Abstract
In this review, we focus on the early events in the process of fibroblast spreading on fibronectin matrices of different rigidities. We present a focused position piece that illustrates the many different tests that a cell makes of its environment before it establishes mature matrix adhesions. When a fibroblast is placed on fibronectin-coated glass surfaces at 37°C, it typically spreads and polarizes within 20-40 min primarily through αvβ3 integrin binding to fibronectin. In that short period, the cell goes through three major phases that involve binding, integrin activation, spreading, and mechanical testing of the surface. The advantage of using the model system of cell spreading from the unattached state is that it is highly reproducible and the stages that the cell undergoes can thus be studied in a highly quantitative manner, in both space and time. The mechanical and biochemical parameters that matter in this example are often surprising because of both the large number of tests that occur and the precision of the tests. We discuss our current understanding of those tests, the decision tree that is involved in this process, and an extension to the behavior of the cells at longer time periods when mature adhesions develop. Because many other matrices and integrins are involved in cell-matrix adhesion, this model system gives us a limited view of a subset of cellular behaviors that can occur. However, by defining one cellular process at a molecular level, we know more of what to expect when defining other processes. Because each cellular process will involve some different proteins, a molecular understanding of multiple functions operating within a given cell can lead to strategies to selectively block a function.
Main Text
Quantitative analysis of the physical factors that underlie cellular behavior has become a fundamental aspect of modern cell biology. This is not a novel notion, since “numerical precision is the very soul of science, and its attainment affords the best, perhaps, the only criterion of the truth of theories and the correctness of experiments” (1). Only in the last couple of decades, however, have micro- and nanofabrication technologies been applied to quantitatively analyze physical traits of the cellular machinery involved in different aspects of cellular physiology. Of particular interest have been the processes that occur during the interaction of cells with their extracellular environment, including chemical sensing and mechanosensing. A series of studies using fibronectin as a substrate for cell-extracellular matrix (ECM) adhesion defined the early steps of fibroblast spreading from suspension. When plated on fibronectin-coated glass, the cells go through three major phases of behavior from the unsuspended state to the flat and polarized state: 1), initial attachment (phase 0 (P0)); 2), a rapid increase in cell spread area through depletion of membrane reservoirs (P1); and 3), a slower spreading phase that includes periodic protrusion/retraction of the cell edge and an increase in membrane area (P2) (2,3) (Fig. 1). This phase behavior defines critical checkpoints for the progression to a spread and growing cell, and reveals important aspects of initial adhesion formation, actin polymerization, myosin contractions, changes in membrane tension, and actin rearward flow.
Figure 1.
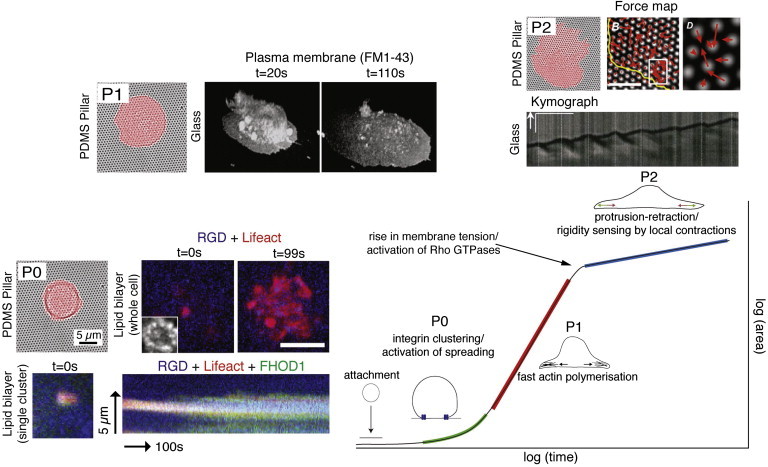
Early steps of fibroblast spreading on fibronectin substrates. A fibroblast cell goes through several distinct phases when plated on a fibronectin surface. Attachment is followed by the formation of initial integrin clusters, best observed on supported lipid bilayer, using the mobile Arg-Gly-Asp (RGD) peptide as the integrin ligand (P0; figure modified from Yu et al. (27) (top panel) and Iskratsch et al. (28) (bottom panel). Cluster formation is accompanied by binding of the actin assembly protein FHOD1 and actin assembly (see kymograph). Myosin II then contracts the clusters (not shown), activating fast actin polymerization and cell spreading. As the cell spreads out, the membrane folds and blebbs flatten out and feed into the expanding membrane surface (P1, epifluorescence images of the membrane dye FM1-43; images modified from Gauthier et al. (38). Additionally, activation of exocytosis increases the availability of phospholipids. A decrease in the pool of available lipids leads to a sharp rise in membrane tension and a switch to the subsequent protrusion-retraction phase (P2). During the P2 phase, the cell tests its environment with local contractions (see the force map on PDMS pillar arrays from Ghassemi et al. (34)) during the periodic protrusion-retractions (kymograph of the spreading cell edge from Giannone et al. (3)). To see this figure in color, go online.
Notably, many of the molecules involved in these steps are shown to be involved in cancer, including integrins (4), Rho GTPases (5), tyrosine kinases (6,7), and bona fide cytoskeletal proteins (8,9). This is perhaps not surprising since one of the hallmarks of cancer cells is their ability to ignore signals from the matrix and grow under anchorage-independent conditions. The picture that emerges from these studies is that the timing and location of action of the different proteins are very important for the progression of the overall process, and thus it is not only the up-/downregulation of specific proteins that affects processes such as cancer progression.
Integrin clustering: minimal adhesion unit
Integrin clustering is a critical step in the early formation of cell-ECM adhesions because the multimeric nature of the clusters provides increased strength of binding to matrix. Thus, when the pentameric fibronectin type III domain 7-10 was used as a ligand, it supported 6- to 9-fold greater force per fibronectin than the corresponding monomeric form (10). The formation of early integrin clusters typically occurs at the cell edge, when the membrane protrudes forward and encounters new matrix, and involves talin and/or kindlin binding (11–13). After the initial clusters form, some grow into mature adhesions in a myosin-II-dependent manner (14–17). Thanks to recent nanotechnological advances, investigators have defined the maximal spacing between integrins that allows the formation of strong adhesions, as well as the minimal number of integrins required. Several studies from the Spatz lab using gold nanodots arranged in a hexagonal lattice functionalized with RGD (Arg-Gly-Asp, the integrin ligand present in ECM proteins such as fibronectin) have shown that the maximal distance between RGD ligands that supports cell spreading is ∼60 nm (18–21). At larger distances between the ligands, the cells do not stay spread and a gradient in the spacing can cause directed cell migration. Using RGD-functionalized gold nanodots arranged in isolated clusters (rather than continuous arrays), Schvartzman et al. (22) showed that a minimal cluster of four dots spaced by 60 nm was needed for adhesion and the clusters could be spaced by at least 400 nm with no loss in cell spreading. The kinetics of the process and which proteins are required for minimal adhesion formation are still not clear; however, it is clear that the cell relies on a minimal adhesion unit to be able to sustain spreading on a surface and to form strong adhesion structures.
Actin polymerization and force generation on very early adhesions
It has been suggested for a number of years that forces applied to small nascent adhesions via actomyosin fibers are required for their growth and stabilization (23). It was shown, for example, that treating cells with inhibitors of myosin contractions prevented the formation of large mature adhesions, and only small adhesions remained at the cell edge (24–26). However, force is not necessary for the initial formation of integrin clusters. This was shown by using myosin-IIA and IIB knockdown cells plated on fibronectin-coated glass (17), as well as wild-type cells plated on lipid bilayers containing lipid-bound RGD ligands (27). In both cases, integrin clusters still formed in the absence of force. In the latter case, the formation of those clusters stimulated actin polymerization from them. The cells were further activated for spreading when barriers were inserted into the lipid bilayers (so that myosin was able to generate force on the clusters), but not when the barriers were absent. Thus, there appears to be a force-dependent step in the early process of adhesion formation that is distinct from the larger contractions that follow. It is the activation of actin polymerization from clusters due to the recruitment of the formin family protein FHOD1 that catalyzes actin polymerization from those sites, followed by myosin contraction of the actin filaments that move the clusters together to activate the next phase of spreading (27,28) (Fig.1). If force is not developed on the clusters, they dissipate and further spreading is prevented. Notably, several reports have indicated that adhesion maturation does not depend on contractile activity by myosin, but rather involves its actin cross-linking function (14,16,17). Still, exceeding a certain force threshold seems to be critical for adhesion reinforcement and growth (29,30). One possible explanation for this conundrum is that on hard surfaces the global forces generated by actin flow could suffice for adhesion maturation (31,32) by generating drag on mechanosensitive molecules, but on soft matrices, localized forces generated by myosin are necessary (33,34). This is supported by the fact that lamellipodia of fish keratocytes can sustain treatment with high concentrations of blebbistatin (35,36) due to their very high actin polymerization activity, which can exert sufficient drag on mechanosensors.
Major spreading of the cells after force-dependent activation
The rapid spreading of cells in the presence of barriers on the lipid bilayers is similar to P1 of the spreading of cells on fibronectin-coated glass, during which the cells rapidly flatten out on the surface. Once activated, this spreading does not require further integrin activation, as evidenced by the fact that the cell edges move out at the same rate on fibronectin-coated glass as on adjacent nonadhesive glass surfaces (37). On the lipid bilayers, the clusters move outward almost concomitantly with the cell edge, and only at the end of spreading does the cell edge move away from the clusters. Throughout this process, clusters near the cell center move away from clusters near the edge, suggesting that actin polymerization from the clusters is driving them apart. As the cell spreads further, the edge polymerization appears to increase relative to the internal polymerization and the distance between the outer clusters and the cell edge increases. This is all consistent with a general activation of actin polymerization at multiple sites that ends when the cell reaches the limit of membrane area (see below).
Interestingly, only a fraction of the fibroblasts spread in this isotropic manner, whereas the rest spread anisotropically through localized extension events of their edges (1–4 μm of the cell edge extending for 30–90 s) that are followed by brief contraction events. It is not clear what decides whether a cell will spread isotropically or anisotropically, although the fraction of isotropic spreading is increased by serum starvation (2). In general, rapid isotropic spreading serves as a better experimental model because there is a clear separation of the spreading and contraction steps over the whole cell, whereas in anisotropic spreading, those behaviors occur in local regions asynchronously over the cell.
Membrane tension drives activation of contraction
The rapid expansion phase of spreading in P1 ends because of the physical barrier posed by the plasma membrane as the cell changes from a sphere to a flattened morphology (38). Thus, it appears that the cell volume and plasma membrane surface area are critical in determining the spread area of a given cell. When cells are in suspension, the plasma membrane has many folds that flatten during spreading until they are depleted. At the end of P1, there is a loss of membrane folds and a rise in membrane tension, which presumably signals for an increase in myosin contractility and an increase in plasma membrane area by ∼40% (38) (Fig. 1). A hypoosmotic shock also ends P1 through the activation of contraction and exocytosis, presumably because of a similar increase in membrane tension (38). Interestingly, if myosin II is inhibited, membrane tension rises dramatically as well, causing the increase in exocytosis. This is consistent with the finding that enhancing myosin activity (using calyculin A) leads to reduced tension (39). How an abrupt change in membrane tension is converted into a biochemical signal that activates exocytosis and myosin II contraction is not understood, but may involve tension-dependent activation of a GTPase exchange factor (GEF) that causes a local rise in GTP-Rho or some other small G protein, which then causes myosin activation and membrane exocytosis. In addition to myosin, the Rho GTPases act through different actin assembly factors to regulate both membrane exocytosis (40,41) and protrusion-retraction coordination (42–46). This is consistent with the spatiotemporal coordination of GTPase activity, which follows a sequence of RhoA, Cdc42, and Rac1 activation during the protrusion-retraction cycles in migrating cells (47). This indicates a cross-coordination between the Rho-GTPases, which Vega et al. (42) studied more closely by examining the distinct roles of RhoA and RhoC in cell migration, where RhoA, but not RhoC, acts through Rho kinase 2 (ROCK2) to inhibit Rac1 activity.
Periodic contractions and rigidity sensing
Once myosin contraction is activated to end P1, the cells on rigid substrates often begin to exhibit periodic lamellipodial contractions that signal the start of P2 of spreading (3,48). These contractions seem to be involved in sensing rigidity, since if the surface is soft, the cells do not exhibit periodic contractions and stop spreading at that point. Further, there is a rapid flow of actin inward without the development of adhesions (3). However, if the surface is rigid, the cells continue to spread through continuous cycles of periodic contractions approximately every 25 s, during which initial adhesions form at the cell edge, which then can mature over time into more substantial adhesions. A mechanical process for the periodic contractions has been proposed that involves movement of an upper layer of actin filaments over the adhesions to myosin filaments behind the adhesions, which then initiates the contractile cycle (49).
On rigid surfaces, the protrusion-retraction cycles lead to the organization of adhesions that mature over time. Saez et al. (50) reported that when they used elastomeric pillars of different rigidities as substrates for cell spreading to measure cellular forces, 4–16 h after spreading, the average displacements were constant at 130 nm over a 100-fold range of rigidity. Further, during spreading, the cells produced local contractions of 100–130 nm irrespective of rigidity that were transient and correlated with rigidity sensing. This indicates that cells sense rigidity by displacing substrates to a constant distance. Thus, the signal for rigidity should be related to the force needed to produce a given displacement.
Rigidity sensing by cellular contractions
Because of the importance of rigidity sensing for fibroblast viability as well as stem cell differentiation, rigidity sensing of deformable matrices has been extensively studied. Laser tweezers, traction force microscopy, and PDMS pillar arrays have been used to measure force generation during rigidity sensing. In early laser-tweezers studies, a force greater than 20 pN for several seconds was needed to activate reinforcement of the adhesion contacts (30). In large-pillar studies of epithelial cells in steady-state conditions (up to 16 h after plating), pillar displacements, as a measure of the traction forces, were constant over a 100-fold rigidity range (50). In traction force microscopy studies, transient contractions of large focal adhesions were correlated with rigidity sensing (52). At the finer resolution afforded by 500-nm-diameter pillars, local contraction units were observed that transiently pulled on the matrix to a maximal displacement of ∼100–130 nm per contractile unit independently of rigidity. In all of these studies, the reinforcement of adhesion contacts required that the force on the matrix contact exceed a certain level (∼20 pN) in a certain time (estimated to be 2–5 s) whether the force was externally generated by laser tweezers or internally by local cellular contractions of matrix attachments. Thus, rigidity sensing involves the initial reinforcement of matrix adhesions.
From studies of the early displacements of both large and small pillars, it was evident that two different contractile processes take place in the fibroblasts: one involving local contractile units 2–3 μm in length and the other involving coupling of adhesions to inward actin flow by a stick-slip mechanism (also called a clutch) (34,53). When rigidity sensing was analyzed on submicrometer diameter pillars, the pillars were locally displaced to a constant distance of ∼50 nm independently of the substrate rigidity (34). For larger pillars (>1 μm), however, the displacements scaled with the pillar height and thus the substrate stiffness. This indicated that cells use special rigidity-sensing contractile units that stretch between two (or more) submicrometer pillars to cause displacements to a constant distance and thereby generate the force needed to activate adhesion reinforcement. This seems to be a universal mechanism and such units are present in many different cell types. The equidistant pulling of two pillars over 25 s (the time period of the periodic contractions (49)) with high forces (>15 nN/μm2) indicates an antiparallel actomyosin structure that can be likened to a basic muscle sarcomere. Alternatively, the cell can generate adhesions by using the flow of actin inward to generate force on the matrix contacts as in the earlier laser-tweezers studies. The two different actin contractile systems are distinct and could well be used differentially in normal cell-matrix interactions. What is not clear is when the flow of actin or the local contraction units will provide the predominant method of rigidity sensing. Since adhesions are formed around the cell edge and over many contractile cycles, they will produce a cumulative signal that will also influence nuclear signaling and ultimately influence cellular processes such as cell growth and differentiation (Y. Cui, F. Hameed, B. Yank, K. Lee, S. Park, M. Sheetz, unpublished) (55,56).
Enzyme pathways in rigidity sensing
Several kinases and phosphatases, such as RPTPα, Src family kinases (SFKs), and other tyrosine kinases, have been implicated in rigidity sensing (57–59). These and other enzymes provide signals for the assembly and turnover of the contractile units and presumably are also involved in the sensing of force. When the matrix forces exceed the force threshold, adhesion components that are needed for the generation of matrix signals accumulate. It appears that phospho-tyrosine kinase (PTK) signaling is especially critical (59). Distinct PTKs influence adhesion formation and stress fiber assembly or disassembly. This signaling ensures that cells rapidly disassemble adhesions on soft substrates, which prevents them from migrating or proliferating. Other PTKs cause the stabilization of adhesion components on rigid substrates. SFKs influence actin assembly from adhesions, retrograde flow, adhesion maturation, and adhesion turnover (27,28,60–62). Thus, enzymatic pathways are key regulators of the rigidity-sensing mechanisms and could be critical for determining the tissue-specific rigidity response.
Stabilization and maturation of adhesions by force
Adhesion stabilization and maturation involve a host of different structural and signaling components (57,59,64–66). The adhesion lifetime, including assembly, stability phase, and disassembly, is typically in the range of tens of minutes and depends on both force signals and actin assembly pathways (17,28,61,67,68). In contrast, individual proteins exchange between the adhesion and cytoplasm within seconds, highlighting the dynamic nature of the adhesion (26,69,70). Again, the exchange rates correlate with myosin forces, and many critical components exchange more slowly and less extensively in the presence of blebbistatin (e.g., paxillin), whereas others exchange more readily (e.g., vinculin) (26). Such exchange rates are known only for a few proteins out of several hundred proteins of the adhesome (64,65). However, quantitative proteomic studies indicate that a significant number of the components are bound in a force-dependent manner and are lost after myosin contractility inhibition (65).
Sensing by actin flow
Assembly of actin at the cell edge is balanced by depolymerization farther inward and results in retrograde actin flow from the edge to the central regions of the cell (71). Recent studies have shown that actin assembly by formins at the cell edge or at adhesions is tension sensitive and may add to nuclear signaling via the MRTF-A or YAP/TAZ pathways (54,55,72–74). Moreover, several of the adhesion proteins connect transiently (directly or indirectly) to retrograde flowing actin and are repeatedly stretched in this process (53). The resulting exposure of cryptic sites on talin, p130Cas, and other mechanosensitive proteins leads to the activation of signaling pathways to the nucleus or other parts of the cell (53,62,75,76). This signaling presumably involves LIM domain proteins, many of which can shuttle to the nucleus and bind to adhesions in a force-dependent manner (65,77,78). Thus, the adhesions are signaling centers that through periodic assembly and disassembly ensure constant signaling to enable cell growth and inhibition of apoptotic pathways on permissive substrates. Both the different matrix proteins and the process of adhesion assembly and disassembly will vary among different cell types and tissues to determine the proper cell behavior in the proper matrix environment.
Conclusions
Cells communicate with their physical microenvironment through matrix adhesions to control cellular responses. Using fibroblast spreading as a model system, the process of mechanosensing through adhesions can be studied with high precision. Adhesion assembly is a stepwise process during which protein complexes form local mechanical tools to locally test different matrix properties for a short time before they move to the next step. Each test creates signals that will lead to an if/then decision for the cell to move (or not) to a further test of matrix properties. At steady state, the frequency of the matrix tests is controlled by internal (e.g., cell growth) or external (mechanical or hormonal) signals. The formation of tissue-type-specific tools results in tissue-specific signals that are integrated over time to determine the proper cellular response, which enables the cell to dynamically react to changes in its environment.
Acknowledgments
H.W. was supported by a Marie Curie International Outgoing Fellowship within the Seventh European Commission Framework Programme (PIOF-GA-2012-332045). T.I. was supported by a Postdoctoral Fellowship from the American Heart Association. M.P.S was partially supported by the Mechanobiology Institute, National University of Singapore and NIH.
Footnotes
Haguy Wolfenson and Thomas Iskratsch contributed equally to this work.
References
- 1.Thompson D.A.W. University Press; Cambridge, UK: 1917. On Growth and Form. [Google Scholar]
- 2.Dubin-Thaler B.J., Giannone G., Sheetz M.P. Nanometer analysis of cell spreading on matrix-coated surfaces reveals two distinct cell states and STEPs. Biophys. J. 2004;86:1794–1806. doi: 10.1016/S0006-3495(04)74246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannone G., Dubin-Thaler B.J., Sheetz M.P. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 4.Desgrosellier J.S., Cheresh D.A. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vega F.M., Ridley A.J. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 6.Mitra S.K., Schlaepfer D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Kim L.C., Song L., Haura E.B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 8.Schramek D., Sendoel A., Fuchs E. Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas. Science. 2014;343:309–313. doi: 10.1126/science.1248627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raval G.N., Bharadwaj S., Prasad G.L. Loss of expression of tropomyosin-1, a novel class II tumor suppressor that induces anoikis, in primary breast tumors. Oncogene. 2003;22:6194–6203. doi: 10.1038/sj.onc.1206719. [DOI] [PubMed] [Google Scholar]
- 10.Roca-Cusachs P., Gauthier N.C., Sheetz M.P. Clustering of α(5)β(1) integrins determines adhesion strength whereas α(v) β(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang G., Giannone G., Sheetz M.P. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- 12.Bachir A.I., Zareno J., Horwitz A.R. Integrin-associated complexes form hierarchically with variable stoichiometry in nascent adhesions. Curr. Biol. 2014;24:1845–1853. doi: 10.1016/j.cub.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moser M., Legate K.R., Fässler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 14.Stricker J., Beckham Y., Gardel M.L. Myosin II-mediated focal adhesion maturation is tension insensitive. PLoS ONE. 2013;8:e70652. doi: 10.1371/journal.pone.0070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oakes P.W., Beckham Y., Gardel M.L. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol. 2012;196:363–374. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi C.K., Vicente-Manzanares M., Horwitz A.R. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalcanti-Adam E.A., Volberg T., Spatz J.P. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007;92:2964–2974. doi: 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selhuber-Unkel C., López-García M., Spatz J.P. Cooperativity in adhesion cluster formation during initial cell adhesion. Biophys. J. 2008;95:5424–5431. doi: 10.1529/biophysj.108.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschfeld-Warneken V.C., Arnold M., Spatz J.P. Cell adhesion and polarisation on molecularly defined spacing gradient surfaces of cyclic RGDfK peptide patches. Eur. J. Cell Biol. 2008;87:743–750. doi: 10.1016/j.ejcb.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold M., Hirschfeld-Warneken V.C., Spatz J.P. Induction of cell polarization and migration by a gradient of nanoscale variations in adhesive ligand spacing. Nano Lett. 2008;8:2063–2069. doi: 10.1021/nl801483w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schvartzman M., Palma M., Wind S.J. Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett. 2011;11:1306–1312. doi: 10.1021/nl104378f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riveline D., Zamir E., Bershadsky A.D. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nayal A., Webb D.J., Horwitz A.R. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 2006;173:587–589. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaidel-Bar R., Milo R., Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J. Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 26.Wolfenson H., Bershadsky A., Geiger B. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J. Cell Sci. 2011;124:1425–1432. doi: 10.1242/jcs.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu C.H., Law J.B., Sheetz M.P. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc. Natl. Acad. Sci. USA. 2011;108:20585–20590. doi: 10.1073/pnas.1109485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iskratsch T., Yu C.H., Sheetz M. FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev. Cell. 2013;27:545–559. doi: 10.1016/j.devcel.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choquet D., Felsenfeld D.P., Sheetz M.P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 30.Jiang G., Huang A.H., Sheetz M.P. Rigidity sensing at the leading edge through αvβ3 integrins and RPTPα. Biophys. J. 2006;90:1804–1809. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexandrova A.Y., Arnold K., Verkhovsky A.B. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE. 2008;3:e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardel M.L., Sabass B., Waterman C.M. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 2008;183:999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shutova M., Yang C., Svitkina T. Functions of nonmuscle myosin II in assembly of the cellular contractile system. PLoS ONE. 2012;7:e40814. doi: 10.1371/journal.pone.0040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghassemi S., Meacci G., Hone J. Cells test substrate rigidity by local contractions on submicrometer pillars. Proc. Natl. Acad. Sci. USA. 2012;109:5328–5333. doi: 10.1073/pnas.1119886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson C.A., Tsuchida M.A., Theriot J.A. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaub S., Bohnet S., Verkhovsky A.B. Comparative maps of motion and assembly of filamentous actin and myosin II in migrating cells. Mol. Biol. Cell. 2007;18:3723–3732. doi: 10.1091/mbc.E06-09-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossier O.M., Gauthier N., Sheetz M.P. Force generated by actomyosin contraction builds bridges between adhesive contacts. EMBO J. 2010;29:1055–1068. doi: 10.1038/emboj.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauthier N.C., Fardin M.A., Sheetz M.P. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc. Natl. Acad. Sci. USA. 2011;108:14467–14472. doi: 10.1073/pnas.1105845108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lieber A.D., Yehudai-Resheff S., Keren K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr. Biol. 2013;23:1409–1417. doi: 10.1016/j.cub.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 40.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim. Biophys. Acta. 2003;1641:175–181. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 41.Croisé P., Estay-Ahumada C., Ory S. Rho GTPases, phosphoinositides, and actin: a tripartite framework for efficient vesicular trafficking. Small GTPases. 2014;5:e29469. doi: 10.4161/sgtp.29469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vega F.M., Fruhwirth G., Ridley A.J. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J. Cell Biol. 2011;193:655–665. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arthur W.T., Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ten Klooster J.P., Evers E.E., Collard J.G. Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. Biochem. J. 2006;397:39–45. doi: 10.1042/BJ20051957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch M.D. The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 1999;9:423–427. doi: 10.1016/s0962-8924(99)01651-7. [DOI] [PubMed] [Google Scholar]
- 46.Le Clainche C., Carlier M.F. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 47.Machacek M., Hodgson L., Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Döbereiner H.G., Dubin-Thaler B.J., Sheetz M.P. Lateral membrane waves constitute a universal dynamic pattern of motile cells. Phys. Rev. Lett. 2006;97:038102. doi: 10.1103/PhysRevLett.97.038102. [DOI] [PubMed] [Google Scholar]
- 49.Giannone G., Dubin-Thaler B.J., Sheetz M.P. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saez A., Buguin A., Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys. J. 2005;89:L52–L54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reference deleted in proof.
- 52.Plotnikov S.V., Pasapera A.M., Waterman C.M. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margadant F., Chew L.L., Sheetz M. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 2011;9:e1001223. doi: 10.1371/journal.pbio.1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reference deleted in proof.
- 55.Dupont S., Morsut L., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 56.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 57.Kostic A., Sheetz M.P. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol. Biol. Cell. 2006;17:2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Wichert G., Jiang G., Sheetz M.P. RPTP-α acts as a transducer of mechanical force on αv/β3-integrin-cytoskeleton linkages. J. Cell Biol. 2003;161:143–153. doi: 10.1083/jcb.200211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prager-Khoutorsky M., Lichtenstein A., Bershadsky A.D. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011;13:1457–1465. doi: 10.1038/ncb2370. [DOI] [PubMed] [Google Scholar]
- 60.Huveneers S., Danen E.H. Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 61.Webb D.J., Donais K., Horwitz A.F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X., Moore S.W., Sheetz M.P. N-WASP-directed actin polymerization activates Cas phosphorylation and lamellipodium spreading. J. Cell Sci. 2014;127:1394–1405. doi: 10.1242/jcs.134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reference deleted in proof.
- 64.Zaidel-Bar R., Itzkovitz S., Geiger B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schiller H.B., Friedel C.C., Fässler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12:259–266. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roca-Cusachs P., Iskratsch T., Sheetz M.P. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 2012;125:3025–3038. doi: 10.1242/jcs.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berginski M.E., Vitriol E.A., Gomez S.M. High-resolution quantification of focal adhesion spatiotemporal dynamics in living cells. PLoS ONE. 2011;6:e22025. doi: 10.1371/journal.pone.0022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolfenson H., Lavelin I., Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev. Cell. 2013;24:447–458. doi: 10.1016/j.devcel.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolfenson H., Lubelski A., Geiger B. A role for the juxtamembrane cytoplasm in the molecular dynamics of focal adhesions. PLoS ONE. 2009;4:e4304. doi: 10.1371/journal.pone.0004304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann J.E., Fermin Y., Zamir E. Symmetric exchange of multi-protein building blocks between stationary focal adhesions and the cytosol. eLife. 2014;3:e02257. doi: 10.7554/eLife.02257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ponti A., Machacek M., Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 72.Courtemanche N., Lee J.Y., Greene E.C. Tension modulates actin filament polymerization mediated by formin and profilin. Proc. Natl. Acad. Sci. USA. 2013;110:9752–9757. doi: 10.1073/pnas.1308257110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jégou A., Carlier M.F., Romet-Lemonne G. Formin mDia1 senses and generates mechanical forces on actin filaments. Nat. Commun. 2013;4:1883. doi: 10.1038/ncomms2888. [DOI] [PubMed] [Google Scholar]
- 74.Young K.G., Copeland J.W. Formins in cell signaling. Biochim. Biophys. Acta. 2010;1803:183–190. doi: 10.1016/j.bbamcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 75.del Rio A., Perez-Jimenez R., Sheetz M.P. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sawada Y., Tamada M., Sheetz M.P. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hotta K., Ranganathan S., Sawada Y. Biophysical properties of intrinsically disordered p130Cas substrate domain—implication in mechanosensing. PLOS Comput. Biol. 2014;10:e1003532. doi: 10.1371/journal.pcbi.1003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kadrmas J.L., Beckerle M.C. The LIM domain: from the cytoskeleton to the nucleus. Nat. Rev. Mol. Cell Biol. 2004;5:920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]


