Abstract
Non-targeted metabolite profiling can identify biological markers of dietary exposure that lead to a better understanding of interactions between diet and health. In this study, pigs were used as an animal model to discover changes in metabolic profiles between regular basal and high fat/high cholesterol diets. Extracts of plasma, fecal and urine samples from pigs fed high fat or basal regular diets for 11 weeks were analyzed using ultra-high performance liquid chromatography with high-resolution mass spectrometry (UHPLC-HRMS) and chemometric analysis. Cloud plots from XCMS online were used for class separation of the most discriminatory metabolites. The major metabolites contributing to the discrimination were identified as bile acids (BAs), lipid metabolites, fatty acids, amino acids and phosphatidic acid (PAs), phosphatidylglycerol (PGs), glycerophospholipids (PI), phosphatidylcholines (PCs) and tripeptides. These results suggest the developed approach can be used to identify biomarkers associated with specific feeding diets and possible metabolic disorders related to diet.
Keywords: metabolomics, diet pattern, ultra-high performance liquid chromatography, high-resolution mass spectrometry
1. Introduction
Nutritional metabolomics is a rapidly developing sub-branch of metabolomics, used to profile small-molecules to support integration of diet and nutrition in complex bio-systems research. (Jones, Park, & Ziegler, 2012) Recently, the concept of “food metabolome” was introduced and defined as all metabolites derived from food products (Fardet, Llorach, Orsoni, Martin, Pujos-Guillot, Lapierre, et al., 2008). Chemical components in foods are absorbed either directly or after digestion, undergo extensive metabolic modification in the gastrointestinal tract and liver and then appear in the urine and feces as final metabolic products (Primrose, Draper, Elsom, Kirkpatrick, Mathers, Seal, et al., 2011). It is well known that diet has a close relationship with the long-term health and well-being of individuals. Hence, investigation of the “food metabolome” in biological samples, after feeding specific diets, has the potential to give objective information about the short- and long-term dietary intake of individuals, and to identify potential biomarkers of certain dietary patterns (Primrose, et al., 2011). Previous studies have identified potential biomarkers after consumption of specific fruits (Carvalho, Franceschi, Feller, Palmieri, Wehrens, & Martens, 2013; Pujos-Guillot, Hubert, Martin, Lyan, Quintana, Claude, et al., 2013), vegetables (Bernal, Martin-Pozuelo, Lozano, Sevilla, Garcia-Alonso, Canovas, et al., 2013; Pujos-Guillot, et al., 2013), cocoa (Llorach, Urpi-Sarda, Jauregui, Monagas, & Andres-Lacueva, 2009; Moco, Martin, & Rezzi, 2012) and juices (Knab, Nieman, Gillitt, Shanely, Cialdella-Kam, Henson, et al., 2013; van Dorsten, Grun, van Velzen, Jacobs, Draijer, & van Duynhoven, 2010). More metabolites were revealed by using metabolomic approaches compared with the detection of pre-defined chemicals found in those foods.
Eating a high-fat and high cholesterol diet is strongly associated with conditions of obesity, diabetes and metabolic syndrome, that are increasingly recognized as worldwide health concerns (Li & Chiang, 2012). For example, a high fat diet is a major risk factor for childhood obesity (Johnson, Mander, Jones, Emmett, & Jebb, 2008), cardiovascular diseases (Burgueno, Gianotti, Mansilla, Pirola, & Sookoian, 2013) and hyperlipidemia (Aubin, Cardin, Comtois, Clement, Gosselin, Gillis, et al., 2010; Ma, Wang, Zhang, Lu, Wu, Yan, et al., 2012). Little is known on the extent to which changes in nutrient content of the human diet elicit changes in metabolic profiles. There are several reports of metabolomic profiling studies on plasma, serum, urine and liver from high fat-diet induced obese mice (Cheng, Benson, Grimsditch, Reid, Connor, & Griffin, 2010; H. J. Kim, Kim, Noh, Hur, Sung, Hwang, et al., 2011; Spagou, Theodoridis, Wilson, Raikos, Greaves, Edwards, et al., 2011), rats (S. H. Kim, Yang, Kim, Kim, Park, & Choi, 2009; Song, Wang, Wang, Tian, Yang, & Kong, 2013) and humans (Lehtonen, Lindstedt, Jarvinen, Sinkkonen, Graca, Viitanen, et al., 2013). Several potential biomarkers of obesity and related diseases, including lysophosphatidylcholines (lysoPCs), fatty acids and branched-amino acids (BCAAs) have been reported.
Levels of endogenous and exogenous metabolites in plasma, urine and tissues reflect different dietary treatments. These differences in metabolite patterns can provide insight into underlying molecular mechanisms related to diet (Gu, Chen, Pan, Jackson, Talaty, Xi, et al., 2007; Jones, Park, & Ziegler, 2012). Experimental platforms that are sensitive to both individual and environmental variations in diet are needed to produce reliable and robust data. Pigs have been well-recognized in biomedical research for physiological and anatomical similarities to humans (Leucht, Leuthold, & Stier, 1977; Simon & Maibach, 2000). To model the metabolite response to diet in humans, pigs were fed a high fat diet for 11 weeks and the metabolite profiles in plasma, urine and feces were analyzed. Non-targeted ultra high performance liquid chromatography tandem with high resolution mass spectrometry (UHPLC-MS) was utilized for metabolomic profiling. Bile acids (BAs), lipid metabolites, fatty acids, amino acids and phosphatidic acid (PAs), phosphatidylglycerol(PGs), glycerophospholipids(PI), phosphatidylcholines (PCs), tripeptides and isoflavone conjugates were found to be the final dietary metabolites that differentiated pigs fed a high-fat and high cholesterol diet versus a basal diet. The results of this study illustrate the capacity of this metabolomic profiling approach to identify new metabolites and to recognize different metabolic patterns associated with diet.
2. Materials and methods
2.1. Animals and sampling
All animal experiments and procedures were conducted in accordance with guidelines established and approved by the Beltsville Area Animal Care and Use Committee. Eleven 4-week-old female pigs were obtained from the experimental farm at the Beltsville Agricultural Research Center, Beltsville, MD. Pigs were derived from boars from a four-way crossbred composite BX line (Duroc X maternal Landrace X terminal Landrace X Yorkshire) designed by scientists at the USDA/ARS/US Meat Animal Research Center, Clay Center, NE to be genetically similar to genetics in the commercial swine industry at the time they were born; the genetics of the gilts are predominantly of the BX composite line. Pigs were from a herd screened yearly for porcine reproductive and respiratory syndrome virus (PRRSV), influenza (H1N1 and H3N2), pseudorabies and brucellosis by the Veterinary Services Group at the Beltsville Agricultural Research Center and have been negative for these infections. They were individually housed in stalls with a non-absorptive concrete floor with ad libitum access to water. Pigs were weighed and randomized into two groups of eleven, and seventeen pigs fed either A) a basal regular diet containing 21% of total kcal from protein, 68% from carbohydrates, and 11% from fat; or B) a high fat/high cholesterol diet containing 16% of total kcal from protein, 40.0% from carbohydrates, and 44% from fat including 2% cholesterol (Table S1). Corn starch was used as a carbohydrate source, and soy protein, L-threonine, tryptophan and methionine were used as protein sources in all diets. Soybean oil was used for diet A and soybean oil, coconut oil, lard and cholesterol were used for diet B. Pigs received a fixed amount (0.6 kg) of feed. This amount was increased gradually up to 1.2 kg to maintain linear growth. Pigs on all diets received an equivalent volume of feed for the 11 weeks of the study. Pigs consumed all feed each day with no appreciable waste.
The four-week old pigs were weighed at 0, 3, 6, 9 and 11 wks of the study. Fecal samples were collected each morning after release by the pigs at weeks 0, 2, 4 and 6 and from the distal colon and proximal colon at necropsy on week 11. Blood samples were drawn from pigs fasted for 24 hours into serum separation tubes or into EDTA containing tubes for the collection of plasma on week 11. Urine was collected aseptically at necropsy from the bladder using a 50 ml syringe and 18 gauge needle. Fecal, plasma, serum and urine samples were stored at −80 °C until analysis. Serum tryglicerydes (TG) and cholesterol (CHOL) levels were analyzed using a commercial clinical diagnostic laboratory service under GLP standards (Antech Diagnostics GLP, Morrisville,NC).
2.2. Sample preparation
Frozen aliquots of urine were thawed, centrifuged at 7,000 g for 10 min and then filtered with a 0.20 µm PVDF filter. Two microliters were injected into UHPLC-MS for analysis.
Plasma samples were prepared for UPLC-MS analysis by methanol protein precipitation. Cold methanol (150 µl) was added to 50 µl of plasma, vortexed for 30 s, incubated at −20 °C for 20 min, centrifuged at 14 000 rpm for 10 min, and the supernatant transferred to a HPLC vial. Two microliters were injected into UHPLC-MS for analysis.
Fecal and intestinal content samples and were lyophilized and then 1 g of each sample was ground and extracted in 3 fold of methanol. The mixture was vortexed strongly and subsequently centrifuged at 14 000 rpm at 4 °C for 10 min. Two microliters were injected into UHPLC-MS and analyzed.
2.3. Materials and chemicals
Cholic acid, lithocholic acid, deoxycholic acid, chenodeoxycholic acid, hyodeoxycholic acid, ursodeoxycholic acid and sodium glycochenodeoxycholate were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Standards were dissolved in a water/methanol solution (50/50 v/v) to obtain solutions of 50 µg·ml−1 before LC-MS analysis. Formic acid, HPLC grade methanol and acetonitrile, were purchased from VWR International, Inc. (Clarksburg, MD). HPLC grade water was prepared from distilled water using a Milli-Q system (Millipore Lab., Bedford, MA).
2.4. UHPLC-PDA-ESI/HRMS/MSn conditions
The UHPLC-HRMS system consisted of an LTQ Orbitrap XL mass spectrometer with an Accela 1250 binary Pump, a PAL HTC Accela TMO autosampler, a PDA detector (ThermoFisher Scientific, San Jose, CA), and a G1316A column compartment (Agilent, Palo Alto, CA). Separation was carried out on a Hypersil Gold AQ RP- C18 UHPLC column (200 mm×2.1 mm i.d., 1.9 µm, ThermoFisher Scientific) with an UltraShield pre-column filter (Analytical Scientific Instruments, Richmond, CA) at a flow rate of 0.4 ml/min. The mobile phase consisted of a combination of A (0.1% formic acid in water, v/v) and B (0.1% formic acid in acetonitrile, v/v). The linear gradient was from 2% to 20% B (v/v) at 10 min, to 50% B at 25 min and to 98% B at 30 min, and held at 98% B to 32 min. Both positive and negative ionization modes were used and the conditions were set as follows: sheath gas at 80 (arbitrary units), aux and sweep gas at 15 (arbitrary units), spray voltage at 4.5 kV for positive mode and 4.0 kV for negative mode, capillary temp at 250 °C, capillary voltage at 15 V and tube lens at 70 V. The mass range was from 100 to 2000 m/z with a resolution of 15,000, FTMS AGC target at 2e5, FT- MS/MS AGC target at 1e5, isolation width of 1.5 amu, and max ion injection time of 500 ms. The most intense ion was selected for the data-dependent scan to offer their MS2 to M S4 product ions, respectively, with the a normalization collision energy at 35%.
2.5. Data analysis
2.5.1. LC−MS data pre-treatment and handling
Acquired UHPLC-HRMS raw files were converted into mzXML format using Proteowizard 3.0. 3569 (http://proteowizard.sourceforge.net/) and then XCMS (Tautenhahn, Patti, Rinehart, & Siuzdak, 2012) was employed for peak detection, noise filtering, and peak alignment. A 2D data matrix was generated from XCMS including variable index (paired m/z-retention time), sample names (observations) and peak intensities. Multivariate analysis was performed on the output table from XCMS using Sigma 13.0 (Umetrics, Umeå, Sweden). Principal component analysis (PCA) was performed to visualize group clustering, trends, or outliers among the observations. Pareto scaling was used for data normalization before multivariate data analysis (MVDA). PC1 and PC2 scores were used for the PCA models as these principal components seemed to reflect the main variation and separation in data. The score plot of PCA was used to observe clustering and trends among all samples. The loadings plot was applied to evaluate the variables causing separation between the groups. R2X represents the cumulative modeled variation in X, where the value approaching 1.0 suggest the model is stable and reliable. Q2 is used to estimate the predictive ability of the model. Supervised partial least-squares-discriminant analysis (PLS-DA) models were carefully validated by an iterative seven-round cross-validation with 1/7 of the samples being excluded from the mode in each round and 100 random permutations testing. The structure of the potential biomarkers was annotated by searching the free databases of HMDB (http://www.hmdb.ca/) and Scripps Metlin (http://metlin.scripps.edu/) using exact mass and MS/MS spectra and structures. Available standards were used for the confirmation and identity of the compounds.
3. Results
3.1. Animal response to diet
Body weight, cholesterol and triglycerides were measured for all the pigs studied (Table S2). There was no significant body weight gain between pigs fed diet A and diet B after 11 weeks of treatment. The serum cholesterol and triglyceride levels were significantly higher in pigs fed with diet B compared with the control group at the end of experiment.
3.2. Metabolomics analysis
Plasma, urine and fecal samples were analyzed in both positive and negative ionization mode. To obtain reliable and high-quality metabolomic data, a pooled sample was used as a quality control (QC) sample to monitor the run (Gika, Theodoridis, Wingate, & Wilson, 2007). The QC sample (a composite of equal volume from 10 real samples) was processed as real samples and placed in the sample queue to monitor the stability of the system. All the samples were submitted in random for analysis. The intensity variances of the major ion features in QC samples from plasma, fecal and urine samples are shown in Table S3. The variation of these ion features across the QC samples was less than 15%. The ion features from each possible metabolite were annotated by XCMS online to confirm the possible fragment ions, isotopic ions and possible adduct ions. The reproducibility of the chromatography was determined by the retention time variation profiles that were generated by XCMS. The retention time deviation was less than 0.3 min for plasma samples, less than 0.3 min for fecal samples, and less than 0.2 min for urine samples, respectively. On the basis of these results of data quality assessment, the differences between the test samples from different pigs proved more likely to reflect varied metabolite profiles rather than analytical variation. The multivariate analysis results from the QC sample showed the deviation of the analytical system was acceptable and the differences in metabolite profiles between the groups were due to different treatments. PCA was firstly employed to visualize the sample clustering, trends, and outliers among the observations. The interactive cloud plot integrated in XCMS online was used for exploring the metabolites of potential biochemical interest from the complex dataset (Patti, Tautenhahn, Rinehart, Cho, Shriver, Manchester, et al., 2013). Data characteristics such as p-value, fold change, retention time, mass-to-charge ratio, and signal intensity of features can be evaluated for interactions. The utility of the cloud plot for interpreting untargeted metabolomic results is demonstrated in this study.
3.3. Plasma sample
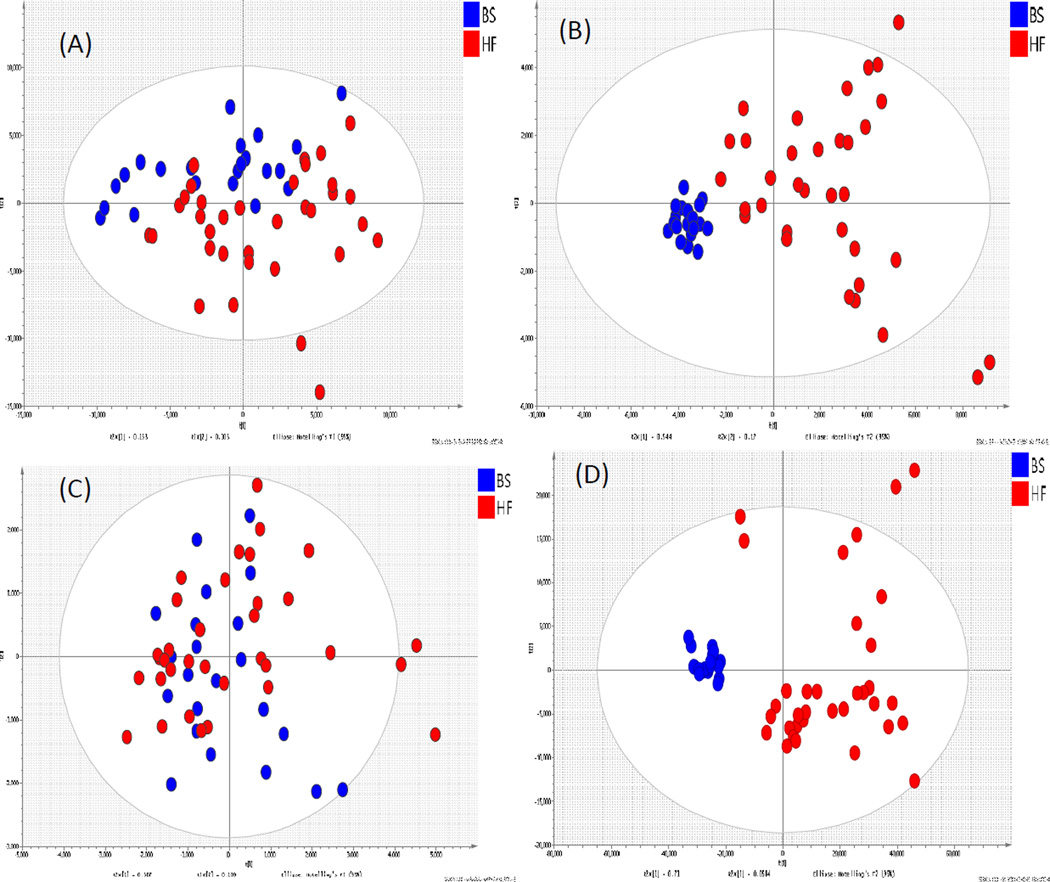
After data-preprocessing using XCMS, the data sets contained 643 metabolite ion features in positive and 357 ion features in negative ESI mode. The preprocessed data were further investigated using PCA and subdivided by diet. PCA reflects the main variation in the data. No discrimination between high fat fed and regular fed pigs could be observed in both the positive and negative ESI mode when all ion features were evaluated (Figure 1A,C), and the quality of prediction from the PCA models based on PC1 and PC2 scores were found to be 0.06 and 0.45 in positive and negative ionization mode, respectively. A further PCA analysis was carried out using selected ion features with p < 0.05 and fold change > 2. Good separation can be observed between pigs on the two diets, which is also reflected in the goodness of prediction (Q2), of 0.64 (Figure 1B) using data from the positive ionization mode. For negative ionization mode data, better separation appears with a Q2of 0.73 (Figure 1D).
Figure 1.
The PCA score plot of Plasma (A)(+)ESI data with all the ion features; (B) (−)ESI data with selected ion features; (C) (−)ESI data with all ion features; (D) (−)ESI data with selected ion features .Samples were taken from pigs fed diet A (BS, blue) and diet B (HF, red)
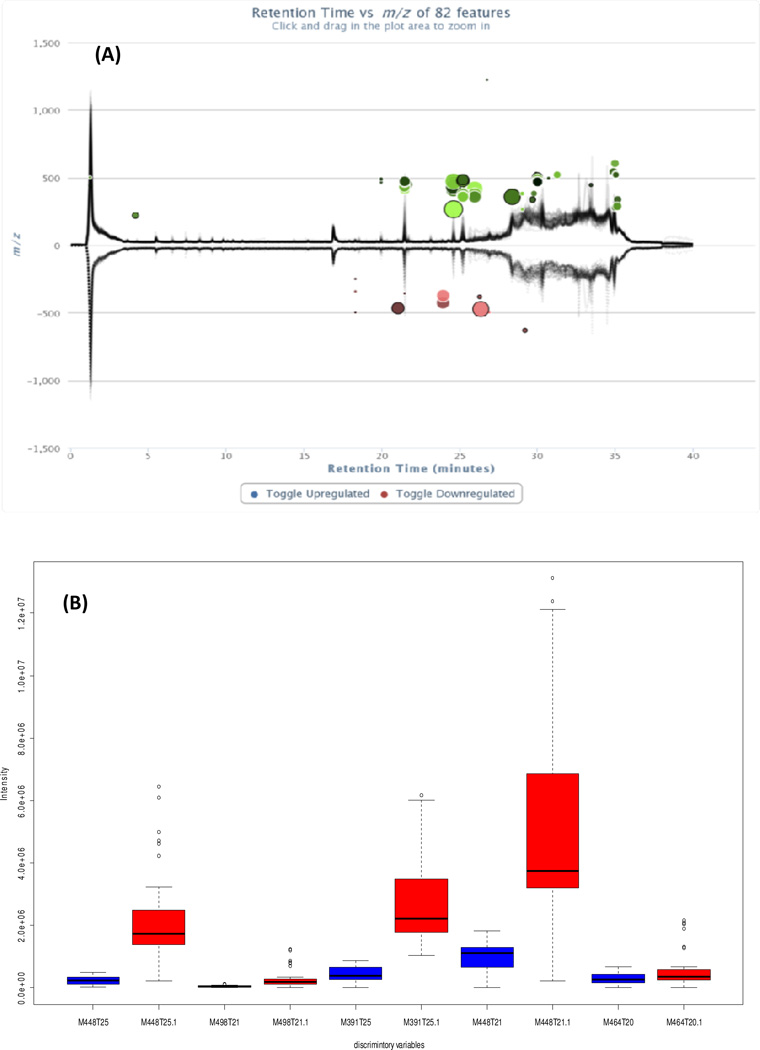
Cloud plot is a new multidimensional data visualization method for global metabolomic data (Patti, et al., 2013). Data characteristics, such as the p-value, fold change, retention time, mass-to-charge ratio and signal intensity of features, can be presented simultaneously using the cloud plot. In this study, the cloud plot was used to illustrate the ion features causing the group separation. In Figure 2, 82 features with p < 0.05 and fold change> 2, including visualization of the p-value, the directional fold change, the retention time and the mass to charge ratio of features, are shown. Also, the total ion chromatograms for each sample were shown. The upper panel in (2A) shows the chromatograms of plasma samples from pigs fed the high fat diet, while the lower panel shows the chromatograms of samples from pigs fed the regular diet. Features whose intensity is increased are shown in green, whereas features whose intensity is decreased are shown in pink (2A). The size of each bubble corresponds to the log fold change of the feature: the larger the bubble, the larger the fold changes. The statistical significance of the fold change, as calculated by a Welch t-test with unequal variances, is represented by the intensity of the feature’s colour where features with low p-values are brighter compared to features with high p-values. The Y coordinate for each feature corresponds to the mass-to-charge ratio of the compound, as determined by mass spectrometry. Each feature is also colour coded, such as features that are shown with a black outline have database hits in METLIN, whereas features shown without a black outline do not have any database hits.
Figure 2.
(A) Cloud plot showing 82 discriminatory ion features (negative ion data) in plasma, and (B) box-plot of data set of the five most abundant bile acids identified in plasma (negative ion data) samples.
From the cloud plot (Figure 2A), 82 discriminating ion features from positive data and 48 discriminating ions features from negative data were considered as of great importance for class separation. After filtering out the fragment ions, isotope annotations, and adduct ions, thirty one metabolites were tentatively assigned using a Metlin library search (Table S4).
Among the assigned metabolites detected, five of the highest abundant metabolites were identified as bile acid and bile acid conjugates (Figure 2B). This series of compounds shared the following characteristics; the unconjugated bile acids showed [M-H]− ion as base peak in the negative mode. For example, the ion feature m/z 391.2854 with a retention time of 25.3 min, gave the formula as C24H39O4 ([M-H]−, 0.12 ppm) while the ion at m/z 375.2779 [M+H-2H2O]+ peaks were observed as the base peak. This characteristic was consistent with bile acid hyodeoxycholic acid (HDCA) and was confirmed with a reference standard. For the conjugated bile acids (usually with glycine and taurine), the [M-H]− and [M+H]+ are always observed as the base peaks. For example, the ion feature m/z 448.3065 at 21.18 min was identified as chenodeoxycholic acid glycine conjugate. The neutral loss of 62 amu (H2O+CO2) was considered as a characteristic fragmentation pathway for bile acid glycine conjugates. This above mentioned characteristic can easily identify a series of bile acids compounds. The five metabolite ions detected in plasma were significantly different between pigs fed the high fat diet (Figure 2B, red bars) and regular diet (Figure 2B, blue bars) for 11 weeks, and were identified as chenodeoxycholic acid glycine conjugate, tauroursodeoxycholic acid, hyodeoxycholic acid, deoxycholic acid glycine conjugate and glycocholicacid; chenodeoxycholic acid glycine and hyodeoxycholic acid. These were confirmed with reference standards for their identical mass fragmentation and retention behaviour (a 5.9 to 11.2 times change were observed for these bile acids).
3.4. Fecal samples
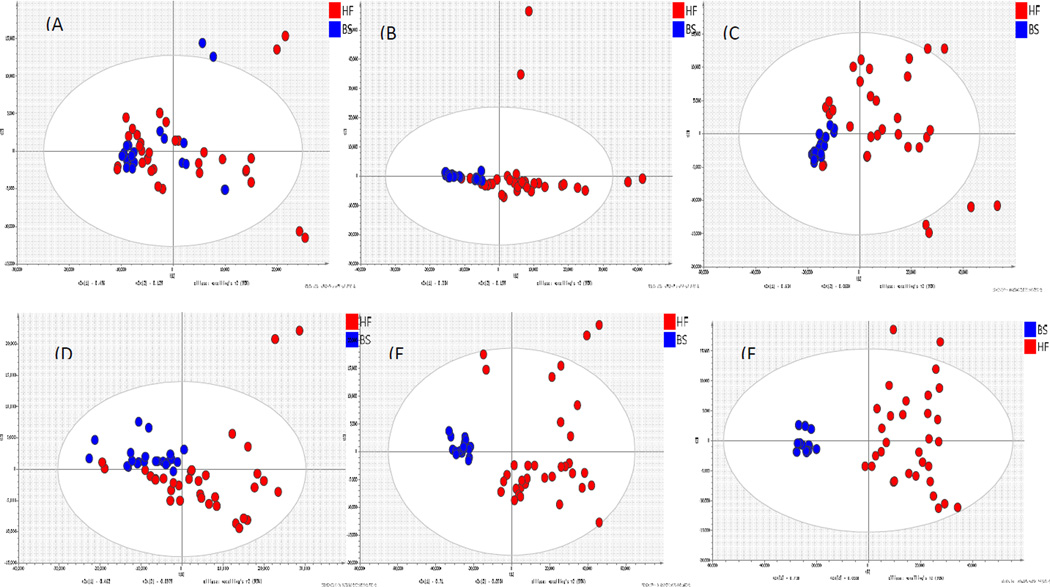
A series of PCA score plots were used to characterize changes in metabolites in pigs fed the different diets. Little separation between the two groups was observed in the PCA score plot in the first week. On week 2, some separation between the groups was observed. From week 4 to week 6, the metabolic profiling in high fat fed pigs had changed considerably, which led to further separation between the groups at the end of the study (week 11). Notably, data from the basal regular fed pigs clustered tighter than from pigs fed the high fat diet. This trend suggests that potential biomarkers of a high fat diet would be dependent on the time scale of feeding (Figure 3).
Figure 3.
PCA score plot of fecal samples from pigs fed diet A (BS, blue) and diet B (HF, red) (A) Week 0, (B) Week 2, (C) Week 4 (D) Week 6, (E) Week 11 for distal samples(F) Week 11 for proximal colon samples
The high intensive discriminating metabolites were mostly identified as bile acids, fatty acids and lipids (Table S5). The bile acids were significantly increased by feeding the high-fat diet. Other metabolites, such as phosphatidic acid (PAs), phosphatidylglycerol (PGs), glycerophospholipids (PIs) and some tripeptides, which indicates high relevance to the difference between sample groups, are major fecal metabolites that contribute to the discrimination between pigs fed the regular and high-fat diets. Bile acids are closely related with the metabolism of cholesterol; however, cholesterol was not significantly different between the two groups. Bile acids and lipids excretion was appreciably higher in the feces of pigs fed the high fat diet. Among all metabolites, hyodeoxycholic acid and deoxycholic acid were confirmed with reference compounds.
3.5. Urine samples
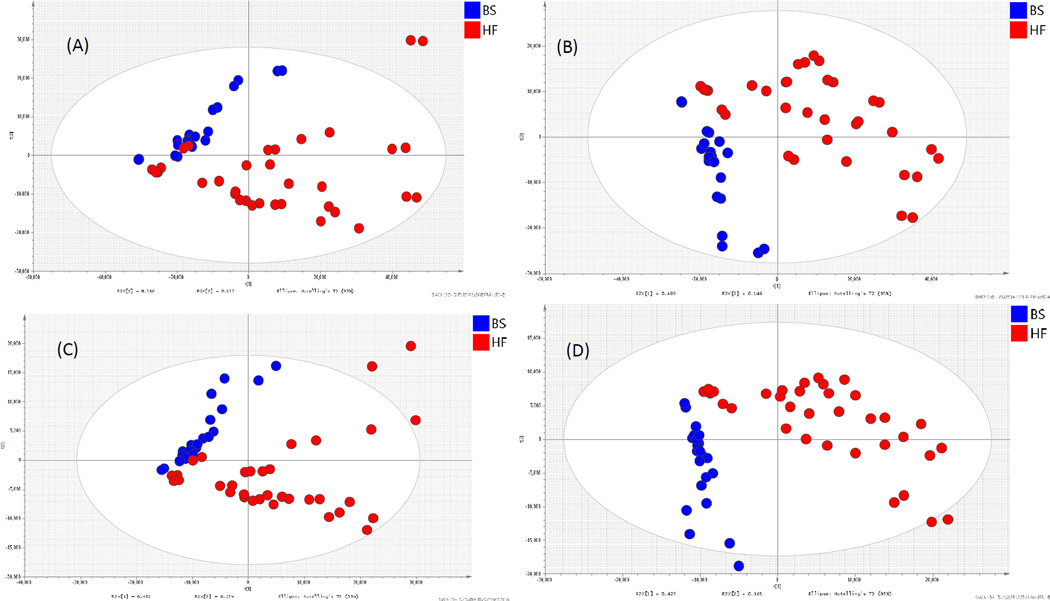
Metabolites in urine samples, taken at the end of the study, showed more complex chromatograms that revealed a change of endogenous and final metabolic products from the diet. After data-preprocessing using XCMS, the data sets contained 2046 metabolite ion features in positive and 828 ion features in negative ESI mode. A certain separation can be observed from a PCA score plot of (+) ESI data (Figure 4A and 4C). For exploration on the difference between pigs fed the high- and low-fat diets , PLS-DA, a supervised method, was further applied for identifying the reliable metabolites responsible for each (−) ESI data separation (Figure 4B and Figure 4D). For UHPLC-(+)ESI-MS data, the classification of models and controls resulted in the cross-validated predictive ability Q2 of 69.8%. A value of 63.4% of the variance in X [R2(X)] was used to account for 72.7% of the variance of Y [R2(Y)]. To investigate the validation of this model, a random permutation test was performed with the PLS-DA model corresponding to the PLS-DA model across the first components. Validation with a permutation number of 100 generated intercepts of R2 = 149 and Q2 = −0.0175. For UHPLC-(−) ESI-MS data, the classification of models and controls resulted in the cross-validated predictive ability Q2(Y) of 76.3%. A value of 58.8% of the variance in X [R2(X)] was used to account for 76.3% of the variance in Y [R2(Y)]. Validation with a permutation number of 100 generated intercepts of R2 = 0.154 and Q2 = −0.196.
Figure 4.
PCA and PLS-DA score plot of urine samples from (+)ESI-data (A, C) and (−)ESI-data (B, D) taken at the end of the study (week 11) from pigs pigs fed diet A (BS, blue) and diet B (HF, red).
Among 147 discriminative metabolites, several phase II conjugates via glucuronidation and sulfation had increased in the urine of pigs fed diet B (Table S6). The metabolites derived from soy isoflavones exhibited the greatest differences between the two diet groups. Pigs fed the high fat diet had a high content of isoflavone related metabolites such as genistein -O-glucuronide, equolglucuronide, daidzein-O-glucuronide, dihydrodaidzin, dihydrodaidzein, dihydrogenistein, and hydroxyldaidzein, and also had increased bile acid conjugates, such as chenodeoxycholic acid 3-glucuronide and cholestane-3,7,12,25-tetrol-3-glucuronideacid; which could indicate an increased uptake of lipids and cholesterol.
4. Discussion
Plasma, fecal and urine metabolites from pigs fed either a high-fat or regular diet were investigated using a UHPLC-HRMS based metabolomic approach. Their metabolic profiles were compared by multivariate statistical analysis. Diet is logically believed to have a close relationship with metabolic profiles. Feeding a high fat and high cholesterol diet to pigs for 11 weeks resulted in an increase in bile acids and their derivatives in plasma, fecal and urine samples, though at this stage, there was no significant weight gain observed.
In a previous study, a significantly higher level of muricholic acid, but not cholic acid, was found in pigs fed a high fat diet. The gut microbiota of these pigs were altered by diet and considered to regulate bile acid metabolism by reducing the levels of tauro-beta-muricholicacid (Sayin, Wahlstrom, Felin, Jantti, Marschall, Bamberg, et al., 2013). In our study, the unconjugated bile acids, hyodeoxycholic acid and deoxycholic acid were found to be significantly higher in the fecal samples of pigs fed a high-fat diet.
Chenodeoxycholic acid glycine was 8.6 times higher in pigs fed a high fat and high cholesterol diet compared to those fed a regular diet. These results confirm that feeding a high fat and high cholesterol diet leads to a changing metabolomic pattern over time, represented by excretion of certain bile acids in the feces. We also found that several metabolites associated with lipid metabolism were increased in the feces of pigs fed the high-fat diet. Feeding the high fat diet to pigs for 11 weeks did not induce any overt expression of disease, except for significantly higher levels of circulating cholesterol and triglycerides in the blood. It is likely, however, that longer periods of feeding would increase expression of metabolic syndrome disorders and features of cardiovascular disease in pigs, as have been previously demonstrated. Products of lipid metabolism that changed early in the dietary treatment could be useful as biomarkers. This may be important because the composition of the fats in the diet, used in this study, was complex and from multiple sources including lard, soybean oil and coconut oil. The complexity of the human diet could be reproduced in this model with the expectation of similarly defining stable metabolic profiles.
Pigs fed the high fat diet also had significantly higher levels of metabolites derived from soy isoflavones in the urine. This may simply be due to the high fat diet containing more soy protein and soybean oil than the regular diet (22.5% versus 18.9% and 2.8% versus 0.5%, respectively), or through interactions between the high fat in the diet and absorption and metabolisms of soy isoflavones, or both. These features of metabolic profiling would not be easily detected using less robust and sensitive methods of analysis and detection, and could be predictive of complex interactions within the food metabolome.
5. Conclusions
In summary, a number of metabolite differences were detected in the plasma, urine and feces of pigs fed a high fat and high cholesterol diet versus a regular diet that significantly increased over time. PCA showed a clear separation of metabolites in all biological samples tested from pigs fed the different diets. This methodology could be used to associate metabolic profiles with early markers of disease expression or the responsiveness of metabolic profiles to alterations in the diet. The ability to identify metabolites from bio-fluids, feces, and tissues that change with alterations in the diet has the potential to identify new biomarkers and to better understand mechanisms related to diet and health.
Supplementary Material
We have developed a metabolomic approach to study to pigs fed with high-fat/high cholesterol and regular diet.
A number of metabolite differences were detected in the plasma, urine, and feces of pigs.
We have found that alterations in diet led to metabolic profile changes.
This methodology could be used to identify biomarkers.
Acknowledgments
This research is supported by the Agricultural Research Service of the U.S. Department of Agriculture and an Interagency Agreement with the Office of Dietary Supplements (ODS) of the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Data are attached as Table S1–S6
References
- Aubin MC, Cardin S, Comtois P, Clement R, Gosselin H, Gillis MA, Le Quang K, Nattel S, Perrault LP, Calderone A. A high-fat diet increases risk of ventricular arrhythmia in female rats: enhanced arrhythmic risk in the absence of obesity or hyperlipidemia. J Appl Physiol. 2010;108(4):933–940. doi: 10.1152/japplphysiol.01281.2009. [DOI] [PubMed] [Google Scholar]
- Bernal C, Martin-Pozuelo G, Lozano AB, Sevilla A, Garcia-Alonso J, Canovas M, Periago MJ. Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J Nutr Biochem. 2013;24(11):1870–1881. doi: 10.1016/j.jnutbio.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Burgueno AL, Gianotti TF, Mansilla NG, Pirola CJ, Sookoian S. Cardiovascular disease is associated with high-fat-diet-induced liver damage and up-regulation of the hepatic expression of hypoxia-inducible factor 1alpha in a rat model. Clin Sci (Lond) 2013;124(1):53–63. doi: 10.1042/CS20120151. [DOI] [PubMed] [Google Scholar]
- Carvalho E, Franceschi P, Feller A, Palmieri L, Wehrens R, Martens S. A targeted metabolomics approach to understand differences in flavonoid biosynthesis in red and yellow raspberries. Plant Physiol Biochem. 2013 doi: 10.1016/j.plaphy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Cheng KK, Benson GM, Grimsditch DC, Reid DG, Connor SC, Griffin JL. A metabolomic study of the LDL receptor null mouse fed a high-fat diet reveals profound perturbations in choline metabolism that are shared with ApoE null mice. Physiol Genomics. 2010 doi: 10.1152/physiolgenomics.00188.2009. [DOI] [PubMed] [Google Scholar]
- Fardet A, Llorach R, Orsoni A, Martin JF, Pujos-Guillot E, Lapierre C, Scalbert A. Metabolomics provide new insight on the metabolism of dietary phytochemicals in rats. J Nutr. 2008;138(7):1282–1287. doi: 10.1093/jn/138.7.1282. [DOI] [PubMed] [Google Scholar]
- Gika HG, Theodoridis GA, Wingate JE, Wilson ID. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: application to human urine. J Proteome Res. 2007;6(8):3291–3303. doi: 10.1021/pr070183p. [DOI] [PubMed] [Google Scholar]
- Gu H, Chen H, Pan Z, Jackson AU, Talaty N, Xi B, Kissinger C, Duda C, Mann D, Raftery D, Cooks RG. Monitoring diet effects via biofluids and their implications for metabolomics studies. Anal Chem. 2007;79(1):89–97. doi: 10.1021/ac060946c. [DOI] [PubMed] [Google Scholar]
- Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. Energy-dense, lowfiber, high-fat dietary pattern is associated with increased fatness in childhood. Am J Clin Nutr. 2008;87(4):846–854. doi: 10.1093/ajcn/87.4.846. [DOI] [PubMed] [Google Scholar]
- Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu Rev Nutr. 2012;32:183–202. doi: 10.1146/annurev-nutr-072610-145159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim JH, Noh S, Hur HJ, Sung MJ, Hwang JT, Park JH, Yang HJ, Kim MS, Kwon DY, Yoon SH. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10(2):722–731. doi: 10.1021/pr100892r. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yang SO, Kim HS, Kim Y, Park T, Choi HK. 1H-nuclear magnetic resonance spectroscopy-based metabolic assessment in a rat model of obesity induced by a high-fat diet. Anal Bioanal Chem. 2009;395(4):1117–1124. doi: 10.1007/s00216-009-3054-8. [DOI] [PubMed] [Google Scholar]
- Knab AM, Nieman DC, Gillitt ND, Shanely RA, Cialdella-Kam L, Henson DA, Sha W. Effects of a flavonoid-rich juice on inflammation, oxidative stress, and immunity in elite swimmers: a metabolomics-based approach. Int J Sport Nutr Exerc Metab. 2013;23(2):150–160. doi: 10.1123/ijsnem.23.2.150. [DOI] [PubMed] [Google Scholar]
- Lehtonen HM, Lindstedt A, Jarvinen R, Sinkkonen J, Graca G, Viitanen M, Kallio H, Gil AM. 1H NMR-based metabolic fingerprinting of urine metabolites after consumption of lingonberries (Vaccinium vitis-idaea) with a high-fat meal. Food Chemistry. 2013;138(2–3):982–990. doi: 10.1016/j.foodchem.2012.10.081. [DOI] [PubMed] [Google Scholar]
- Leucht W, Leuthold G, Stier H. [The newborn pig as an experimental animal. III. Oxygen uptake in newborn miniature pigs] Z Versuchstierkd. 1977;19(4):206–210. [PubMed] [Google Scholar]
- Li T, Chiang JY. Bile Acid signaling in liver metabolism and diseases. J Lipids. 2012;2012:754067. doi: 10.1155/2012/754067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorach R, Urpi-Sarda M, Jauregui O, Monagas M, Andres-Lacueva C. An LC-MS-based metabolomics approach for exploring urinary metabolome modifications after cocoa consumption. J Proteome Res. 2009;8(11):5060–5068. doi: 10.1021/pr900470a. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang W, Zhang J, Lu Y, Wu W, Yan H, Wang Y. Hyperlipidemia and atherosclerotic lesion development in Ldlr-deficient mice on a long-term high-fat diet. PLoS One. 2012;7(4):e35835. doi: 10.1371/journal.pone.0035835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moco S, Martin FP, Rezzi S. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. J Proteome Res. 2012;11(10):4781–4790. doi: 10.1021/pr300581s. [DOI] [PubMed] [Google Scholar]
- Patti GJ, Tautenhahn R, Rinehart D, Cho K, Shriver LP, Manchester M, Nikolskiy I, Johnson CH, Mahieu NG, Siuzdak G. A view from above: cloud plots to visualize global metabolomic data. Anal Chem. 2013;85(2):798–804. doi: 10.1021/ac3029745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose S, Draper J, Elsom R, Kirkpatrick V, Mathers JC, Seal C, Beckmann M, Haldar S, Beattie JH, Lodge JK, Jenab M, Keun H, Scalbert A. Metabolomics and human nutrition. Br J Nutr. 2011;105(8):1277–1283. doi: 10.1017/S0007114510004812. [DOI] [PubMed] [Google Scholar]
- Pujos-Guillot E, Hubert J, Martin JF, Lyan B, Quintana M, Claude S, Chabanas B, Rothwell JA, Bennetau-Pelissero C, Scalbert A, Comte B, Hercberg S, Morand C, Galan P, Manach C. Mass Spectrometry-based Metabolomics for the Discovery of Biomarkers of Fruit and Vegetable Intake: Citrus Fruit as a Case Study. J Proteome Res. 2013 doi: 10.1021/pr300997c. [DOI] [PubMed] [Google Scholar]
- Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Simon GA, Maibach HI. The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations--an overview. Skin Pharmacol Appl Skin Physiol. 2000;13(5):229–234. doi: 10.1159/000029928. [DOI] [PubMed] [Google Scholar]
- Song X, Wang J, Wang P, Tian N, Yang M, Kong L. (1)H NMR-based metabolomics approach to evaluate the effect of Xue-Fu-Zhu-Yu decoction on hyperlipidemia rats induced by high-fat diet. J Pharm Biomed Anal. 2013;78–79:202–210. doi: 10.1016/j.jpba.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Spagou K, Theodoridis G, Wilson I, Raikos N, Greaves P, Edwards R, Nolan B, Klapa MI. A GC-MS metabolic profiling study of plasma samples from mice on low- and high-fat diets. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(17–18):1467–1475. doi: 10.1016/j.jchromb.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem. 2012;84(11):5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorsten FA, Grun CH, van Velzen EJ, Jacobs DM, Draijer R, van Duynhoven JP. The metabolic fate of red wine and grape juice polyphenols in humans assessed by metabolomics. Mol Nutr Food Res. 2010;54(7):897–908. doi: 10.1002/mnfr.200900212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.