Abstract
The currently fielded pre-hospital therapeutic regimen for the treatment of organophosphorus (OP) poisoning in the United States (U.S.) is the administration of atropine in combination with an oxime antidote (2-PAM Cl) to reactivate inhibited acetylcholinesterase (AChE). Depending on clinical symptoms, an anticonvulsant, e.g., diazepam, may also be administered. Unfortunately, 2-PAM Cl does not offer sufficient protection across the range of OP threat agents, and there is some question as to whether it is the most effective oxime compound available. The objective of the present study is to identify an oxime antidote, under standardized and comparable conditions, that offers protection at the FDA approved human equivalent dose (HED) of 2-PAM Cl against tabun (GA), sarin (GB), soman (GD), cyclosarin (GF), and VX, and the pesticides paraoxon, chlorpyrifos oxon, and phorate oxon. Male Hartley guinea pigs were subcutaneously challenged with a lethal level of OP and treated at approximately 1 min post challenge with atropine followed by equimolar oxime therapy (2-PAM Cl, HI-6 DMS, obidoxime Cl2, TMB-4, MMB4-DMS, HLö-7 DMS, MINA, and RS194B) or therapeutic-index (TI) level therapy (HI-6 DMS, MMB4-DMS, MINA, and RS194B). Clinical signs of toxicity were observed for 24 hours post challenge and blood cholinesterase [AChE and butyrylcholinesterase (BChE)] activity was analyzed utilizing a modified Ellman’s method. When the oxime is standardized against the HED of 2-PAM Cl for guinea pigs, the evidence from clinical observations, lethality, quality of life (QOL) scores, and cholinesterase reactivation rates across all OPs indicated that MMB4 DMS and HLö-7 DMS were the two most consistently efficacious oximes.
Keywords: oxime, efficacy, guinea pig, intramuscular, toxicity
1. Introduction
Organophosphorus (OP) compounds, including pesticides and chemical warfare nerve agents (CWNAs), represent a threat to the general population, not only as possible weapons of terrorism (Okumura, 2005; Zurer, 1998; Hubbard et al., 2013; Baker, 2013; Dolgin, 2013), but also as chemicals that could be released from transportation and storage facilities during industrial accidents. Given the rapid onset of symptoms and toxicity of OP nerve agents, a quick-acting therapeutic regimen that is efficacious over the broad spectrum of OPs is needed. To provide the most effective therapy, medical countermeasures must be administered as soon as possible post-exposure.
The current U.S. therapy regimen includes the administration of atropine in combination with the oxime acetylcholinesterase (AChE) reactivator pralidoxime chloride (2-PAM Cl) (Inchem.org, 1989, 1999), followed by the anticonvulsant diazepam depending on whether convulsive symptoms are observed. This approach is accomplished with the use of the DuoDote® autoinjector kit (Meridian Medical Technologies™, Columbia, MD; https://www.duodote.com/meridian.aspx#) by trained emergency medical services personnel. The DuoDote® is a two-chambered, self-propelled syringe used for the intramuscular (IM) injection of atropine (2.1 mg free base) and 2-PAM Cl (600 mg) through the same needle.
Although the current treatment approach does protect against some OP toxicities, this protection does not extend across all OP CWNAs, i.e., it is not a broad-spectrum antidote (Worek and Thiermann, 2013; Thiermann et al., 2013). Unfortunately, when OP pesticides are included as potential intoxicants, the spectrum of therapeutic effectiveness is even less. Additionally, most leading oxime reactivators currently available worldwide, including 2-PAM Cl, are ionized with a hydrophilic bis-pyridinium quaternary backbone. As such, they are capable of reactivating cholinesterases (ChEs) in peripheral tissues, but not in the central nervous system (CNS) because they do not readily cross the blood brain barrier (BBB) (Voicu et al., 2013, Shih et al., 2012). Consequently, more effective oxime therapies, including a broader spectrum of activity and/or the capacity to cross the BBB, are being investigated to identify a more effective treatment than 2-PAM Cl. As the only true antidote, i.e., one that reactivates the target molecule AChE, a better oxime therapy would improve the nation’s medical response capabilities.
While many oxime compounds have already been synthesized and tested for broad-spectrum efficacy (Bajgar, 2010; Shih et al., 2009; Voicu et al., 2013; Worek et al., 2007) as well as BBB penetration capabilities (Sit et al., 2011; Radić et al., 2012), an actual head-to-head and rigorous comparison of efficacy entailing quality of life (QOL) evaluation after treatment, peripheral blood cholinesterase reactivation, and lethality endpoints has been absent. The few studies to assess comparative efficacy in animals have typically been confined only to oximes within the same chemical class or moiety developed within a particular laboratory, rather than what is currently approved and fielded worldwide. Since those studies are also often conducted under non-standardized experimental conditions and lack other methodological controls to increase scientific rigor, the unintentional introduction of bias remains a possibility when interpreting the results.
The currently fielded oximes 2-PAM Cl (USA, UK, France), obidoxime Cl2 (LüH-6; Germany, Netherlands), TMB-4 (trimedoxime bromide; Israel), and HI-6 DMS (Canada, Sweden) are efficacious against specific OP CWNAs (Antonijevic and Stojiljkovic, 2007; Bajgar, 2004; 2009; 2010; Cabal et al., 2004; Calic et al., 2006; Delfino et al., 2009; Eyer et al., 2008; Kassa, 1998; 2002; 2005; Kuca et al., 2007a; Kuca et al., 2009; Lundy et al., 2006). Although there are few studies assessing the efficacies of oximes against OP pesticides, obidoxime Cl2 is currently regarded as the most efficacious against pesticides (Worek et al., 2007). The search for a centrally acting oxime to maintain brain AChE activity has produced MINA and RS194B. MINA is a relatively small (molecular weight, or MW = 87.1 Da) AChE reactivator that has been shown to improve survivability against GB (Rutland, 1958; Askew, 1956; Dultz et al., 1957; Myers, 1959; and Shih et al., 2009, 2010, 2012). RS194B (MW = 213.3 Da) and has been shown to reactivate human AChE in vitro and protect mice against VX, GB, and paraoxon (Radić et al., 2012).
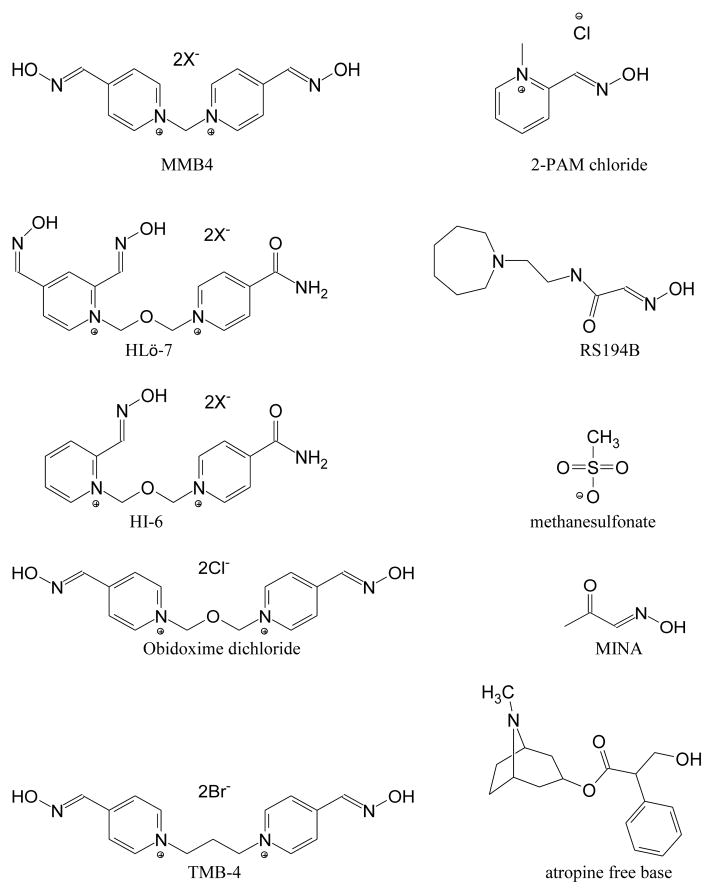
HLö-7, HI-6, and obidoxime are bis-pyridinium oximes, each containing two charged pyridine rings (requisite in an oxime for optimal reactivation of VX-inhibited AChE; Esposito, 2014) joined by a dimethyl ether (-CH2-O-CH2-) linker (Figure 1). The structural differences among HLö-7, HI-6, and obidoxime are in the number and position(s) of aldoximes on the pyridine rings (Kuca et al., 2006; Ekström et al., 2009). Also, one of the ring groups in HLö-7 and HI-6 is an isonicotinamide, which was included in their original synthesis to reduce toxicity (Oldiges and Schoene, 1970) but which, as molecular dynamic studies suggest, may also enhance ChE reactivation (Maxwell et al., 2008). HLö-7 and obidoxime are the most potent reactivators of phosphonylated and phosphorylated AChE, respectively (Worek et al., 2004). MMB4 and TMB-4 are the same 4-position bis-pyridinium aldoxime, except that MMB4 has a -CH2- linker while TMB-4 (a dibromide salt) has a -C3H6- linker. TMB-4 originated in 1958 and was the first bis-pyridinium oxime to be effective against GA (Schoene and Oldiges, 1973; Inns and Leadbeater, 1983). The difference between these two similar compounds in terms of toxicity to the Hartley guinea pig by IM injection is remarkable: the 24-hour LD50 (median lethal dose) is 679 mg/kg (1514 μmol/kg) for MMB4 DMS (unpublished data), and 80 mg/kg (179 μmol/kg) for TMB-4 (Shih et al., 2009).
Figure 1.
Structures of Eight Oximes Tested and Atropine Free Base
The overall objective of this study was to compare rigorously the efficacy of currently fielded and select promising novel AChE oxime reactivators under strict standardized experimental conditions to enable an accurate and unbiased assessment of their efficacies against OP CWNAs and pesticides. To accomplish this, the human equivalent FDA-approved dose of 2-PAM Cl was used as the experimental standard and the equimolar oxime therapy was administered to atropinized guinea pigs after an LD85 challenge of each OP CWNA or pesticides (data not shown). The LD85 was selected as the challenge level across OPs because it maximized the power of the test to discriminate among the oximes in terms of lethality. Additionally, those oximes with a safety index greater than 2-PAM Cl, i.e., MMB4 DMS, HI-6 DMS, MINA, and RS194B were also evaluated at an additional ‘therapeutic dose’ level equal to the median lethal dose (LD50) for the oxime divided by the TI for 2-PAM Cl. Overall efficacy was determined specifically in terms of QOL, blood cholinesterase levels in 24-hour survivors, and lethality.
2. Materials and Methods
Organophosphorus Compounds
The five CWNAs evaluated were tabun (GA; O-ethyl N,N-dimethyl phosphoramidocyanidate), sarin (GB; O-isopropyl methylphosphonofluoridate), soman (GD; O-pinacolyl methylphosphonofluoridate), cyclosarin (GF, cyclohexyl methylphosphonofluoridate), and VX (O-ethyl S-(2-diisopropylaminoethyl) methylphosphonothiolate). They were obtained from the U.S. Army Edgewood Chemical Biological Center (Aberdeen Proving Ground, MD). The purity values of the CWNAs were >98.5 percent as determined by gas chromatography. Chlorpyrifos oxon (purity ≥98 percent) and paraoxon (purity ≥98 percent) were purchased from Chem Service, Inc, West Chester, PA. Phorate oxon (purity ≥97.1 percent) was synthesized at Battelle’s Analytical Chemistry Development Department (Columbus, OH). GB, GD, GF, and VX were diluted in 0.9 percent saline; GA and phorate oxon were diluted in multisol (a biocompatible solution of 48.5 percent water, 40 percent propylene glycol, 10 percent ethanol, and 1.5 percent benzyl alcohol, all v/v); and chlorpyrifos oxon and paraoxon were diluted in ethanol (99.96 percent), with the dosing solution concentration of each pesticide being limited to that which would allow the total volume of ethanol injected to be no more than 0.06 percent (v/w) of the body mass.
Oximes
2-PAM Cl (pralidoxime chloride, 2-hydroxyiminomethyl-1-methylpyridinium chloride; supplied as an injectable drug at 100 mg/mL) and MMB4 DMS (methoxime dimethanesulfonate; 1,1-methylene bis[4(hydroxyimino) methyl]pyridinium) dimethanesulfonate; purity 100 percent) were supplied by the U.S. Department of Defense. HI-6 DMS (4-carbamoyl-1-[({2-[(E)-(hydroxyimino)methyl)]pyridinium-1-yl}methoxyl)methyl] pyridinium dimesylate; purity 98.7 percent), MINA ((1E)-1-(hydroxyimino)propan-2-one; purity 98.7 percent), TMB-4 (trimedoxime bromide; 1′-propane-1,3-diylbis{4-[(E)-(hydroxyimino)methyl]pyridinium} dibromide; purity 98.5 percent), and HLö-7 DMS (pyridinium,1-(((4-(aminocarbonyl) pyridinio)methoxy)methyl)-2,4- bis((hydroxyimino)methyl), dimesylate; purity 96.73 percent) were procured from Southwest Research Institute, San Antonio, TX. RS194B (N-(2-(azepan-1-yl)ethyl)-2-(hydroxyimino)acetamide; purity 96±2 percent) was procured from Skaggs School of Pharmacy & Pharmaceutical Sciences (University of California, San Diego). Obidoxime Cl2, LüH-6 (oxo-[[1-[[4-(oxoazaniumylmethylidene)pyridin-1-yl]methoxymethyl]pyridin-4-ylidene]methyl]azanium dichloride; purity 97.1 percent) was procured from Sigma Aldrich. MMB4 DMS, HI-6 DMS, MINA, TMB-4, HLö-7 DMS, and obidoxime Cl2 were formulated as dosing solutions for intramuscular (IM) injection in normal (0.9 percent, w/v) saline. RS194B was prepared as per supplier instruction by dissolving in a mixture of concentrated (37 percent, w/w) hydrochloric acid diluted 1:1 (v/v) with distilled water, and adjusting the final oxime solution to pH 7 using 6.25 percent (w/v) aqueous sodium hydroxide. This brought the sodium chloride concentration in the RS194B solution to 1.6 percent, w/v.
Chemical Analysis
The concentrations of the OP agent stock solutions were checked by gas chromatography (GC) using an Agilent 6890 GC equipped with a flame photometric detector (FPD) in phosphorus mode, prior to and after administration. Additionally, OP pesticides and oxime analysis was performed by high performance liquid chromatography (HPLC) using an Agilent 1200 series LC and ultraviolet (UV) detector to confirm solution concentration. Prior to use on study, each oxime was determined to be stable for the concentrations required on study at both room temperature (25°C) and within refrigeration (4°C) for 96 hours. Chemical verification and concentration analysis of atropine (King Pharmaceuticals, St. Louis, MO, Batch #RP-526-1) were performed using HPLC. Target dosing concentrations were prepared at 259 mg/mL (equimolar) and 400 mg/mL (TI) for MMB4 DMS, 270 mg/mL (equimolar) and 400 mg/mL (TI) for HI-6 DMS, 50 mg/mL (equimolar) and 100 mg/mL (TI) for MINA, 100 mg/mL (equimolar) and 500 mg/mL (TI) for RS194B, 102 mg/mL 2-PAM Cl, 205 mg/mL obidoxime Cl2, 63 mg/mL TMB-4, and 302 mg/mL HLö-7 DMS.
Preparation of Animals
Male Hartley guinea pigs were purchased from Charles River (Raleigh, NC & Saint-Constant, QC, Canada) and utilized in accordance with protocol specifications approved by the Institutional Animal Care and Use Committee (IACUC). The guinea pig was selected based on having low levels of plasma carboxylesterase relative to other rodent species, which is more similar to humans (Bahar et al, 2012), similarity of AChE protein sequence to that of humans (Cadieux et al, 2010), affordability, and historical use. Animals were quarantined for 3 to 5 days prior to randomization by body weight. Body weights, used to determine challenge doses and treatment volumes, were taken the day prior to challenge. Weights ranged from 250 to 500 g with a mean of 330 g among the 1920 guinea pigs placed on study. Baseline bloods were also collected via the vena cava in chilled K3 EDTA (Covidien, Mansfield, MA) tubes and processed to determine a baseline AChE and BChE activity in whole blood.
OP Challenges and Oxime Therapies
On the day of study, guinea pigs were injected subcutaneously (SC) to ensure an accurate exposure level, with vehicle or an LD85 dose of one OP (Table 1) via a 29G ½” needle/syringe system between the scapulae. OPs were administered in vehicle at the LD85 dose after atropine only treatment as determined in preparatory work (Table 2a). An LD85 was selected as the optimal challenge level across all OPs as this exposure level maximizes the ability to discriminate among oxime efficacies in terms of lethality while conserving resources. Power calculations were performed to ensure group size was sufficient for 80 percent statistical power between oxime groups. At 1 minute after challenge, either saline or atropine (0.4 mg atropine free base/kg from a solution of 1.64 mg atropine free base/mL) was given IM immediately followed by treatment with either an oxime in vehicle or vehicle. Both administrations were via a 29G ½” needle/syringe system in contralateral thighs. An atropine free base level of 0.4 mg/kg in the guinea pig was selected for this study based on the body surface area-corrected equivalent dose given to a human victim of OP poisoning in a first responder setting after administration of three DuoDote® autoinjectors (USD HHS, FDA, CDER, 2005). The FDA recommends that no more than three injections be administered to victims without adequate supportive care, e.g., ventilator assistance. Oximes, 2-PAM Cl, MMB4 DMS, HI-6 DMS, MINA, RS194B, obidoxime Cl2, HLö-7 DMS, with the exception of TMB-4, were administered at the equimolar dose of 2-PAM Cl available in three DuoDote® autoinjectors given to a 70-kg human, equivalent to 25.7 mg/kg (146 μmol/kg) in guinea pigs (Table 2b). Due to toxicity concerns, only TMB-4 could not be administered at the equimolar level. The therapy level for TMB-4 was revised based on the approved human dose of 2.56 μmol/kg and converted to 11.8 μmol/kg based on the FDA conversion factor for guinea pigs (4.6 to adjust for body surface area, guinea pig/human, USDHHS, 2005). This was equivalent to 5.26 mg/kg then multiplied by three autoinjectors (maximum pre-hospital dose) for a final dose of 15.8 mg/kg (35 μmol/kg). HI-6 DMS, MMB4 DMS, RS194B and MINA were evaluated at an additional dose level equal to the median lethal dose (LD50) for the oxime divided by the Therapeutic Index (TI) for 2-PAM Cl (Table 2c). Specifically, TI2-PAM Cl is the ratio of the 24-hr LD50 (168 mg/kg; Fleisher et al 1970) to the FDA-approved human therapeutic dose (i.e., median effective dose, ED50 = 25.7 mg/kg; Koplovitz et al. 1992) or
Table 1.
Treatment Groups Study Design
| Group | SC Injection at t=0 min (between scapulae) | IM Injection at t=1 ± 0.25 min (right thigh) | IM Injection immediately post atropine (left thigh) | |||
|---|---|---|---|---|---|---|
| OP | OP Vehicle | Atropine, free base 0.4 mg/kg | Atropine Vehicle, 0.25 mL/kg | Oxime | Oxime Vehicle | |
| 1 | - | X | - | X | - | X |
| 2 | - | X | - | X | X | - |
| 3 | X | - | X | - | - | X |
| 4 | X | - | X | - | X | - |
Table 2a.
Dose Levels for OPs in Atropinized Guinea Pig
| OP | OP Dose Level (LD85) |
|---|---|
| Tabun (GA) | 240 μg/kg |
|
| |
| Sarin (GB) | 52 μg/kg |
|
| |
| Soman (GD) | 49 μg/kg |
|
| |
| Cyclosarin (GF) | 75 μg/kg |
|
| |
| VX | 8 μg/kg |
|
| |
| Chlorpyrifos oxon | 100 mg/kg |
| Paraoxon | 3.37 mg/kg |
|
| |
| Phorate oxon | 2.48 mg/kg |
Table 2b.
Therapy Levels of Oxime Treatments
| Oxime | Oxime Equimolar | IM LD50 (μmol/kg) | Therapy Level as a portion of LD50 | IM LD50 Ref. | |
|---|---|---|---|---|---|
| Therapy (mg/kg) | Level (μmol/kg) | ||||
| 2-PAM Cl | 25.7 | 146 | 951 | 15% | Fleisher et al., 1970 |
|
| |||||
| MMB4 DMS | 65.3 | 146 | 1514 | 10% | unpublished |
|
| |||||
| HI-6 DMS | 69.6 | 146 | 1498 | 10% | unpublished |
|
| |||||
| MINA | 12.7 | 146 | 2492 | 6% | unpublished |
|
| |||||
| RS194B | 31.0 | 146 | 3849 | 4% | unpublished |
|
| |||||
| Obidoxime Cl2 | 52.3 | 146 | 220 | 66% | Fleisher et al., 1970 |
|
| |||||
| TMB-4 | 15.8 | 35 | 179 | 20% | Shih et al., 2009 |
|
| |||||
| HLö-7 DMS | 75.9 | 146 | 993 | 15% | Eyer et al., 1992 |
Table 2c.
TI Dose Levels of Select Oxime Treatments
| Oxime | 24-hr Toxicity (mg/kg) | TI-Based Therapy Level (mg/kg) | Equimolar Therapy Level (mg/kg) | TI-Based/Equimolar Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| n | LD50 | 95% LCL | 95% UCL | Probit Slope | ||||
| MMB4 DMS | 26 | 679 | 635 | 944 | 21 | 104 | 65.3 | 1.6 |
|
| ||||||||
| HI-6 DMS | 31 | 717 | 659 | 766 | 31 | 110 | 69.6 | 1.6 |
|
| ||||||||
| MINA | 31 | 217 | 191 | 243 | 15 | 33 | 12.7 | 2.6 |
|
| ||||||||
| RS194B | 21 | 821 | 733 | 916 | 24 | 126 | 31.0 | 4.1 |
The TI-based dose level for those oximes would be determined using the following method
or 15.3% of the LD50,oxime.
Clinical observations were recorded for 24 hours post challenge by individuals not involved in challenges. Terminal blood samples were collected and processed for all survivors using Hemoglobind™ (McGarry et al. 2013). For each animal, the relative AChE activity level (RAAChE) was calculated as the Ellman assay acetylthiocholine turnover rate in a terminal blood sample divided by the turnover rate in the baseline blood sample (Ellman et al. 1961). A similar calculation was made using butyrylthiocholine turnover rates to determine RABChE for each surviving guinea pig. Cholinesterase activity was normalized to the individual animal’s baselineto determine RAAChE and RABChE, which were compared using t-tests.
Quality of Life Assessment
A QOL scoring system was used to provide an objective value for the clinical signs observed. Increasing scores were indicative of a decrease in the QOL. QOL scores were calculated with group averages at each time-point. The signs and scores associated with the signs are described in Table 3. For the impaired and mild signs, if any of the listed signs were present for that classification (e.g., ataxic, miosis), then the score for that classification was assigned a value of 1 regardless of the presence/absence of other signs in that classification. Moderate and severe signs were scored individually and not as a group. For any time period in which the animal was still alive to include moribund, the highest score that an animal could attain was 11. If death was recorded at any time-point, the total score for that period was assigned a value of 12. The lower the animal’s QOL score, the closer its exhibited behavior was to that prior to challenge. QOL scores were compared using non parametric Wilcoxon Mann Whitney tests. Fisher’s exact tests at a one-sided α = 0.05 decision level were used to contrast lethality between control and each treatment group.
Table 3.
Quality of Life (QOL) Scoring System
| Classification of Signs | Signs | Weighted Score |
|---|---|---|
| Normal | N/A | 0 |
|
| ||
| Impaired | Hunched Posture | 1 |
| Lethargic | ||
| Ataxic | ||
| Weak | ||
| Chewing | ||
| Miosis | ||
| Mydriasis | ||
|
| ||
| Mild | Fasciculations | 1 |
| Lacrimation | ||
| Nystagmus | ||
| Salivation | ||
| Hyperaesthetic | ||
| Other (comment) | ||
|
| ||
| Moderate | Tremors | 1 |
|
| ||
| Severe | Convulsions | 2 |
| Non-Responsive | 2 | |
| Prostration | 2 | |
| Respiratory Distressa | 2 | |
|
| ||
| Terminal | Moribund or Found Dead | 12 |
Abbreviation: N/A, not applicable.
Includes any severe respiratory related signs (i.e., shallow respirations, abdominal respirations, deep abdominal respirations, labored respirations, labored and audible respirations, agonal breathing).
3. Results
Effects of OPs at LD85 Challenges in Positive Control Animals
Clinical signs of cholinesterase intoxication for control OP animals began within 5 minutes post challenge for all G agents, CPO, and phorate oxon and by 30 minutes post challenge for VX and paraoxon. In all OP control animals, salivation or lacrimation, ataxia, fasciculations, respiratory distress, tremors, and prostration were the most prevalent signs. Target LD85 challenges successfully produced lethality between 73 percent and 100 percent for all OPs except VX. The lethality among VX control animals was only 52 percent (50/96).
Mitigation of the Effects of OPs at LD85 Challenges by Oxime Therapies
In Tables 4 through 10, the oxime treatment results for each OP are listed in order of increasing lethality. Significant oxime-related effects (p < 0.05) are indicated with an asterisk. It should be noted that no significant decrease in lethality was seen when treating animals with the equimolar dose relative to the TI dose. However, minor differences were observed in lethality and QOL for select agents when treated with a TI dose of MMB4 DMS, HI-6 DMS or MINA.
Table 4.
Summary of Oximes Challenged with GA
| Oxime Dose | Oxime | Oxime Dose (mg/kg) | 24-hr Lethality | 24-hr Mean QOL Score | 24-hr Mean RAAChE (%) | 24-hr Mean RABChE (%) |
|---|---|---|---|---|---|---|
| Equimolar | MMB4 DMS | 65.3 | 1/8* | 2.0* | 71* | 73 |
| HLö7 DMS | 75.9 | 1/8* | 2.5* | 41 | 50 | |
| Obidoxime | 52.3 | 2/8* | 3.5 | 33 | 26 | |
| HI-6 DMS | 69.6 | 3/8* | 5.6 | 52 | 59 | |
| 2 PAM Cl | 25.7 | 6/8 | 9.6 | 22 | 35 | |
| RS194B | 31 | 7/8 | 10.6 | 32 | 37 | |
| MINA | 12.7 | 7/8 | 11.3 | 28 | 53 | |
| TMB4 | 15.8 | 8/8 | 12.0 | - | - | |
| none | 0.0 | 55/64 = 86% | 10.8† | 35 | 49 | |
|
| ||||||
| Therapeutic | MMB4 DMS | 104 | 0/8* | 0.0* | 75* | 110* |
| HI-6 DMS | 110 | 0/8* | 1.3* | 53* | 58* | |
| MINA | 33.2 | 5/8 | 8.9 | 25 | 34 | |
| RS194B | 126 | 6/8 | 9.8 | 33 | 47 | |
| none | 0.0 | 28/32 = 88% | 11‡ | 24 | 33 | |
Statistically significant at the 0.05 level compared to the control group
pooled across 64 animals challenged as equimolar controls with GA.
pooled across 32 animals challenged as TI controls with GA.
Table 10.
Summary of Oximes Challenged with Phorate oxon
| Oxime Dose | Oxime | Oxime Dose (mg/kg) | 24-hr Lethality | 24-hr Mean QOL Score | 24-hr Mean RAAChE (%) | 24-hr Mean RABChE (%) |
|---|---|---|---|---|---|---|
| Equimolar | HLö7 DMS | 75.9 | 0/8* | 0.1* | 64* | 61 |
| Obidoxime | 52.3 | 0/8* | 1.1* | 75* | 48 | |
| 2 PAM Cl | 25.7 | 1/8* | 1.5* | 48 | 50 | |
| MMB4 DMS | 65.3 | 2/8* | 3.0* | 64* | 55 | |
| TMB4 | 15.8 | 6/8 | 9.6 | 67* | 52 | |
| HI-6 DMS | 69.6 | 7/8 | 10.6 | 48 | 44 | |
| RS194B | 31 | 7/8 | 11.3 | 47 | 49 | |
| MINA | 12.7 | 8/8 | 12.0 | - | - | |
| none | 0.0 | 62/64 = 97% | 11.8† | 32 | 36 | |
|
| ||||||
| Therapeutic | MMB4 DMS | 104 | 1/8* | 1.5* | 82 | 56 |
| HI-6 DMS | 110 | 5/8 | 8.3 | 50 | 43 | |
| RS194B | 126 | 7/8 | 10.5 | 52 | 53 | |
| MINA | 33.2 | 8/8 | 12.0 | - | - | |
| none | 0.0 | 32/32 = 100% | 12.0 | - | - | |
Statistically significant at the 0.05 level compared to the control group
pooled across 64 animals challenged as equimolar controls with phorate oxon
Tabun (GA)
Treatment of GA-challenged animals with either MMB4 DMS or HLö-7 DMS reduced lethality to 13 percent, significantly less than the 86 percent obtained in the control animals. Additionally, both oximes reduced the occurrence of respiratory distress and prostration, with MMB4 DMS-treated animals primarily exhibiting only ataxia between 1 and 8 hours post challenge. Although lethality for GA-challenged animals treated with TMB-4 was 100 percent, the clinical presentations of respiratory distress and prostration were reduced. MMB4 DMS and HLö-7 DMS treatment resulted in QOL scores that were significantly reduced in treatment group animals compared to control group animals from 30 minute post challenge through the 24 hour observation. Although other oximes provided some benefit at various time points, only MMB4 DMS and HLö-7 DMS treatment limited clinical signs to the mild or moderate classification at the 24 hour observation time point. As shown in Table 4, MMB4 DMS-treated animals exhibited relatively uninhibited activity for both AChE and BChE (greater than 70 percent) at 24 hours post challenge. This activity level for both ChEs was more than 20 percent higher than the activity level of the GA-challenged control animals. Only MMB4 DMS and HI-6 DMS offered greater mitigation of OP effects when the oximes were given at TI-based levels relative to equimolar levels. Both provided significant ChE reactivation and MMB4 DMS animals were asymptomatic at the 24 hour observation.
Sarin (GB)
All GB-challenged animals survived when treated with either MMB4 DMS or 2-PAM Cl, and the effect was significant (p < 0.05) relative to the 73 percent lethality obtained in the control animals (Table 5). The oxime therapy in these two groups resulted in the majority of animals returning to normal by 24 hours post challenge. Both oximes delayed the time to onset of signs by 25 minutes and reduced the frequencies of respiratory distress and prostration. MMB4 DMS, 2-PAM Cl, and HI-6 DMS provided sufficient protection against GB that QOL scores in treatment group animals compared to control group animals were significantly reduced from 30 minutes post challenge through the 24 hour observation. Although other oximes provided some statistical significance at various time-points, only MMB4 DMS and 2-PAM Cl treatment resulted in QOL scores at the minimal “impaired” level at the 24 hour observation time point. 2-PAM Cl, MMB4 DMS, HI-6 DMS, and TMB-4 significantly mitigated both AChE and BChE inhibition. As shown in Table 5, only MINA had significant improvement of therapy at the TI dose with zero lethality and animals being asymptomatic at the 24 hour observation.
Table 5.
Summary of Oximes Challenged with GB
| Oxime Dose | Oxime | Oxime Dose (mg/kg) | 24-hr Lethality | 24-hr Mean QOL Score | 24-hr Mean RAAChE (%) | 24-hr Mean RABChE (%) |
|---|---|---|---|---|---|---|
| Equimolar | MMB4 DMS | 65.3 | 0/8* | 0.5* | 92* | 78* |
| 2 PAM Cl | 25.7 | 0/8* | 0.4* | 69* | 89* | |
| HI-6 DMS | 69.6 | 1/8* | 2.8* | 84* | 79* | |
| HLö7 DMS | 75.9 | 2/8* | 3.1* | 67* | 70* | |
| RS194B | 31 | 2/8* | 3.1* | 43 | 56 | |
| Obidoxime | 52.3 | 3/8 | 5.1* | 80* | 83* | |
| MINA | 12.7 | 3/8 | 6.9 | 25 | 38 | |
| TMB4 | 15.8 | 4/8 | 6.6 | 53* | 63 | |
| none | 0.0 | 47/64 = 73% | 9.8† | 28 | 42 | |
|
| ||||||
| Therapeutic | MINA | 33.2 | 0/8* | 0.0* | 48* | 56 |
| MMB4 DMS | 104 | 1/8* | 2.8* | 84* | 94* | |
| RS194B | 126 | 3/8* | 4.5* | 74* | 74* | |
| HI-6 DMS | 110 | 3/8* | 5.8 | 74* | 76* | |
| none | 0.0 | 29/32 = 91% | 11.3‡ | 19 | 29 | |
Statistically significant at the 0.05 level compared to the control group
pooled across 64 animals challenged as equimolar controls with GB
pooled across 32 animals challenged as TI controls with GB
Soman (GD)
When tested against a GD challenge, none of the oximes tested showed any significant differences in the measured endpoints between the treatment and control groups (data not shown). It may be of interest that HLö-7 DMS delayed the time to onset of signs by 25 minutes, although none of the animals in this group survived to the 24 hour post challenge time point.
Cyclosarin (GF)
Treatment of GF-challenged animals with MMB4 DMS significantly reduced lethality to 13 percent compared to the 89 percent lethality in the control group (Table 6). In addition, half of the MMB4 DMS-treated animals became asymptomatic by 24 hours post challenge. MMB4 DMS also reduced the frequency of salivation/lacrimation, fasciculations, tremors, and prostration as compared to control animals. MMB4 DMS provided sufficient protection against GF that QOL scores in treatment group animals compared to control group animals were significantly reduced from 30 minutes post challenge through the 24 hour observation, when signs were mild to moderate in severity. MMB4 DMS offered statistically significant reactivation of both AChE and BChE. HI-6 DMS also provided significant reactivation; however those survivors, as well as the HLö-7 DMS survivors, had QOL scores that reflected moderate to severe signs at the end of the observation period. No improvements in therapy were seen with the TI dose with any of the oximes.
Table 6.
Summary of Oximes Challenged with GF
| Oxime Dose | Oxime | Oxime Dose (mg/kg) | 24-hr Lethality | 24-hr Mean QOL Score | 24-hr Mean RAAChE (%) | 24-hr Mean RABChE (%) |
|---|---|---|---|---|---|---|
| Equimolar | MMB4 DMS | 65.3 | 1/8* | 2.4* | 88* | 97* |
| HI-6 DMS | 69.6 | 2/8* | 5.0* | 65 | 57 | |
| HLö7 DMS | 75.9 | 2/8* | 5.4* | 51 | 59 | |
| 2 PAM Cl | 25.7 | 6/8 | 10.3 | 35 | 67 | |
| MINA | 12.7 | 6/8 | 10.6 | 19 | 29 | |
| TMB4 | 15.8 | 7/8 | 10.5 | 74 | 85 | |
| Obidoxime | 52.3 | 7/8 | 11.3 | 48 | 70 | |
| RS194B | 31 | 7/8 | 11.9 | 23 | 37 | |
| none | 0.0 | 57/64 = 89% | 11.1† | 36 | 50 | |
|
| ||||||
| Therapeutic | MMB4 DMS | 104 | 3/8* | 6.0 | 82* | 95* |
| HI-6 DMS | 110 | 3/8* | 6.6* | 53* | 57* | |
| MINA | 33.2 | 5/8 | 8.6 | 32 | 39 | |
| RS194B | 126 | 7/8 | 11.4 | 40 | 52 | |
| none | 0.0 | 27/32 = 84% | 10.9‡ | 23 | 35 | |
Statistically significant at the 0.05 level compared to the control group
pooled across 64 animals challenged as equimolar controls with GF
pooled across 32 animals challenged as TI controls with GF
VX
Although VX lethality in controls was only 52 percent, the model was able to detect significant efficacy and differentiate among the oximes. The LD85 of VX used in this study was based on a dose/lethality probit curve with a slope of 34 (p = 0.041), determined in preparation for this work (data not shown). All animals treated with 2-PAM Cl, MMB4 DMS, HLö-7 DMS, or TMB-4 survived. Treatment with those oximes, as well as treatment with obidoxime Cl2, resulted in QOL scores at the minimal “impaired” level (i.e., ataxia) at the 24 hour observation time point. Although the 24 hour QOL scores for both TMB-4 and obidoxime Cl2 appeared to be low, the means were not statistically different from that for the control animals due to an inadvertently low challenge level across all groups. Animal groups treated with those oximes had statistically significant reactivation of AChE compared to the control group animals (Table 7). All animals treated with 2-PAM Cl were normal at every observation, and those treated with MMB4 DMS exhibited reduced frequencies of fasciculations, respiratory distress, and prostration. Both oximes provided adequate therapy for animals to be asymptomatic by the 24 hour observation. Additionally, with the TI dose of MINA, zero lethality was reported with improvement in the QOL score at 24 hours.
Table 7.
Summary of Oximes Challenged with VX
| Oxime Dose | Oxime | Oxime Dose (mg/kg) | 24-hr Lethality | 24-hr Mean QOL Score | 24-hr Mean RAAChE (%) | 24-hr Mean RABChE (%) |
|---|---|---|---|---|---|---|
| Equimolar | 2 PAM Cl | 25.7 | 0/8* | 0.0* | 86* | 87* |
| MMB4 DMS | 65.3 | 0/8* | 0.0* | 104* | 93* | |
| HLö7 DMS | 75.9 | 0/8* | 1.0* | 73* | 77 | |
| TMB4 | 15.8 | 0/8* | 1.0 | 68* | 67 | |
| Obidoxime | 52.3 | 1/8* | 1.5 | 92* | 96* | |
| HI-6 DMS | 69.6 | 2/8 | 3.6* | 103* | 94* | |
| MINA | 12.7 | 2/8 | 3.8 | 49 | 64 | |
| RS194B | 31 | 4/8 | 7.0 | 53 | 57 | |
| none | 0.0 | 33/64 = 52% | 6.9† | 46 | 62 | |
|
| ||||||
| Therapeutic | MMB4 DMS | 104 | 0/8* | 0.3* | 96* | 97* |
| MINA | 33.2 | 0/8* | 0.9* | 53 | 64 | |
| HI-6 DMS | 110 | 2/8 | 3.8 | 85* | 75* | |
| RS194B | 126 | 3/8 | 6.0 | 69* | 64 | |
| none | 0.0 | 17/32 = 53% | 7.8‡ | 44 | 52 | |
Statistically significant at the 0.05 level compared to the control group
pooled across 64 animals challenged as equimolar controls with VX
pooled across 32 animals challenged as TI controls with VX
Chlorpyrifos oxon (CPO)
Treatment of CPO-challenged animals with obidoxime Cl2, MMB4 DMS, HLö-7 DMS, and 2-PAM Cl significantly reduced lethality in the treatment group animals to ≤38 percent compared to 78 percent in the control group animals (Table 8). Additionally, obidoxime Cl2, MMB4 DMS, HLö-7 DMS, 2-PAM Cl, and RS194B significantly reduced the frequencies of lacrimation, fasciculations, respiratory distress, and prostration. Obidoxime Cl2, MMB4 DMS, and HLö-7 DMS treatment significantly improved QOL scores in treatment groups compared to the control group at 24 hours post challenge, at which time clinical signs in the treatment groups were limited to the mild and moderate categories. Among the oximes offering significantly improved survivability, only obidoxime Cl2 also provided statistically significant reactivation of both ChEs. Although 2-PAM Cl appeared to improve ChE activities, statistical significance could not be determined.
Table 8.
Summary of Oximes Challenged with Chlorpyrifos oxon
| Oxime Dose | Oxime | Oxime Dose (mg/kg) | 24-hr Lethality | 24-hr Mean QOL Score | 24-hr Mean RAAChE (%) | 24-hr Mean RABChE (%) |
|---|---|---|---|---|---|---|
| Equimolar | MMB4 DMS | 65.3 | 1/8* | 2.4* | 37 | 19 |
| Obidoxime | 52.3 | 1/8* | 1.8* | 43 | 17 | |
| HLö7 DMS | 75.9 | 2/8* | 3.1* | 19 | 11 | |
| 2 PAM Cl | 25.7 | 3/8 | 4.5 | 92* | 68* | |
| RS194B | 31 | 4/8 | 7.0 | 37 | 37 | |
| TMB4 | 15.8 | 5/8 | 8.5 | 43 | 10 | |
| HI-6 DMS | 69.6 | 6/8 | 9.9 | 14 | 8 | |
| MINA | 12.7 | 7/8 | 10.5 | 60 | 76 | |
| none | 0.0 | 50/64 = 78% | 10.1† | 13 | 8 | |
|
| ||||||
| Therapeutic | MMB4 DMS | 104 | 0/8* | 0.5* | 19 | 8 |
| RS194B | 126 | 2/8* | 4.3* | 9 | 3 | |
| MINA | 33.2 | 5/8 | 7.6 | 11 | 4 | |
| HI-6 DMS | 110 | 5/8 | 8.9 | 7 | 3 | |
| none | 0.0 | 27/32 = 84% | 10.3‡ | 9 | 4 | |
Statistically significant at the 0.05 level compared to the control group
pooled across 64 animals challenged as equimolar controls with chlorpyrifos oxon
pooled across 32 animals challenged as TI controls with chlorpyrifos oxon
Paraoxon and Phorate oxon (PHO)
When treated with 2-PAM Cl, HLö-7 DMS, obidoxime Cl2, or MMB4 DMS, the lethality for the pesticides paraoxon and phorate oxon were significantly reduced to rates between 0 and 25 percent. HI-6 DMS and TMB-4 also provided significant protection against paraoxon with 13 percent and 25 percent lethality, respectively. Control group animals challenged with paraoxon and phorate oxon had lethality of 84 percent and 97 percent, respectively (Tables 9 and 10). There was also a significant reduction in frequencies of salivation, fasciculations, respiratory distress, and prostration with 2-PAM Cl, HLö-7 DMS, obidoxime Cl2, or MMB4 DMS. QOL scores for the animals treated with 2-PAM Cl, HLö-7 DMS, obidoxime Cl2, or MMB4 DMS were significantly reduced relative to the control animals at 24 hours post challenge, with oxime-treated animals showing only impaired to moderate signs. Against paraoxon, MMB4 DMS and TMB-4 provided reactivation of both ChEs, while HLö-7 DMS reactivated only AChE, relative to control animals. There was no significant difference in cholinesterase activity of survivors within the phorate oxon-challenged animals at 24 hours.
Table 9.
Summary of Oximes Challenged with Paraoxon
| Oxime Dose | Oxime | Oxime Dose (mg/kg) | 24-hr Lethality | 24-hr Mean QOL Score | 24-hr Mean RAAChE (%) | 24-hr Mean RABChE (%) |
|---|---|---|---|---|---|---|
| Equimolar | 2 PAM Cl | 25.7 | 0/8* | 0.1* | 49* | 45* |
| HLö7 DMS | 75.9 | 0/8* | 1.0* | 38 | 30 | |
| Obidoxime | 52.3 | 1/8* | 1.5* | 80* | 47* | |
| MMB4 DMS | 65.3 | 1/8* | 2.3* | 71* | 52* | |
| HI-6 DMS | 69.6 | 1/8* | 3.3* | 25 | 19 | |
| TMB4 | 15.8 | 2/8* | 4.9* | 52* | 31 | |
| MINA | 12.7 | 7/8 | 11.3 | 16 | 14 | |
| RS194B | 31 | 8/8 | 12.0 | - | - | |
| none | 0.0 | 54/64 = 84% | 10.8† | 23 | 21 | |
|
| ||||||
| Therapeutic | MMB4 DMS | 104 | 0/8* | 0.0* | 35 | 22 |
| HI-6 DMS | 110 | 5/8 | 8.6 | 26 | 19 | |
| RS194B | 126 | 5/8 | 7.6 | 16 | 11 | |
| MINA | 33.2 | 7/8 | 11.3 | 5 | 4 | |
| none | 0.0 | 28/32 = 88% | 11.1‡ | 13 | 7 | |
Statistically significant at the 0.05 level compared to the control group
pooled across 64 animals challenged as equimolar controls with paraoxon
pooled across 32 animals challenged as TI controls with paraoxon
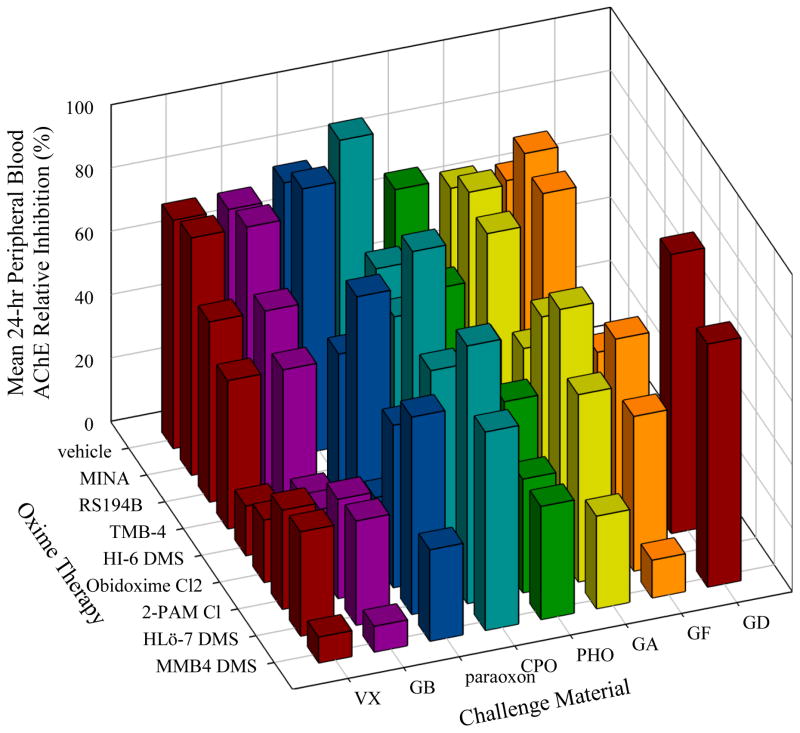
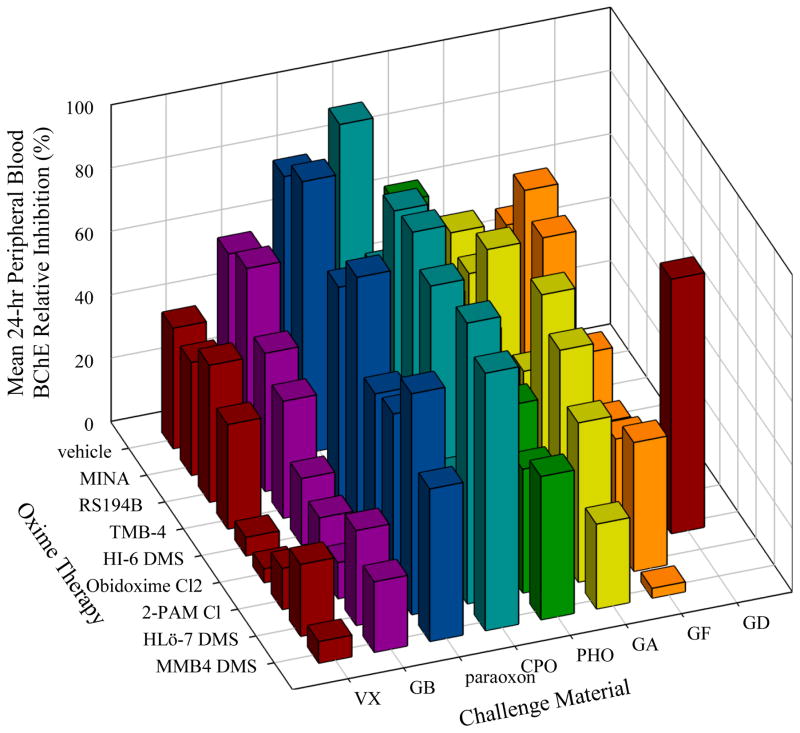
Figure 2 presents the 24-hour lethality data collated across the eight OPs tested, and illustrates that MMB4 DMS and HLö-7 DMS offered protection against all OPs except GD, and that 2-PAM Cl and obidoxime Cl2 were effective against all but GD, GF, and (for 2-PAM Cl) GA. A comparison with the equimolar (Figure 2) and TI lethality (Figure 3) shows that no significant difference is seen in the lethality results for any agents except a slight improvement for GB when treating with MINA. Figure 4 presents the mean equimolar QOL scores at the 24-hour observation, and is consistent with the efficacy pattern of the lethality data. Figure 5 collates the complement of the equimolar relative AChE activity at 24 hours (100 – RAAChE = relative AChE inhibition) in order to represent effective oximes with short bars, as in Figures 3 and 4. A similar plot for the equimolar 24-hour BChE is presented in Figure 6. The fact that ChE activity in the blood did not correlate well with lethality can be seen by contrasting Figures 5 and 6 with Figure 3.
Figure 2.
24-hour Lethality for Each OP/Oxime Equimolar Combination
Figure 3.
24-hour Lethality for Each OP/Oxime TI Combination
Figure 4.
Mean 24-hour Quality of Life Score for Each OP/Oxime Equimolar Combination
Figure 5.
Mean 24-hour Peripheral Blood Acetylcholinesterase Relative Inhibition for Each OP/Oxime Equimolar Combination
Figure 6.
Mean 24-hour Peripheral Blood Butyrylcholinesterase Relative Inhibition for Each OP/Oxime Equimolar Combination
There was a clear division of oxime efficacy in animals challenged with tabun (GA), cyclosarin (GF), and phorate oxon (PHO). Against GA and GF, MMB4-DMS, HLö-7 DMS, and HI-6 DMS showed statistically significant protection against lethality (lethality ≤ 38 percent) relative to control animals, while obidoxime Cl2 was statistically significant against lethality (25 percent lethality) only for GA. MMB4-DMS, HLö-7 DMS, 2-PAM Cl, and obidoxime Cl2 showed statistically significant protection against lethality (lethality ≤38 percent) against both phorate oxon and CPO-challenged animals. All other oximes (TMB-4, RS194B, and MINA), when used as therapy against GA, GF, CPO, or phorate oxon, demonstrated low efficacy with generally ≥50 percent lethality.
GB and VX had much higher ChE reactivation rates and no more than 50 percent lethality in all oxime-treated groups. For oxime therapy against GB, MMB4-DMS, HLö-7 DMS, HI-6 DMS, 2-PAM Cl, and RS194B all exhibited statistically significant improvement in lethality relative to the controls. In pesticide oxon-challenged animals, MMB4-DMS, HLö-7 DMS, and obidoxime Cl2 were each significantly efficacious relative to lethality in control animals. Obidoxime Cl2 demonstrated the best overall protection for pesticide oxons with significant ChE reactivation, improvement in QOL scores, and survival through the 24 hour period. Obidoxime Cl2 has been shown to be one of the most efficacious reactivators against OP pesticides by other laboratories (Worek et al., 2007), and, in the present study, the oxime performed well against the nerve agents GA, GB, and VX as well.
MMB4 DMS and HLö-7 DMS were the two most consistently efficacious oximes across all challenge OPs. MMB4 DMS treatment resulted in an average 80 percent survivability while HLö-7 DMS treatment resulted in an average of 77 percent. The 24 hour average QOL score was ≤3.0 for MMB4 DMS and ≤5.4 for HLö-7 DMS on a 0 to 12 scale, excluding GD data. Additionally, reactivation of both AChE and BChE was more than 50 percent with MMB4 DMS for all OP challenges with the exception of chlorpyrifos oxon and GD. Peripheral blood ChE inhibition by GF and VX was significantly mitigated by MMB4 DMS, with total cholinesterase reactivation at 88 to 100 percent. Reactivation of AChE and BChE among survivors with HLö-7 DMS was not as significant when compared to that of MMB4 DMS; however, both enzymes were reactivated above 30 percent for all OPs with the exception of chlorpyrifos oxon and GD.
4. Discussion
Oxime efficacy is an amalgamation of somewhat unrelated physicochemical and pharmacologic factors. A favored, effective oxime has a relatively high therapeutic/safety index, quickly biodistributes to organs targeted by OPs (Voicu et al., 2013), effectively reactivates inhibited AChE within those target organs, and ensures permanent rapid elimination of the poison. Many research articles have discussed the merits of particular reactivator compounds against specific CWNAs, and several review articles have described the history and protective ratios of medical counter measures to OP intoxication (Dawson, 1994; Stojiljković and Jokanović, 2006; Worek et al., 2007; Antonijevic and Stojiljkovic, 2007). In the following discussion, we review each of the oximes tested in the present study within the context of those historical data. It is important to note that since these historical data have been obtained under vastly different experimental conditions, e.g., different animal species, doses, timing and routes of administration of the oxime, adjuvants, or challenge materials, the results may not be directly comparable. It is the result of this variability in procedures that necessitated the evaluation of promising oximes within a single study in a standardized and comparable manner.
2-PAM Cl, first synthesized in 1955 (Childs et al., 1955), is a monopyridinium oxime with the aldoximide in the 2-position. In clinical settings, the use of 2-PAM Cl is contraindicated (Wille et al., 2013) or at least controversial (Rosman et al., 2009) against some pesticide intoxication. In the present study, 2-PAM Cl offered significant survival protection against LD85 challenges of GB, VX, and the pesticide oxons; however significant AChE reactivation was observed only for GB and VX. These data are consistent with the less than optimal utility of 2-PAM Cl against GA, GD, and GF observed in this study, and underscore the need for a second generation reactivator for use in the U.S. In vitro reactivation studies using human AChE indicated that 2-PAM Cl was generally the least effective against GA, GB, GF, and VX relative to HLö-7, HI-6, MMB4, and obidoxime (Worek et al., 2007) when compared to the other oximes. A similar study by Cadieux et al. (2010) also indicated less than optimal in vitro reactivation against GA, GB, GF, VX, and Russian VX (VR) relative to HI-6 and MMB4.
In the present study, MMB4 DMS offered significant protection in terms of survivability against all of the OPs at both the equimolar and TI dose levels except GD, likely due to rapid “aging” (irreversible dealkylation of an alkoxy chain on the phosphorus atom) of the GD/AChE conjugate (Vale, 2009). In terms of reactivation of blood AChE and BChE 24-hour post-challenge, MMB4 DMS was superior to the other seven oximes tested.
Similar to MMB4 DMS, HLö-7 DMS (at 146 μmol/kg given at 1 min after challenge) was significantly effective against every OP challenge except GD as well. These results concur with the literature in that HLö-7 DMS is a very good candidate for treatment of most OPs (Eyer et al., 1992). Even prophylactically, HLö-7 diiodide at 43 μmol/kg by intraperitoneal injection (IP) was effective for reactivating diaphragm ChE inhibition by the G agents in atropinized mice (Clement et al., 1992).
HI-6 is available both as a dichloride or dimethanesulfonate salt. The dichloride form of HI-6 is moderately effective in vitro at reactivating GB-inhibited rat AChE (Esposito et al., 2014), whereas the dimethanesulfonate salt was shown to be superior in terms of both solubility in biocompatible vehicles and biodistribution (Kuca et al., 2007b). HI-6 historically is a potent in vitro reactivator of GD- and GF- but not GA-inhibited AChE (Lundy et al., 1992; Clement et al., 1992; Worek et al., 2007; Esposito et al., 2014). Some have even stated that HI-6, despite poor activity against GA, is as close to a broad-spectrum oxime as any (Soukup et al., 2013). In the present study, HI-6 DMS at 146 μmol/kg was significantly effective in promoting survival against GA, GB, GF, and paraoxon, but did not possess as broad a spectrum of activity as did MMB4 DMS or HLö-7 DMS. At the TI dose level (245 μmol/kg) similar results were seen as compared to the equimolar treatment, except with paraoxon where the TI therapy was not effective.
Obidoxime dichloride offered significant survival protection against GA, (nearly GB, p = 0.0515), VX, and each of the pesticide oxons, confirming historical data. In vitro tests showed that obidoxime was a relatively poor reactivator of rat GA/AChE and GF/AChE conjugates, was a moderate reactivator against GB, but performed well against VX (Esposito et al., 2014). Obidoxime has exhibited ChE reactivation activity against the pesticides chlorpyrifos (Musilek et al., 2005), parathion, and oxydemeton-methyl (Thiermann et al., 1997).
RS194B is a relatively new compound, the most effective among a class of uncharged N-substituted 2-hydroxyiminoacetamido alkylamine compounds tested in mice (Radić et al., 2012). However, at the equi-molar to 2-PAM Cl level of 146 μmol/kg, a significant increase in survival was observed only against GB in the present study. However, significant survival was seen against GB and chlorpyrifos oxon at the TI dose level (281 μmol/kg).
Since TMB-4 was lethal at 146 μmol/kg in atropinized guinea pigs in the present study, the treatment dose was reduced to 35 μmol/kg (20% of the IM LD50) for evaluations. TMB-4 at 35 μmol/kg significantly improved survival rates only against LD85 challenge doses of VX and paraoxon, but significant reactivation of blood AChE was observed only against VX, paraoxon, GB, and CPO. These observations are partially in agreement with those observed by others, where TMB-4 offered high reactivation of rat AChE inhibited by either GA, GB, or VX but not GF (Esposito et al., 2014).
MINA was the only non-heterocyclic oxime tested in the present study. This oxime is also capable of diffusion across the BBB (Skovira et al., 2010). Here, protection by MINA alone at the equimolar dose did not reach statistical significance against all OPs tested. However, at the TI dose level (74 μmol/kg) significant protection was seen with complete survival against both GB and VX. MINA has been shown to offer significant protection (the lower 95 percent confidence limits for each PR were >1) against GB, GF, and VX both with and without atropine therapy, but only at much higher levels (402, 689, and 1148 μmol/kg). While MINA affords protection against GB and VX, it is a relatively poor reactivator of both AChE and BChE. Protection by MINA was evident when coadministered with atropine and 2-PAM Cl therapies, though this may be related to concurrent reactivation of peripheral and central cholinesterase by 2-PAM Cl and MINA, respectively (Skovira et al., 2010).
Conclusion
Collectively, the oxime reactivators MMB4 and HLö-7 were the most efficacious of all the oximes evaluated across the spectrum of eight subcutaneously administered OPs tested. In terms of overall best efficacy performance based on 24-hour survivability, QOL, and blood cholinesterase levels, using the standardized equimolar (146 μmol/kg) approach, treatments of the dimethanesulfonate salts of MMB4 and HLö-7 offered the most protection. Additionally, none of the oximes evaluated at their TI dose exhibited protection levels matching those exhibited by MMB4 and HLö-7 at the standardized equimolar dose. 2-PAM chloride, obidoxime dichloride, and HI-6 DMS were identified in the second efficacy tier, and RS194B and MINA offered the least general protection in a third efficacy tier. TMB-4 was tested at 35 μmol/kg due to its toxicity and offered survival protection between the second and third tiers.
Highlights.
First comprehensive evaluation of leading AChE oxime reactivators
All oximes are compared against current U.S. therapy 2-PAM Cl
Relative therapeutic oxime efficacies against OP CWNA and pesticides
Contribution to more effective antidotes for civilian and military populations
Acknowledgments
The authors wish to recognize the excellent technical assistance of Jennifer Webb, Ashley Robertson, Richard Morosco, Beth Reed, Kevin McGarry, Ernest Johnson, and Benjamin Carper. A special thanks to the work of Rakesh K. Sit and Valery V. Fokin (Department of Chemistry and the Skaggs Institute for Chemical Biology, The Scripps Research Institute, La Jolla, CA) for the design, synthesis, and characterization work on RS194B. Additionally, we thank the Medical Countermeasure Systems Joint Project Management Office, Department of Defense, for providing the oxime MMB-4 DMS through an agency to agency material transfer.
Abbreviations
- 2-PAM Cl
pralidoxime chloride
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
- BBB
blood brain barrier
- ChE
cholinesterase
- CNS
central nervous system
- CWNA
chemical weapon nerve agent
- FDA
Food and Drug Administration
- HI-6 DMS
1-(((4-(aminocarbonyl) pyridinio)methoxy) methyl)-2-((hydroxyimino)methyl) pyridinium dimethanesulfonate
- HLö-7 DMS
pyridinium,1-(((4-(aminocarbonyl) pyridinio)methoxy)methyl)-2,4-bis((hydroxyimino)methyl), dimesylate
- HPLC
high performance liquid chromatography
- IM
Intramuscular
- LD50
median lethal dose
- MINA
monoisonitrosoacetone
- MMB4 DMS
methoxime dimethanesulfonate; 1,1-methylene bis(4(hydroxyimino-methyl)pyridinium) dimethanesulfonate
- MW
molecular weight
- OP
organophosphorus
- PR
protective ratio
- QOL
quality of life
- RAAChE
baseline-normalized activity of acetylcholinesterase in peripheral blood
- RABChE
baseline-normalized activity of butyrylcholinesterase in peripheral blood
- RS194B
N-(2-(azepan-1-yl)ethyl)-2-(hydroxyimino)acetamide
- SC
subcutaneous
- TMB-4
trimedoxime bromide, TMB-4, 1′-propane-1,3-diylbis{4-[(E)-(hydroxyimino)methyl]pyridinium} dibromide
- USDHHS
U.S. Department of Health and Human Services
Footnotes
Declaration of Interest
The authors have no known conflicts of interest. This work was supported by the National Institutes of Health (NIH) Office of the Director through an interagency agreement (OD#: Y1-OD-0387-01) between the National Institute of Allergy and Infectious Diseases (NIAID) and Department of Defense (DoD) and prepared under the auspices of the NIH, NIAID, National Institute of Neurological Disorders and Stroke (NINDS), and the DoD Defense Technical Information Center (DTIC) under the Chemical, Biological, Radiological & Nuclear Defense Information Analysis Center (CBRNIAC) program, Contract No. SP0700-00-D-3180, Delivery Order Number 0687, CBRNIAC Task 832/CB-IO-OOI2.
The views expressed in this article are those of the authors and do not reflect the official policy of the NIH, Department of Health and Human Services, or the U.S. Government. No official support or endorsement of this article by the NIAID, NINDS, or NIH is intended or should be inferred. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Battelle. All procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89–544), as amended.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Thomas H. Snider, Email: snidert@battelle.org.
Michael C. Babin, Email: babinm@battelle.org.
David A. Jett, Email: jettd@ninds.nih.gov.
Gennady E. Platoff, Jr., Email: platoffg@niaid.nih.gov.
David T. Yeung, Email: dy70v@nih.gov.
References
- Antonijevic B, Stojiljkovic MP. Unequal efficacy of pyridinium oximes in acute organophosphate poisoning. Clin Med Res. 2007;5(1):71–82. doi: 10.3121/cmr.2007.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew BM. Oximes and hydroxamic acids as antidotes in anticholinesterase poisoning. Br J Pharmacol Chemother. 1956;11(4):417–423. doi: 10.1111/j.1476-5381.1956.tb00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar FG, Ohura K, Ogihara T, Imai T. Species difference of esterase expression and hydrolase activity in plasma. J Pharm Sci. 2012;101(10):3979–3988. doi: 10.1002/jps.23258. [DOI] [PubMed] [Google Scholar]
- Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv Clin Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- Bajgar J. Treatment and prophylaxis of nerve agent/organophosphates intoxication. Therap Pharmacol Clin Toxicol. 2009;13:247–253. [Google Scholar]
- Bajgar J. Optimal Choice of Acetylcholinesterase Reactivators for Antidotal Treatment of Nerve Agent Intoxication. ACTA MEDICA. 2010;53(4):207–211. doi: 10.14712/18059694.2016.78. [DOI] [PubMed] [Google Scholar]
- Baker A. Syrian Opposition Says Deadly Chemical Attack Kills Hundreds in Damascus. Time. 2013 Aug 21 [Google Scholar]
- Cabal J, Kuca K, Kassa J. Specification of the structure of oximes able to reactivate tabun-inhibited acetylcholinesterase. Basic Clin Pharmacol Toxico. 2004;95:81–86. doi: 10.1111/j.1742-7843.2004.950207.x. [DOI] [PubMed] [Google Scholar]
- Cadieux CL, Broomfield CA, Kirkpatrick MG, Kazanski ME, Lenz DE, Cerasoli DM. Comparison of human and guinea pig acetylcholinesterase sequences and rates of oxime-assisted reactivation. Chem Biol Interact. 2010;187(1–3):229–233. doi: 10.1016/j.cbi.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Calic M, Lucic-Vrdoljak A, Radic B, Jelic D, Jun D, Kuca K, Kovarik Z. In vitro and in vivo evaluation of pyridinium oximes: mode of interaction with acetylcholinesterase, effect on tabun- and soman-poisoned mice and their cytotoxicity. Toxicology. 2006;219:85–96. doi: 10.1016/j.tox.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Childs AF, Davies DR, Green AL, Rutland JP. The reactivation by oximes and hydroxamic acids of cholinesterase inhibited by organo-phosphorus compounds. Br J Pharmacol Chemother. 1955;10(4):462–5. doi: 10.1111/j.1476-5381.1955.tb00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement JG, Hansen AS, Boulet CA. Efficacy of HLö-7 and pyrimidoxime as antidotes of nerve agent poisoning in mice. Arch Toxicol. 1992;66(3):216–9. doi: 10.1007/BF01974018. [DOI] [PubMed] [Google Scholar]
- Dawson RM. Review of oximes available for treatment of nerve agent poisoning. J Appl Toxicol. 1994;14(5):317–331. doi: 10.1002/jat.2550140502. [DOI] [PubMed] [Google Scholar]
- Delfino RT, Ribeiro TS, Figueroa-Villar JD. Organophosphorus compounds as chemical warfare agents: a review. J Braz Chem Soc. 2009;20:407–428. [Google Scholar]
- Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med. 2013;19 (10):1194–1195. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- Dultz L, Epstein MA, Freeman G, Gray EH, Weil WB. Studies on a group of oximes as therapeutic compounds in sarin poisoning. J Pharmacol Exp Ther. 1957;119(4 April):522–31. [PubMed] [Google Scholar]
- Ekström F, Hörnberg A, Artursson E, Hammarström LG, Schneider G, Pang YP. Structure of HI-6*sarin-acetylcholinesterase determined by X-ray crystallography and molecular dynamics simulation: reactivator mechanism and design. PLoS One. 2009;4(6):e5957. doi: 10.1371/journal.pone.0005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Esposito EX, Stouch TR, Wymore T, Madura JD. Exploring the Physicochemical Properties of Oxime-Reactivation Therapeutics for Cyclosarin, Sarin, Tabun, and VX Inactivated Acetylcholinesterase. Chem Res Toxicol. 2014;27(1):99–110. doi: 10.1021/tx400350b. [DOI] [PubMed] [Google Scholar]
- Eyer P, Hagedorn I, Klimmek R, Lippstreu P, Löffler M, Oldiges H, Spöhrer U, Steidl I, Szinicz L, Worek F. HLö 7 dimethanesulfonate, a potent bispyridinium-dioxime against anticholinesterases. Arch Toxicol. 1992;66(9):603–21. doi: 10.1007/BF01981499. [DOI] [PubMed] [Google Scholar]
- Eyer P, Radtke M, Worek F. Reactions of isodimethoate with human red cell acetylcholinesterase. Biochem Pharmacol. 2008;75:2045–53. doi: 10.1016/j.bcp.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Fleisher JH, Harris LW, Miller GR, Thomas NC, Cliff WJ. Antagonism of sarin poisoning in rats and guinea pigs by atropine, oximes, and mecamylamine. Toxicol Appl Pharmacol. 1970;16(1):40–47. doi: 10.1016/0041-008x(70)90160-2. [DOI] [PubMed] [Google Scholar]
- Harvilchuck JA, Hong SP, Richey JS, Osheroff MR, Johnson JD. In vivo acetylcholinesterase reactivation in male guinea pigs and rhesus macaques following cyclosarin exposure and treatment with 1,1′-methylenebis{4-[(hydroxyimino)methyl] pyridinium} dimethanesulfonate. Int J Toxicol. 2013;32(4 Suppl):99S–107S. doi: 10.1177/1091581813498778. [DOI] [PubMed] [Google Scholar]
- Hubbard B, Mazzetti M, Landler M. Blasts in the night, a smell, and a flood of Syrian victims. The New York Times. 2013 Aug 26 [Google Scholar]
- Inns RH, Leadbeater L. The efficacy of bispyridinium derivatives in the treatment of organophosphate poisoning in the guinea-pig. J Pharm Pharmacol. 1983;35:427–433. doi: 10.1111/j.2042-7158.1983.tb04316.x. [DOI] [PubMed] [Google Scholar]
- International Programme on Chemical Safety. Organophosphorus pesticides. World Health Organization; Geneva: 1989, 1999. Poisons Information Monograph G001. [Google Scholar]
- Kassa J. A comparison of the efficacy of new asymmetric bispyridinium oxime BI-6 with other oximes (obidoxime, HI-6) against soman in rats. Hum Exp Toxicol. 1998;17(6 June):331–5. doi: 10.1177/096032719801700608. [DOI] [PubMed] [Google Scholar]
- Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol. 2002;40(6):803–16. doi: 10.1081/clt-120015840. [DOI] [PubMed] [Google Scholar]
- Kassa J. The influence of the time of antidotal treatment administration on the potency of newly developed oximes to counteract acute toxic effects of tabun in mice. Acta Medica (Hradec Králové) 2005;48(2):87–90. [PubMed] [Google Scholar]
- Koplovitz, Harris LW, Anderson DR, Lennox WJ, Stewart JR. Reduction of pyridostigmine pretreatment of the efficacy of atropine and 2-PAM Cl treatment of sarin and VX poisoning in rodents. Fundam Appl Toxicol. 1992;18:102–106. doi: 10.1016/0272-0590(92)90201-r. [DOI] [PubMed] [Google Scholar]
- Kuca K, Cabal J, Jun D, Bajgar J, Hrabinova M. Potency of new structurally different oximes to reactivate cyclosarin-inhibited human brain acetylcholinesterases. J Enzyme Inhib Med Chem. 2006;21(6):663–666. doi: 10.1080/14756360600850916. [DOI] [PubMed] [Google Scholar]
- Kuca K, Jun D, Bajgar J. Structural factors influencing potency of currently used acetylcholinesterase reactivators for treatment of cyclosarin intoxications. Curr Pharm Des. 2007a;13(33):3445–52. Review. [PubMed] [Google Scholar]
- Kuca K, Musilek K, Stodulka P, Marek J, Hanusova P, Jun D, Hrabinova M, Kassa J, Dolezal B. Twelve different HI-6 salts and their potency to reactivate cyclosarin inhibited AChE in vitro. Lett Drug Des Discov. 2007b;4:510–512. [Google Scholar]
- Kuca K, Musilek K, Jun D, Pohanka M, Karasova JZ, Novotny L, Musilova L. Could oxime HI-6 really be considered as “broad-spectrum” antidote? J Applied Biomedicine. 2009;7:143–149. [Google Scholar]
- Lundy PM, Hansen AS, Hand BT, Boulet CA. Comparison of several oximes against poisoning by soman, tabun and GF. Toxicology. 1992;72(1):99–105. doi: 10.1016/0300-483x(92)90089-w. [DOI] [PubMed] [Google Scholar]
- Lundy PM, Raveh L, Amitai G. Development of the bisquaternary oxime HI-6 toward clinical use in the treatment of organophosphate nerve agent poisoning. Toxicol Rev. 2006;25(4):231–43. doi: 10.2165/00139709-200625040-00004. [DOI] [PubMed] [Google Scholar]
- Maxwell DM, Brecht KM. The role of carboxylesterase in species variation of oxime protection against soman. Neurosci Biobehav Rev. 1991;15(1):135–139. doi: 10.1016/s0149-7634(05)80105-8. [DOI] [PubMed] [Google Scholar]
- Maxwell DM, Koplovitz I, Worek F, Sweeney RE. A structure-activity analysis of the variation in oxime efficacy against nerve agents. Toxicol Appl Pharmacol. 2008;231(2):157–64. doi: 10.1016/j.taap.2008.04.007. [DOI] [PubMed] [Google Scholar]
- McGarry KG, Bartlett RA, Machesky NJ, Snider TH, Moyer RA, Yeung DT, Brittain MK. Evaluation of HemogloBind™ treatment for preparation of samples for cholinesterase analysis. Adv Biosci Biotechnol. 2013;4(12):1020–1023. doi: 10.4236/abb.2013.412136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musilek K, Kuca K, Jun D, Dohnal V, Dolezal M. Synthesis of a novel series of bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. J Enzyme Inhib Med Chem. 2005;20:409–415. doi: 10.1080/14756360500179762. [DOI] [PubMed] [Google Scholar]
- Myers DK. Mechanism of the prophylactic action of diacetylmonoxime against sarin poisoning. Biochim Biophys Acta. 1959;34 (August):555–7. doi: 10.1016/0006-3002(59)90311-7. [DOI] [PubMed] [Google Scholar]
- Okumura T, Hisaoka T, Naito T, Isonuma H, Okumura S, Miura K, Maekawa H, Ishimatsu S, Takasu N, Suzuki K. Acute and chronic effects of sarin exposure from the Tokyo subway incident. Environ Toxicol Pharmacol. 2005;3:447–450. doi: 10.1016/j.etap.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Oldiges H, Schoene K. Antidotal effects of pyridinium and imidazolium salts on soman and paraoxon poisoning in mice. Arch Toxikol. 1970;26:293–305. [PubMed] [Google Scholar]
- Radić Z, Sit RK, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Fokin VV, Sharpless KB, Taylor P. Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol Chem. 2012;287(15):11798–11809. doi: 10.1074/jbc.M111.333732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radić Z, Dale T, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Duggan BM, Ajami D, Rebek J, Taylor P. Catalytic detoxification of nerve agent and pesticide organophosphates by butyrylcholinesterase assisted with non-pyridinium oximes. Biochem J. 2013;450(1):231–242. doi: 10.1042/BJ20121612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosman Y, Makarovsky I, Bentur Y, Shrot S, Dushnistky T, Krivoy A. Carbamate poisoning: treatment recommendations in the setting of a mass casualties event. Am J Emerg Med. 2009;27(9):1117–1124. doi: 10.1016/j.ajem.2009.01.035. [DOI] [PubMed] [Google Scholar]
- Rutland JP. The effect of some oximes in sarin poisoning. Br J Pharmacol Chemother. 1958;13(4 December):399–403. doi: 10.1111/j.1476-5381.1958.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Skovira JW, O’Donnell JC, McDonough JH. Evaluation of nine oximes on in vivo reactivation of blood, brain, and tissue cholinesterase activity inhibited by organophosphorus nerve agents at lethal dose. Toxicol Mech Methods. 2009;19(6–7):386–400. doi: 10.1080/15376510903213892. [DOI] [PubMed] [Google Scholar]
- Shih TM, Skovira JW, O’Donnell JC, McDonough JH. In vivo reactivation by oximes of inhibited blood, brain and peripheral tissue cholinesterase activity following exposure to nerve agents in guinea pigs. Chemico-Biological Interactions. 2010;187:207–214. doi: 10.1016/j.cbi.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Shih TM, Koplovitz I, Kan RK, McDonough JH. In Search of an Effective in vivo Reactivator for Organophosphorus Nerve Agent-Inhibited Acetylcholinesterase in the Central Nervous System. Advanced Studies in Biology. 2012;10 (4):451–478. [Google Scholar]
- Schoene K, Oldiges H. Efficacy of pyridinium salts in tabun and sarin poisoning in vivo and in vitro. Arch Int Pharmacodyn Ther. 1973;204(1):110–123. [PubMed] [Google Scholar]
- Sekowski JW, Dillman JF., III . Application of genomic, proteomic, and metabolomic technologies to the development of countermeasures against chemical warfare agents. In: Romano JA Jr, Lukey BJ, Salem H, editors. Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology And Therapeutics. 2. CRC Press; Boca Raton: 2008. pp. 123–143. [Google Scholar]
- Sit R, Radic Z, Gerardi V, Zhang L, Garcia E, Katalinic M, Amitai G, Kovarik Z, Fokin V, Sharpless KB, Taylor P. New structural scaffolds for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol Chem. 2011;286(22):19422–30. doi: 10.1074/jbc.M111.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovira JW, O’Donnell JC, Koplovitz I, Kan RK, McDonough JH, Shih TM. Reactivation of brain acetylcholinesterase by monoisonitrosoacetone increases the therapeutic efficacy against nerve agents in guinea pigs. Chem Biol Interact. 2010;187(1–3):318–324. doi: 10.1016/j.cbi.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Soukup O, Jun D, Tobin G, Kuca K. The summary on non-reactivation cholinergic properties of oxime reactivators: the interaction with muscarinic and nicotinic receptors. Arch Toxicol. 2013;87(4):711–719. doi: 10.1007/s00204-012-0977-1. [DOI] [PubMed] [Google Scholar]
- Stojiljković MP, Jokanović M. Pyridinium oximes: rationale for their selection as causal antidotes against organophosphate poisonings and current solutions for auto-injectors. Arh Hig Rada Toksikol. 2006;57(4):435–443. [PubMed] [Google Scholar]
- Thiermann H, Mast U, Klimmek R, Eyer P, Hibler A, Pfab R, Felgenhauer N, Zilker T. Cholinesterase status, pharmacokinetics and laboratory findings during obidoxime therapy in organophosphate poisoned patients. Hum Exp Toxicol. 1997;16:473–480. doi: 10.1177/096032719701600809. [DOI] [PubMed] [Google Scholar]
- Thiermann H, Worek F, Kehe K. Limitations and challenges in treatment of acute chemical warfare agent poisoning Chem. Biol Interact. 2013;206(3):435–43. doi: 10.1016/j.cbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Pharmacology and Toxicology. 2005 Jul; [Google Scholar]
- Vale JA. Nerve Agents: Why They Are So Toxic and Can Poisoning from These Agents Be Treated? Toxicol. 2009;240:141–142. [Google Scholar]
- Voicu V, Rădulescu FŞ, Medvedovici A. Toxicological considerations of acetylcholinesterase reactivators. Expert Opin Drug Metab Toxicol. 2013;9(1):31–50. doi: 10.1517/17425255.2013.736489. [DOI] [PubMed] [Google Scholar]
- Wille T, Kaltenbach L, Thiermann H, Worek F. Investigation of kinetic interactions between approved oximes and human acetylcholinesterase inhibited by pesticide carbamates. Chem Biol Interact. 2013;206(3):569–572. doi: 10.1016/j.cbi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol. 2004;68:2237–2248. doi: 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Worek F, Eyer P, Aurbek N, Szinicz L, Thiermann H. Recent advances in evaluation of oxime efficacy in nerve agent poisoning by in vitro analysis. Toxicol Appl Pharmacol. 2007;219(2–3):226–234. doi: 10.1016/j.taap.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Worek F, Thiermann H. The value of novel oximes for treatment of poisoning by organophosphorus compounds. Pharmacol Ther. 2013;139(2):249–259. doi: 10.1016/j.pharmthera.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Zurer P. Japanese cult used VX to slay member. Chem Eng News. 1998;76 (35):7. [Google Scholar]