toxicity profile of currently available chemotherapeutic agents for MTC, the lack of curative intent, and the need for chronic use for disease control, systemic chemotherapeutic agents, such as vandetanib or cabozantinib, should not be initiated in patients with indolent MTC, regardless of the presence of metastatic disease. For patients who meet the indication for treatment (progressive or symptomatic MTC without viable surgical or targeted treatment options), it is extremely important for a clinician to recognize that a patient-centered approach should be taken concerning which drug to initiate, which includes a careful review of the patient's medical history, physical examination, concomitant medications, EKG and laboratory results, and tumor characteristics. Careful monitoring of patients on these drugs is important in order to keep patients safe, manage AEs efficiently and proactively, and determine when the drug is no longer effective.
Acknowledgments
We thank Kathryn Hale for her editorial services.
This work was supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672.
Disclosure Summary: M.E.C. has received research funding and consulting fees from Exelixis and consulting fees from Astra Zeneca. M.I.H. has received research support from AstraZeneca. C.J. has nothing to disclose.
Footnotes
- AE
- adverse event
- CEA
- carcinoembryonic antigen
- DT
- doubling time
- EKG
- electrocardiogram
- GI
- gastrointestinal
- MTC
- medullary thyroid cancer
- PFS
- progression-free survival
- QTcF
- corrected QT interval (Fridericia formula)
- TKI
- tyrosine kinase inhibitor.
References
- 1. Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134–2142. [DOI] [PubMed] [Google Scholar]
- 2. Boostrom SY, Grant CS, Thompson GB, et al. Need for a revised staging consensus in medullary thyroid carcinoma. Arch Surg. 2009;144:663–669. [DOI] [PubMed] [Google Scholar]
- 3. Meijer JA, le Cessie S, van den Hout WB, et al. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin Endocrinol (Oxf). 2010;72:534–542. [DOI] [PubMed] [Google Scholar]
- 4. Calcitonin and carcinoembryonic antigen (CEA) doubling time calculator. American Thyroid Association. http://www.thyroid.org/thyroid-physicians-professionals/calculators/thyroid-cancer-carcinoma Accessed August 11, 2014.
- 5. Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab. 2005;90:2029–2034. [DOI] [PubMed] [Google Scholar]
- 6. Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol. 2012;167:643–650. [DOI] [PubMed] [Google Scholar]
- 7. Cabanillas ME, Waguespack SG, Bronstein Y, et al. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab. 2010;95:2588–2595. [DOI] [PubMed] [Google Scholar]
- 8. Fromigué J, De Baere T, Baudin E, Dromain C, Leboulleux S, Schlumberger M. Chemoembolization for liver metastases from medullary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91:2496–2499. [DOI] [PubMed] [Google Scholar]
- 9. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 10. Tuttle RM, Haddad R, Ball DW, et al. NCCN clinical practice guidelines in oncology: thyroid carcinoma. J Natl Compr Canc Netw. 2014;1.2014. [Google Scholar]
- 11. Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sherman SI, Cohen EE, Schoffski P, et al. Efficacy of cabozantinib (Cabo) in medullary thyroid cancer (MTC) patients with RAS or RET mutations: Results from a phase III study. In: Proceedings from the American Society of Clinical Oncology; May 31–June 4, 2013; Chicago, IL Abstract 6000. [Google Scholar]
- 14. Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene. 2004;23:6056–6063. [DOI] [PubMed] [Google Scholar]
- 15. Caprelsa (vandetanib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2013. [Google Scholar]
- 16. Cometriq (cabozantinib) [package insert]. South San Francisco, CA: Exelixis Pharmaceuticals Inc; 2012. [Google Scholar]
- 17. Baudry C, Paepegaey AC, Groussin L. Reversal of Cushing's syndrome by vandetanib in medullary thyroid carcinoma. N Engl J Med. 2013;369:584–586. [DOI] [PubMed] [Google Scholar]
- 18. Nella AA, Lodish MB, Fox E, et al. Vandetanib successfully controls medullary thyroid cancer-related Cushing syndrome in an adolescent patient. J Clin Endocrinol Metab. 2014:99:3055–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carhill AA, Cabanillas ME, Jimenez C, et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: establishing a standard for patient safety and monitoring. J Clin Endocrinol Metab. 2013;98:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massicotte MH, Borget I, Broutin S, et al. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. J Clin Endocrinol Metab. 2013;98:2401–2408. [DOI] [PubMed] [Google Scholar]