Abstract
Purpose
Estimate whether low to moderate prenatal alcohol exposure is associated with selected birth outcomes.
Methods
Low to moderate prenatal alcohol drinking and effects on low birth weight, preterm delivery, intrauterine growth restriction (IUGR), and selected neonatal outcomes were evaluated among 4,496 women and singleton infants. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using multivariable logistic regression, controlling for confounding variables.
Results
Early pregnancy drinking was associated with reduced odds of low birth weight, OR 0.66 (95% CI 0.46, 0.96) and birth length < 10th percentile, OR 0.74 (95% CI 0.56, 0.97). Drinking during the first 3 months showed lower odds for birth length and head circumference < 10th percentile, OR 0.56 (95% CI 0.36, 0.87) and OR 0.69 (95% CI 0.50, 0.96), respectively. Third trimester drinking was associated with lower odds for low birth weight, OR 0.56 (95% CI 0.34, 0.94) and preterm delivery, OR 0.60 (95% CI 0.42, 0.87).
Conclusions
Our results suggest low to moderate alcohol exposure during early and late gestation is not associated with increased risk of low birth weight, preterm delivery, IUGR and most selected perinatal outcomes.
Keywords: alcohol, birth outcomes, low birth weight, preterm delivery, IUGR
Introduction
Alcohol use during pregnancy has historically been associated with a range of negative birth outcomes and developmental effects that include fetal alcohol syndrome (FAS), alcohol related birth defects (ARBD) and alcohol related neurodevelopmental disorders (ARND) [1, 2], often characterized at birth by facial dysmorphology, poor growth, and neurologic functional and structural abnormalities, including reduced head circumference [3]. While epidemiological research has delineated adverse effects of heavy or chronic drinking on the fetus, reported effects of low to moderate prenatal alcohol which represents the majority of exposures, are inconsistent. Previous studies have documented increased risks between alcohol and infertility [4], miscarriage [5], stillbirth and infant mortality [6, 7], congenital anomalies [8], low birth weight [9], reduced gestational age [10], preterm delivery [11], and intrauterine growth restriction (IUGR) or small-for-gestational age (SGA) [8, 12, 13], but at relatively higher consumption levels. Conversely, other research demonstrated no increase in risk from light to moderate alcohol consumption for selected perinatal or developmental outcomes [14–18], and several studies have reported reductions in risk of adverse pregnancy outcomes, including a curvilinear effect for increasing levels of prenatal alcohol exposure [9, 19–21]. A systematic review of low to moderate prenatal drinking reported lacking evidence of increased risk for selected birth outcomes including IUGR, prematurity, birth weight, and malformations [22] yet results overall were inconclusive.
Methodological difficulties related to study design, including retrospective exposure assessment, potential exposure misclassification, and inadequate control for potential confounders have resulted in limited high-quality analyses of low to moderate prenatal alcohol drinking. The current study is a prospective investigation of alcohol use during pregnancy and IUGR, low birth weight, preterm delivery, and other selected neonatal outcomes among a cohort of 4,496 women and their newborns.
Materials and Methods
Sample
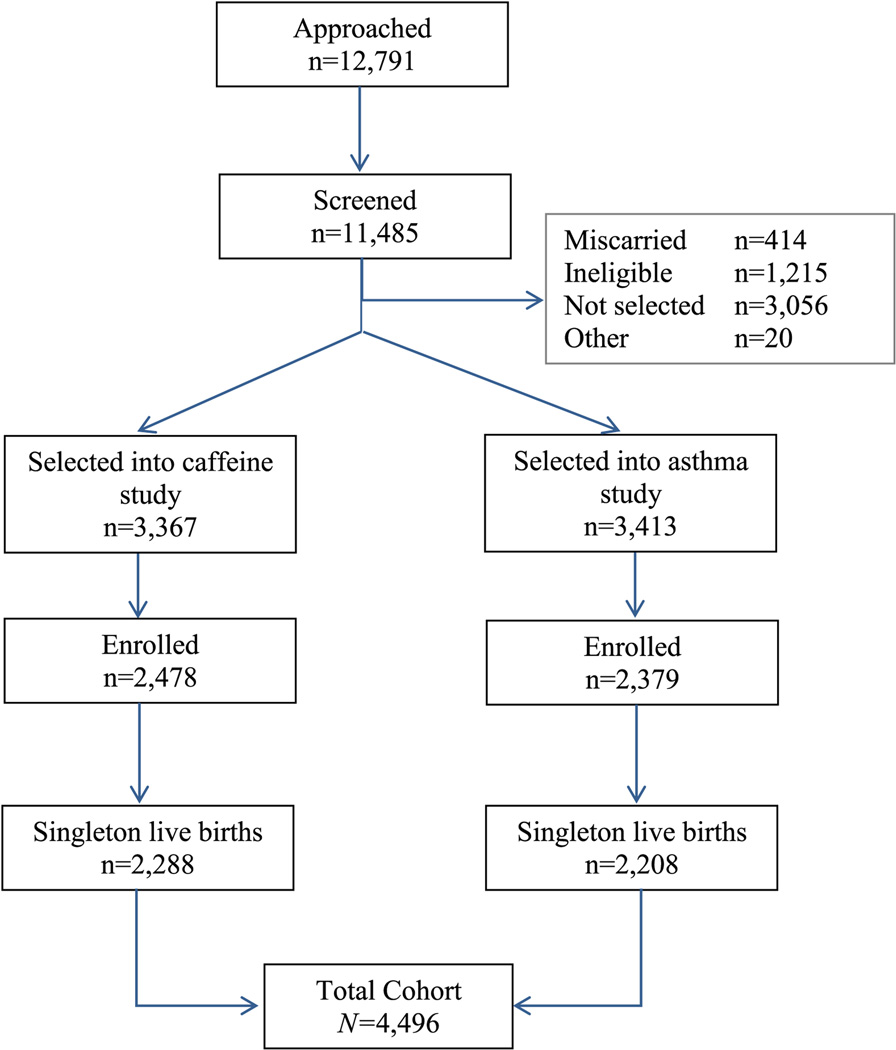
The study population included women enrolled in two related and almost concurrent prospective longitudinal cohorts: one examining prenatal caffeine exposure and the other investigating asthma in pregnancy; see Figure 1. Pregnant women were recruited from 56 obstetric practices and 15 clinics associated with six hospitals in Connecticut and Massachusetts during the period of September 1996 to June 2000. Study design for each cohort was similar with respect to methodology, timing and content of structured interviews [23, 24]. The final sample was restricted to singleton live births, yielding a total study sample of N=4,496 for the current analyses.
Figure 1.
Number of subjects approached, screened, and enrolled into the total cohort. Low to moderate alcohol use in pregnancy and birth outcomes: Connecticut/Massachusetts, 1996–2000.
All women completed a baseline interview prior to 24 weeks gestation. Information was collected on multiple risk factors through the pregnancy, including comprehensive maternal characteristics and potential confounding variables. Detailed pregnancy history was collected, including pre-existing medical conditions. The postpartum interview was conducted following delivery, typically in the hospital during the postpartum stay or within 1 month of delivery. Medical records for both the mother and infant were reviewed to collect detailed information related to labor and delivery, selected medical risk factors and potential confounders.
Exposure ascertainment
Alcohol consumption information was collected for specific months of pregnancy during two study visits: baseline prenatal interview and postpartum interview. In the baseline prenatal interview, participants were asked in detail about alcohol use during months 1–3 of gestation, in addition to any alcohol exposure up to the baseline interview; median gestational age at baseline interview was 14 weeks (range 6–24 weeks). During the postpartum interview an assessment of drinking was completed for gestational month 7 and the third trimester. Second trimester alcohol use was not assessed. For each beverage type (wine, beer, liquor), women were asked how often they drank alcohol and how many drinks they consumed during the specific time period. Using a previously established algorithm [25], alcohol content values for each beverage were summed for a total exposure score expressed as daily ounces of absolute alcohol (oz AA/day) for each month/trimester. Drinking levels were categorized as: abstinent, <0.1 oz AA/day, 0.1<0.25 oz/AA day, 0.25<0.50 oz AA/day, 0.50<1.0 oz AA/day, and ≥1.0 oz AA/day. It is estimated that 0.5 oz (14g) AA/day is approximately equal to 1 standard drink; therefore, alcohol exposure categories correspond approximately to: 0 drinks, <1.5 drinks/week, 1.5<3.5 drinks/week, 3.5<7 drinks/week, 7<14 drinks/week, and 14+ drinks/week. First trimester exposure was categorized into: abstinent, drinking in month 1 only (with no subsequent drinking in months 2 or 3), and other alcohol exposure during months 1–3. Binge drinking was classified as consuming 4+ drinks at one time [26].
Outcomes of interest
Birth weight obtained from the delivery log was used to categorize infants considered low birth weight (<2,500 g). Gestational age was based on last menstrual period (LMP) or an ultrasound estimate if LMP was uncertain or inaccurate: 55.3% were confirmed by early ultrasound, 37.2% based on LMP, and 7.5% based on a newborn clinical exam. Preterm delivery was defined as <37 weeks. IUGR was classified as <10th percentile of birth weight for gestational age according to 1999 US birth standards [27], adjusted for gender and mother’s ethnicity. Birth length and head circumference were analyzed as continuous outcomes using the lowest 10th percentile according to Centers for Disease Control and Prevention (CDC) standards [28] to define low birth length and reduced head circumference. Additional clinical outcomes included: major selected congenital malformations [29]; Apgar score <7 at 1 and 5 minutes; ventilation (including need for continuous positive airway pressure, CPAP); placement in the neonatal intensive care unit, NICU (observation or admission); and neonatal jaundice.
Potential Confounding Variables
Demographic covariates included maternal age, ethnicity, marital status, education, parity, and employment. Smoking, caffeine intake, prenatal and multivitamin use, passive smoke exposure, illicit drug use prior to conception, pre-pregnancy body mass index, work, and exercise before and during pregnancy were also evaluated. Obstetric and medical variables assessed included hypertension, preterm labor, bleeding during pregnancy, placental problems, gestational diabetes, incompetent cervix, respiratory disease, maternal asthma, induction or augmentation of labor, and infant gender.
Statistical Analysis
Analysis focused on patterns of drinking throughout pregnancy in first and third trimesters. Bivariate analyses were performed for selected maternal characteristics and alcohol exposure compared to primary dichotomous outcomes of interest using the χ² statistic. Multivariable analysis of prenatal alcohol exposure and birth outcomes was performed using logistic regression modeling in PC-SAS 9.3 (SAS Institute, Cary, NC). Odds ratio (OR) estimates for alcohol exposure were obtained for each assessment period and each outcome of interest. Models were developed using multiple logistic regression with backwards selection at α=0.10 level of significance, including the exposure of interest, potential confounders, and independent risk factors. Final models included the selected measure of alcohol exposure, indicator variable for study cohort, as well as confounding variables that changed alcohol β estimates more than 10%. Continuous outcomes were analyzed using generalized linear modeling and backwards selection at α=0.10 level of significance, with transformation of the response variable using log base 10 to account for lack of normal distribution.
Protocols for both investigations were approved by the Human Investigations Committee at Yale University and all participating institutions. Written or oral consent was obtained from each participant per the guidelines of the local human investigations committee.
Results
Maternal alcohol exposure was most prevalent during the first month of pregnancy (29%), and declined in the second and third months to 9% and 7%, respectively; median exposure among women who drank during months 1, 2, and 3 was 0.07oz AA/day (about 1 drink/week), 0.03oz AA/day (slightly less half a drink/week), and 0.02oz AA/day (slightly less than one-third of a drink/week), respectively. Alcohol consumption demonstrated a curvilinear pattern, becoming less frequent following recognition of pregnancy, and then modestly increasing through the third trimester, to 11% in month 7 (median consumption of 0.02oz AA/day among drinkers, slightly less than one-third of a drink/week) and reaching 27% with any alcohol exposure during the third trimester.
Selected maternal characteristics and first gestational month alcohol exposure are presented in Table 1. Women drinking in month 1 of pregnancy were more likely to be nulliparous, over age 25, Caucasian, married, normal pre-pregnancy body mass index (BMI) (18.5–24.9), and completed higher education. A strong relationship is shown between caffeine (mg/day) and alcohol consumption in month 1. Similarly, women smoking during pregnancy were more likely to be drinking alcohol compared to non-smokers. Women who exercise, work and take multivitamins are more likely to have consumed alcohol in month 1. Additional associated maternal variables are presented in Supplemental Table 1.
Table 1.
Selected Characteristics of the Study Cohort by Absolute Alcohol (AA) Drinking Month 1 – n (%)
| Characteristic | Na | Levels of alcohol exposure | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| oz AA/dayb | 0 | <0.10 | 0.10<.25 | 0.25<0.50 | 0.50<1.00 | ≥1.00 | |||
| (drinks/week) | 0 | (<1.5) | (1.5<3.5) | (3.5<7.0) | (7.0<14.0) | (≥14.00) | |||
| 4,496 | 3183 (71.2) | 733 (16.4) | 245 (5.5) | 155 (3.5) | 96 (2.2) | 59 (1.3) | |||
| Parity | |||||||||
| 0 | 1981 | 1363 (68.8) | 328 (16.6) | 129 (6.5) | 80 (4.0) | 47 (2.4) | 34 (1.7) | P=0.0294 | |
| 1 | 1553 | 1128 (72.6) | 253 (16.3) | 79 (5.1) | 40 (2.6) | 37 (2.4) | 16 (1.0) | ||
| 2 | 663 | 491 (74.1) | 105 (15.8) | 26 (3.9) | 27 (4.1) | 8 (1.2) | 6 (0.9) | ||
| 3+ | 270 | 200 (74.1) | 45 (16.7) | 11 (4.1) | 8 (3.0) | 4 (1.5) | 2 (0.7) | ||
| Age | |||||||||
| <25 | 1052 | 872 (82.9) | 83 (7.9) | 35 (3.3) | 21 (2.0) | 22 (2.1) | 19 (1.8) | P<0.0001 | |
| 25<30 | 1150 | 826 (71.8) | 193 (16.8) | 66 (5.7) | 38 (3.3) | 17 (1.5) | 10 (0.9) | ||
| 30<35 | 1465 | 976 (66.6) | 295 (20.1) | 94 (6.4) | 50 (3.4) | 34 (2.3) | 16 (1.1) | ||
| ≥35 | 803 | 509 (63.4) | 162 (20.1) | 50 (6.2) | 46 (5.7) | 23 (2.9) | 13 (1.6) | ||
| Education | |||||||||
| < 12 yrs | 627 | 542 (86.4) | 46 (7.3) | 11 (1.8) | 8 (1.3) | 8 (1.3) | 12 (1.9) | P<0.0001 | |
| 12 years | 792 | 611 (77.2) | 91 (11.5) | 36 (4.6) | 17 (2.2) | 18 (2.3) | 19 (2.4) | ||
| 13–16 yrs | 2131 | 1425 (66.9) | 389 (18.3) | 148 (6.9) | 94 (4.4) | 54 (2.5) | 21 (1.0) | ||
| 17+ yrs | 916 | 603 (65.9) | 205 (22.4) | 50 (5.5) | 36 (3.9) | 16 (1.7) | 6 (0.7) | ||
| BMIc (kg/m²) | |||||||||
| Underweight | 231 | 169 (73.2) | 38 (16.5) | 9 (3.9) | 5 (2.2) | 8 (3.5) | 2 (0.9) | P=0.0088 | |
| Normal weight | 2617 | 1791 (68.44) | 471 (18.0) | 158 (6.0) | 104 (4.0) | 61 (2.3) | 32 (1.2) | ||
| Overweight | 927 | 677 (73.0) | 133 (14.4) | 51 (5.5) | 32 (3.5) | 19 (2.1) | 15 (1.6) | ||
| Obese | 583 | 450 (77.2) | 82 (14.1) | 24 (4.1) | 12 (2.1) | 7 (1.2) | 8 (1.4) | ||
| Marital Status | |||||||||
| Married | 3114 | 2119 (68.1) | 590 (19.0) | 190 (6.1) | 122 (3.9) | 67 (2.2) | 26 (0.8) | P<0.0001 | |
| Single | 1164 | 927 (79.6) | 115 (9.9) | 46 (4.0) | 24 (2.1) | 25 (2.2) | 27 (2.3) | ||
| Divorced, separated | 191 | 136 (71.2) | 28 (14.7) | 9 (4.7) | 9 (4.7) | 4 (2.1) | 5 (2.6) | ||
| Ethnicity - mother | |||||||||
| Caucasian | 3048 | 1982 (65.0) | 605 (19.9) | 207 (6.8) | 131 (4.3) | 82 (2.7) | 41 (1.4) | P<0.0001 | |
| Black, African American | 399 | 329 (82.5) | 44 (11.0) | 11 (2.8) | 8 (2.0) | 5 (1.3) | 2 (0.5) | ||
| Hispanic | 842 | 719 (85.4) | 70 (8.3) | 19 (2.3) | 13 (1.5) | 9 (1.1) | 12 (1.4) | ||
| Asian | 77 | 64 (83.1) | 9 (11.7) | 2 (2.6) | 2 (2.6) | 0 (0.0) | 0 (0.0) | ||
| Other | 96 | 82 (85.4) | 5 (5.2) | 6 (6.3) | 0 (0.0) | 0 (0.0) | 3 (3.1) | ||
| Caffeine (mgs/day) - first trimester | |||||||||
| 0 | 1888 | 1463 (77.5) | 285 (15.1) | 81 (4.3) | 32 (1.7) | 20 (1.1) | 7 (0.4) | P<0.0001 | |
| 1–149 mgs/day | 2193 | 1458 (66.5) | 391 (17.8) | 144 (6.6) | 104 (4.7) | 62 (2.8) | 34 (1.6) | ||
| 150–299 mgs/day | 265 | 177 (66.8) | 43 (16.2) | 14 (5.3) | 11 (4.2) | 7 (2.6) | 13 (4.9) | ||
| 300+ mgs/day | 123 | 85 (69.1) | 13 (10.6) | 6 (4.9) | 8 (6.5) | 7 (5.7) | 4 (3.3) | ||
| Smoking History | |||||||||
| Never | 2754 | 2046 (74.3) | 453 (16.5) | 128 (4.7) | 74 (2.7) | 39 (1.4) | 14 (0.5) | P<0.0001 | |
| Quit before pregnancy | 945 | 642 (67.9) | 168 (17.8) | 61 (6.5) | 45 (4.8) | 21 (2.2) | 8 (0.9) | ||
| First trimester only | 475 | 290 (61.1) | 77 (16.2) | 35 (7.4) | 29 (6.1) | 20 (4.2) | 24 (5.1) | ||
| First and third trimester | 293 | 202 (68.9) | 35 (11.5) | 21 (7.2) | 7 (2.4) | 16 (5.5) | 12 (4.1) | ||
| Exercise since becoming pregnant | |||||||||
| No | 2058 | 1553 (75.5) | 302 (14.7) | 95 (4.6) | 52 (2.5) | 32 (1.6) | 24 (1.2) | P<0.0001 | |
| Yes | 2410 | 1628 (67.6) | 431 (17.9) | 150 (6.2) | 103 (4.3) | 64 (2.7) | 34 (1.4) | ||
| Worked since becoming pregnant | |||||||||
| No | 1121 | 892 (79.6) | 131 (11.7) | 38 (3.4) | 29 (2.6) | 17 (1.5) | 14 (1.3) | P<0.0001 | |
| Yes | 3349 | 2291 (68.4) | 602 (18.0) | 207 (6.2) | 126 (3.8) | 79 (2.4) | 44 (1.3) | ||
| Prenatal vitamin use month 1 | |||||||||
| No | 2650 | 1858 (70.1) | 426 (16.1) | 157 (5.9) | 94 (3.6) | 67 (2.5) | 48 (1.8) | P=0.0006 | |
| Yes | 1820 | 1325 (72.8) | 307 (16.9) | 88 (4.8) | 61 (3.4) | 29 (1.6) | 10 (0.6) | ||
| Multivitamin use month 1 | |||||||||
| No | 3579 | 2639 (73.7) | 549 (15.3) | 169 (4.7) | 104 (2.9) | 74 (2.1) | 44 (1.2) | P<0.0001 | |
| Yes | 871 | 525 (60.3) | 183 (21.0) | 76 (8.7) | 51 (5.9) | 22 (2.5) | 14 (1.6) | ||
| Nausea/vomiting 1st trimester | |||||||||
| No | 790 | 520 (65.8) | 136 (17.2) | 64 (8.1) | 30 (3.8) | 21 (2.7) | 19 (2.4) | P<0.0001 | |
| Yes | 3679 | 2663 (72.4) | 596 (16.2) | 181 (4.9) | 125 (3.4) | 75 (2.0) | 39 (1.1) | ||
| Bleeding/spotting | |||||||||
| No | 3522 | 2485 (70.6) | 601 (17.1) | 191 (5.4) | 129 (3.7) | 72 (2.0) | 44 (1.3) | P=0.1759 | |
| Yes | 751 | 555 (73.9) | 102 (13.6) | 44 (5.9) | 21 (2.8) | 18 (2.4) | 11 (1.5) | ||
| Hypertension | |||||||||
| No | 3880 | 2760 (71.1) | 641 (16.5) | 217 (5.6) | 131 (3.4) | 86 (2.2) | 45 (1.2) | P=0.0811 | |
| Yes | 409 | 293 (71.6) | 63 (15.4) | 20 (4.9) | 19 (4.6) | 4 (1.0) | 10 (2.4) | ||
| Gestational diabetes | |||||||||
| No | 3547 | 2519 (71.0) | 575 (16.2) | 198 (5.6) | 131 (3.7) | 82 (2.3) | 42 (1.2) | P=0.1119 | |
| Yes | 736 | 533 (72.4) | 126 (17.1) | 37 (5.0) | 19 (2.6) | 8 (1.1) | 13 (1.8) | ||
| Preterm labor | |||||||||
| No | 3831 | 2689 (70.2) | 648 (16.9) | 219 (5.7) | 142 (3.7) | 81 (2.1) | 52 (1.4) | P=0.0103 | |
| Yes | 426 | 335 (78.6) | 52 (12.2) | 19 (4.5) | 8 (1.9) | 9 (2.1) | 3 (0.7) | ||
| Beverage type | |||||||||
| Wine only | 478 | 366 (76.6) | 59 (12.3) | 36 (7.5) | 12 (2.5) | 5 (1.1) | P<0.0001 | ||
| Beer only | 204 | 143 (70.1) | 27 (13.2) | 18 (8.8) | 11 (5.4) | 5 (2.5) | |||
| Liquor only | 136 | 83 (61.0) | 34 (25.0) | 7 (5.2) | 6 (4.4) | 6 (4.4) | |||
| Drinks in combination | 469 | 141 (30.1) | 125 (26.7) | 94 (20.0) | 67 (14.3) | 42 (9.0) | |||
Numbers may not sum to total N due to missing values
AA= absolute alcohol ounces; 0.5 oz AA is approximately equal to 1 standard drink
BMI: Underweight(<18.5); normal weight (18.5–24.9); overweight (25–29.9); obese (30+)
The association between maternal characteristics and birth weight, preterm delivery, and IUGR are presented in Table 2. Among the study cohort, 4.7% of infants were low birth weight, 6.9% were delivered preterm, and 7.9% were diagnosed with IUGR. Risks of low birth weight, preterm delivery and IUGR were increased among women who were nulliparous, under age 25, with less than high school education, and smoked during pregnancy; married and Caucasian women had decreased risk for these birth outcomes. Women who were underweight (pre-pregnancy BMI <18.5) or reported caffeine consumption, were at increased risk for low birth weight, preterm delivery and IUGR, while exercise decreased the risk of low birth weight, and early prenatal vitamin use decreased the risk of each outcome. Additional associations are presented in Supplemental Table 2.
Table 2.
Maternal Characteristics and Low Birth Weight, Preterm Delivery, and IUGR
| Na | LBW (%) | p value | N | PTD (%) | p value | N | IUGR (%)b | p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 4488 | 209 (4.7) | 4495 | 310 (6.9) | 4486 | 353 (7.9) | |||||
| Parity | ||||||||||
| 0 | 1988 | 119 (6.0) | P=0.0010 | 1990 | 160 (8.0) | P=0.0112 | 1978 | 214 (10.8) | P<0.0001 | |
| 1 | 1561 | 56 (3.6) | 1564 | 97 (6.2) | 1556 | 81 (5.2) | ||||
| 2 | 666 | 20 (3.0) | 667 | 31 (4.7) | 664 | 40 (6.0) | ||||
| 3+ | 270 | 14 (5.2) | 271 | 22 (8.1) | 270 | 20 (7.4) | ||||
| Age | ||||||||||
| <25 | 1048 | 70 (6.7) | P=0.0032 | 1054 | 92 (8.7) | P=0.0453 | 1044 | 96 (9.2) | P=0.1284 | |
| 25<30 | 1153 | 48 (4.2) | 1154 | 67 (5.8) | 1146 | 99 (8.6) | ||||
| 30<35 | 1479 | 64 (4.3) | 1479 | 96 (6.5) | 1476 | 103 (7.0) | ||||
| 35+ | 807 | 27 (3.4) | 807 | 55 (6.8) | 804 | 57 (7.1) | ||||
| Education | ||||||||||
| < 12 yrs | 625 | 60 (9.6) | P<0.0001 | 629 | 74 (11.8) | P<0.0001 | 624 | 73 (11.7) | P=0.0024 | |
| 12 years | 794 | 35 (4.4) | 796 | 54 (6.8) | 788 | 59 (7.5) | ||||
| 13–16 yrs | 2143 | 80 (3.7) | 2143 | 126 (5.9) | 2135 | 152 (7.1) | ||||
| 17+ yrs | 921 | 33 (3.6) | 922 | 56 (6.1) | 919 | 70 (7.6) | ||||
| BMIc (kg/m²) | ||||||||||
| Underweight | 230 | 16 (7.0) | P=0.2965 | 231 | 25 (10.8) | P=0.0045 | 229 | 27 (11.8) | P=0.0047 | |
| Normal weight | 2637 | 118 (4.5) | 2640 | 159 (6.0) | 2629 | 2124 (8.5) | ||||
| Overweight | 927 | 39 (4.2) | 928 | 65 (7.0) | 922 | 68 (7.4) | ||||
| Obese | 582 | 30 (5.2) | 583 | 53 (9.1) | 579 | 29 (5.0) | ||||
| Marital Status | ||||||||||
| Married | 3133 | 103 (3.3) | P<0.0001 | 3135 | 177 (5.7) | P<0.0001 | 3126 | 221 (7.1) | P=0.0037 | |
| Single | 1161 | 87 (7.5) | 1166 | 112 (9.6) | 1152 | 113 (9.8) | ||||
| Divorced, separated | 192 | 19 (9.9) | 192 | 21 (10.9) | 191 | 21 (11.0) | ||||
| Ethnicity - mother | ||||||||||
| White, Caucasian | 3065 | 97 (3.2) | P<0.0001 | 3068 | 179 (5.8) | P=0.0001 | 3061 | 227 (7.4) | P=0.3474 | |
| Black, African-American | 401 | 33 (8.2) | 401 | 31 (7.7) | 399 | 36 (9.0) | ||||
| Hispanic | 841 | 68 (8.1) | 844 | 83 (9.8) | 838 | 75 (9.0) | ||||
| Asian | 77 | 4 (5.2) | 77 | 4 (5.2) | 77 | 9 (11.7) | ||||
| Other | 96 | 7 (7.3) | 97 | 13 (13.4) | 96 | 8 (8.3) | ||||
| Caffeine (mgs/day) - first trimester | ||||||||||
| 0 | 1897 | 73 (3.9) | P=0.0051 | 1901 | 117 (6.2) | P=0.0060 | 1893 | 134 (7.1) | P=0.0061 | |
| 1–149 mgs/day | 21990 | 106 (4.8) | 2201 | 152 (6.9) | 2187 | 183 (8.4) | ||||
| 150–299 mgs/day | 266 | 18 (6.8) | 266 | 24 (9.0) | 266 | 19 (7.1) | ||||
| 300+ mgs/day | 123 | 12 (9.8) | 124 | 17 (13.7) | 122 | 19 (15.6) | ||||
| Smoking History | ||||||||||
| Never | 2764 | 112 (4.1) | P<0.0001 | 2770 | 169 (6.1) | P=0.0040 | 2754 | 197 (7.2) | P<0.0001 | |
| Quit before pregnancy | 949 | 35 (3.7) | 950 | 66 (7.0) | 946 | 61 (6.5) | ||||
| First trimester only | 477 | 33 (7.0) | 477 | 43 (9.0) | 473 | 38 (8.0) | ||||
| First and third trimester | 294 | 29 (9.9) | 294 | 32 (10.9) | 294 | 59 (20.1) | ||||
| Exercise since becoming pregnant | ||||||||||
| No | 2057 | 109 (5.3) | P=0.0621 | 2063 | 156 (7.6) | P=0.1082 | 2052 | 160 (7.8) | P=0.7328 | |
| Yes | 2427 | 100 (4.1) | 2428 | 154 (6.3) | 2415 | 195 (8.1) | ||||
| Worked since becoming pregnant | ||||||||||
| No | 1123 | 63 (5.6) | P=0.0804 | 1127 | 81 (7.2) | P=0.6581 | 1119 | 100 (8.9) | P=0.1552 | |
| Yes | 3364 | 146 (4.3) | 3367 | 229 (6.8) | 3351 | 255 (7.6) | ||||
| Prenatal vitamin use month 1 | ||||||||||
| No | 2653 | 146 (5.5) | P=0.0012 | 2658 | 198 (7.5) | P=0.0794 | 2642 | 235 (8.9) | P=0.0046 | |
| Yes | 1834 | 63 (3.4) | 1836 | 112 (6.1) | 1828 | 120 (6.6) | ||||
| Multivitamin use month 1 | ||||||||||
| No | 3590 | 171 (4.8) | P=0.4969 | 3597 | 260 (7.2) | P=0.0871 | 3574 | 289 (8.1) | P=0.5194 | |
| Yes | 876 | 37 (4.2) | 876 | 49 (5.6) | 875 | 65 (7.4) | ||||
| Nausea/vomiting 1st trimester | ||||||||||
| No | 793 | 37 (4.7) | P=0.9907 | 794 | 48 (6.1) | P=0.2960 | 788 | 65 (8.3) | P=0.7255 | |
| Yes | 3694 | 172 (4.7) | 3700 | 263 (7.1) | 3682 | 290 (7.9) | ||||
| Bleeding/spotting | ||||||||||
| No | 3546 | 135 (3.8) | P<0.0001 | 3546 | 185 (5.2) | P<0.0001 | 3535 | 279 (7.9) | P=0.9965 | |
| Yes | 753 | 64 (8.5) | 753 | 101 (13.4) | 748 | 59 (7.9) | ||||
| Hypertension | ||||||||||
| No | 3904 | 159 (4.1) | P<0.0001 | 3904 | 244 (6.3) | P<0.0001 | 3887 | 288 (7.4) | P<0.0001 | |
| Yes | 411 | 44 (10.7) | 411 | 47 (11.4) | 411 | 53 (12.9) | ||||
| Gestational diabetes | ||||||||||
| No | 3573 | 164 (4.6) | P=0.5030 | 3573 | 228 (6.4) | P=0.0440 | 3558 | 282 (7.9) | P=0.8836 | |
| Yes | 736 | 38 (5.2) | 736 | 62 (8.4) | 734 | 57 (7.8) | ||||
| Preterm Labor | ||||||||||
| No | 3853 | 109 (2.8) | P<0.0001 | 3853 | 117 (3.0) | P<0.0001 | 3842 | 292 (7.6) | P=0.0268 | |
| Yes | 428 | 92 (21.5) | 428 | 171 (40.0) | 422 | 45 (10.7) | ||||
numbers may not sum to total N due to missing values
IUGR based on 1999 US singletons adjusted for gender and ethnicity
Underweight(<18.5); normal weight (18.5–24.9); overweight (25–29.9); obese (30+)
Analyses to estimate the effect for early pregnancy drinking and birth outcomes are presented in Table 3. Alcohol consumption among low to moderate drinkers in month 1, evaluated as discrete levels of daily drinking (oz AA/day), does not confer any increased risk compared to the non-drinking group. For first trimester exposure, drinking in month 1 only had a significantly lower odds for low birth weight, odds ratio (OR) 0.63 (95% confidence interval (CI) 0.43, 0.94). Tests for trend across level of drinking and low birth weight were statistically significant for month 1 drinking (P=0.03) and first trimester drinking (P=0.007). Low to moderate prenatal drinking up to the baseline interview was associated with significantly reduced odds of low birth weight, OR 0.58 (95% CI 0.42, 0.80). Low to moderate drinking in early pregnancy was not associated with preterm delivery or IUGR.
Table 3.
Unadjusted and Adjusted Estimates for Early Pregnancy Drinking and Low Birth Weight, Preterm Delivery, and IUGR
| Low birth weight |
Preterm delivery |
IUGR |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | OR | 95% CI | aOR | 95% CI | N (%) | OR | 95% CI | aOR | 95% CI | N (%) | OR | 95% CI | aOR | 95% CI | |
| Month 1 | |||||||||||||||
| None | 163 (5.1) | 1.00 | 1.00a | 226 (7.1) | 1.00 | 1.00d | 257 (8.1) | 1.00 | 1.00g | ||||||
| <0.10 oz AA/day | 28 (3.8) | 0.74 | 0.49–1.10 | 0.96 | 0.61–1.52 | 48 (6.6) | 0.92 | 0.66–1.27 | 1.05 | 0.74–1.51 | 58 (7.9) | 0.97 | 0.72–1.31 | 0.98 | 0.71–1.35 |
| 0.10<0.25oz AA/day | 8 (3.3) | 0.62 | 0.30–1.28 | 0.57 | 0.24–1.36 | 17 (6.9) | 0.98 | 0.59–1.63 | 0.79 | 0.42–1.47 | 13 (5.3) | 0.64 | 0.36–1.13 | 0.60 | 0.33–1.08 |
| ≥0.25oz AA/day | 10 (3.2) | 0.62 | 0.32–1.18 | 0.52 | 0.24–1.12 | 19 (6.2) | 0.86 | 0.53–1.39 | 0.79 | 0.46–1.36 | 23 (7.5) | 0.91 | 0.59–1.42 | 0.69 | 0.42–1.13 |
| First trimester | |||||||||||||||
| Abstinent | 157 (5.3) | 1.00 | 1.00b | 215 (7.3) | 1.00 | 1.00e | 240 (8.2) | 1.00 | 1.00h | ||||||
| 1st month only | 31 (3.4) | 0.63 | 0.43–0.94 | 0.67 | 0.43–1.06 | 58 (6.4) | 0.88 | 0.65–1.18 | 0.89 | 0.63–1.25 | 63 (7.0) | 0.84 | 0.63–1.13 | 0.79 | 0.57–1.08 |
| Drinking months 1–3 | 21 (3.6) | 0.66 | 0.41–1.04 | 0.78 | 0.47–1.31 | 37 (6.4) | 0.87 | 0.60–1.24 | 0.79 | 0.51–1.20 | 46 (7.8) | 0.95 | 0.69–1.32 | 0.83 | 0.58–1.20 |
| To baseline interview | |||||||||||||||
| Abstinent | 156 (5.5) | 1.00 | 1.00c | 212 (7.4) | 1.00 | 1.00f | 236 (8.3) | 1.00 | 1.00i | ||||||
| Drinking | 53 (3.3) | 0.58 | 0.42–0.80 | 0.66 | 0.46–0.96 | 98 (6.0) | 0.80 | 0.62–1.02 | 0.79 | 0.60–1.05 | 119 (7.3) | 0.87 | 0.69–1.10 | 0.81 | 0.63–1.04 |
n=4116, adjusted for parity, age, ethnicity, study cohort, height, marital status, smoking, exercise (before/during pregnancy), multivitamin use, preterm labor, hypertension, anomalies
n=4105; adjusted for parity, age, ethnicity, study cohort, height, marital status, education, smoking, exercise (before/during pregnancy), hypertension, preterm labor, anomalies, incompetent cervix, placental problems, infant gender, induction
n=4157; adjusted for parity, age, ethnicity, study cohort, height, smoking, exercise (before/during pregnancy), hypertension, preterm labor, anomalies, incompetent cervix, placental problems, infant gender, induction
n=4090; adjusted for parity, age, ethnicity, study cohort, education, BMI, smoking, caffeine, marijuana use, bleeding, hypertension, nausea/vomiting, incompetent cervix, placental problem, STD, diabetes
n=4028; adjusted for parity, age, ethnicity, study cohort, BMI, smoking, caffeine, bleeding, hypertension, nausea/vomiting, incompetent cervix, placental problem, STD, anomalies, induction
n=4115; adjusted for parity, age, ethnicity, study cohort, BMI, smoking, bleeding, hypertension, nausea/vomiting, incompetent cervix, placental problems, STD, diabetes, induction
n=4053; adjusted for parity, age, ethnicity, study cohort, marital status, exercise during pregnancy, caffeine, smoking, work, prenatal vitamins/multivitamins (month 1), hypertension, preterm labor, anomalies
n=4035; adjusted for parity, age, ethnicity, study cohort, BMI, smoking, prenatal vitamins (month 1), work, caffeine, hypertension, preterm labor, anomalies
n=4122; adjusted for parity, age, ethnicity, study cohort, BMI, smoking, prenatal vitamins (month 1), work, hypertension, preterm labor
Following multivariable analysis, no increased risk from low to moderate levels of alcohol drinking in the first trimester on selected birth outcomes was observed. Month 1 estimates for levels ≥0.10 oz AA/day were stronger after adjustment, and early pregnancy drinking showed a significant, yet attenuated, odds for low birth weight, OR 0.66 (95% CI 0.46, 0.96). Estimates do not demonstrate any increase in risk for preterm delivery or IUGR.
Third trimester drinking estimates were modeled to determine any differences in risk based on timing of alcohol exposure (Table 4). Due to small cell numbers, analysis was limited to evaluation of alcohol as a bivariate exposure: mothers who abstained or drank. Drinking in month 7 demonstrated significant reduced odds of low birth weight; following multivariate adjustment, the estimate is attenuated and non-significant. Third trimester drinking was associated with a significant reduction in odds of low birth weight, and was statistically significant following multivariate adjustment, OR 0.56 (95% CI 0.34, 0.94). Month 7 drinking did not appear to be associated with preterm delivery and adjusted estimates for third trimester drinking showed a decreased risk with preterm delivery, OR 0.60 (95% CI 0.42, 0.87). No increased risk of IUGR is observed for month 7 or third trimester drinking.
Table 4.
Unadjusted and Adjusted Estimates for Third Trimester Drinking and Low Birth Weight, Preterm Delivery, and IUGR
| Low birth weight |
Preterm delivery |
IUGR |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | OR | 95% CI | aOR | 95% CI | N (%) | OR | 95% CI | aOR | 95% CI | N (%) | OR | 95% CI | aOR | 95% CI | |
| Month 7 | |||||||||||||||
| Abstinent | 154 (4.4) | 1.00 | 1.00a | 227 (6.5) | 1.00 | 1.00c | 284 (8.2) | 1.00 | 1.00e | ||||||
| Drinking | 9 (2.1) | 0.45 | 0.23–0.89 | 0.67 | 0.33–1.39 | 24 (5.5) | 0.83 | 0.54–1.28 | 0.89 | 0.55–1.44 | 31 (7.1) | 0.85 | 0.58–1.26 | 0.91 | 0.60–1.38 |
| Third trimester | |||||||||||||||
| Abstinent | 143 (5.0) | 1.00 | 1.00b | 209 (7.3) | 1.00 | 1.00d | 238 (8.3) | 1.00 | 1.00f | ||||||
| Drinking | 20 (1.9) | 0.36 | 0.22–0.58 | 0.56 | 0.34–0.94 | 42 (3.9) | 0.52 | 0.37–0.72 | 0.60 | 0.42–0.87 | 78 (7.3) | 0.86 | 0.66–1.12 | 0.91 | 0.68–1.22 |
n=3726; adjusted for parity, ethnicity, marital status, height, study cohort, smoking, exercise (before/during pregnancy), bleeding, hypertension, preterm labor, placental problems, infant gender, induction
n=3672; adjusted for parity, ethnicity, marital status, height, study cohort, smoking, exercise (before/during pregnancy), hypertension, preterm labor, placental problems, infant gender, induction
n=3746; adjusted for parity, ethnicity, age, marital status, education, study cohort, smoking, bleeding, hypertension, nausea/vomiting, incompetent, cervix, placental problems, diabetes, maternal asthma, STD, induction
n=3773; adjusted for parity, education, study cohort, smoking bleeding, hypertension, incompetent cervix, placental problem, STD, diabetes, induction
n=3641; adjusted for parity, ethnicity, education, BMI, study cohort, smoking, work, bleeding, hypertension, preterm labor, maternal asthma, anomalies
n=3689; adjusted for parity, age, education, BMI, study cohort, smoking, work, exercise during pregnancy, cocaine use (year before pregnancy), prenatal/multivitamin use (month 1), hypertension, maternal asthma, anomalies
Table 5 presents adjusted odds ratio estimates for early pregnancy alcohol use and selected neonatal outcomes. Following multivariable analysis, alcohol exposure was not associated with a significant increased risk for major congenital malformations, Apgar score <7 at 5 minutes, admission or observation in the NICU, or jaundice. In addition, low to moderate drinking was not associated with an increased risk for reduced head circumference or reduced birth length, markers often associated with fetal alcohol spectrum disorders. However, month 1 drinking at ≥0.25 oz AA/day was associated with an increased risk of need for ventilation, OR 2.10 (95% CI 1.16–3.79). Analysis of neonatal ventilation and month 1 drinking stratified into higher drinking levels demonstrated that while drinking 0.25<0.50oz AA/day showed a significantly increased risk, estimates for higher levels (0.50<1.00 oz AA/day and ≥1.00 oz AA/day) were attenuated and not significant. Apgar score <7 at 5 minutes, as well as 1 minute Apgar (not presented) show similar but statistically nonsignificant increases. First trimester drinking in months 1–3 or drinking ever in early pregnancy was associated with reduced risk of short birth length, OR 0.56 (95% CI 0.36–0.87) and OR 0.74 (95% CI 0.56–0.97), respectively. Lower odds ratio estimates were observed for drinking in months 1–3 and small head circumference, OR 0.69 (95% CI 0.50–0.96). Analysis of continuous outcomes demonstrated longer infant birth length among those women drinking in the first 3 months than among those who were abstinent, 20.3cm vs. 20.1cm (β=0.0031; p=0.02); newborns of women who consumed 0.10<0.25 oz AA/day in month 1 had mean head circumference of 34.04cm compared to 33.77cm among those who were abstinent (β=0.0034; p=0.03); see Supplemental Table 3.
Table 5.
Adjusted estimates for selected clinical measures and early pregnancy drinkinga
| Major congenital malformationsb |
Apgar 5 Min (<7) | Ventilation/CPAPc | NICUd | Jaundice | Birth Lengthe | Head Circumferencee |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome n (%): | 190 (4.4) | 41 (1.0) | 139 (3.3) | 519 (12.3) | 1230 (28.8) | 338 (8.0) | 576 (14.0) | |||||||
| Adjusted estimates: | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI |
| Month 1 drinking | ||||||||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| < 0.10 oz AA/day | 0.78 | 0.40–1.50 | 1.61 | 0.67–3.84 | 1.43 | 0.89–2.31 | 1.12 | 0.86–1.46 | 1.17 | 0.97–1.42 | 1.10 | 0.78–1.54 | 1.08 | 0.83–1.42 |
| 0.10<0.25oz AA/day | 1.20 | 0.65–2.22 | - | NAC | 0.28 | 0.07–1.19 | 0.67 | 0.41–1.09 | 0.87 | 0.64–1.20 | 0.71 | 0.38–1.32 | 0.78 | 0.49–1.25 |
| ≥0.25 oz AA/day | 1.15 | 0.77–1.71 | 2.32 | 0.86–6.28 | 2.10 | 1.16–3.79 | 1.25 | 0.87–1.79 | 1.01 | 0.79–1.37 | 0.70 | 0.41–1.20 | 0.83 | 0.56–1.24 |
| First Trimester drinking | ||||||||||||||
| Abstinent | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| 1st month only | 1.07 | 0.73–1.57 | 1.12 | 0.47–2.66 | 1.27 | 0.81–2.00 | 1.08 | 0.84–1.38 | 1.01 | 0.85–1.21 | 0.92 | 0.67–1.27 | 0.99 | 0.77–1.28 |
| Drinking months 1–3 | 1.28 | 0.83–1.96 | 1.28 | 0.48–3.43 | 1.12 | 0.64–1.97 | 0.96 | 0.70–1.31 | 1.09 | 0.88–1.35 | 0.56 | 0.36–0.87 | 0.69 | 0.50–0.96 |
| Drinking up to baseline interview | ||||||||||||||
| Abstinent | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Drinking | 1.17 | 0.86–1.60 | 1.19 | 0.59–2.40 | 1.23 | 0.84–1.80 | 0.99 | 0.81–1.23 | 1.06 | 0.92–1.23 | 0.74 | 0.56–0.97 | 0.81 | 0.66–1.01 |
Adjusted models included investigation of the following potential confounders and risk factors: parity, maternal age, education, BMI, marital status, ethnicity, caffeine, smoking, exercise, work, prenatal and multivitamin use, passive smoke exposure, marijuana use, cocaine use, study cohort, preterm labor, respiratory problem, infant gender, bleeding, nausea/vomiting, hypertension, incompetent cervix, placental problems, sexually transmitted disease, induction/augmentation, maternal asthma, gestational diabetes. Total observations included in adjusted multivariable models: Month 1 across (n=4047; n=4017; n=3988; n=3983; n=3999; n=3985; n=3892); First trimester across (n=4009; n=3979; n=3950; n=3945; n=3961; n=3947; n=3856); Drinking to baseline interview, across (n=4066; n=4036; n=4007; n=4002; n=4018; n=4003; n=3910)
Based on definition of major congenital malformations 28
Includes both ventilation and continuous positive airway pressure (CPAP)
Includes NICU observation and admission
10th percentile based on CDC 2000 growth standards 27
NAC=Not able to calculate
Binge drinking was also analyzed in this cohort. During month 1, 139 women (3%) reported drinking 4+ servings on one occasion, and 159 (3.5%) reporting binge drinking during the first trimester. Adjusted models for binge drinking in the first trimester, while imprecise, did not suggest an increased risk for low birth weight, OR=0.64 (0.24, 1.69), preterm delivery, OR=0.55 (0.22, 1.38), or IUGR, OR=0.75 (0.39, 1.44) compared to non-drinkers.
Discussion
Our findings provide no support for an increased risk for low birth weight, preterm delivery, IUGR, and selected birth outcomes, consistent with earlier studies of low to moderate alcohol exposure [14, 16, 17, 30] and a systematic review of low to moderate drinking [22]. Similar to a previous meta-analysis of low to moderate prenatal drinking and malformations [30] we did not observe an increase in the risk for major congenital malformations. Risk for reduced birth length, reduced head circumference, and lower Apgar scores was not increased with low to moderate drinking in this cohort, consistent with an earlier analysis [17]. Previous studies have also reported significant reductions in risk of preterm delivery, low birth weight, and IUGR with low to moderate prenatal drinking and a curvilinear effect [19–21]. In the current analysis, significant reductions in risk were observed for low birth weight, head circumference <10th percentile, and birth length <10th percentile with low to moderate drinking. While an increased risk for neonatal ventilation was observed, stratified analysis of drinking levels ≥0.50 did not demonstrate a dose-response effect. Whether this is a real association or owed to multiple comparisons is unclear. Previous report of an increased risk for jaundice with maternal drinking [31] was not observed in our analysis; while rates of jaundice in our cohort were lower than some literature reports [32], almost one-third of the newborns were jaundiced overall. Earlier investigation using record linkage data demonstrated increased newborn care admission among infants born to mothers with alcohol-related diagnoses, however this represented a high-risk group [33]. We observed no significant increase in NICU admission with low to moderate prenatal alcohol exposure.
Comprehensive analysis of alcohol exposure throughout the first and third trimesters of pregnancy is a major strength of this study. Alcohol exposure was assessed in the first trimester prospective to birth outcomes, avoiding potential recall bias. Second trimester exposure was not assessed but is unlikely to have deviated in a meaningful way from the first and third trimester assessments. Third trimester exposures were measured retrospectively but concordance of results across trimesters suggests that recall bias was not a major factor in third trimester reporting. Exposure assessment was objectively quantified, included alcohol type, frequency, and volume, from which a validated alcohol score could be constructed to determine daily absolute alcohol exposure [25]. In addition, we adjusted for numerous potential confounders, collected primarily during prospective interviews.
There are several study limitations. The cohort was primarily of women reporting lower levels of drinking during pregnancy, precluding evaluation of risk estimates for higher levels of drinking. However, analysis of this cohort is important as it permits interpretation of effects attributable to more typical levels of low to moderate alcohol exposure among women of childbearing age. The cohort included a diverse population of urban and suburban pregnant women from hospital clinics, community clinics, and private obstetrical offices, supporting the generalizability of findings. Underreporting is a concern in studies involving maternal alcohol exposure [34]; however 29% of women in this cohort reported some alcohol exposure in month 1 which does not suggest underreporting, and exposure was lower in months 2 (9%) and 3 (7%), reflective of drinking patterns prior to and following pregnancy recognition. Prospective ascertainment of maternal drinking, including beverage type, frequency, and amount, may improve validity of self-reported drinking [35, 36]; with the current cohort, we assessed alcohol exposure both prospectively and by beverage type, frequency and amount. Cohort follow-up continued until immediately postpartum, thus evaluation of neurodevelopmental and pediatric outcomes previously reported [18, 37, 38] was not performed.
Different standards used to define IUGR make it difficult to draw comparisons across existing studies of prenatal drinking and fetal growth restriction. We constructed the most widely used formulation: the lowest 10th percentile of birth weight for gestational age, and did so according to the 1999 US birth standards [27], aligning with cohort recruitment from 1997–2001; percentiles were further adjusted for gender and ethnicity to refine population standards. CDC standards (2000) establishing the lowest 10th percentile of birth length and head circumference [28] were appropriate for the study period. While the potential for misclassification exists, further multivariable analysis of birth length and head circumference as a continuous outcome confirmed no risk increase with low to moderate levels of prenatal alcohol. Additional adjustment for gestational age within these models did not materially affect risk estimates.
We also performed a sensitivity analysis for first trimester drinking and alcohol exposure up to the baseline interview, to account for participants entering the study at different weeks during early gestation. This analysis excluded those who completed the baseline interview less than 9 weeks gestation and therefore could not provide exposure information for month 3. Both unadjusted and adjusted odds ratio estimates were similar in magnitude and statistical significance was unchanged for all outcomes.
Reported lower odds ratio estimates may be attributable to a “healthy lifestyle” effect where low levels of drinking are associated with specific lifestyles or behaviors. We observed that low to moderate drinkers in month 1 were significantly more likely to consume multivitamins and prenatal vitamins during the first trimester, work, exercise, and have normal pre-pregnancy BMI. Women may abstain from alcohol for medical reasons (which could increase risk); however, our analysis evaluated potential confounders in detail, including maternal medical history and obstetrical factors. While the observed effect may be due to unmeasured confounders, they would need to be protective and independent of variables included in our multivariable models. Effects due to beverage type have been of interest; previous analysis of supermarket transactions found an association between wine and healthier food purchases [39]. Among the current cohort, wine was the predominant source of alcohol, consumed by 68% of women drinking in month 1. Multivariable modeling for beverage type did not demonstrate a significant increase in risk for low birth weight, IUGR, or preterm delivery.
Specific birth outcomes investigated in this analysis, including IUGR, birth length, and reduced head circumference can be hallmark features of FAS. We found no association of low to moderate alcohol with these outcomes. Evaluation of infant medical records identified two newborns with ICD-9 diagnostic code 760.7, defined as “Noxious influences affecting fetus or newborn via placenta or breast milk.” One had no adverse birth outcomes and exposure to 0.25<0.50oz AA/day (approximately 3.5 to 7 drinks per week) in the first trimester; the other newborn had IUGR, with head circumference and birth length below the 10th percentile, yet no first trimester alcohol exposure. However, due to lack of systematic evaluation of specific structural features and longitudinal follow-up to assess cognitive, motor and neurological functioning, we were not able to specifically evaluate FAS or ARND in this cohort.
Conclusions
National and international guidelines advise women to abstain from drinking during pregnancy [40–43]. As lower level drinking represents a more prevalent exposure among pregnant women especially before pregnancy recognition, scientific research regarding lower level exposures is a priority; yet published study findings remain inconsistent and qualitatively varied. This study adds to accumulating evidence regarding a lack of increased risk from low to moderate maternal alcohol consumption during pregnancy, selected perinatal outcomes and measures of fetal growth.
Supplementary Material
ACKNOWLEDGEMENTS
Funding: This work was supported by the National Institutes of Health (Grants DA05484 and AI41040).
LIST OF ABBREVIATIONS AND ACRONYMS
- AA
absolute alcohol
- ADH1B
alcohol dehydrogenase 1B
- ARBD
alcohol related birth defects
- ARND
alcohol related neurodevelopmental disorders
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CPAP
continuous positive airway pressure
- FAS
fetal alcohol syndrome
- ICD-9
International Classification of Diseases, Ninth Revision
- IUGR
intrauterine growth restriction
- LMP
last menstrual period
- NICU
neonatal intensive care unit
- OR
odds ratio
- SGA
small-for-gestational age;
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interests: The authors state no conflict of interest.
Contribution to Authorship: The contributions of the authors are as follows: Lisbet Lundsberg wrote the draft of the manuscript and performed the statistical analysis; Jessica Illuzzi evaluated the draft and statistical plan, providing input regarding her clinical expertise and overall analysis; Kathleen Belanger† was instrumental in original study design and data collection, and reviewed the paper to reflect her experience with the cohort; Elizabeth Triche advised the statistical analysis, was responsible for development of selected variable constructs, and contributed to oversight and management of the original research project; Michael Bracken was PI on both NIH grants that supported the cohorts involved, and revised the first, subsequent, and final drafts of the paper. All authors have contributed to and read the final version of this manuscript, approve its submission and accept responsibility for its content.
Contributor Information
Lisbet S. Lundsberg, Email: lisbet.lundsberg@yale.edu.
Jessica L. Illuzzi, Email: jessica.illuzzi@yale.edu.
Elizabeth W. Triche, Email: Elizabeth_Triche@brown.edu.
Michael B. Bracken, Email: michael.bracken@yale.edu.
References
- 1.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 2.Gray R, Mukherjee RA, Rutter M. Alcohol consumption during pregnancy and its effects on neurodevelopment: what is known and what remains uncertain. Addiction. 2009;104(8):1270–1273. doi: 10.1111/j.1360-0443.2008.02441.x. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Fetal Alcohol Spectrum Disorders (FASDs) National Center on Birth Defects and Developmental Disorders; 2012. [accessed 9 March 2013]. http://www.cdc.gov/ncbddd/fasd/index.html. [Google Scholar]
- 4.Tolstrup JS, Kjaer SK, Holst C, Sharif H, Munk C, Osler M, et al. Alcohol use as predictor for infertility in a representative population of Danish women. Acta Obstet Gynecol Scand. 2003;82(8):744–749. doi: 10.1034/j.1600-0412.2003.00164.x. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health. 1992;82(1):85–87. doi: 10.2105/ajph.82.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strandberg-Larsen K, Gronboek M, Andersen AM, Andersen PK, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiol. 2009;20(6):884–891. doi: 10.1097/EDE.0b013e3181bbd46c. [DOI] [PubMed] [Google Scholar]
- 7.O’Leary C, Jacoby P, D’Antoine H, Bartu A, Bower C. Heavy prenatal alcohol exposure and increased risk of stillbirth. BJOG. 2012;119(8):945–952. doi: 10.1111/j.1471-0528.2012.03333.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosett HL, Weiner L, Lee A, Zuckerman B, Dooling E, Oppenheimer E. Patterns of alcohol consumption and fetal development. Obstet Gynecol. 1983;61(5):539–546. [PubMed] [Google Scholar]
- 9.Passaro KT, Little RE, Savitz DA, Noss J. The effect of maternal drinking before conception and in early pregnancy on infant birthweight. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Epidemiol. 1996;7(4):377–383. doi: 10.1097/00001648-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Sulaiman ND, Florey CD, Taylor DJ, Ogston SA. Alcohol consumption in Dundee primigravidas and its effects on outcome of pregnancy. Br Med J (Clin Res Ed) 1988;296(6635):1500–1503. doi: 10.1136/bmj.296.6635.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Leary CM, Nassar N, Kurinczuk JJ, Bower C. The effect of maternal alcohol consumption on fetal growth and preterm birth. BJOG. 2009;116(3):390–400. doi: 10.1111/j.1471-0528.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead N, Lipscomb L. Patterns of alcohol use before and during pregnancy and the risk of small-for-gestational-age birth. Am J Epidemiol. 2003;158(7):654–662. doi: 10.1093/aje/kwg201. [DOI] [PubMed] [Google Scholar]
- 13.Mills JL, Graubard BI, Harley EE, Rhoads GG, Berendes HW. Maternal alcohol consumption and birth weight. How much drinking during pregnancy is safe? JAMA. 1984;252(14):1875–1879. [PubMed] [Google Scholar]
- 14.Bakker R, Pluimgraaff LE, Steegers EA, Raat H, Tiemeier H, Hofman A, et al. Associations of light and moderate maternal alcohol consumption with fetal growth characteristics in different periods of pregnancy: the Generation R Study. Int J Epidemiol. 2010;39(3):777–789. doi: 10.1093/ije/dyq047. [DOI] [PubMed] [Google Scholar]
- 15.Olsen J, Bolumar F, Boldsen J. Does moderate alcohol intake reduce fecundability? A European multicenter study on infertility and subfecundity. Alc Clin Exp Res. 1997;21(2):206–212. [PubMed] [Google Scholar]
- 16.Shiono PH, Klebanoff MA, Rhoads GG. Smoking drinking during pregnancy. Their effects on preterm birth. JAMA. 1986;255(1):82–84. [PubMed] [Google Scholar]
- 17.Walpole I, Zubrick S, Pontre J. Is there a fetal effect with low to moderate alcohol use before or during pregnancy? J Epidemiol Community Health. 1990;44(4):297–301. doi: 10.1136/jech.44.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesmodel US, Bertrand J, Stovring H, Skarpness B, Denny CH, Mortensen EL. The effect of different alcohol drinking patterns in early to mid pregnancy on the child’s intelligence, attention, and executive function. BJOG. 2012;119(10):1180–1190. doi: 10.1111/j.1471-0528.2012.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesmodel U, Olsen SF, Secher NJ. Does alcohol increase the risk of preterm delivery? Epidemiol. 2000;11(5):512–518. doi: 10.1097/00001648-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Lundsberg LS, Bracken MB, Saftlas AF. Low-to-moderate gestational alcohol use and intrauterine growth retardation, low birthweight, and preterm delivery. Ann Epidemiol. 1997;7(7):498–508. doi: 10.1016/s1047-2797(97)00081-1. [DOI] [PubMed] [Google Scholar]
- 21.McDonald AD, Armstrong BG, Sloan M. Cigarette, alcohol, and coffee consumption and prematurity. Am J Public Health. 1992;82(1):87–90. doi: 10.2105/ajph.82.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG. 2007;114(3):243–252. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- 23.Bracken MB, Triche EW, Belanger K, Saftlas A, Beckett WS, Leaderer BP. Asthma symptoms, severity, and drug therapy: a prospective study of effects on 2205 pregnancies. Obstet Gynecol. 2003;102(4):739–752. doi: 10.1016/s0029-7844(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 24.Bracken MB, Triche EW, Belanger K, Hellenbrand K, Leaderer BP. Association of maternal caffeine consumption with decrements in fetal growth. Am J Epidemiol. 2003;157(5):456–466. doi: 10.1093/aje/kwf220. [DOI] [PubMed] [Google Scholar]
- 25.Jessor R, Graves TD, Hanson RC, Jessor SL. Society, Personality, and Deviant Behavior: a study of a tri-ethnic community. New York: Holt, Reinhard and Winston; 1968. [Google Scholar]
- 26.National Institute on Alcohol Abuse and Alcoholism. [accessed 27 March 2013];Moderate and Binge Drinking. 2013 http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- 27.1999 Natality Detail File: National Center for Health Statistics [database on the Internet] National Center for Health Statistics; 1999. Available from: http://www.nber.org/data/natality.html. [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 29.Kulaga S, Berard A. Congenital malformations: agreement between diagnostic codes in an administrative database and mothers’ reports. J Obstet Gynaecol Can. 2010;32(6):549–554. doi: 10.1016/S1701-2163(16)34523-6. [DOI] [PubMed] [Google Scholar]
- 30.Polygenis D, Wharton S, Malmberg C, Sherman N, Kennedy D, Koren G, et al. Moderate alcohol consumption during pregnancy and the incidence of fetal malformations: a meta-analysis. Neurotoxicol Teratol. 1998;20(1):61–67. doi: 10.1016/s0892-0362(97)00073-1. [DOI] [PubMed] [Google Scholar]
- 31.Lazzaroni F, Bonassi S, Magnani M, Calvi A, Repetto E, Serra F, et al. Moderate maternal drinking and outcome of pregnancy. Eur J Epidemiol. 1993;9(6):599–606. doi: 10.1007/BF00211433. [DOI] [PubMed] [Google Scholar]
- 32.CDC. Facts about jaundice and kernicterus. Centers for Disease Control and Prevention; 2011. [accessed 27 March 2013]. http://www.cdc.gov/ncbddd/jaundice/facts.html. [Google Scholar]
- 33.Burns L, Mattick RP, Cooke M. Use of record linkage to examine alcohol use in pregnancy. Alcohol Clin Exp Res. 2006;30(4):642–648. doi: 10.1111/j.1530-0277.2006.00075.x. [DOI] [PubMed] [Google Scholar]
- 34.Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S. Underreporting of alcohol use in pregnancy. Alcohol Clin Exp Res. 1988;12(4):506–511. doi: 10.1111/j.1530-0277.1988.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109(5):815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 36.Feunekes GI, van ‘t Veer P, van Staveren WA, Kok FJ. Alcohol intake assessment: the sober facts. Am J Epidemiol. 1999;150(1):105–112. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- 37.Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcohol Clin Exp Res. 2014;38(1):214–226. doi: 10.1111/acer.12214. [DOI] [PubMed] [Google Scholar]
- 38.Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy, a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38(1):129–140. doi: 10.1093/ije/dyn230. [DOI] [PubMed] [Google Scholar]
- 39.Johansen D, Friis K, Skovenborg E, Gronbaek M. Food buying habits of people who buy wine or beer: cross sectional study. BMJ. 2006;332(7540):519–522. doi: 10.1136/bmj.38694.568981.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Surgeon General. U.S. Surgeon General Releases Advisory on Alcohol Use in Pregnancy. Office of the Surgeon General; 2005. [accessed 6 December 2011]. http://www.cdc.gov/ncbddd/fasd/documents/surgeongenbookmark.pdf. [Google Scholar]
- 41.CDC. [accessed 6 December 2011];What you should know about alcohol and pregnancy. 2010 http://www.cdc.gov/Features/AlcoholAndPregnancy/
- 42.The American College of Obstetricians and Gynecologists (ACOG) [accessed 12 June 2012];FAQ068: Alcohol and women. 2011 http://www.acog.org/~/media/For%20Patients/faq068.pdf?dmc=1&ts=20120612T1116590083.
- 43.NHS Choices. Can I drink alcohol if I'm pregnant? UK: Gov.; 2012. [accessed 11 April 2013]. http://www.nhs.uk/chq/pages/2270.aspx#close. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.