Abstract
Freshly isolated, human peripheral blood T (PBT) cells are largely resistant to the apoptotic effects of anti-CD3 monoclonal antibody, ionomycin, or phorbol 12-myristate 13-acetate (PMA). We demonstrate here, however, that PBT cells, including both CD4+ and CD8+ cell populations, can be readily induced to undergo apoptosis when cocultured with either autologous or allogeneic monocytes (Mo) in PMA-containing medium. Incubation of PBT cells with Mo at a ratio of 1:1 for 18 hr resulted in maximal levels (80%) of apoptotic cell death. The mechanism whereby Mo enable PBT cells to undergo apoptosis in PMA-containing medium appeared to depend on cell-cell contact or close proximity between Mo and PBT cells rather than solely via soluble mediators. It was demonstrated that Mo acquire the ability to prime PBT cells for apoptosis after treatment with PMA and that treated Mo maintain this ability even after fixation with formaldehyde. It was also found that once PBT cells became primed for apoptosis by incubation with PMA-pretreated Mo, the primed PBT cells were susceptible to apoptosis triggered not only by PMA but also by either ionomycin or by monoclonal antibody crosslinking of T-cell surface molecules such as CD4 and CD3. Interestingly, the degree of apoptosis of CD4+ T cells by crosslinking of CD4 molecules via a combination of gp120, anti-gp120, and goat anti-mouse IgG was significantly greater for T cells primed with PMA-treated Mo than for unprimed T cells. Together, these findings reveal an important role for accessory cells in priming resting PBT cells for apoptosis and suggest a possible Mo-dependent mechanism by which T cells may become primed for apoptosis in human immunodeficiency virus-infected asymptomatic individuals.
Full text
PDF


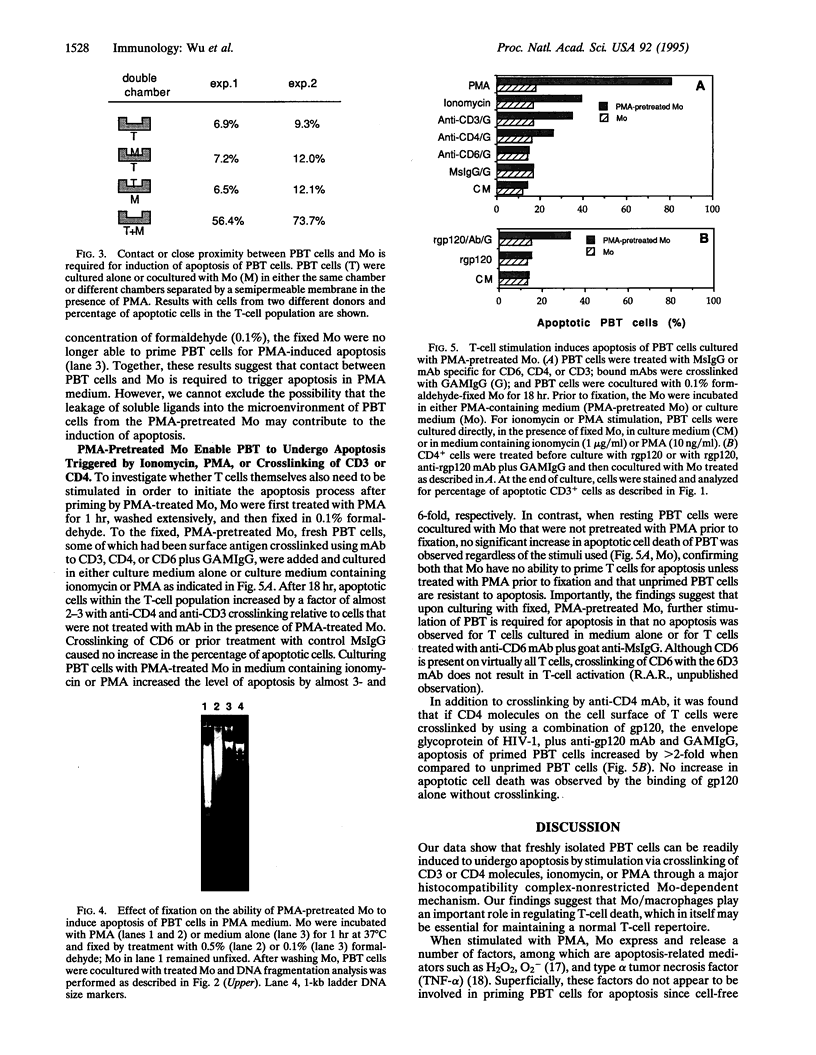

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda N. K., Bernier J., Kurahara D. K., Kurrle R., Haigwood N., Sekaly R. P., Finkel T. H. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992 Oct 1;176(4):1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Debatin K. M., Fahrig-Faissner A., Enenkel-Stoodt S., Kreuz W., Benner A., Krammer P. H. High expression of APO-1 (CD95) on T lymphocytes from human immunodeficiency virus-1-infected children. Blood. 1994 May 15;83(10):3101–3103. [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989 Jul;52(1):10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- Gougeon M. L., Garcia S., Heeney J., Tschopp R., Lecoeur H., Guetard D., Rame V., Dauguet C., Montagnier L. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res Hum Retroviruses. 1993 Jun;9(6):553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- Groux H., Torpier G., Monté D., Mouton Y., Capron A., Ameisen J. C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992 Feb 1;175(2):331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D., Pohl T., Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993 Jul;14(7):338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Wesselborg S. Life and death of a superantigen-reactive human CD4+ T cell clone: staphylococcal enterotoxins induce death by apoptosis but simultaneously trigger a proliferative response in the presence of HLA-DR+ antigen-presenting cells. Int Immunol. 1992 Dec;4(12):1381–1388. doi: 10.1093/intimm/4.12.1381. [DOI] [PubMed] [Google Scholar]
- Kizaki H., Tadakuma T., Odaka C., Muramatsu J., Ishimura Y. Activation of a suicide process of thymocytes through DNA fragmentation by calcium ionophores and phorbol esters. J Immunol. 1989 Sep 15;143(6):1790–1794. [PubMed] [Google Scholar]
- Meyaard L., Otto S. A., Jonker R. R., Mijnster M. J., Keet R. P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., MacIsaac P. D., Torbett B. E., Levy J. A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993 Apr 30;260(5108):689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- Mosier D., Sieburg H. Macrophage-tropic HIV: critical for AIDS pathogenesis? Immunol Today. 1994 Jul;15(7):332–339. doi: 10.1016/0167-5699(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. A., González A., López-Rivas A., Diaz-Espada F., Gambón F. IL-2 protects against anti-CD3-induced cell death in human medullary thymocytes. J Immunol. 1990 Sep 1;145(5):1364–1368. [PubMed] [Google Scholar]
- Rasmussen R. A., Counts S. L., Daley J. F., Schlossman S. F. Isolation and characterization of CD6- T cells from peripheral blood. J Immunol. 1994 Jan 15;152(2):527–536. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C. H., Giorgi J. V. Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry. 1994 Jan 1;15(1):12–20. doi: 10.1002/cyto.990150104. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Shepard H. M., Rothstein J. L., Sugarman B. J., Schreiber H. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis D. J., Sentman C. L., Bach E. A., Korsmeyer S. J. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993 Sep 1;151(5):2546–2554. [PubMed] [Google Scholar]
- Wesselborg S., Janssen O., Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993 May 15;150(10):4338–4345. [PubMed] [Google Scholar]