Abstract
Context:
Obesity is associated with diminished GH secretion, which may result in the overdiagnosis of adult GH deficiency (GHD) in overweight/obese pituitary patients. However, there are no body mass index (BMI)-specific peak GH cutoffs for the glucagon stimulation test (GST), the favored dynamic test for assessing adult GHD in the United States.
Objective:
The objective of the study was to determine a peak GH cutoff level for the diagnosis of adult GHD in overweight/obese individuals using the GST.
Design:
This was a retrospective, cross-sectional study.
Setting:
The study was conducted at Massachusetts General Hospital and Oregon Health and Science University.
Methods:
A total of 108 subjects with a BMI ≥ 25 kg/m2 were studied: healthy controls (n = 47), subjects with total pituitary deficiency (TPD) (n = 20, ≥ 3 non-GH pituitary hormone deficiencies), and subjects with partial pituitary deficiency (PPD) (n = 41, 1–2 non-GH pituitary hormone deficiencies).
Intervention:
The intervention consisted of a standard 4-hour GST.
Main Outcome Measures:
The main outcome measure was peak GH level on GST.
Results:
Using the standard peak GH cutoff of 3 ng/mL, 95% of TPD cases (19 of 20), 80% of PPD (33 of 41), and 45% of controls (21 of 47) were classified as GHD. In receiver-operator characteristic curve analysis (controls vs TPD), a peak GH value of 0.94 ng/mL provided the greatest sensitivity (90%) and specificity (94%). Using a peak GH cutoff of 1 ng/mL, 6% of controls (3 of 47), 59% of PPDs (24 of 41), and 90% of TPDs (18 of 20) were classified as GHD. BMI (R = −0.35, P = .02) and visceral adipose tissue (R = −0.32, P = .03) negatively correlated with peak GH levels in controls.
Conclusion:
A large proportion of healthy overweight/obese individuals (45%) failed the GST using the standard 3 ng/mL GH cutoff. Overweight/obese pituitary patients are at risk of being misclassified as GHD using this cutoff level. A 1-ng/mL GH cutoff may reduce the overdiagnosis of adult GHD in overweight/obese patients.
As compared with other pituitary hormone deficits, the diagnosis of adult GH deficiency (GHD) tends to be more challenging because GH and IGF-1 levels do not strictly correlate (1, 2). In addition, there are other confounders of GH stimulation testing, including age and body mass index (BMI) (3). Studies have shown that in the presence of three or more other pituitary hormone deficiencies, an IGF-1 level of less than 84 ng/mL or a SD score of −3.0 indicates a 99% probability of GHD with no further testing needed (1). However, random IGF-1 levels can be normal in adults with GHD (4). Therefore, when these strict criteria are not met, the GH axis must be further investigated with stimulation testing to confirm the diagnosis of adult GHD.
The insulin tolerance test has historically been accepted as the gold standard test for diagnosis of adult GHD. Due to safety concerns, this test is used less frequently today (5). GHRH-arginine testing is a safe alternative to the insulin tolerance test and well validated for the diagnosis of adult GHD (2). However, the GHRH analog has not been available in the United States since 2008. Since then, the glucagon stimulation test (GST) has grown in popularity as a safe and widely available test of the GH axis (6). The standard GST peak GH cutoff of greater than 3 ng/mL used to distinguish GH deficient patients from those who have sufficient GH production was established by comparing controls with a lower BMI than their hypopituitary counterparts or by excluding obese subjects altogether (7, 8), despite the fact that obesity reduces endogenous GH secretion. Other studies examined the characteristics of the GST in nonhypopituitary controls who were almost exclusively in the normal weight range (9–13). Moreover, obesity is not only prevalent in the general population, but hypopituitarism itself is also complicated by an increased risk for the development of obesity (14).
The effect of obesity on GH levels has been identified as a critical confounder of the diagnosis of GHD in overweight and obese pituitary patients (15). Corneli et al (16) determined BMI-appropriate cutoffs for the GHRH-arginine stimulation test. The difference in these BMI-specific GH peak cutoffs for the GHRH-arginine test is notable, with the proposed cutoff for lean individuals (4.2 ng/mL) being nearly one-third of the cutoff for obese individuals (11.5 ng/mL) (16). These BMI-specific cutoffs for the GHRH-arginine test have been incorporated into formal guidelines for the assessment and treatment of GHD (3, 5). Toogood et al (17) demonstrated a negative relationship between BMI and peak GH by GST in patients with pituitary disease; however, the study did not include controls and was not designed to identify BMI-specific cutoff values for this test. Thus, there is no study to date that has established a BMI-specific peak GH cutoff value for the GST.
Therefore, we hypothesized that a higher BMI would correlate with lower peak GH on GST, leading to overdiagnosis of GHD in otherwise healthy, overweight/obese individuals using the traditional cutoff value of 3 ng/mL. We studied 108 overweight/obese men (BMI ≥ 25 kg/m2) to determine the optimal peak GH cutoffs to diagnose GHD in overweight/obese pituitary patients using the GST. We also sought to define body composition and metabolic variables that might be associated with peak GH levels on GST with the hypothesis that visceral adipose tissue (VAT) would be associated with lower peak GH (18, 19).
Materials and Methods
Subjects
The study was approved by the Partners HealthCare Inc. (Boston, Massachusetts) and Oregon Health and Science University (OHSU) Institutional Review Boards, and written informed consent was obtained from all control subjects. Subjects with pituitary disease who had previously undergone GST were studied retrospectively in a chart review after institutional review board approval.
Total pituitary deficiency (TPD) and partial pituitary deficiency (PPD) subjects
All patients who underwent glucagon stimulation testing at Massachusetts General Hospital (MGH) between September 2009 and August 2013 and OHSU between December 2009 and December 2012 were retrospectively reviewed. Three hundred forty-nine patients were identified (136 from MGH and 214 from OHSU) with mean BMI of 32 kg/m2 (29 kg/m2 at MGH and 33 kg/m2 at OHSU). Subjects included those who were male, had clear pituitary diagnoses, 1–4 non-GH pituitary deficits, and a BMI of 25 kg/m2 or greater. The following were excluded from analysis: women, patients with a BMI less than 25 kg/m2, individuals with traumatic brain injury, individuals without proven pituitary pathology, those with childhood-onset GHD, patients with proven hypothalamic or pituitary diagnoses but with zero pituitary deficiencies, and patients for whom we did not have sufficient data to confirm their diagnoses of hypopituitarism. The case subjects included patients with total hypopituitarism [defined as those with three to four non-GH pituitary hormone deficits (TPDs), n = 20] and patients with partial hypopituitarism [defined as those with one to two non-GH pituitary deficits (PPDs), n = 41]. This categorization was made based on adrenal, gonadal, thyroid, and vasopressin deficiencies, irrespective of peak GH on GST. Pituitary diagnoses, hormonal deficits, hormonal replacement, and prior treatment for pituitary disorders at the time of the GST were determined by chart review. The GST results in a subset of these patients were previously reported (20).
Control subjects
Control subjects were men aged 18–45 years with a BMI of 25 kg/m2 or greater, waist circumference greater than 102 cm (as a definition of central obesity) (21), and stable weight (≤5 lb deviation from baseline in prior 3 mo). Subjects with chronic illness, diabetes mellitus [fasting glucose ≥ 126 mg/dL or 2 h, 75 g oral glucose tolerance test (OGTT) glucose level > 250 mg/dL], smoking, or pituitary disorders were excluded. Because GH decreases with age, a control group of healthy young men, rather than an aged-matched population, was specifically chosen for analysis to avoid a bias toward a lower cutoff due to the effect of age itself; we reasoned that use of an older (age-matched) control group might result in a lower GH peak cutoff due to the effects of age itself, rather than BMI, and sought to determine a BMI-appropriate, age-independent cutoff. Additionally, age-matched cohorts were studied in subgroup analysis. Baseline characteristics (22–24) and peak GH levels (22, 23) in a subset of controls were previously reported.
Glucagon stimulation testing
GST was performed using an identical protocol in the controls and clinic populations. For pituitary patients, testing was performed in the context of routine clinical care. Intramuscular glucagon was administered at a dose of 1 mg (if weight < 90 kg) or 1.5 mg (if weight > 90 kg) (GlucaGen; Novo Nordisk). In both groups, fasting GH levels were measured at time zero and then every 30 minutes throughout the 4-hour GST for a total of nine samples. GH was measured using the Immulite assay (Siemens Medical), with an intraassay coefficient of variation below 7% at both sites. Both MGH and OHSU implemented a restandardization of the Immulite GH assay in 2011 from World Health Organization National Institute for Biological Standards and Control first IS 80/505 (through 2011) to World Health Organization National Institute for Biological Standards and Control second IS 98/574 (2011 to current). After this reagent change, both institutions reported a 20%–25% average decrease in GH levels as compared with the prior reagents. To standardize to the currently used assay and reagents, GH levels performed before this change were adjusted to match the currently used standard. The lower limit of detection was less than 0.05 ng/mL at MGH and ranged between less than 0.5 ng/mL to as low as less than 0.05 ng/mL at OHSU. GH level measurements from all 47 control subjects were run in batch at MGH with a lower limit of detection of less than 0.05 ng/mL. All values reported as less than assay were entered as 1 integer below the reported lower limit (ie, < 0.5 ng/mL as 0.4 ng/mL and < 0.05 ng/mL as 0.04 ng/mL) for the purpose of analysis.
Peak GH values on GST were reported below the lower limit of less than 0.5 ng/mL at OHSU in only two of the TPD cases. Secondary analysis excluding these two study subjects did not change the reported receiver-operator characteristic (ROC) results. Additionally, a secondary analysis using a lower limit of 0.5 ng/mL for all values did not reveal significant differences in analysis. Timing of the peak for the GST was determined by review of the GH stimulation tests. The peak was defined as the measurement time point with the highest GH level. These data were then combined into timing of the peak by test hour, including time zero, hour 1 (30 and 60 min), hour 2 (90 and 120 min), hour 3 (150 and 180 min), and hour 4 (210 and 240 min). Subjects with less than measurable GH at all time points were designated to have no peak on GST for analysis. Of note, three PPD individuals had GH measurements available for only 3 hours after glucagon administration and were excluded from the timing of peak analysis.
Assays
IGF-1 was measured using an Immulite 2000 Immunoassay System (Siemens Medical Systems) by a solid-phase enzyme-labeled chemiluminescent immunometric assay with a coefficient of variation below 5% at both sites. A standard 75-g OGTT was performed in all controls. In addition, controls underwent a measurement of high-sensitivity C-reactive protein by latex particle-enhanced immunoturbidimetric assay on a Hitachi 911 analyzer (Roche Diagnostics).
Computed tomography for body composition analysis
All control subjects underwent single-slice computed tomography of the abdomen at L4 for the assessment of abdominal fat. Single 1-cm axial images were obtained with 144 cm table height, 80 kV, 70 mA, 2-second scan time, and 48 cm field of view. VAT was quantified (square centimeters) as previously described (25).
Biometric measurements
The iliac waist to hip ratio was calculated in the control subjects using measurements taken in a horizontal plane and in accordance with the National Health and Nutrition Examination Survey procedures (26).
Statistical analysis
JMP Pro Statistical Database Software (version 11.0.0; SAS Institute) was used for statistical analyses. Results are expressed as mean ± SD unless otherwise noted. Variables were log transformed and the means compared with Fisher's least significance testing for three-way comparisons (27). ROC analysis was performed to calculate a diagnostic cutoff for peak GH level in response to the GST with controls as the negative condition (not having GHD) and patients with panhypopituitarism (TPD) as the positive condition (GHD). Area under the curve, sensitivity, and specificity of the new cutoff were reported in this analysis using Medcalc Software (version 13.0.0; Ostend). Univariate correlations were performed after log transformation of variables, and Pearson's correlation coefficients were reported. Additionally, multivariate regression models using the standard least square analysis after the log transformation of variables were constructed within the control group to identify determinants of GH peak.
Results
Baseline Characteristics
All baseline characteristics are reported in Table 1. Per the study design, the mean age of the controls was younger than that of the TPD group. Mean BMI was not significantly different between controls and PPD or TPD patients. Mean IGF-1 levels were higher in controls vs both the PPD and TPD groups. No other baseline variable differed significantly between the 2 patients groups.
Table 1.
Baseline Clinical Characteristics
| Variable | Controls (n = 47) | PPD (1–2) (n = 41) | TPD (3–4) (n = 20) |
|---|---|---|---|
| Age, y | 33.3 ± 7.0a | 45.4 ± 12.7 | 44.9 ± 13.9 |
| BMI, kg/m2 | 34.0 ± 3.4 | 32.6 ± 6.5 | 31.9 ± 6.9 |
| IGF-1, ng/mL | 169 ± 55a | 124 ± 68 | 112 ± 65 |
| Hormone deficits, n (%) | |||
| Hypoadrenal | 7 (17) | 17 (85) | |
| Hypothyroid | 23 (56) | 20 (100) | |
| Hypogonadal | 30 (73) | 20 (100) | |
| T use | 26 (63) | 17 (85) | |
| Diabetes insipidus | 2 (5) | 12 (60) | |
| DDAVP use | 1 (2) | 11 (55) | |
| Treatment history, n (%) | |||
| Medical treatment | 6 (14) | 5 (25) | |
| Surgery | 18 (44) | 12 (60) | |
| Radiation | 7 (17) | 6 (30) | |
Abbreviation: DDAVP, desmopressin acetate. Values are reported as mean ± SD.
Value of P < .001 for controls vs PPD and TPD.
Of the TPD patients (n = 20), nine had four hormonal deficits and 11 had three hormonal deficits. Diagnoses included pituitary adenoma (n = 9), craniopharyngioma (n = 5), germinoma (n = 2), and one each of lymphocytic hypophysitis, apoplexy, empty sella, and subarachnoid hemorrhage (SAH). Of the PPD patients (n = 41), 21 had two hormonal deficits and 20 had one hormonal deficit. Pituitary diagnoses in this group included pituitary adenoma (n = 27), arachnoid cyst (n = 3), empty sella (n = 3), apoplexy (n = 3), neurosarcoid (n = 2), and one each of astrocytoma, craniopharyngioma, and other. Hormonal deficits, hormone replacement, and pituitary treatment history for each group are detailed in Table 1.
GST results
GST data are presented in Table 2. The mean GH peak during GST was significantly higher in controls vs both patient groups (4.84 ± 5.04 ng/mL vs 1.94 ± 2.51 in PPD patients, P < .0001, and 0.57 ± 1.04 in TPD patients, P < .0001). GH was not normally distributed in the three groups; therefore, the median peak GH is also reported. Median peak GH was 3.54 ng/mL [95% confidence interval (CI) (0.59–17.24) ng/mL] in controls, 0.83 ng/mL (95% CI 0.04–8.46 ng/mL) in PPD patients, and 0.22 ng/mL (95% CI 0.03–4.20 ng/mL) in TPD patients.
Table 2.
Glucagon Stimulation Test Results
| Variable | Controls (n = 47) | PPD (1–2) (n = 41) | TPD (3–4) (n = 20) |
|---|---|---|---|
| GH time zero, ng/mL | 0.18 ± 0.16 | 0.39 ± 0.45 | 0.30 ± 0.55 |
| Mean GH peak, ng/mLa | 4.84 ± 5.04 | 1.94 ± 2.51 | 0.57 ± 1.04 |
| Median GH peak, ng/mLb | 3.54 (0.59–17.24) | 0.83 (0.04–8.46) | 0.22 (0.03–4.20) |
| Timing of GH Peak, n (%)c | |||
| No peak | 0 (0) | 3 (8) | 9 (45) |
| 0 min | 1 (2) | 1 (3) | 2 (10) |
| 30 min | 0 (0) | 1 (3) | 0 (0) |
| 60 min | 0 (0) | 3 (8) | 2 (10) |
| 90 min | 1 (2) | 0 (0) | 0 (0) |
| 120 min | 7 (15) | 5 (13) | 2 (10) |
| 150 min | 20 (43) | 12 (32) | 1 (5) |
| 180 min | 12 (26) | 6 (16) | 2 (10) |
| 210 min | 4 (9) | 6 (16) | 2 (10) |
| 240 min | 2 (4) | 1 (3) | 0 (0) |
P value for the difference in mean peak GH level was significant between all three groups (P < .0001).
Median reported with 95% CI.
n = 38 for PPD group in timing of peak analysis.
ROC curve analysis
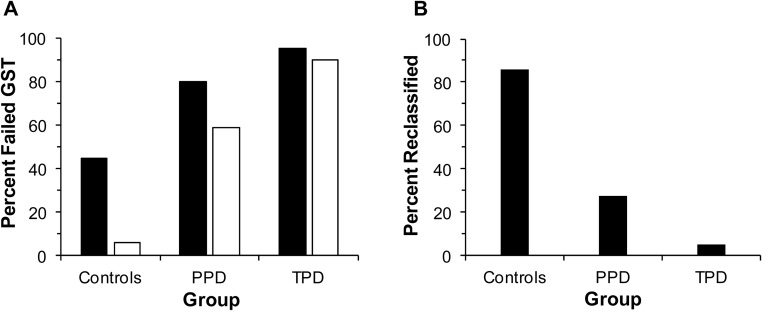
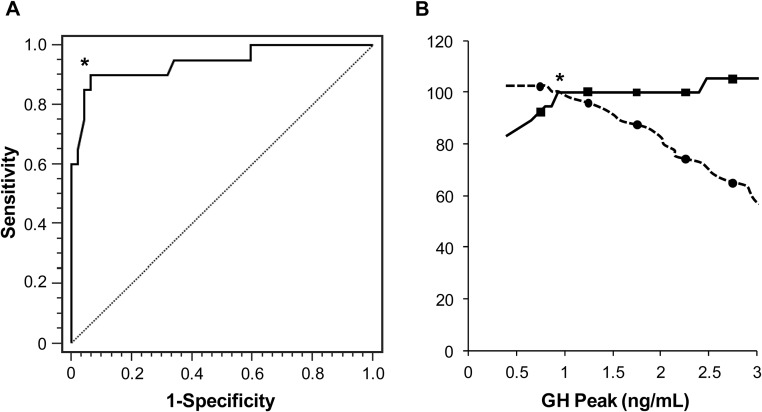
Using the standard peak GH cutoff of 3 ng/mL, 45% of controls (21 of 47), 80% of PPD patients (33 of 41), and 95% of TPD cases (19 of 20) would have been classified as GHD (Figure 1A). In the ROC curve analysis of controls vs TPD cases, a value of 0.94 ng/mL provided the greatest sensitivity (90%) and specificity (94%) for diagnosing GHD in overweight/obese men with an area under the curve (AUC) of 0.94, a value of P < .0001, and 95% CI of 0.87–0.98 (Figure 2). The traditional cutoff value of 3 ng/mL on this ROC curve resulted in a similar sensitivity of 95% but significantly reduced specificity of 53%.
Figure 1.
A, Percentage of subjects with failed GST using a peak GH cutoff of 3 ng/mL (■) vs percentage with failed GST using a peak GH cutoff of 1 ng/mL (□). B, Percentage of subjects per group diagnosed with GHD using the standard cutoff of 3 ng/mL that would be reclassified as GH sufficient using the cutoff of 1 ng/mL for glucagon stimulation testing.
Figure 2.
A, ROC curve of peak GH level on GST to detect GHD. The AUC is 0.93 (P < .0001). *, Designates point of optimized sensitivity (94%) and specificity (90%) on ROC curve corresponding to a cutoff of at 0.94 ng/mL for diagnosis of GHD. B, ROC curve assessment at GH cutoff of 0.94 ng/mL for diagnosis of GHD (designated by an asterisk). Solid line represents sensitivity and dotted line represents specificity.
Using a cutoff value of 1 ng/mL, 6% of controls (3 of 47), 59% of patients with PPD (24 of 41), and 90% of those with TPD (18 of 20) would have been classified as GHD (Figure 1A). Additionally, 86% of controls (18 of 21), 27% of PPD cases (9 of 33), and 5% of TPD cases (1 of 19) who initially failed using the standard cutoff of 3 ng/mL would be reclassified as GH sufficient using the cutoff of 1 ng/mL (Figure 1B).
When a subset of subjects (15 TPD and 47 controls) with comparable mean age and BMI was studied in a secondary analysis, the ROC derived cutoff remained at 0.94 ng/mL for a diagnosis of GHD, with a slightly reduced sensitivity of 87% and a preserved specificity of 94%.
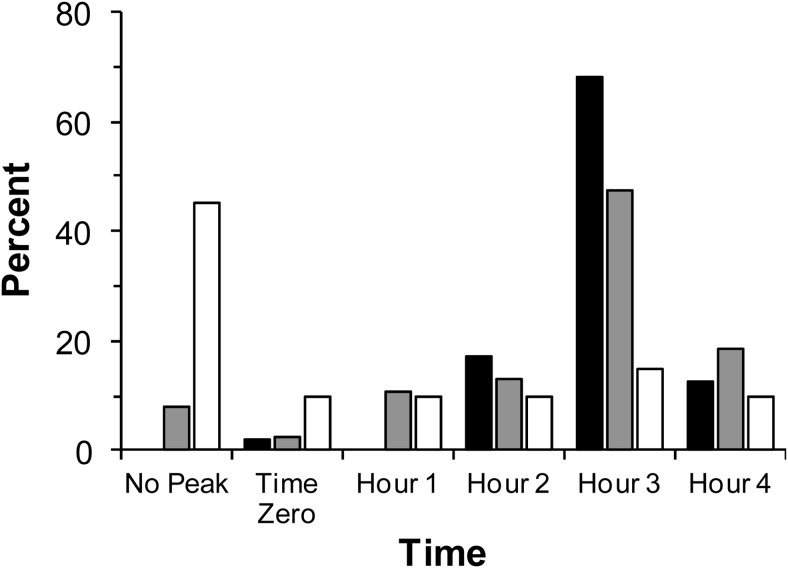
Timing of GH peak
The timing of the GH peak was examined in all 105 subjects (20 TPD, 38 PPD, and 47 controls) with 4-hour GSTs. As outlined in Table 2, 45%, 8%, and 0% of patients with TPD, PPD, and controls, respectively, had no peak after the GST. Most subjects who peaked did so within the first 3 hours of the GST as described in Figure 3. However, 14% of all subjects (15 of 105) and 16% of pituitary patients (9 of 58) had a peak GH level in the fourth hour of the test. Detailed analysis showed that no subjects would be reclassified as failing the GST using the cutoff of 1 ng/mL if GH levels from the first hour of testing (0, 30, and 60 min) were excluded. Additionally, no subjects would be reclassified if only the last half-hour of testing were omitted (240 min). However, three subjects who initially passed the GST would be reclassified as failing if the last full hour of testing (210 and 240 min) were omitted.
Figure 3.
Timing of peak in controls (black), patients with PPD (gray), and TPD (white) represented as a percentage of each group. Of note, no control subject had an absent GH peak.
GST side effects
There were no serious adverse events requiring medical intervention during any of the 108 GSTs. Detailed side effect data were available for 106 subjects. Nausea was the most frequent side effect, which occurred in 19% of all subjects (20 of 106). However, only two subjects vomited during testing. The remaining side effects included transient lightheadedness (n = 3), diaphoresis (n = 1), warmth (n = 1), hunger (n = 3), fatigue (n = 5), and alcoholic taste in mouth (n = 1). There were no instances of symptomatic hypoglycemia or hypotension.
GH peak correlations in control subjects
BMI, VAT, waist circumference, and hip circumference were negatively associated with peak GH levels in controls (Table 3). None of these correlations remained significant after controlling for BMI, with the exception of a trend toward significance of association with VAT (P = .06). In addition, there was a negative association between peak GH and OGTT AUC, which remained significant after controlling for BMI. Because age was not significantly correlated with GH peak, it was not controlled for in further analysis.
Table 3.
Correlation With Peak GH Level in Controls
| Variable | r | P Value |
|---|---|---|
| Age | −0.15 | .33 |
| IGF-1 | 0.27 | .06 |
| BMI | −0.35 | .02 |
| VAT | −0.32 | .03a |
| Iliac waist circumference | −0.31 | .03 |
| Hip circumference | −0.30 | .04 |
| Iliac waist to hip ratio | −0.16 | .30 |
| OGTT glucose AUC | −0.41 | .004b |
Trend (P < .1) after controlling for BMI.
Remained significant (P < .05) after controlling for BMI.
Discussion
Our study demonstrated that using the traditional peak GH cutoff value of 3 ng/mL on GST in otherwise healthy overweight and obese individuals leads to significant overdiagnosis of adult GHD. Moreover, our ROC analysis resulted in a cutoff of 0.94 ng/mL for maximum sensitivity and specificity for the diagnosis of GHD, whether cases were compared with young, healthy controls or a group with comparable mean age. We also showed that a cutoff of 1 ng/mL greatly increases the specificity with preserved sensitivity for the diagnosis of GHD in this population. Additionally, we sought to identify factors within the overweight and obese pituitary population who may be more likely to have physiologically reduced peak GH on stimulation testing. This is important because the overdiagnosis of GHD in pituitary patients may lead to an inappropriate use of GH, with unnecessary patient burden and health care costs related to injections and monitoring.
Obesity has been shown to be a state of relative GH deficiency (18, 19, 28–30). Physiological studies have demonstrated reduced GH half-life and fewer GH pulses, longer intervals between GH pulses, and one-quarter the GH production of normal-weight men. Free fatty acids have been implicated in the pathophysiology of this relative GH deficiency in obesity. Elegant studies using Acipimox to block free fatty acid production showed a return of GH response to stimulation testing in obese individuals (31, 32). Insulin resistance may also be a mechanism of reduced GH levels in obesity, although published data are somewhat contradictory (33, 34). Veldhuis and colleagues (29) also demonstrated that each BMI unit increase (in kilograms per square meter) was associated with a 6% decrement in the rate of daily GH secretion within each age tertile. They estimated an approximately 50% decrease in 24-hour GH secretion in an individual with a BMI of 28 kg/m2 vs 21 kg/m2 (29). These data suggest that BMI should be considered when testing pituitary patients for adult GHD in the clinical setting.
Prior studies have reported decreased peak GH levels on GHRH-arginine testing with increasing BMI, which has resulted in the establishment of BMI-specific cutoffs for this test in the diagnosis of GH deficiency in pituitary patients (2, 16, 35–37). Biller et al (2) noted a 1.4-ng/mL decrease in peak GH level for every 1 kg/m2 BMI in a control population with a mean BMI of approximately 30 kg/m2. Corneli et al (16) determined BMI-specific cut points for the GHRH-arginine test by ROC curve analysis of pituitary cases and controls. The resulting GH peak cutoffs were different between groups, with values of 11.5 ng/mL for lean subjects (BMI < 25 kg/m2, sensitivity 98.7%, specificity 83.7%), 8.0 ng/mL in the overweight group (BMI ≥ 25 kg/m2 and < 30 kg/m2, sensitivity 96.7%, specificity 75.5%), and 4.2 ng/mL in the obese cohort (BMI ≥ 30 kg/m2, sensitivity 93.5%, specificity 78.3%). However, GHRH is not currently available for diagnostic testing in the United States, leading clinicians to use alterative stimulation tests, most commonly the GST (6).
Most prior studies that examine the diagnostic use of the GST for adult GHD either omit BMI information or include only controls with normal BMI (2, 8, 10–13). A few studies have addressed the impact of increasing BMI on GST results, including Gomez et al (7), who reported a negative correlation between BMI and peak GH (r = −0.329, P = .0254) in control patients (mean BMI 25.4 ± 3.4 kg/m2) but not hypopituitary cases (mean BMI 29.4 ± 8.6 kg/m2). All 46 of the control subjects in that study had peak stimulated GH values above the GH peak cutoff of 3 ng/mL. However, because the average BMI of controls was just above normal, the study did not fully address the effects of obesity on peak GH levels. Thus, no studies to date have specifically examined populations of patients with pituitary disease and BMI of 25 kg/m2 or greater compared with a group of controls in this same BMI range. Importantly, BMI-specific cut points have not been established for the GST.
Recent US census data demonstrate that 69% of adults older than 20 years of age are overweight or obese (BMI ≥ 25 kg/m2) and 36% are obese (BMI ≥ 30 kg/m2) (38). Hormone deficiencies, glucocorticoid replacement, and hypothalamic damage may all be potential contributors to obesity in patients with pituitary conditions. In fact, we found that the mean BMI of 349 consecutive patients who underwent a GST at our two institutions was in the obese range at 32 kg/m2. Thus, the lack of BMI-specific cutoffs for the now widely used GST presents a significant clinical problem. Our study demonstrated that a reasonable peak GH cutoff for the GST in individuals with a BMI of 25 kg/m2 or greater is one-third of the current cutoff used in standard clinical care (1 ng/mL vs 3 ng/mL, respectively). Additionally, under the traditional criteria, nearly 45% of otherwise healthy, obese controls in this study would have been classified as having adult GHD by GST. However, using the proposed cutoff of 1 ng/mL, only 6% of these healthy, obese individuals met the diagnosis for GHD. We also demonstrated preserved sensitivity (90% vs 95%) and considerably improved specificity (94% vs 53%) using cutoffs of 1 ng/mL and 3 ng/mL, respectively.
In our study, VAT and the OGTT AUC for glucose predicted lower peak GH in overweight and obese but otherwise healthy subjects. After controlling for BMI, the OGTT glucose AUC retained significance, and the negative correlation between peak GH and VAT remained as a trend. It cannot be determined from this cross-sectional study whether relative GH deficiency is a contributor to body composition abnormalities and insulin resistance, or vice versa, in these overweight and obese subjects. However, these data suggest that obese, hypopituitary patients with visceral adiposity and insulin resistance may be at particularly high risk for the overdiagnosis of GHD. This is consistent with prior literature indicating that relative GHD in obesity is associated specifically with visceral adiposity (18, 19). Therefore, pituitary patients with visceral adiposity may be at risk of overdiagnosis of GHD using unadjusted GST cutoff values.
Another issue our study addresses is the timing of the peak GH response during glucagon stimulation testing. Although most individuals in this study peaked by the third hour of the GST, 14% of all subjects peaked in the fourth hour, including 13% of all controls, 18% of patients with PPD, and 10% of TPD cases. Previous studies of the GST have reported a later peak in weight-based dosing vs fixed dosing of glucagon (20). We observed peak GH levels in the fourth hour, despite the fact that a weight-based dosing regimen was used. Overall, these data would suggest that a 4-hour test would be appropriate to avoid overdiagnosis of GHD.
Limitations of our study include the relatively small sample size, which did not allow for stratification of BMI subgroups (overweight vs obese) in the analysis, and inclusion of male subjects only. However, it should be noted that prior studies using GH stimulation tests suggest that sex does not significantly impact peak stimulated GH cutoffs (2, 35), and sex-specific cutoff values are not currently used in clinical practice for the diagnosis of GHD (3, 5). Therefore, it might have been valid to include female patients with hypopituitarism in the analysis in comparison with the male-only control group to increase the number of subjects analyzed. Another limitation of the study was that our cutoff was developed using the Immulite 2000 GH assay (Siemens Medical). Although this is a commonly used assay, the results may not be generalizable to other assays due to interassay variation (39). Additionally, we were not able to assess the reproducibility of the GST because the test was performed only once per subject. Finally, we did not compare the GST results to a gold standard method of testing such as the insulin tolerance test.
In conclusion, our study suggests that there is likely overdiagnosis of GHD in overweight and obese individuals using currently established peak GH cutoff value of 3 ng/mL for the GST. Instead, a peak GH cutoff of 1 ng/mL allows for preserved sensitivity with much improved specificity for the diagnosis of GHD in overweight and obese pituitary patients. Larger studies are needed to confirm BMI-stratified peak GH cutoff levels to define adult GHD in this population. We also identified visceral adipose tissue mass and insulin resistance as factors that may identify overweight/obese pituitary patients at particular risk for a decreased GH response to the GST. This knowledge is critical in the clinical setting to prevent the overdiagnosis of GHD in overweight and obese patients while providing an accurate diagnosis in those who are truly deficient and might benefit from GH replacement therapy.
Acknowledgments
This study had the Clinical Trial registration number of NCT00131378.
This work was supported by National Institutes of Health Grants R01 HL077674, K24 HL092902, K23 RR023090, and T32 DK007028 as well as the Harvard Clinical and Translational Science Center Grant UL1 RR025758.
Disclosure Summary: K.C.J.Y. received research grants and served on the advisory boards for Pfizer and Novo Nordisk. B.M.K.B. is a consultant for Novo Nordisk and Pfizer. K.K.M. has received investigator-initiated research grants from Pfizer and Ipsen. The other authors have nothing to declare.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- CI
- confidence interval
- GHD
- GH deficiency
- GST
- glucagon stimulation test
- MGH
- Massachusetts General Hospital
- OGTT
- oral glucose tolerance test
- OHSU
- Oregon Health and Science University
- PPD
- partial pituitary deficiency
- ROC
- receiver-operator characteristic
- TPD
- total pituitary deficiency
- VAT
- visceral adipose tissue.
References
- 1. Hartman ML, Crowe BJ, Biller BM, Ho KK, Clemmons DR, Chipman JJ. Which patients do not require a GH stimulation test for the diagnosis of adult GH deficiency? J Clin Endocrinol Metab. 2002;87:477–485. [DOI] [PubMed] [Google Scholar]
- 2. Biller BM, Samuels MH, Zagar A, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2002;87:2067–2079. [DOI] [PubMed] [Google Scholar]
- 3. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609. [DOI] [PubMed] [Google Scholar]
- 4. Yuen KC, Cook DM, Sahasranam P, et al. Prevalence of GH and other anterior pituitary hormone deficiencies in adults with nonsecreting pituitary microadenomas and normal serum IGF-1 levels. Clin Endocrinol (Oxf). 2008;69:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700. [DOI] [PubMed] [Google Scholar]
- 6. Yuen KC, Biller BM, Molitch ME, Cook DM. Clinical review: is lack of recombinant growth hormone (GH)-releasing hormone in the United States a setback or time to consider glucagon testing for adult GH deficiency? J Clin Endocrinol Metab. 2009;94:2702–2707. [DOI] [PubMed] [Google Scholar]
- 7. Gomez JM, Espadero RM, Escobar-Jimenez F, et al. Growth hormone release after glucagon as a reliable test of growth hormone assessment in adults. Clin Endocrinol (Oxf). 2002;56:329–334. [DOI] [PubMed] [Google Scholar]
- 8. Conceicao FL, da Costa e Silva A, Leal Costa AJ, Vaisman M. Glucagon stimulation test for the diagnosis of GH deficiency in adults. J Endocrinol Invest. 2003;26:1065–1070. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell ML, Byrne MJ, Silver J. Growth-hormone release by glucagon. Lancet. 1969;1:289–290. [DOI] [PubMed] [Google Scholar]
- 10. Cain JP, Williams GH, Dluhy RG. Glucagon-initiated human growth hormone release: a comparative study. Can Med Assoc J. 1972;107:617–622. [PMC free article] [PubMed] [Google Scholar]
- 11. Lin T, Tucci JR. Provocative tests of growth-hormone release. A comparison of results with seven stimuli. Ann Intern Med. 1974;80:464–469. [DOI] [PubMed] [Google Scholar]
- 12. Aimaretti G, Baffoni C, DiVito L, et al. Comparisons among old and new provocative tests of GH secretion in 178 normal adults. Eur J Endocrinol. 2000;142:347–352. [DOI] [PubMed] [Google Scholar]
- 13. Rahim A, Toogood AA, Shalet SM. The assessment of growth hormone status in normal young adult males using a variety of provocative agents. Clin Endocrinol (Oxf). 1996;45:557–562. [DOI] [PubMed] [Google Scholar]
- 14. Verhelst J, Abs R. Cardiovascular risk factors in hypopituitary GH-deficient adults. Eur J Endocrinol. 2009;161(suppl 1):S41–S49. [DOI] [PubMed] [Google Scholar]
- 15. Copinschi G, Wegienka LC, Hane S, Forsham PH. Effect of arginine on serum levels of insulin and growth hormone in obese subjects. Metabolism. 1967;16:485–491. [DOI] [PubMed] [Google Scholar]
- 16. Corneli G, Di Somma C, Baldelli R, et al. The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur J Endocrinol. 2005;153:257–264. [DOI] [PubMed] [Google Scholar]
- 17. Toogood A, Brabant G, Maiter D, et al. Similar clinical features among patients with severe adult growth hormone deficiency diagnosed with insulin tolerance test or arginine or glucagon stimulation tests. Endocr Pract. 2012;18:325–334. [DOI] [PubMed] [Google Scholar]
- 18. Pijl H, Langendonk JG, Burggraaf J, et al. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab. 2001;86:5509–5515. [DOI] [PubMed] [Google Scholar]
- 19. Utz AL, Yamamoto A, Sluss P, Breu J, Miller KK. Androgens may mediate a relative preservation of IGF-I levels in overweight and obese women despite reduced growth hormone secretion. J Clin Endocrinol Metab. 2008;93:4033–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuen KC, Biller BM, Katznelson L, et al. Clinical characteristics, timing of peak responses and safety aspects of two dosing regimens of the glucagon stimulation test in evaluating growth hormone and cortisol secretion in adults. Pituitary. 2013;16:220–230. [DOI] [PubMed] [Google Scholar]
- 21. National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 22. Bredella MA, Gerweck AV, Lin E, et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98:3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bredella MA, Lin E, Gerweck AV, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97:4115–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bredella MA, Gill CM, Gerweck AV, et al. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology. 2013;269:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring, MD). 2011;19:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Protocol. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 27. Meier U. A note on the power of Fisher's least significant difference procedure. Pharm Stat. 2006;5:253–263. [DOI] [PubMed] [Google Scholar]
- 28. Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93:4254–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73:1081–1088. [DOI] [PubMed] [Google Scholar]
- 30. Beck P, Koumans JH, Winterling CA, Stein MF, Daughaday WH, Kipnis DM. Studies of insulin and growth hormone secretion in human obesity. J Lab Clin Med. 1964;64:654–667. [PubMed] [Google Scholar]
- 31. Cordido F, Peino R, Penalva A, Alvarez CV, Casanueva FF, Dieguez C. Impaired growth hormone secretion in obese subjects is partially reversed by acipimox-mediated plasma free fatty acid depression. J Clin Endocrinol Metab. 1996;81:914–918. [DOI] [PubMed] [Google Scholar]
- 32. Peino R, Cordido F, Penalva A, Alvarez CV, Dieguez C, Casanueva FF. Acipimox-mediated plasma free fatty acid depression per se stimulates growth hormone (GH) secretion in normal subjects and potentiates the response to other GH-releasing stimuli. J Clin Endocrinol Metab. 1996;81:909–913. [DOI] [PubMed] [Google Scholar]
- 33. Scacchi M, Pincelli AI, Cavagnini F. Growth hormone in obesity. Int J Obes Relat Metab Disord. 1999;23:260–271. [DOI] [PubMed] [Google Scholar]
- 34. Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147:2754–2763. [DOI] [PubMed] [Google Scholar]
- 35. Colao A, Di Somma C, Savastano S, et al. A reappraisal of diagnosing GH deficiency in adults: role of gender, age, waist circumference, and body mass index. J Clin Endocrinol Metab. 2009;94:4414–4422. [DOI] [PubMed] [Google Scholar]
- 36. Bonert VS, Elashoff JD, Barnett P, Melmed S. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. J Clin Endocrinol Metab. 2004;89:3397–3401. [DOI] [PubMed] [Google Scholar]
- 37. Markkanen HM, Pekkarinen T, Valimaki MJ, Alfthan H, Hamalainen E, Stenman UH. Comparison of two growth hormone stimulation tests and their cut-off limits in healthy adults at an outpatient clinic. Growth Horm IGF Res. 2013;23:165–169. [DOI] [PubMed] [Google Scholar]
- 38. National Center for Health Statistics. Health, United States, 2013: With Special Feature on Prescription Drugs. Hyattsville, MD: National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 39. Clemmons DR. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin Chem. 2011;57:555–559. [DOI] [PubMed] [Google Scholar]