Abstract
Holocarboxylase synthetase (HLCS) catalyzes the covalent attachment of biotin to cytoplasmic and mitochondrial carboxylases, nuclear histones, and over a hundred human proteins. Nonhydrolyzable ketophosphonate (β-ketoP) and hydroxyphosphonate (β-hydroxyP) analogs of biotin-5′-AMP inhibit holocarboxylase synthetase (HLCS) with IC50 values of 39.7 μM and 203.7 μM. By comparison, an IC50 value of 7 μM was observed with the previously reported biotinol-5'-AMP. The Ki values, 3.4 μM and 17.3 μM, respectively, are consistent with the IC50 results, and close to the Ki obtained for biotinol-5'-AMP (7 μM). The β-ketoP and β-hydroxyP molecules are competitive inhibitors of HLCS while biotinol-5'-AMP inhibited HLCS by a mixed mechanism.
Keywords: Biotin-5′-AMP; β-ketophosphonate; β-hydroxyphosphonate; holocarboxylase synthetase, biotinylation
Holocarboxylase synthetase (HLCS) is the sole enzyme in the human proteome capable of catalyzing the covalent attachment of biotin to lysine residues.1 HLCS localizes in the cytoplasm, mitochondria, and cell nuclei.1,2 HLCS catalyzes biotinylation of five carboxylases in mitochondria and cytoplasm, which play key roles in gluconeogenesis, fatty acid metabolism, and leucine metabolism.3 In addition, a recent mass spectrometry screen identified 108 novel biotinylated proteins; heat shock proteins and enzymes from glycolysis are overrepresented among these proteins.4 HLCS orchestrates the assembly of a multiprotein gene repression complex in human chromatin, partially mediated through HLCS-dependent methylation of the histone methyltransferase EHMT1 and the nuclear receptor co-repressor N-CoR.5 Consistent with the importance of HLCS in intermediary metabolism and cell function, no living HLCS null person has ever been reported, and persons with HLCS mutations require lifelong treatment with pharmacological doses of biotin.6
HLCS-dependent biotinylation involves basically two steps (Fig. 1). In the first, activation of biotin by ATP generates the mixed anhydride biotin-5′-adenosine monophosphate (Bio-5′-AMP) (1). In the second step, the phosphate anhydride serves as an acylating agent for a target lysine in carboxylases (2), histones (3), and other proteins,3,4,7 covalently linking biotin to the substrate via an amide bond.
Figure 1.
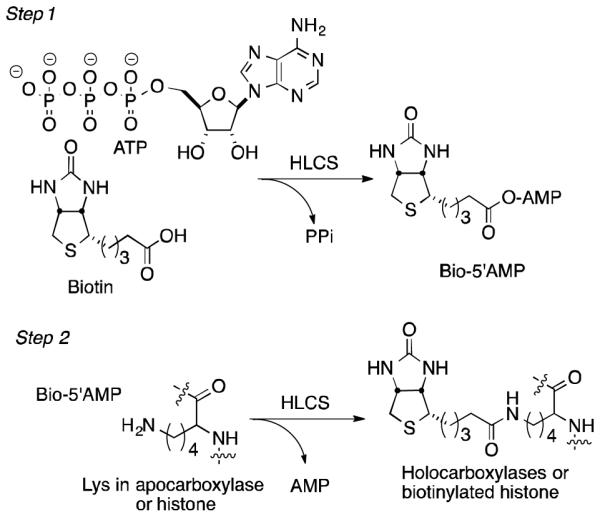
HLCS catalysis
The objective of this study was to develop a new class of synthetic HLCS inhibitors which could potentially be targeted to distinct cellular structures. Such an inhibitor would be a useful analytical tool in studies of HLCS-dependent biotinylation events in the cytoplasm, mitochondria, and nucleus. We based these studies on analogs of biotin-5′-AMP, namely biotin β-ketophosphonate-5′-AMP (β-ketoP) and biotin β-hydroxyphosphonate-5′-AMP (β-hydroxyP), that substitute a hydrolytically stable phosphonate for the acyl phosphate found in biotin-5′-AMP (Figure 2a). The use of phosphonates as unreactive isosteres of phosphates is well established.8 For example, nonhydrolyzable aminoacyl analogs of aspartyl adenylate exhibit potent inhibitory activity against E. coli aspartyl-tRNA synthetase.9 There is precedence for the efficacy of structurally analogous compounds sulfamides, sulfonamides, and 1,2,3-triazoles in the inhibition of a microbial biotin protein ligase, the HLCS ortholog BirA (Fig. 2b).10-13 We also investigated biotinol-5′-AMP, a known phosphate analog of biotin-5′-AMP which replaces the carbonyl oxygen with a methylene (CH2).10,14
Figure 2.
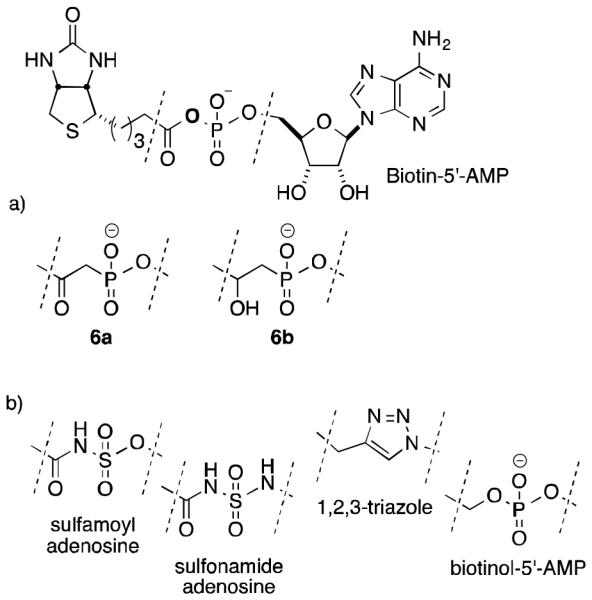
a. Chemical structure of biotin-5′-AMP and ketophosphonate (6a) and hydroxyphosphonate (6b) analogs; b. reported sulfamoyl, sulfonamide, triazole, and phosphate inhibitors.
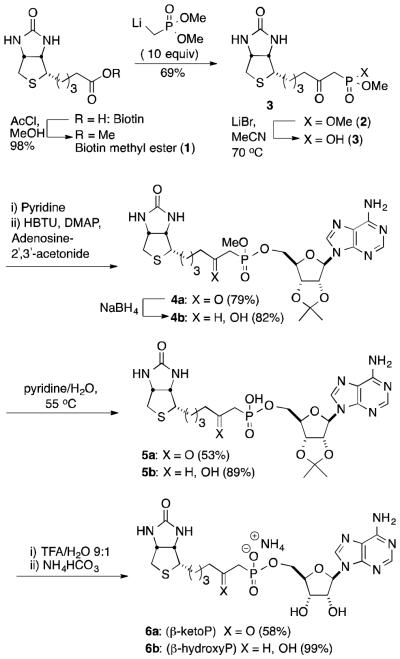
Inhibitor synthesis: The central element of the synthesis is the formation of a protected version of a biotin ketophosphonate (4a) via condensation of a biotin-derived ketophosphonic acid (3) with a protected adenosine (Scheme 1). The synthesis begins with biotin methyl ester (1), prepared via the acid-catalyzed esterification of biotin.15 Reaction with the carbanion derived from methyl phosphonate was anticipated to offer a convenient route to a precursor of the desired phosphonates. However, reaction of ester 1 with the lithiated methylphosphonate, generated using lithium bis(trimethylsilyl)amide (LiHMDS) or n-butyl lithium (n-BuLi), resulted in poor yields. Fortunately, reaction of the ester with a large excess of the lithiated phosphonate, followed by quenching with deionized water to minimize demethylation of the phosphonate diester product, produced a 69% yield of dimethyl β-ketophosphonate 2.16 Selective monodemethylation with lithium bromide provides a good yield of mono ester 3. The pyridinium salt of 3 underwent coupling with 2′, 3′-isopropylidineadenosine (i-PrA) in the presence of O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) to provide a mixed phosphonate diester (4a) in 79% yield for two steps.17,18 No coupling was observed if N,N'-dicyclohexylcarbodiimide (DCC) was substituted for HBTU. The acidic methylene of the ketone phosphonate was observed (NMR) to readily undergo H/D exchange upon dissolution in d4-MeOH.
Scheme 1.
Synthesis of ketophosphonate (β-ketoP) and hydroxyphosphonate (β-hydroxyP) analogs of biotin-5′-AMP
Reduction of the ketone with NaBH4 resulted in formation of the corresponding alcohol (4b) as a mixture of diastereomers at the newly formed stereocenter. Stirring the ketone diester (4a) or the alcohol diester (4b) in pyridine/water resulted in selective demethylation to afford monoesters 5a or 5b, respectively. Removal of the acetonide protecting group from the sugar followed by neutralization with ammonium bicarbonate provided the target biotin β-ketophosphonate (β-ketoP) 6a in 58% yield for two steps. The same procedure, when applied to alcohol 5b, furnished biotin-β-hydroxyphosphonate (β-hydroxyP) 6b in 99% yield.
Inhibition of HLCS: Biotin β-ketophosphonate-5′-AMP (β - ketoP, 6a) inhibited HLCS in a dose-dependent manner. The polypeptide p67 is a substrate for biotinylation by HLCS.19 When recombinant p67 was incubated with recombinant HLCS and 20 μM biotin in the presence of 50 to 500 μM β-ketoBP, HLCS inhibition was maximal at the highest inhibitor concentration tested. For example, the inhibition at 500 μM β-ketoBP was 81.3±11.1% (P<0.01; n=4) compared with vehicle control (Fig. 3A). Data are presented as mean±SD of 4 replicates. Incubation of p67 in the absence of HLCS produced no detectable signal (lane 6 in Fig. 3B). Under the conditions used here, IC50 and Ki for β-ketoBP equaled 39.7±1.9 μM and 2.0 ±0.1 μM, respectively; see Supplementary Materials for details regarding assays and calculations. When tested under identical conditions, β-hydroxyP inhibited HLCS activity by 67.7 ±10.0% (P=0.0001; n=4) at concentrations of 500 μM inhibitor. The IC50 and Ki values calculated under these conditions were 203.7 ±3.7μM and 10.4±0.2μM, respectively. β-KetoP is a competitive inhibitor of HLCS, based on competition studies with biotin. In these studies, the concentration of the inhibitor was held constant at 250 μM while that of biotin was varied from 0-320 μM; a second curve was generated in the absence of inhibitor (Fig. 3C). The apparent Vmax was similar for incubations with and without inhibitor [31.5±1.6 vs. 22.3±1.51 pmol biotinylated p67/(nmol HLCS x s); n=4] whereas the apparent Km for biotin was increased by the addition of inhibitor (77.9±10.3 vs. 1.6±1.8 μM biotin; N=4). β-HydroxyP (6b) also acts as a competitive inhibitor as evidenced by similar apparent Vmax values with and without inhibitor [20.6±1.8 vs. 22.6±1.4 pmol biotinylated p67/nmol HLCS x s); n=4] whereas reactions incubated with inhibitor increased the apparent Km for biotin (82.5±20.4 vs. 1.9±1.8 μM biotin; n=4). As a negative control we conducted incubations with a biotin ketophosphonic acid (compound 3 in Scheme 1). This substrate incorporates an electrophilic carbonyl carbon beta to a charged phosphonate but lacks the adenosyl fragment hypothesized as essential for mediating HLCS inhibition. Consistent with this theory, the ketophosphonic acid compound did not inhibit HLCS (data not shown).
Figure 3.
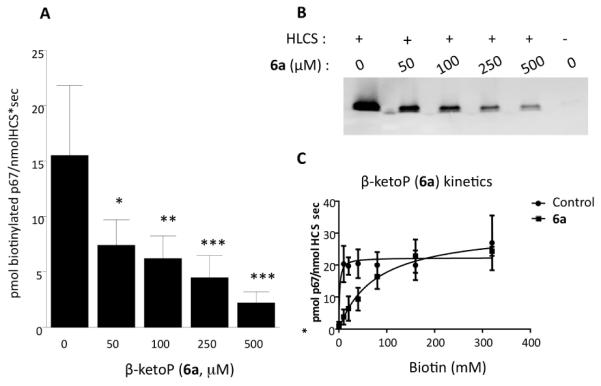
Inhibition of HLCS by biotin β-ketophosphonate-5′-AMP. (A) HLCS activity was quantified using infrared absorbance in the presence of 20 μM biotin and various concentrations of biotin β-ketophosphonate-5′-AMP (One way-ANOVA; *P<0.05; N=4; Dunnett’s multiple t-test *p<0.05, **p<0.01, ***p<0.001). (B) Representative gel, depicting biotinylation of p67 in the presence of various concentrations of biotin β-ketophosphonate-5′-AMP. Lane 6 shows p67 incubated in the absence of HLCS. (C) Competitive inhibition of HLCS by biotin β-ketophosphonate-5-AMP.
The results were compared against assays conducted with biotinol-AMP, a known phosphate analog of biotin-5′-AMP which has previously been employed for inhibition of BirA (biotin protein ligase).10,11 Biotinol-AMP reduces HLCS activity by 98.01±0.1% at concentrations of 500 μM and has an IC50 value and Ki of 8.8±3.6μM and 754±303nM, respectively. When reactions incubated with biotinol-AMP were challenged with increasing amounts of up to 320 μM biotin, the apparent Vmax decreased compared to reactions without inhibitor (22.6±1.4 vs. 5.9±1.4; n=4) and Km increased (1.9±1.8 vs. 146±79; n=4) indicating the biotinol-AMP most likely acts as a mixed inhibitor. There remains limited knowledge regarding the structure of HLCS and the mechanism of catalysis.11,13b,20 Similarly, little is known about the basis for selectivity between the classic carboxylase targets of HLCS and novel targets in chromatin and other proteins. The objective of this study was to develop a synthetic HLCS inhibitor capable of penetrating cell membranes and which could potentially be targeted to distinct cellular structures. Such an inhibitor would be a useful analytical tool in studies of carboxylase biotinylation in cytoplasm and mitochondria, studies of chromatin protein biotinylation and HLCS-dependent formation of multiprotein gene repression complexes in nuclei, and studies of newly discovered species of biotinylated proteins throughout the cell.
Consistent with the importance of HLCS in intermediary metabolism and epigenetics, no living HLCS null individual has ever been reported, suggesting embryonic lethality. HLCS knockdown in Drosophila melanogaster (~30% residual activity) produces phenotypes such as decreased life span and reduced heat resistance.21 Mutations and single nucleotide polymorphisms have been identified and characterized in the human HLCS gene; these mutations cause a substantial decrease in HLCS activity, aberrant gene regulation and metabolic abnormalities.6,22 Unless diagnosed and treated at an early stage, homozygous severe HLCS deficiency is characteristically fatal.23 Three independent cancer and patent databases correlate HLCS loss or mutation with an increase in detected tumors.24
Several classes of biotin-5′-AMP analogs have been applied to study the function of biotin protein ligases (BPLs), exemplified by HLCS as well as BirA, an enzyme catalyzing biotinylation of acyl carrier protein in prokaryotes.10,13,14 BirA from E. coli has 21% sequence similarity to HLCS.25 Biotinol-5′-AMP, a phosphate ester lacking the acyl carbonyl of biotin-5′-AMP, binds tightly to the Escherichia coli biotin repressor (KD = 1.5 ± 0.2 nM)11,20 and inhibits biotin transfer to the acceptor protein.10,14 Biotinol-5′-AMP also binds tightly to Staphylococcus aureus BPL (Ki = 0.03 ± 0.01 μM). The activity of this analog would appear to suggest the key role of the phosphate moiety and the relative lack of importance of hydrogen-bonding interactions to the acyl carbonyl of biotin-5′-AMP. However, other work has demonstrated that a sulfamoyl-containing bisubstrate analog, replacing the acyl phosphate with an acyl sulfonamide, strongly binds Mycobacteria tuberculosis BPL (MtBPL); a co-crystal revealing multiple hydrogen-bonding interactions between the protein and the acyl sulfonamide.12 More recently, a biotin-5′-AMP analog replacing the acyl phosphate with a 1,2,3-triazole was found to bind tightly to BPL and exhibit >1100-fold selectivity for the S. aureus BPL over the human homologue.13 This suggests the possibility of designing potent inhibitors of bacterial BPL. However, no similar approach has been used to study the function of HLCS or human BPL.
A model of the HLCS/biotin-5′-AMP complex as well as the crystal structure of biotin-5′-AMP with BPL from Pyrococcus horikoshii OT3 (pdb:1wqw) suggests the importance of hydrogen bonding involving the carbonyl and phosphonate oxygen (Figure S1).13b,26 The β-ketophosphonate and β-hydroxyphosphonate analogs introduced here maintain the natural charge state of biotin-AMP and place a basic oxygen atom beta to the phosphonate group. However, in contrast to the BirA inhibitors described above, the ketophosphonate (β-ketoP, 6) incorporates an electrophilic carbon at the location of the original acyl group in biotin-5′-AMP. Although the reduced activity of the new inhibitors compared with biotinol-AMP suggests that preservation of an electrophilic center (C=O) or hydrogen bonding group (CHOH) beta to phosphonate is of limited importance in inhibitor design, we note that the 1,2,3-triazole analogs completely lacking a carbonyl group show no inhibition toward human BPL. It is also possible that conformational differences between the acyl phosphate of biotin-5′-AMP and the phosphonate of 6a and 6b might also contribute to the reduced binding observed.
In conclusion, we have described a new class of inhibitors of holocarboxylase synthetase HLCS based upon replacement of the ester of biotin-5′-AMP with a ketone or a secondary alcohol. The analogs produce significant levels of inhibition with isolated enzyme. Efficacy of the new inhibitors in vivo has not been tested and further investigations are warranted.
Supplementary Material
Acknowledgments
A contribution of the University of Nebraska Agricultural Research Division, supported in part by funds provided through the Hatch Act (to PD and JZ). Additional support was provided by NIH grants DK063945 and P20GM104320 (to JZ). Research was conducted, in part, in facilities remodeled with support from NIH (RR016544).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Synthetic protocols, spectral listings, and assay conditions.
References and notes
- 1.(a) Chiba Y, Suzuki Y, Aoki Y, Ishida Y, Narisawa K. Arch. Biochem. Biophys. 1994;313:8. doi: 10.1006/abbi.1994.1351. [DOI] [PubMed] [Google Scholar]; (b) Suzuki Y, Aoki Y, Ishida Y, Chiba Y, Iwamatsu A, Kishino T, Niikawa N, Matsubara Y, Narisawa K. Nat. Genet. 1994;8:122. doi: 10.1038/ng1094-122. [DOI] [PubMed] [Google Scholar]
- 2.(a) Bailey LM, Wallace JC, Polyak SW. Arch. Biochem. Biophys. 2010;496:45–52. doi: 10.1016/j.abb.2010.01.015. [DOI] [PubMed] [Google Scholar]; (b) Bao B, Wijeratne SS, Rodriguez-Melendez R, Zempleni J. Biochem. Biophys. Res. Commun. 2011;412:115. doi: 10.1016/j.bbrc.2011.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zempleni J, Wijeratne SSK, Kuroishi T, Erdman JW., Jr. In: Biotin. In Present Knowledge in Nutrition. Macdonald I, Zeisel SH, editors. 10th. International Life Sciences Institute; Washington, D.C.: 2012. pp. 587–609. [Google Scholar]
- 4.Li Y, Malkaram SA, Zhou J, Zempleni J. J. Nutr. Biochem. 2014;25:475. doi: 10.1016/j.jnutbio.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Li Y, Hassan YI, Moriyama H, Zempleni J. J. Nutr. Biochem. 2013;24:1446. doi: 10.1016/j.jnutbio.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xue J, Wijeratne S, Zempleni J. Epigenetics. 2013;8(5):504. doi: 10.4161/epi.24449. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu D, Zempleni J. Biochem J. 2014;461:477. doi: 10.1042/BJ20131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki Y, Yang X, Aoki Y, Kure S, Matsubara Y. Hum. Mutat. 2005;26:285. doi: 10.1002/humu.20204. [DOI] [PubMed] [Google Scholar]
- 7.Lane MD, Young DL, Lynen F. J. Biol. Chem. 1964;239:2858. [PubMed] [Google Scholar]
- 8.a) Thatcher RJ, Campbell AS. J. Org. Chem. 1993;58:2272. [Google Scholar]; b) Engel R. Chem. Rev. 1977;77:349. [Google Scholar]
- 9.Bernier S, Akochy PM, Lapointe J, Chênevert R. Bioorg Med Chem. 2005;13:69. doi: 10.1016/j.bmc.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 10.Brown PH, Cronan JE, Grøtli M, Beckett D. J. Mol. Biol. 2004;337:857. doi: 10.1016/j.jmb.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Brown PH, Beckett D. Biochemistry. 2005;44:3112–21. doi: 10.1021/bi047792k. [DOI] [PubMed] [Google Scholar]
- 12.Duckworth BP, Geders TW, Tiwari D, Boshoff HI, Sibbald PA, Barry CE, Schnappinger D, Finzel BC, Aldrich CC. Chem. Biol. 2011;18:1432. doi: 10.1016/j.chembiol.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Soares da Costa T, Tieu W, Yap M, Pendini N, Polyak S, Pedersen D, Morona R, Turnidge J, Wallace J, Wilce M, Booker G, Abell A. J. Biol. Chem. 2012;287:17823. doi: 10.1074/jbc.M112.356576. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Paparella AS, Soares da Costa T, Yap MY, Tieu W, Wilce MCJ, Booker GW, Abell AD, Polyak SW. Curr. Top. Med. Chem. 2014;14:4. doi: 10.2174/1568026613666131111103149. [DOI] [PubMed] [Google Scholar]
- 14.Wood ZA, Weaver LH, Brown PH, Beckett D, Matthews BW. J Mol Biol. 2006;357:509. doi: 10.1016/j.jmb.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 15.Slavoff SA, Chen I, Choi Y-A, Ting AY. J. Am. Chem. Soc. 2008;130:1160. doi: 10.1021/ja076655i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maloney KM, Chung JYL. J. Org. Chem. 2009;74:7574. doi: 10.1021/jo901552k. [DOI] [PubMed] [Google Scholar]
- 17.Balg C, Blais SP, Bernier S, Huot JL, Couture M, Lapointe J, Chênevert R. Biorg. Med. Chem. 2007;15:295. doi: 10.1016/j.bmc.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 18.Campagne J-M, Coste J, Jouin P. J. Org. Chem. 1995;60:5214. [Google Scholar]
- 19.Kobza K, Sarath G, Zempleni J. BMB Rep. 2008;41:310. doi: 10.5483/bmbrep.2008.41.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naganathan S, Beckett DJ. Molec. Biol. 2007;373:96. doi: 10.1016/j.jmb.2007.07.020. doi:10.1016/j.jmb.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camporeale G, Giordano E, Rendina R, Zempleni J, Eissenberg JC. J. Nutr. 2006;136:2735. doi: 10.1093/jn/136.11.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) National Center for Biotechnology Information Online Mendelian Inheritance in Man. 2008 http://www.ncbi.nlm.nih.gov/omim (accessed:10/29/2014); (b) Esaki S, Malkaram SA, Zempleni J. Eur. J. Hum. Genet. 2012;20:428. doi: 10.1038/ejhg.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thuy LP, Belmont J, Nyhan WL. Prenat. Diagn. 1999;19:108. doi: 10.1002/(sici)1097-0223(199902)19:2<108::aid-pd476>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.(a) UniProt UniProtKB. www.uniprot.org/uniprot/P50747 (accessed:10/29/2014);); (b) Massague J, Bos P. Metastasis promoting genes and proteins. www.faqs.org/patents/app/20100029748 (accessed:10/29/2014);); (c) Institute for Biomedical Technologies Genes-to-system breast cancer database. www.itb.cnr.it/breastcancer/php/showMostCorrelated.php?id=6664 (accessed:10/29/2014);)
- 25.Hassan YI, Moriyama H, Olsen LJ, Bi X, Zempleni J. Mol Genet Metab. 2009;96:183. doi: 10.1016/j.ymgme.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esaki S, Malkaram SA, Zempleni J. Eur. J. Hum. Genet. 2012;20:428. doi: 10.1038/ejhg.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.