Abstract
The standard respiratory function test for case detection of chronic obstructive pulmonary disease (COPD) is spirometry. The criterion for diagnosis defined in guidelines is based on the FEV1/FVC ratio forced expiratory ratio (FER) and its severity is based on forced expiratory volume in one second (FEV1) from measurements obtained during maximal forced expiratory manoeuvres. Spirometry is a safe and practical procedure, and when conducted by a trained operator using a spirometer that provides quality feedback, the majority of patients can be coached to provide acceptable and repeatable results. This allows potentially wide application of testing to improve recognition and diagnosis of COPD, such as for case finding in primary care. However, COPD remains substantially under diagnosed in primary care and a major reason for this is underuse of spirometry. The presence of symptoms is not a reliable indicator of disease and diagnosis is often delayed until more severe airflow obstruction is present. Early diagnosis is worthwhile, as it allows risk factors for COPD such as smoking to be addressed promptly and treatment optimised. Paradoxically, investigation of the patho-physiology in COPD has shown that extensive small airway disease exists before it is detectable with conventional spirometric indices, and methods to detect airway disease earlier using the flow-volume curve are discussed.
Keywords: Spirometry, chronic obstructive pulmonary disease (COPD), case finding, flow-volume curve
Pathology of chronic obstructive pulmonary disease (COPD)
Relatively early research in the 1950s and 1960s into what was by then recognized as a smoking-related disease (1) focused on pathology, and especially tissue remodeling changes in the airways and lungs. It was observed that throughout the airways there was some element of inflammation, but sub-mucosal mucous gland hyperplasia, epithelial goblet cell hyperplasia and epithelial squamous metaplasia were prominent. The characteristic lung lesion was usually peri-bronchial, centri-lobular parenchymal destruction, termed emphysema (2-4). An important conclusion from the detailed pathological analysis of this epoch was that the airway pathological component in COPD was universal and generalized, while emphysema usually developed later, perhaps as a secondary phenomenon, and only in some individuals but by no means all. This is different from the diffuse primary pan-acinar emphysema that occurs in the younger-onset alpha-1 anti-trypsin (anti-proteinase) deficiency lung disease, for example (5).
The next research epoch involved innovative physiological laboratory work in the late 1960s into the 1970s, which defined the obstructive consequences of smoking-related airway disease and the anatomical site of increased airway resistance that ultimately lead to symptoms (6,7). From this work, construction of a series of iso-volume pressure-flow curves gave rise to development of the now widely used flow-volume curve, but then without the sophisticated, sensitive and computerized equipment now available, and which we will be discussing later in some detail. However, even by that time and using the relatively crude bellows-based spirometer, the standard measure for defining airway obstruction had been specified as a reduction in the ratio of forced expiratory volume in one second (FEV1) to forced vital capacity (FVC), the forced expiratory ratio (FER); indeed in that regard little has changed over the last fifty years or so, in spite of improved understanding of physiology. Paradoxically, the seminal work of Macklem and others in clinical physiology showed that the first change in spirometry in COPD was actually a reduction in FVC due to air trapping, rather than a change in FEV1 (8). Importantly, they showed that this in turn is caused by fixed small airway narrowing, in airways less than 2 mm internal diameter. To demonstrate this, they used flow-volume studies in patients and volunteers with gases of different densities, and also measured flow resistance in different parts of the airway with retrograde catheters in resected lungs. Normal small airways have low resistance to air flow but this is markedly increased in COPD (9-11). In contrast, in asthma the main pattern is one of non-uniformly distributed larger airways obstruction, except in older asthmatics and those that smoke in whom a peripheral distribution of resistive change was common, similar to COPD.
Notably, it was shown in this epoch of physiological research, that there could be a great deal of peripheral increase in flow resistance before there was any indication on traditional spirometric measures. Patho-physiological correlation studies followed, indicating that in small airways in COPD there is indeed narrowing due to wall thickening, fibrosis and indeed airway obliteration (10,12,13). This was a new and startling insight, which is now confirmed by more sophisticated methodology (14), that there can be extensive small airway disease, damage and obliteration before it is detectable with conventional spirometric tests.
Over the years, new physiologic methods were developed to try and pick up these early small airway changes in smokers before overt COPD, defined by the FER emerged. However none was robust or practical enough at the concurrent stage of technological development to be suitable for clinical laboratory or medical office use. Such attempts continue with increasingly sophisticated techniques, and this is dealt with in a separate article in this volume; the FER remains the standard. In this article, we will review and discuss how useful in clinical practice this standard measure is, what we know about its pitfalls in clinical application and especially in primary care practice. We will also look anew at how the use of all the information available in the current standard flow-volume curve, which is now routinely obtained at the time of FER measurement but largely ignored, can potentially be harnessed and give a better overview of the status of the airways. This might contribute to recognizing early physiological impairment in smokers, perhaps as an alternative to the need to develop more expensive and complex tests.
Epidemiology and prevalence of COPD
The prevalence of COPD varies across countries; accurate estimates based on standardised population-based sampling of adults aged 40 and over in 12 sites in the burden of obstructive lung disease (BOLD) survey indicated an overall COPD prevalence (GOLD stage II or higher, FEV1 <80% predicted) (15) of 10.1% (SE 4.8) (16). Prevalence increased with age and smoking history, but other factors were also thought to be important in explaining the variation. In the Australian BOLD survey conducted in six centres, prevalence was 7.5% (95% CI, 5.7-9.4%) overall, but was greater among those aged above 75 years at 29.2% (95% CI, 18.1-40.2%) (17). The Australian survey showed large variations between centres the causes of which are being investigated (unpublished data).
Estimates of the population attributable fraction of tobacco smoking as a cause of COPD vary by age and population setting (18), although more recent estimates in those aged 30-69 years, 54% for men and 24% for women, are probably accurate and less than the widely quoted 80-90% in the 1984 US Surgeon General Report (19). Attributable fractions are higher in industrialized countries than developing countries (18), and other risk factors are also important, including exposure to biomass smoke, occupational exposures to dust and fumes, history of pulmonary tuberculosis, outdoor air pollution, and poor socioeconomic status (20) or chronic asthma (21). However smoking remains the most important cause of COPD in western countries. Around 50% of smokers eventually develop COPD, although the risk falls by about half following smoking cessation (19).
Diagnosis of COPD
As discussed previously, spirometry is accepted as the diagnostic test to assess airflow obstruction and classify severity of disease, based on specific cut points for FER (FEV1/FVC <0.7 after bronchodilator) and FEV1 (mild ≥80% predicted, moderate 50-80%, severe 30-49% predicted, very severe <30% predicted) (15). FEV1 normally decreases with age, and the rate of fall is an important spirometric indicator of disease progression in COPD. In healthy non-smoking adults the decrease is about 30 mL/year with an upper limit of about 50 mL/year (22-24); a decrease greater than this is considered abnormally rapid. There is debate on the use of a single fixed cut-off for FER to confirm the presence of airflow obstruction in COPD, because the lower limit of normal for FER decreases with age (25). Thus, this may misclassify some older patients as having COPD (26). Similarly, basing the classification of COPD on FEV1 as percentage predicted may misclassify patients especially the elderly and those in global initiative for chronic obstructive lung disease (GOLD) stages I and II (27). It has been proposed that classification should be based on a lower limit of normal (LLN) i.e., more than 1.64·SD below the predicted level (5th percentile) (28), although international guidelines still recommend use of the fixed FER for diagnosis (15).
Maximum flow achieved during forced expiration decreases progressively as lung volume falls and is most evident in the expiratory flow-volume curve where flow is plotted as a function of volume. Although flow and volume are complex biological signals, the curve is highly repeatable in both healthy and obstructed individuals and the shape of the curve can be helpful as it reflects the underlying mechanics limiting maximal flow. In healthy younger adults the shape of the flow-volume curve usually approximates a straight-sided triangle with maximum flows decreasing linearly with lung volume. In people with obstructive lung disease key physiologic features of the flow-volume curve are reduced expiratory flows in proportion to disease severity and the presence of a concavity in the descending limb; the latter indicating an abnormal decrease in maximal flow as lung volume falls.
Flow measurements derived from spirometry such as the forced expiratory flow over the middle half of the FVC (FEF25-75%) and forced expiratory flow at 75% of the FVC (FEF75%) may be more specific to small airway function, particularly in the presence of a normal FEV1, but they have not proved particularly helpful because they are dependent on the measurement of FVC, lack the repeatability of FEV1, have a wide normal range, and are reduced in the presence of narrowing occurring in proximal airways (29,30).
COPD recognition and detection
However, in spite of spirometric standards for diagnosis, a high proportion of COPD in the community remains undiagnosed; estimates of non-diagnosis in the 1990’s were 66% in the US (31) to 78% in Spain (32). Under-recognition is related to the severity of airflow obstruction; 50% of those with FEV1 <40% predicted reported a physician diagnosis of COPD, but only 19% of those with FEV1 between 60-79% predicted in the Obstructive Lung Disease in Northern Sweden study (33). More recently, only 5.2% of BOLD population survey participants in Australia reported having been diagnosed with COPD compared to the 7.5% prevalence detected (17). Undetected COPD or asthma is common in primary care; over half those aged between 25-70 years in general practices in the Netherlands had symptoms or signs (34). There is also consistent evidence of misclassification of COPD in general practice. Substantial misclassification (31% and 42%) based on practice records COPD diagnosis was found in two studies in Australia (35,36). This probably relates to the diagnosis not being based on objective spirometry testing criteria.
Increased detection of COPD may result from a community-based screening programme; 27% of participants aged over 40 years had airflow obstruction based on FER <85% predicted in outpatient clinics in Poland (37). However such screening has not been widely implemented; a US Preventive Services Task Force assessment of the evidence did not recommend screening with spirometry and concluded with moderate certainty that there was no net benefit (38).
A more cost effective strategy using opportunistic case finding in primary care based on the presence of risk factors (age and smoking) and symptoms is recommended in the UK Update Guideline on COPD (39). A substantial amount of undiagnosed clinically significant COPD was demonstrated in the Health Survey for England 1995-6 (40). In over half these cases of unrecognized COPD management guidelines recommend treatment, either with combination inhaled corticosteroid/long-acting beta agonist or anticholinergic inhaler to reduce hospitalisation and mortality, or pulmonary rehabilitation to improve quality of life (40). Case finding can be effective when conducted opportunistically for patients attending general practice for any reason (41), compared with only a small improvement for ‘targeted’ case finding using pre-attendance practice register searches and mail out invitations to selected patients (42).
In many health systems, primary care provides the most accessible and most frequently accessed health care and efforts to increase recognition and diagnosis of COPD have mainly focussed on general practice (43,44). Spirometry testing should focus on those at risk particularly from smoking; thus spirometry was able to detect unrecognised airflow obstruction (FEV1 <80% predicted) in 22% of current smokers aged 35 to 70 years with at least one typical COPD symptom in the Netherlands (41). The proportion of COPD of at least GOLD grade II (FEV1 <80% predicted) in smokers aged over 40 in general practices varies, from 25% in a Canadian study (45) to 47% in a study in Belgium (46), with only around a third in both already having a COPD diagnosis.
An alternative approach is to base spirometry testing on respiratory-relevant symptom screening using a questionnaire (47), with the cut-off score for subsequent spirometry chosen to maximise sensitivity and specificity (48). In this way, using a COPD screening questionnaire (48) and a cut-off score of 17 or above (range, 0-40) in patients over 40 years attending general practice in Greece, the sensitivity for new COPD diagnosis was 93% but specificity was only 39% (49). Simple inexpensive hand-held spirometers are available for use in general practice; they display FEV1, forced expiratory volume in 6 seconds (FEV6) as a surrogate for FVC, and the ratio FEV1/FEV6. Applying a cut off ratio FEV1/FEV6 <0.7 after bronchodilator in the same study in Greek general practices, increased the specificity to 94% with sensitivity of 80% for COPD diagnosis (49).
A further refinement for identifying COPD in general practice is to combine a COPD symptom questionnaire with measurement of FEV1/FEV6 ratio. In the Greek study quoted above, the combination of the questionnaire with its high negative predictive value and the hand-held spirometer with its high positive predictive value had a sensitivity of 74% and specificity of 97% for COPD diagnosis, while the negative predictive value was 95% and positive predictive value was 82% (49). In a scenario when individuals at risk of COPD in primary care were screened with a hand-held spirometer before full spirometry testing, a cut off point corresponding to FEV1/FEV6 <0.75 was found to offer optimal sensitivity (81%) and specificity (71%) for diagnosis in current and former smokers aged over 50 years (50). Linking symptom screening to case finding for COPD is ideal if the intention is to commence treatment in symptomatic individuals, but this approach is less suitable if the aim is to reduce end-organ disease.
Symptoms and a diagnosis of COPD
The place of symptoms in the diagnostic criteria for COPD has been debated (51) and there is some inconsistency between GOLD (15) and NICE (39) guidelines, with NICE advising not diagnosing COPD in the absence of symptoms in patients with mild airflow obstruction (FER <0.7, FEV1 >80% predicted) (52). However, there is substantial evidence that reported symptoms are unreliable for diagnosis, although in general the symptom burden in COPD increases with severity of airflow obstruction. There is wide variation in the degree of breathlessness, health status and exercise capacity within GOLD stages; thus even when airflow obstruction is severe in COPD, some people do not report symptoms or exercise limitation (53). There is also under-presentation by patients with potential chronic respiratory disease who do not raise respiratory symptoms with their general practitioner; 46% of patients with spirometrically confirmed COPD had not paid a single visit for respiratory health problems during a 10-year observational study in the Netherlands (54). Patients may attribute their symptoms to ageing and attribute multi-casual explanations that lessen the importance of obtaining a diagnosis (55). On the other hand, respiratory symptoms typical of COPD may be noted in practice records for long periods prior to diagnosis (56), with varying attitudes and degrees of vigilance among general practitioners to early diagnosis (56,57). Thus diagnosis of COPD may be delayed and indeed often does not occur until an acute exacerbation results in admission and hospital-based diagnosis (57).
Early diagnosis
Early diagnosis is a contentious issue, but it optimises the opportunities to prevent worsening of disease and prevention of comorbidities. Guidelines for COPD emphasise that it is a multi-system disease requiring a multidimensional approach to treatment (52). There is a strong emphasis on smoking cessation in both NICE (39) and GOLD (15) guidelines as the intervention with the greatest capacity to influence the natural history of COPD (58). Although a review in 2007 of randomised controlled studies on the value of spirometry itself as a motivational tool to increase smoking cessation was inconclusive (59), telling smokers their ‘lung age’ based on spirometry testing increased 12 months sustained quitting by over 7%, irrespective of the actual deficit in ‘lung age’ (60).
An increased risk of lung cancer in COPD was found in a long term US observational study in moderate or severe COPD (61) and in a case control study in lung cancer (62). The increased risk with COPD is present even when allowance is made for cigarette smoking history.
Similarly, the association of reduced FEV1 with increased overall mortality has been recognized in studies in non-smokers (63) and smokers, with the effect of reduced FEV1 independent of smoking history (64). The potential importance of the FVC was highlighted in a USA general population cohort without chronic respiratory diagnoses or persistent respiratory symptoms, in which survival was associated with higher FVC in both men and women after adjustment for smoking and demographic factors (65). Such associations underlie the need for an earlier awareness of abnormality on spirometry as a part of a general health screening approach, such as was taken in cardiovascular disease to reduce the high burden of mortality that existed 40 years ago (66).
Value of current diagnostic tools for COPD: spirometry
Spirometry is a safe, practical and reproducible maximum breathing test that can be used in primary care to objectively determine the ventilatory capacity of the lungs. As already emphasised earlier in this article, it is the ‘gold standard’ for detecting and quantifying airflow obstruction (15) and as discussed, is the core component of clinical guidelines for the diagnosis and management of COPD (67). The test is relatively quick to perform, well tolerated by most patients and the results are immediately available to clinician. It is important to appreciate that the clinical value of spirometry is critically dependent on the correct operation and accuracy of the spirometer, performance of the correct maximal breathing manoeuvre, selection of the best test results to use and correct interpretation. When a trained and experienced operator using modern equipment conducts the test, at least 90% of adults are able to provide acceptable and repeatable results (68). In the primary care setting the rate is lower but can still be reasonable at about 80%, especially when the spirometer grades each test and provides feedback relating to test quality (69).
Development of spirometry
A spirometer is a medical device that allows measurement of how much air is expelled and how quickly the lungs can be emptied, in a maximal expiration from full inflation. Modern spirometry has its origins in the 1840’s when the English surgeon, John Hutchinson, developed the spirometer and described the measurement of slow vital capacity as a means of detecting lung disease (70). One hundred years later Tiffeneau and Pinelli from France revolutionised spirometry by describing the forced expiratory timed spirogram and introducing an obstructive index, the ratio FEV1/inspiratory vital capacity (IVC) which is still used today, albeit with IVC most commonly replaced with FVC (71) or expiratory vital capacity (72). It was only a few years later in 1960 that the American physiologists, Fry and Hyatt, in a landmark study of lung mechanics, replotted the data contained in the timed spirogram in the form of the flow-volume curve (73) which is now universally accepted as the preferred method of graphically displaying spirometric data. The flow-volume curve is now available in almost all commercially available spirometers and is displayed in real-time as the patient performs the test.
Modern spirometers
Almost all modern spirometers utilise a sensitive real-time flow sensor to directly measure respired flow and obtain volume by electronic or numerical integration. Manual volume-displacement spirometers are still in limited use, especially in primary care (74), such as the iconic wedge bellows Vitalograph which over many decades has played a very significant role in popularising the measurement and application of spirometry beyond the expert laboratory, but this genre of spirometer usually lacks portability, is difficult to clean and disinfect, can be difficult to calibrate and requires spirometric variables to be calculated manually and does not produce the flow-volume curve.
There are many spirometers on the market today and most are robust, portable, accurate and reliable and specifically designed for use in either a lung function laboratory or a physician’s office (74). Most, if not all, modern spirometers meet minimum international performance standards and validation procedures that were developed jointly by the American Thoracic Society and European Respiratory Society (75). These include meeting accuracy requirements for volume, flow and time signals using specifically developed test signals, and applying the back-extrapolation technique to identify both sluggish starts to the blow and the zero time point from which timed volumes such as FEV1 are calculated. Modern spirometers also have the added advantages of infection control, automatic calculation of all lung function indices including correction for temperature, pressure and water-saturation conditions. Many will also provide immediate computer-generated feedback to the operator on the test quality and repeatability as well as real-time graphical display of the spirogram and flow-volume curve, will select the best results to report, calculate normal reference values including the lower limit of normal, and can automatically upload results to medical records.
Primary care spirometry
Spirometry is commonly performed outside the lung function laboratory. A survey of primary care practices in Australia found that 64% owned a spirometer with almost 70% performing at least one test per week mainly for the diagnosis and management of asthma and COPD (76). The high spirometer ownership was not surprising given that a large number of patients with lung disease are first seen and subsequently managed in primary care.
Opinion is divided as to whether the quality of spirometry performed outside expert laboratories meets adequate minimum standards (75) with the potential for high rates of misclassification, especially when the results are near the lower limit of normal (69,77-79). The measurement of spirometry requires a motivated and enthusiastic operator to coach the patient to perform a number of very rigorous maximally forced and sustained breathing manoeuvres (80). It is not surprising therefore that unlike most other medical tests such as the measurement of blood pressure and the electrocardiogram, the quality of spirometry tests are crucially dependent on the operator and cooperation of the patient and thus spirometry performed in primary care is often of poor quality (81). Although the key to obtaining quality spirometry is attending a comprehensive training course, the importance of testing experience cannot be overstated and may well be the most important factor.
The concave pattern on flow-volume curve
Current guideline criteria for airway obstruction and its severity essentially rely on just two variables FEV1 and FVC, and their ratio the FER. Although these variables have played an important role in developing our understanding of the mechanisms and functional effects of COPD, we have emphasised that they are relatively insensitive to early obstructive small airway pathology, because these cause FVC to fall first (8) with initial preservation of the FER. Spirometry has thus been of limited use as a screening tool for early disease; this is disappointing as it is the most practical and widely performed test of lung health and should therefore be ideal to screen for early disease. We present a case that relying solely on the FEV1 and FER potentially misses information contained in the whole flow-volume curve, particularly the concave pattern, which may provide greater sensitivity in detecting and monitoring early disease.
The development of concavity in the descending limb of the maximum expiratory flow-volume curve is a recognised feature of airflow obstruction, with greater concavity reflecting increased obstruction, and the first indication of a concavity is frequently seen in the tail of the curve (30,72). This is explicitly acknowledged in the ATS/ERS statement on the interpretation of lung function (82), but has largely been ignored in practice because none of the measurements taken currently to reflect this concavity are robust enough.
The functional information provided by the FEV1 is necessarily limited to the first second of the forced expiratory manoeuvre when the lung is relatively fully inflated and the small airways exposed to significant distending forces. This means that in older people with a normal FER, as much as 40% of the flow-volume curve is not assessed, all in the terminal portion of the curve, and a greater proportion in people with airflow obstruction. In contrast, the concave pattern seen in people with airflow obstruction is not limited to the first second but often extends over most of the curve, reflecting a global pattern of airway dysfunction. In early airflow obstruction, when the FEV1 is normal, a concavity is often present and may well be mostly confined to the terminal portion of the curve where lung volume is relatively low and the distending forces on the small airways are significantly reduced, resulting in a higher peripheral airway resistance and non-uniform emptying in peripheral lung regions. It is notable that the latter may well be the major spirometric defect signalling early disease, and requires better quantitative assessment.
The concave pattern develops when lung compartments have widely differing expiratory time constants causing regional inhomogeneity (83) as is certainly the case in obstructive lung disease with peripheral increase in airway resistances, with the slowest emptying compartments contributing disproportionally to flows near residual volume, resulting in a curve with the familiar exaggerated ‘tail’. It is not surprising, therefore, that even though the underlying mechanics determining FEV1 and the concave pattern overlap, they are not necessarily equivalently strong physiological signals at different disease stages. They may however be quite complementary, not only in assessing airflow obstruction overall but especially in detecting early obstructive small airway disease (84).
It seems reasonable that to detect and assess early disease we need a method that is sensitive to inhomogeneous airway emptying because this almost certainly precedes the development of the more advanced obstruction for which we use currently the standard FER. Highly sophisticated technology is currently being developed to measure this inhomogeneous lung emptying, but it could well be that much of this information is already available in the expiratory flow-volume curve if only it can be harnessed.
Strong evidence that a concavity confined in the terminal portion of the curve is most likely to be associated with small airways dysfunction came from further studies that compared flow-volume curves obtained breathing gases of widely differing gas density which showed that maximal flows near the terminal portion of the flow-volume curve predominantly reflect small airway function (85). This is also consistent with studies using wave speed mechanics (86) and the equal pressure point theory (72) which predicts that the flow-limiting segment developed during forced expiration moves peripherally into progressively smaller airways as lung volume falls and especially when peripheral airway resistance increases.
The clinical value of quantifying concavity has been under-appreciated although demonstrated spirometrically in different populations (84,87-92). The study by Kraan et al. (89) was of particular interest as it provided strong evidence that although the reduction in FEV1 and the degree of concavity are related, they do not necessarily measure the same things; for example they were differentially affected by bronchodilator and anti-inflammatory treatment. Schachter et al. (87) showed that although cotton workers had abnormal spirometry, the concave pattern was only present in current cigarette smokers. Another study showed the degree of concavity was greater in those with a smoking history and people with breathlessness and wheezes (88).
New indices to quantify concavity
Visual assessment of concavity in the flow-volume curve is highly subjective and cannot reliably be used to assess an abnormal degree of concavity. What is needed is a practical and easily understood numerical index to quantify concavity with clearly defined limits of normal. Although a number of methods have been described (83,87,93) they are complex or difficult to apply routinely and none has been incorporated into commercial spirometry software or clinical guidelines.
However, we describe two indices for estimating concavity (global and peripheral) with preliminary data comparing these with conventional spirometric variables, in a randomly selected population of adults aged >40 years in Australia. The global index is based on FEF50% and quantifies concavity that usually involves the entire descending limb, whilst the peripheral index is based on the FEF75% and independently quantifies concavity present near the terminal portion of the curve. The degree of concavity is obtained by calculating the percentage decrease of the measured flows from the corresponding idealised reference flows (Figure 1).
Figure 1.
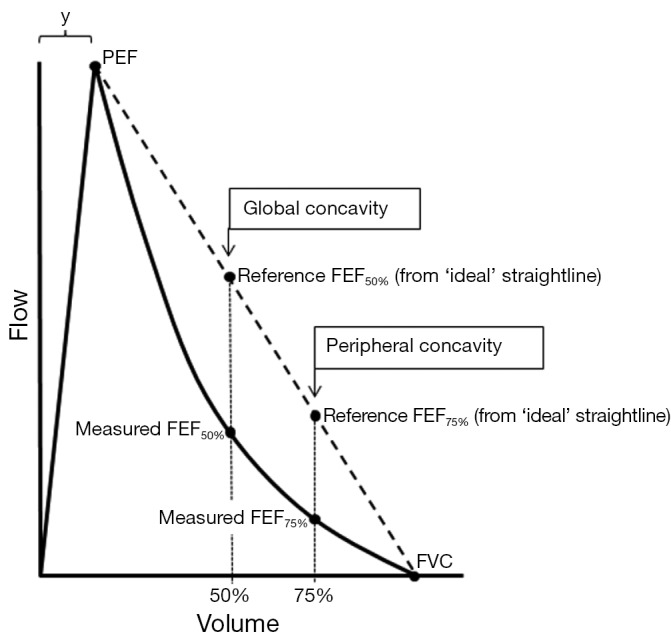
Variables used to quantify global and peripheral concavity (see text). Measured FEF50% and measured FEF75% are the forced expired flows when 50% and 75% of the FVC has been expired. Reference FEF50% and Reference FEF75% are the reference flows that would be obtained if the flow-volume curve had zero curvature i.e., a linear descending limb (dotted line). The variable, y, is the volume to peak expiratory flow (PEF); a value of 0.6 L can be assumed for this. In this example, global concavity is approximately 50 Units and peripheral concavity is approximately 65 Units.
| Global Concavity =100* (reference FEF50%—measured FEF50%)/reference FEF50% |
| Peripheral Concavity =100* (reference FEF75%—measured FEF75%)/reference FEF75% |
The measured FEF50% and measured FEF75% are obtained from the subject’s flow-volume curve. The two corresponding reference flows are calculated assuming that the descending limb is a straight line from PEF to end-expiration (Figure 1) and therefore has no curvature:
| Reference FEF50% = PEF*(FVC/2)/(FVC-y) |
| Reference FEF75% = PEF*(FVC/4)/(FVC-y) |
The variable, y, is the volume expired to PEF (Figure 1) and although ideally should be measured from the curve, assuming a fixed value of 0.6 litres leads to little error. The calculated indices are dimensionless with units ranging from zero (no concavity) to a theoretical limiting value approaching 100 (maximum concavity). Negative values are possible and indicate that that the curvature of the descending limb is convex (no concavity). These indices (Concavity Units) are easily incorporated into spirometry software, are independent of the size of the flow-volume curve and closely mirror the intuitive way many clinicians visually assess the degree of concavity, mentally adding the straight line, but with more objectivity.
Our exploratory analysis of global and peripheral concavity involved 387 (223 females, 164 males) randomly selected subjects from Tasmania who had participated in the BOLD Australia study (17). Spirometry and the degree of concavity were obtained from baseline and post-bronchodilator flow-volume curves measured using the Easyone ultrasonic spirometer (ndd Medizintechnik) that met ATS/ERS acceptability and repeatability criteria (75). The age range of subjects was 42-87 years, with mean age 59.4 years for males and 58.3 for females. A higher proportion of males had ever smoked (60%) compared with females (48%) and males had substantially higher lifetime tobacco consumption (median 24 versus 11 pack years). Overall subjects who had ever smoked, especially males with the highest lifetime tobacco consumption, had a greater degree of global and peripheral concavity compared with never smokers (Table 1). The degree of global and peripheral concavity decreased after the administration of a bronchodilator, in both the male and female subjects who had ever smoked or had never smoked. Of note, even in people who had never smoked the presence of both patterns of concavity was a common finding in this older population.
Table 1. Spirometry and concavity data mean and standard deviation (SD) from the Tasmanian BOLD population (17).
| Males (n=164); mean (SD) |
Females (n=223); mean (SD) |
||||
|---|---|---|---|---|---|
| Never smoked (n=66) | Ever smoked (n=98) | Never smoked (n=115) | Ever smoked (n=108) | ||
| Post-BD spirometry | |||||
| FEV1, % predicted | 101.5 (13.2) | 93.3 (15.5) | 98.8 (16.6) | 99.0 (16.3) | |
| FVC, % predicted | 101.3 (12.4) | 97.5 (14.1) | 99.5 (15.2) | 99.4 (13.2) | |
| FER, absolute % | 76.0 (6.8) | 72.5 (9.3) | 77.2 (8.0) | 73.8 (9.5) | |
| Acute reversibility | |||||
| FEV1, % change | 4.4 (4.1) | 3.7 (6.6) | 3.2 (7.0) | 3.6 (5.8) | |
| FER, absolute change | 2.3 (4.2) | 2.8 (4.5) | 2.4 (4.2) | 2.2 (4.5) | |
| Global concavity units | |||||
| Pre-BD | 32.7 (21.6) | 40.4 (22.3) | 22.9 (24.2) | 32.0 (26.9) | |
| Post-BD | 27.5 (22.6) | 35.2 (23.6) | 13.4 (26.4) | 25.6 (29.4) | |
| Peripheral concavity units | |||||
| Pre-BD | 67.1 (19.0) | 74.0 (14.0) | 63.9 (19.4) | 69.5 (18.5) | |
| Post-BD | 59.1 (24.1) | 68.3 (18.0) | 56.9 (21.6) | 63.3 (21.4) | |
BD, bronchodilator; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FER, forced expiratory ratio.
The limits of normal for concavity were estimated separately for males and females using post-BD data from subjects who had never smoked, with FER >0.7 and reversibility of FEV1 <10%. Thus, an abnormal degree of concavity was defined as present in males if global concavity >34.8 Units or peripheral concavity >61.2 Units, and in females if global concavity >26.3 Units and peripheral concavity >63.1 Units. The LLN for FEF25-75% was based on reference values from Hankinson et al. (25).
In this Tasmanian population, the prevalence of abnormal global and peripheral concavity was far higher than estimated based on either GOLD criteria or FEF25-75% (Table 2). It is of interest that the presence of an abnormal degree of concavity confined solely to the terminal portion of the curve (global > ULN plus peripheral < ULN) was not uncommon. This pattern was present in 73 (19%) of participants overall of whom only four had abnormal FER (<0.7).
Table 2. Comparison of prevalence rates of abnormal conventional spirometry indices and abnormal concavity for the Tasmanian BOLD population (17).
| Index | Criterion for abnormal | n, prevalence (%) |
|
|---|---|---|---|
| Males (n=164) | Females (n=223) | ||
| FER (± FEV1% pred.) | FER <0.7 (GOLD any stage) | 46 (28.0) | 47 (21.1) |
| FER <0.7 + FEV1 ≥80% pred. (GOLD stage I) | 31 (18.9) | 27 (12.1) | |
| FER <0.7 + FEV1 ≥50% to <80% pred. (GOLD stage II) | 14 (8.5) | 17 (7.6) | |
| FEF25-75% | FEF25-75% < LLN (25) | 11 (6.7) | 20 (9.0) |
| Global Concavity Units | > ULN | 76 (46.3) | 89 (39.9) |
| Peripheral Concavity Units | > ULN | 108 (65.9) | 110 (49.3) |
| Pure peripheral Concavity | Global < ULN + peripheral > ULN | 37 (22.6) | 36 (16.1) |
GOLD, global initiative for chronic obstructive lung disease (15).
Both the FER and degree of concavity are independent of the size of the flow-volume curve. Figure 2 shows that there is a strong non-linear relationship between FER and our measures of concavity. The horizontal and vertical lines are the limits of normal for FER (GOLD) and concavity, respectively. According to clinical guidelines, subjects falling to the right of the vertical line have a normal FER (no airflow obstruction) and from our data those above the horizontal line have an abnormal degree of concavity. The upper right quadrant (shaded area in Figure 2) is of special interest because it identifies subjects without airflow obstruction defined by the FER but who have an abnormal degree of concavity. This may be useful in detecting airflow obstruction that is unseen by conventional analysis of spirometric data. This requires further investigation as does the relationship between concavity and symptom scores, and whether the association is stronger than between symptoms and FEV1. The ability to fully utilize the large amount of information obtained in modern spirometry could have great potential, opening a way to introduce the insights about early small airway dysfunction from classical physiology into the clinic without a need for additional complex equipment.
Figure 2.
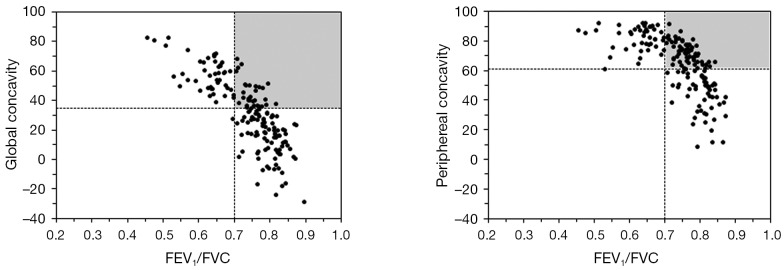
Post-bronchodilator forced expiratory ratio (FER) of FEV1/FVC plotted against global and peripheral concavity in male participants. The horizontal and vertical lines are the limits of normal for FER (15) and concavity, respectively. The shaded quadrant identifies subjects with normal FER but an abnormal degree of concavity (see text).
Conclusions
The standard respiratory function test for case detection of COPD is spirometry, with the criterion for diagnosis defined in guidelines being based on FER and the severity being based on FEV1. However, using this approach is poor at detecting early disease in the small airways. Improved, although more complex, tests are being developed to recognise such early cases but we have shown that by using all the information available from the spirometric expiratory flow-volume curve, and especially by quantifying the degree of concavity, that this may be in itself more sensitive and specific for small airways disease. However, even the current means of diagnosing relatively more severe disease that is detectable by the FER threshold is poorly taken up in primary care, despite the benefits that could be achieved with smoking cessation and pharmacological and non-pharmacological interventions to improve patients’ well-being. The reasons are not completely understood but include attitudes of both doctors and patients to COPD. The potential importance of detecting early fixed airway obstruction for prevention of lung cancer and non-respiratory end-organ disease also needs to be better highlighted in public health campaigns.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Anderson DO, Ferris BG. Role of Tobacco Smoking in the Causation of Chronic Respiratory Disease. N Engl J Med 1962;267:787-94. [DOI] [PubMed] [Google Scholar]
- 2.Reid L.Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis. Thorax 1960;15:132-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurlbeck WM, Angus GE. A distribution curve for chronic bronchitis. Thorax 1964;19:436-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunnill MS. The classification and quantification of emphysema. Proceedings of the Royal Society of Medicine 1969;62:1024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flotte TR, Mueller C. Gene therapy for alpha-1 antitrypsin deficiency. Hum Mol Genet 2011;20:R87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macklem PT. The physiology of small airways. Am J Respir Crit Care Med 1998;157:S181-3. [DOI] [PubMed] [Google Scholar]
- 7.Macklem PT. A Century of the Mechanics of Breathing. Am J Respir Crit Care Med 2004;170:10-5. [DOI] [PubMed] [Google Scholar]
- 8.Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J 2010;35:676-80. [DOI] [PubMed] [Google Scholar]
- 9.Despas PJ, Leroux M, Macklem PT. Site of airway obstruction in asthma as determined by measuring maximal expiratory flow breathing air and a helium-oxygen mixture. J Clin Invest 1972;51:3235-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968;278:1355-60. [DOI] [PubMed] [Google Scholar]
- 11.Martin RR, Lindsay D, Despas P, et al. The early detection of airway obstruction. Am Rev Respir Dis 1975;111:119-25. [DOI] [PubMed] [Google Scholar]
- 12.Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978;298:1277-81. [DOI] [PubMed] [Google Scholar]
- 13.Macklem PT, Thurlbeck WM, Fraser RG. Chronic Obstructive Disease of Small Airways. Ann Intern Med 1971;74:167-77. [DOI] [PubMed] [Google Scholar]
- 14.McDonough JE, Yuan R, Suzuki M, et al. Small-Airway Obstruction and Emphysema in Chronic Obstructive Pulmonary Disease. N Engl J Med 2011;365:1567-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD 2013. Available online: http://www.goldcopd.org/
- 16.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741-50. [DOI] [PubMed] [Google Scholar]
- 17.Toelle BG, Xuan W, Bird TE, et al. Respiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) study. Med J Aust 2013;198:144-8. [DOI] [PubMed] [Google Scholar]
- 18.Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;182:693-718. [DOI] [PubMed] [Google Scholar]
- 19.Lundbäck B, Lindberg A, Lindström M, et al. Not 15 but 50% of smokers develop COPD?--Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2003;97:115-22. [DOI] [PubMed] [Google Scholar]
- 20.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009;374:733-43. [DOI] [PubMed] [Google Scholar]
- 21.Perret JL, Dharmage SC, Matheson MC, et al. The interplay between the effects of lifetime asthma, smoking, and atopy on fixed airflow obstruction in middle age. Am J Respir Crit Care Med 2013;187:42-8. [DOI] [PubMed] [Google Scholar]
- 22.Tager IB, Segal MR, Speizer FE, et al. The natural history of forced expiratory volumes. Effect of cigarette smoking and respiratory symptoms. Am Rev Respir Dis 1988;138:837-49. [DOI] [PubMed] [Google Scholar]
- 23.Sherman CB, Xu X, Speizer FE, et al. Longitudinal lung function decline in subjects with respiratory symptoms. Am Rev Respir Dis 1992;146:855-9. [DOI] [PubMed] [Google Scholar]
- 24.Kerstjens HA, Rijcken B, Schouten JP, et al. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax 1997;52:820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric Reference Values from a Sample of the General U.S. Population. Am J Respir Crit Care Med 1999;159:179-87. [DOI] [PubMed] [Google Scholar]
- 26.Vaz Fragoso CA, Concato J, McAvay G, et al. The ratio of FEV1 to FVC as a basis for establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:446-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fragoso CA, Concato J, McAvay G, et al. Staging the severity of chronic obstructive pulmonary disease in older persons based on spirometric Z-scores. J Am Geriatr Soc 2011;59:1847-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schermer TR, Quanjer PH. COPD screening in primary care: who is sick? Prim Care Respir J 2007;16:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olive JT, Jr, Hyatt RE. Maximal expiratory flow and total respiratory resistance during induced bronchoconstriction in asthmatic subjects. Am Rev Respir Dis 1972;106:366-76. [DOI] [PubMed] [Google Scholar]
- 30.Hyatt RE, Scanlon PD, Nakamura M. Interpretation of pulmonary function tests: a practical guide. Philadelphia: Lippincott-Raven, 1997:23-4. [Google Scholar]
- 31.Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2000;160:1683-9. [DOI] [PubMed] [Google Scholar]
- 32.Peña VS, Miravitlles M, Gabriel R, et al. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest 2000;118:981-9. [DOI] [PubMed] [Google Scholar]
- 33.Lindberg A, Bjerg A, Rönmark E, et al. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2006;100:264-72. [DOI] [PubMed] [Google Scholar]
- 34.van den Boom G, van Schayck CP, van Mollen MP, et al. Active detection of chronic obstructive pulmonary disease and asthma in the general population. Results and economic consequences of the DIMCA program. Am J Respir Crit Care Med 1998;158:1730-8. [DOI] [PubMed] [Google Scholar]
- 35.Walters JA, Walters EH, Nelson M, et al. Factors associated with misdiagnosis of COPD in primary care. Prim Care Respir J 2011;20:396-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust 2011;195:168-71. [DOI] [PubMed] [Google Scholar]
- 37.Zieliñski J, Bednarek M, Know the Age of Your Lung Study Group . Early detection of COPD in a high-risk population using spirometric screening. Chest 2001;119:731-6. [DOI] [PubMed] [Google Scholar]
- 38.U. S. Preventive Services Task Force . Screening for Chronic Obstructive Pulmonary Disease Using Spirometry: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2008;148:529-34. [DOI] [PubMed] [Google Scholar]
- 39.National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. Available online: http://guidance.nice.org.uk/CG101/Guidance/pdf/English
- 40.Jordan RE, Lam KB, Cheng KK, et al. Case finding for chronic obstructive pulmonary disease: a model for optimising a targeted approach. Thorax 2010;65:492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Schayck CP, Loozen JM, Wagena E, et al. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ 2002;324:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haroon S, Adab P, Griffin C, et al. Case finding for chronic obstructive pulmonary disease in primary care: a pilot randomised controlled trial. Br J Gen Pract 2013;63:e55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy ML, Fletcher M, Price DB, et al. International Primary Care Respiratory Group (IPCRG) Guidelines: Diagnosis of respiratory diseases in primary care. Prim Care Respir J 2006;15:20-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J 2009;18:216-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ 2010;182:673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandevoorde J, Verbanck S, Gijssels L, et al. Early detection of COPD: a case finding study in general practice. Respir Med 2007;101:525-30. [DOI] [PubMed] [Google Scholar]
- 47.Price DB, Tinkelman DG, Halbert RJ, et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration 2006;73:285-95. [DOI] [PubMed] [Google Scholar]
- 48.Price DB, Tinkelman DG, Nordyke RJ, et al. Scoring system and clinical application of COPD diagnostic questionnaires. Chest 2006;129:1531-9. [DOI] [PubMed] [Google Scholar]
- 49.Sichletidis L, Spyratos D, Papaioannou M, et al. A combination of the IPAG questionnaire and PiKo-6® flow meter is a valuable screening tool for COPD in the primary care setting. Prim Care Respir J 2011;20:184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frith P, Crockett A, Beilby J, et al. Simplified COPD screening: validation of the PiKo-6 in primary care. Prim Care Respir J 2011;20:190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parkes G.Asymptomatic COPD and NICE guidelines. Br J Gen Pract 2011;61:294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gruffydd-Jones K, Loveridge C.The 2010 NICE COPD Guidelines: how do they compare with the GOLD guidelines? Prim Care Respir J 2011;20:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albers M, Schermer T, Molema J, et al. Do family physicians’ records fit guideline diagnosed COPD? Fam Pract 2009;26:81-7. [DOI] [PubMed] [Google Scholar]
- 55.Hansen EC, Walters J, Baker RW. Explaining chronic obstructive pulmonary disease (COPD): perceptions of the role played by smoking. Sociol Health Illn 2007;29:730-49. [DOI] [PubMed] [Google Scholar]
- 56.Walters JA, Hansen E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician 2005;34:201-3. [PubMed] [Google Scholar]
- 57.Walters JA, Hansen E, Walters EH, et al. Under-diagnosis of Chronic obstructive Pulmonary Disease: a qualitative study in primary care. Respir Med 2008;102:738-43. [DOI] [PubMed] [Google Scholar]
- 58.Fletcher C, Peto R.The natural history of chronic airflow obstruction. BMJ 1977;1:1645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilt TJ, Niewoehner D, Kane RL, et al. Spirometry as a motivational tool to improve smoking cessation rates: A systematic review of the literature. Nicotine and Tobacco Research 2007;9:21-32. [DOI] [PubMed] [Google Scholar]
- 60.Parkes G, Greenhalgh T, Griffin M, et al. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008;336:598-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mannino DM, Aguayo SM, Petty TL, et al. Low lung function and incident lung cancer in the united states: Data from the first national health and nutrition examination survey follow-up. Arch Intern Med 2003;163:1475-80. [DOI] [PubMed] [Google Scholar]
- 62.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. [DOI] [PubMed] [Google Scholar]
- 63.Strachan DP. Ventilatory function, height, and mortality among lifelong non-smokers. J Epidemiol Community Health 1992;46:66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005;127:1952-9. [DOI] [PubMed] [Google Scholar]
- 65.Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax 2011;66:49-54. [DOI] [PubMed] [Google Scholar]
- 66.Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009;374:721-32. [DOI] [PubMed] [Google Scholar]
- 67.The COPD-X Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease V2.30. 2011. Available online: www.copdx.org.au
- 68.Enright PL, Beck KC, Sherrill DL. Repeatability of Spirometry in 18,000 Adult Patients. Am J Respir Crit Care Med 2004;169:235-8. [DOI] [PubMed] [Google Scholar]
- 69.Yawn BP, Enright PL, Lemanske RF, Jr, et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest 2007;132:1162-8. [DOI] [PubMed] [Google Scholar]
- 70.Hutchinson J.On the capacity of the lungs, and on the respiratory functions, with a view of establishing a precise and easy method of detecting disease by the spirometer. Med Chir Trans 1846;29:137-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiffeneau R, Pinelli A.Air circulant et air captive dans l’exploration de la fonction ventilatrice pulmonaire. Paris Med 1947;37:624-28. [PubMed] [Google Scholar]
- 72.Mead J, Turner J, Macklem P, et al. Significance of the relationship between lung elastic recoil and maximum expiratory flows. J Appl Physiol 1967;22:95-108. [DOI] [PubMed] [Google Scholar]
- 73.Fry DL, Hyatt RE. Pulmonary mechanics. A unified analysis of the relationship between pressure, volume and gasflow in the lungs of normal and diseased human subjects. Am J Med 1960;29:672-89. [DOI] [PubMed] [Google Scholar]
- 74.Buffels J, Degryse J, Heyrman J, et al. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO Study. Chest 2004;125:1394-9. [DOI] [PubMed] [Google Scholar]
- 75.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS task force: Standardisation of spirometry. Eur Respir J 2005;26:319-38. [DOI] [PubMed] [Google Scholar]
- 76.Johns DP, Burton D, Walters JA, et al. National survey of spirometer ownership and usage in general practice in Australia. Respirology 2006;11:292-8. [DOI] [PubMed] [Google Scholar]
- 77.Enright P, Schermer T.Don’t pay for poor quality spirometry tests. Prim Care Respir J 2013;22:15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eaton T, Withy S, Garrett JE, et al. Spirometry in primary care practice: the importance of quality assurance and the impact of spirometry workshops. Chest 1999;116:416-23. [DOI] [PubMed] [Google Scholar]
- 79.Tuomisto L, Jarvinen V, Laitinen J, et al. Asthma Programme in Finland: the quality of primary care spirometry is good. Prim Care Respir J 2008;17:226-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferguson GT, Enright PL, Buist AS, et al. Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest 2000;117:1146-61. [DOI] [PubMed] [Google Scholar]
- 81.Walters JA, Hansen E, Johns D, et al. A mixed methods study to compare models of spirometry delivery in primary care for patients at risk of Chronic Obstructive Pulmonary Disease. Thorax 2008;63:408-14. [DOI] [PubMed] [Google Scholar]
- 82.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [DOI] [PubMed] [Google Scholar]
- 83.Mead J.Analysis of the configuration of maximum expiratory flow-volume curves. J Appl Physiol Respir Environ Exerc Physiol 1978;44:156-65. [DOI] [PubMed] [Google Scholar]
- 84.Ohwada A, Takahashi K. Concave pattern of a maximal expiratory flow-volume curve: a sign of airflow limitation in adult bronchial asthma. Pulm Med 2012;2012:797495. [DOI] [PMC free article] [PubMed]
- 85.Dosman J, Bode F, Urbanetti J, et al. The Use of a Helium-Oxygen Mixture during Maximum Expiratory Flow to Demonstrate Obstruction in Small Airways in Smokers. J Clin Invest 1975;55:1090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dawson SV, Elliott EA. Wave-speed limitation on expiratory flow-a unifying concept. J Appl Physiol Respir Environ Exerc Physiol 1977;43:498-515. [DOI] [PubMed] [Google Scholar]
- 87.Schachter EN, Kapp MC, Maunder LR, et al. Smoking and cotton dust effects in cotton textile workers: an analysis of the shape of the maximum expiratory flow volume curve. Environmental health perspectives 1986;66:145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kapp MC, Schachter EN, Beck GJ, et al. The shape of the maximum expiratory flow volume curve. Chest 1988;94:799-806. [DOI] [PubMed] [Google Scholar]
- 89.Kraan J, van der Mark TW, Koeter GH. Changes in maximum expiratory flow-volume curve configuration after treatment with inhaled corticosteroids. Thorax 1989;44:1015-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omland O, Sigsgaard T, Pedersen OF, et al. The shape of the maximum expiratory flow-volume curve reflects exposure in farming. Ann Agric Environ Med 2000;7:71-8. [PubMed] [Google Scholar]
- 91.Nève V, Matran R, Baquet G, et al. Quantification of shape of flow-volume loop of healthy preschool children and preschool children with wheezing disorders. Pediatr Pulmonol 2012;47:884-94. [DOI] [PubMed] [Google Scholar]
- 92.Wildhaber JH, Sznitman J, Harpes P, et al. Correlation of spirometry and symptom scores in childhood asthma and the usefulness of curvature assessment in expiratory flow-volume curves. Respir Care 2007;52:1744-52. [PubMed] [Google Scholar]
- 93.Zheng CJ, Adams AB, McGrail MP, et al. A proposed curvilinearity index for quantifying airflow obstruction. Respir Care 2006;51:40-5. [PubMed] [Google Scholar]


