Abstract
OBJECTIVES:
Vardenafil enhances dilatation of vascular smooth muscle and inhibits platelet aggregation. The purpose of this study was to evaluate the clinical effects of vardenafil and pentoxifylline administration in an experimental model of ischemic colitis.
METHODS:
Forty female Wistar albino rats weighing 250–300 g were randomized into five experimental groups (each with n = 8) as follows:1) a sham group subjected to a sham surgical procedure and administered only tap water; 2) a control group subjected to a standardized surgical procedure to induce ischemic colitis and administered only tap water; 3) and 4) treatment groups subjected to surgical induction of ischemic colitis followed by the postoperative administration of 5 mg/kg or 10 mg/kg vardenafil, respectively; and 5) a treatment group subjected to surgical induction of ischemic colitis followed by postoperative administration of pentoxifylline at 50 mg/kg/day per day as a single dose for a 3-day period. All animals were sacrificed at 72 h post-surgery and subjected to relaparotomy. We scored the macroscopically visible damage, measured the ischemic area and scored histopathology to determine the severity of ischemia. Tissue malondialdehyde levels were also quantified.
RESULTS:
The mean Gomella ischemic areas were 63.3 mm2 in the control group; 3.4 and 9.6 mm2 in the vardenafil 5 and vardenafil 10 groups, respectively; and 3.4 mm2 in the pentoxifylline group (p = 0.0001). The mean malondialdehyde values were 63.7 nmol/g in the control group; 25.3 and 25.6 nmol/g in the vardenafil 5 and vardenafil 10 groups, respectively; and 22.8 nmol/g in the pentoxifylline group (p = 0.0001).
CONCLUSION:
Our findings indicate that vardenafil and pentoxifylline are effective treatment options in an animal model of ischemic colitis. The positive clinical effects produced by these drugs are likely due to their influence on the hemodynamics associated with vascular smooth muscle and platelet functions.
Keywords: Ischemic Colitis, PDE–5 Inhibitors, Pentoxifylline, Vardenafil
INTRODUCTION
When oxygen and energy delivery is below the metabolic requirement of the intestine or when there is mesenteric circulation failure, the resultant condition is called mesenteric ischemia. Ischemic colitis (IC) is a type of mesenteric ischemia involving failure in colonic circulation 1. IC is one of the most common colon diseases affecting the elderly population and the annual incidence rate in the general population is 4.5-44/100.000. While the incidence of ischemic colitis following elective aortic surgeries is 1-2%, it increases to 60% following emergency surgeries 2. In emergency surgeries, the mortality rate is 50–70%. Diabetes mellitus, chronic renal failure, various rheumatic diseases, vasculitis and renal transplantation are other known causes of IC 3,4, which results from injury to the colonic wall, leading to insufficient blood flow. The actual mechanism responsible for the pathophysiology of IC is ischemia-reperfusion damage. In most cases, colonic tissue hypoxia results in local hypoperfusion. If not properly treated, this may lead to multiple organ dysfunction 4,5. The majority of IC cases are asymptomatic. Clinical presentation usually begins with sudden cramping and abdominal pain, mostly localized in the left lower quadrant and an intense need to defecate. Within 24 hours, bright red or maroon-colored stools mixed with blood are observed 1,3. Physical examination findings include mild to moderate degrees of sensitivity in the intestinal segment, abdominal distension, mild fever, tachycardia and positive test results for occult blood in stool samples. In 10-20% of cases, signs of peritoneal irritation may occur, depending on the development of necrosis in the colon. The emergence of peritoneal irritation in acute onset ischemic colitis indicates the development of gangrene or perforation and requires explorative laparotomy 1,2,4,6. No published studies have provided a sufficient level of evidence to create an appropriate treatment model for IC 7. A majority of the literature describes IC cases associated with mesenteric infarction. Some retrospective studies have focused on specific treatments and have concluded that an extended colectomy or surgery is not recommended in most cases. In serious cases, prolonged surgery and aggressive surgery are recommended to reduce mortality 8. PDE–5 inhibitors are used to induce dilatation of the vascular smooth muscle and to inhibit platelet aggregation in the treatment of erectile dysfunction and testicular torsion 24, pulmonary hypertension 37, coronary artery disease, diabetes mellitus 38, deep vein thrombosis and in anti-anginal therapy 39. Vardenafil is a phosphodiesterase-5 inhibitor 9. Pentoxifylline (PTX) and its metabolites increase the blood fluidity by reducing blood viscosity. By increasing microcirculatory blood flow, these drugs have a positive effect on the oxygen uptake of tissue and are thus used to treat patients with chronic peripheral arterial diseases 10. Considering the above, we hypothesized that vardenafil and pentoxifylline might be effective treatment options in models of ischemic colitis. The purpose of this study was to evaluate the therapeutic effects of vardenafil and pentoxifylline administration in an experimental rat model of ischemic colitis.
MATERIALS AND METHODS
Ethics
This experimental study was conducted at the Marmara University School of Medicine Experimental and Animal Research Center (DEHAMER) between January and June 2013. The study was approved by the Ethics Committee of the Faculty of Medicine (dated 5/27/2013 with Ethics Committee approval n°. 332.2013.Mar).
Animals
Forty 28–32-week-old female albino Wistar rats, ranging in weight from 200 g to 250 g, were evaluated. The rats were maintained under a 12-hour/12-hour light/dark cycle; they were housed three to a cage at maximum and kept at a room temperature of approximately 23±2°C. Throughout the study, the rats were provided with aged tap water (rested tap water) and standard pellet feed.
Experimental design and administration of drugs
A homogeneous solution was prepared by dissolving vardenafil (Levitra; Bayer, Ümraniye-İstanbul) tablets (10 mg) in water. The first round of drug administration occurred via orogastric tube within 2 hours after surgery.
Pentoxifylline (Hemopene, Ibrahim Etem, Topkapi-Istanbul) ampoules (100 mg, 5 cc) were mixed with physiological saline solution and subsequently administered intramuscularly at 50 mg/kg/day. The drug was also administered intramuscularly following surgery. The study animals were sacrificed at 3 days post-surgery using cervical dislocation and U-shaped incisions were used to open their abdominal cavities. Colonic structures were removed for macroscopic and microscopic evaluation and the necessary scoring was performed (Table 1).
Table 1.
-Management of the ischemic colitis rat model.
| Groups | Operation | Procedure | Euthanasia | |
| Sham group | n:8 | Only laparotomy | Oral 10 mg/kg 0.9% NaCl at the same time for 3 days | Cervical dislocation with 50 mg/kg ketamine after 72 hours. |
| Control group | n:8 | Laparotomy and IC model | Oral 10 mg/kg 0.9% NaCl at the same time for 3 days | |
| Vardenafil 5 mg group | n:8 | Laparotomy and IC model | Oral 5 mg/kg vardenafil at the same time for 3 days | |
| Vardenafil 10 mg group | n:8 | Laparotomy and IC model | Oral 10 mg/kg vardenafil at the same time for 3 days | |
| Pentoxifylline group | n:8 | Laparotomy and IC model | I.M. 50 mg/kg PTX at the same time for 3 days | |
The animals were randomized into five experimental groups (each with n = 8). Group 1 animals were subjected to a sham operation (n = 8) and received only tap water. Group 2 animals were subjected to surgical induction of IC and also received only tap water. Group 3 and group 4 animals were subjected to surgical induction of IC and received either 5 mg/kg/body weight or 10 mg/kg/body weight oral vardenafil, respectively. Group 5 animals were subjected to surgical induction of IC and received 50 mg/kg/body weight intramuscular PTX. The indicated drug was administered to all treatment groups as a single daily dose for 3 days.
Surgical procedure
During surgery, anesthesia was administered intraperitoneally at doses of 50 mg/kg (for ketamine) and 10 mg/kg (for chlorpromazine). Rats were shaved following the administration of anesthesia and the skin was prepared with 10% povidone-iodine. The method described by Griffen and Hagihara 11 was used to induce experimental IC (Figure 1). According to this method, a median laparotomy was performed, the left colon was carefully removed from the abdomen and ischemic colitis was induced by connecting the marginal arteries of the 4-cm-long segment of the left colon at two separate places and all of the vasa recta with 4/0 silk (Ethicon, Edinburgh, UK; Figure 1). The left colon was then anatomically placed into the abdomen and the abdomen was closed via continuous suturing using 3/0 silk sutures (Silk, Dogsan, Istanbul, Turkey).
Figure 1.
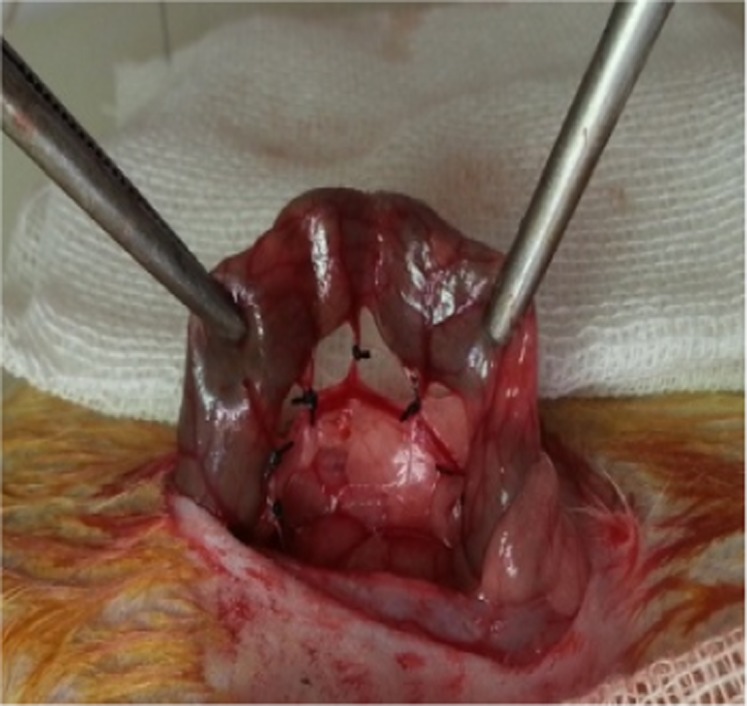
Ischemic colitis model.
The animals were returned to their cages upon regaining consciousness and were allowed to drink water and eat. At 72 hours post-surgery, the animals were euthanized (cervical dislocation) under anesthesia and relaparotomy was performed. Colonic tissues were resected for examination. There were no observable fistula or perforations in the binding sites of the marginal artery and branches of the colon.
Macroscopic evaluation of the damage
Prior to euthanasia, the rats were anesthetized and reopened via median laparotomy. The abdominal cavities were examined to determine the presence of acidic liquid, intestinal dilatation, serosal change and perforation. The devascularized segment of the colon was excised and opened longitudinally from the antimesenteric side.
A pathologist who was blinded to the sample origin examined the collected tissues. Ischemic changes in the colonic mucosa (excised according to the method of Gomella et al. 12) were examined on clean graphene paper. The following scoring system developed by Wallace 13 was used to assess the severity of macroscopically visible damage (MVD): 0, normal; 1, local ischemia without ulceration; 2, ulceration without hyperemia; 3, ulceration (+) and hyperemia in the area; 4, ulceration (≤2 cm; +) and hyperemia in the area; 5, >2 cm ulcers in many areas 13 (Figure 2).
Figure 2.
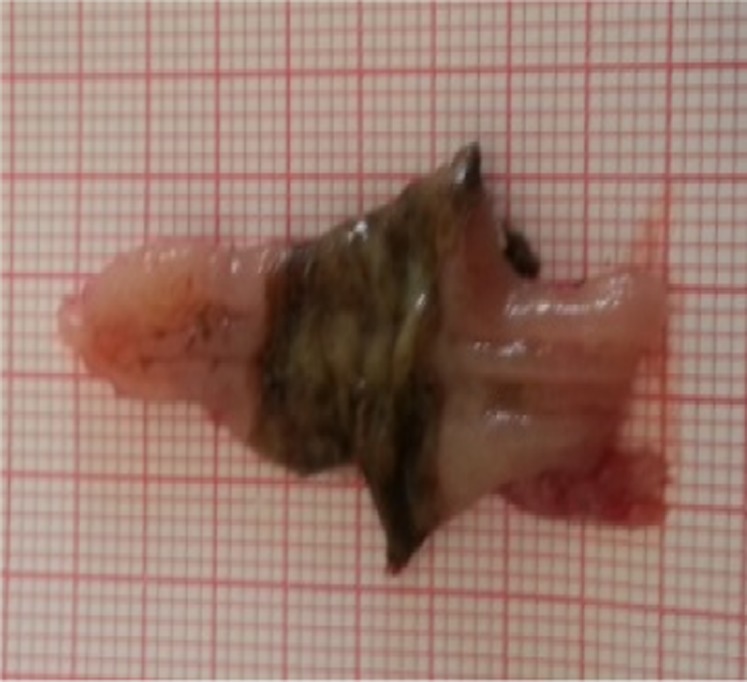
Measurement of ischemic areas in the control group.
Histopathological analysis
A histopathological examination was performed at the Marmara University School of Medicine, Department of Pathology. Tissue samples from the left colon that were fixed in a 10% formaldehyde solution were divided into 5 µm pieces and separated for examination. A pathologist who was blinded to sample origin examined the collected tissues. Histopathological analysis and measurement of the severity of ischemic damage were performed using the following ischemic damage classification system developed by Chiu 14: 0, normal mucosal villus; 1, subepithelial Gruenhagen's area congestion; 2, epithelial layer moderately separated from the lamina propria, 3, massive epithelial separation of villus slices and peeling in a few places; 4, villus separated from lamina propria and stretched with dilated capillaries; 5, digested and diffuse lamina propria with bleeding and ulceration.
Measurement of malondialdehyde in tissue
Malondialdehyde (MDA) is a secondary product of lipid peroxidation and is thus used as a parameter to determine the extent of lipid peroxidation. Uchiyama and Mihara 15 described how MDA measurements can be used to assess the levels of oxidative damage within tissues. In our study, colonic tissues were frozen at -20°C and malondialdehyde content was quantified. The tissue samples were homogenized in 50 mmol Tris-HCl (pH 7.4) containing 180 mmol KCL and 10 mmol EDTA and subsequently weighed. The MDA concentration was analyzed via high-performance liquid chromatography (HPLC).
Statistical analysis
The data obtained in this study were analyzed using the SPSS 20 Package (Statistical Package for the Social Sciences, Chicago, IL). Paired group comparisons were performed using the Mann-Whitney U test and the Bonferroni-corrected Kruskal-Wallis H test was employed for comparisons involving 3 or more groups. The results are expressed as the mean ± standard deviation (min-max). The correlation between the variables was examined using Fisher's Exact Test. P-values of <0.05 indicated a significant difference. The Bonferroni test was utilized for multiple comparisons and p<0.01 was considered statistically significant.
RESULTS
None of the rats died during the study.
Macroscopic damage
The statistical comparison between sham and control groups, significant differences were found with respect to the presence of acidic liquid, bowel dilatation and serosal changes (p<0.05). However, no significant differences were observed when comparing either perforation or adhesion between these groups (p>0.05). In comparing the vardenafil 5 mg and 10 mg groups and the pentoxifylline group with the control group, significant differences were found with respect to the presence of acidic liquid, bowel dilatation and serosal changes (p<0.05; Table 2).
Table 2.
-Comparison of macroscopic damage between groups.
| Acidic liquid | Bowel dilatation | Serosal changes | Perforation | Adhesion | |||||||
| GROUPS | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | |
| A = Sham group | n | 8 | 0 | 8 | 0 | 8 | 0 | 8 | 0 | 8 | 0 |
| % | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | |
| B = Control group | n | 0 | 8 | 0 | 8 | 0 | 8 | 5 | 3 | 0 | 100 |
| % | 0 | 100 | 0 | 100 | 0 | 100 | 62.5 | 37.5 | 0 | 100 | |
| C = Vardenafil 10 mg group | n | 5 | 3 | 6 | 2 | 6 | 2 | 8 | 0 | 2 | 6 |
| % | 62.5 | 37.5 | 75 | 25 | 75 | 25 | 100 | 0 | 25 | 75 | |
| D = Vardenafil 5 mg group | n | 6 | 2 | 5 | 3 | 8 | 0 | 8 | 0 | 2 | 6 |
| % | 75 | 25 | 62.5 | 37.5 | 100 | 0 | 100 | 0 | 25 | 75 | |
| E = Pentoxifylline group | n | 8 | 0 | 6 | 2 | 8 | 0 | 8 | 0 | 3 | 5 |
| % | 100 | 0 | 75 | 25 | 100 | 0 | 100 | 0 | 37.5 | 62.5 | |
| Statistics | p and binary comparison | A-B (0,001), B-C (0.026), B- (0.007), B- (0.0001) | A-B (0.001), B-C (0.007), B-D (0.026), B-E (0.007) | A-B (0.0001), B-C (0.007), B-D, (0001), B- (0.0001) | A-B (0.201), B-C (0.201), B-D (0.201), B-E (0.201) | A- (0.467), B-C (0.467), B-D (0.467), B-E (0.201) | |||||
Macroscopic damage was measured according to the method described by Gomella et al. 12. The ischemic areas were 63.3 mm2 in the control group, 3.4 and 9.6 mm2 in the vardenafil 5 mg and 10 mg groups, respectively and 2.8 mm2 in the PTX group. A significant difference was observed when comparing the Gomella ischemic areas (p = 0.0001) of the sham and control groups. Comparison of the vardenafil 5 mg and 10 mg groups and the pentoxifylline group with the control group also revealed significant differences in Gomella ischemic areas (p = 0.0001; Table 3).
Table 3.
-Comparisons of MDA, Wallace macroscopic damage scores and Chiu classification in rats.
| MDA (nmol/gr) | Wallace macroscopic damage scoring (MVD) | Chiu classification | Gomella ischemic areas (IAs) (mm2) | ||||||||||||
| Groups | n | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | ||
| A = Sham group | 8 | 18.0 | 8 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| B = Control group | 8 | 63.7 | 0 | 1 | 3 | 2 | 1 | 1 | 0 | 1 | 3 | 0 | 1 | 3 | 63.3 |
| C = Vardenafil 10 mg | 8 | 25.6 | 2 | 5 | 1 | 0 | 0 | 0 | 2 | 5 | 0 | 1 | 0 | 0 | 9.6 |
| D = Vardenafil 5 mg | 8 | 25.3 | 2 | 6 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 3.4 |
| E = Pentoxifylline group | 8 | 22.7 | 3 | 5 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 2.8 |
| Binary group comparisons (p-value) | A-B, B-C, B-D, B-E (p = 0.0001) | A-B (0.0001), A-C (0.003), A-D (0.003), A-E (0.009), B-C (0.003), B-D (0.001), B-E (0.001), C-D (0.679), C-E (0.424), D-E (0.602) | A-B (0.0001), A-C (0.003), A-D (0.009), A-E (0.009), B-C (0.006), B-D (0.001), B-E (0.001), C-D (0.424), C-E (0.424), D-E (0.333) | A-B, A-C, A-D, A-E, B-C, B-D, B-E p = 0.0001 | |||||||||||
We also evaluated the colonic mucosa to identify hyperemia, ulceration and inflammation, and resected colons were macroscopically evaluated using the Wallace macroscopic damage scoring system 13. A significant difference was observed in the average Wallace scores obtained for the sham and control groups (p<0.01). Comparison of the vardenafil 5 mg and 10 mg groups and the pentoxifylline group with the control group also revealed a significant difference in the average Wallace score (p<0.01). However, there were no statistically significant differences in the average Wallace scores between the vardenafil 5 mg and 10 mg groups or between the vardenafil groups and the pentoxifylline group (p>0.01; Table 3).
Histopathological evaluation
We employed the ischemic damage classification system developed by Chiu 14 to perform histopathological evaluation of the resected tissues. A significant difference was found between the average Chiu scores of the sham and control groups (p<0.01). Statistically significant differences (p<0.01) were also found when comparing the average Chiu scores of the vardenafil 5 mg and 10 mg groups or the pentoxifylline group to that of the control group. However, there was no statistically significant difference observed in the average Chiu scores of the vardenafil groups or between the vardenafil groups and the pentoxifylline group (Table 3) (Figure 3).
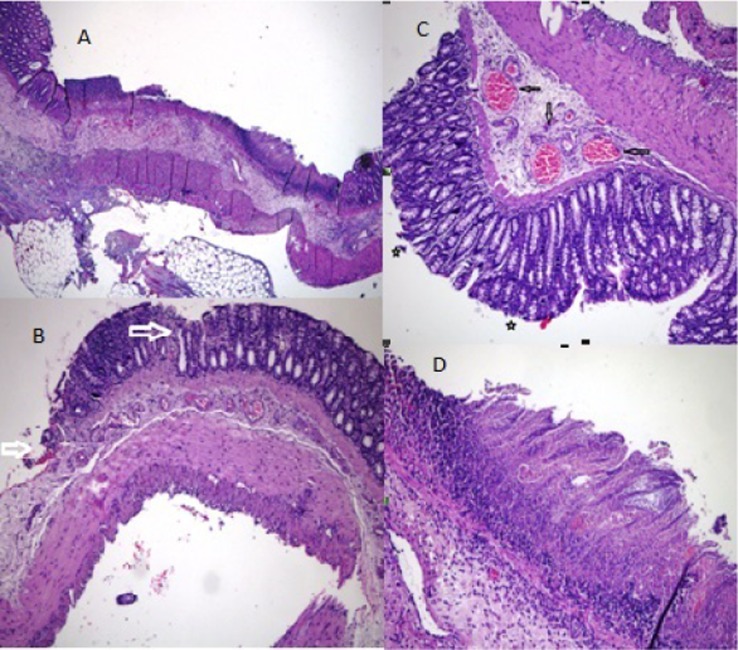
Figure 3.
Group histopathology. A. Common massive areas of epithelial detachment and presence of transmural necrosis. [“Chiu” classification grade 5] (H&E x40). B. Massive areas of epithelial detachment (shown with an arrow) [“Chiu” classification grade 3] (H&E x100). C. Subepithelial “Gruenhagen's space” (shown with an asterisk) and capillary congestion (shown with an arrow) [“Chiu” classification grade 1] (H&E x200). D. Area of mucosal ischemic necrosis (H&E x200).
Measurement of malondialdehyde in tissue (MDA)
The mean MDA values were 18 and 63.7 nmol/g in the sham and control groups, respectively; 25.3 and 25.6 nmol/g in the vardenafil 5 mg and 10 mg groups, respectively; and 22.7 nmol/g in the pentoxifylline group. A significant difference was found in the mean MDA values (p = 0.0001) of the sham and control groups. When comparing the vardenafil 5 mg and 10 mg groups and the pentoxifylline group to the control group, significant differences in mean MDA values were also observed (p = 0.0001; Table 3).
DISCUSSION
Ischemic colitis is the most common ischemic colon disease 1,2. Boley et al. 3 were the first to describe ischemic colitis in the 1960 s. The annual incidence rate of IC is 4.5-44/100,000 individuals in the general population and the disease is present in 1 out of every 2000 patients admitted to emergency units 1-3. IC frequently occurs following aortic surgery and abdominal aortic surgery 3,4; it generally results from colonic damage as a result of hypoperfusion and has various causes. The main factor leading to colonic damage is a decrease in oxygen diffusion due to insufficient tissue perfusion 16,17,39. IC occurs most commonly in the left colon, especially in the splenic flexure. The incidence rate in the right colon is also gradually increasing. The splenic flexure has the disadvantage of limited blood flow, as it is located between two mesenteric arteries that in effect function as a “watershed area” 18. While ischemia may only affect the mucosa, transmural colitis can affect all layers of the colon and lead to stricture. In fulminant cases, it may further progress and cause gangrene 16-18. Experimental studies have demonstrated that colonic damage caused by microcirculation disorders is similar to that caused by ischemic colitis in humans 19.
The clinical spectrum of IC is broad, varying from the self-limiting mild form to the fast-progressing life-threatening form 1,2. Symptomology frequently includes the sudden onset of abdominal pain, nausea, vomiting and diarrhea (often bloody). Systemic inflammatory response syndrome (SIRS), abdominal distention and abdominal tenderness may also occur. In the mild form of IC, the mucosa and the submucosa are damaged; however, the damage is limited and generally responds to medical intervention. Oral feeding is interrupted, the intestines are allowed to rest and supportive drug treatment may be initiated using parenteral antibiotics administered via a nasogastric tube 1,2,4,5,7. In severe forms of IC, the colon is completely damaged, patient prognosis is poor and surgical treatment is often required 20,21. PDE–5 inhibitors induce vascular smooth muscle dilatation and inhibit platelet aggregation by preventing the breakdown of nitric oxide (NO)-dependent cGMP. Vardenafil, sildenafil and tadalafil are potent PDE-5 inhibitors that can be used in the treatment of erectile dysfunction, pulmonary artery hypertension, coronary artery disease, diabetes mellitus, testicular torsion, deep vein thrombosis, anti-anginal therapy 39 and acute renal ischemia 22,23,36.
Hotta et al. 24 demonstrated the efficacy of vardenafil in the treatment of acute arterial erectile dysfunction in rats.
PTX inhibits microvascular construction and blocks erythrocyte and platelet aggregation. It also increases fibrinolysis and suppresses leukocyte hyperactivity by reducing superoxide release and neutrophil adhesion 10,33. In an experimental study, Parra-Membrives et al. 25 demonstrated that pentoxifylline administration has a positive impact on the recovery of ischemic anastomosis by reducing anastomotic leakage and increasing bursting pressure. Moreover, Tireli et al. 26 showed that pentoxifylline reduces ischemic reperfusion injury in small intestine anastomosis through its additive effect on collagen synthesis and anastomotic strength. Additionally, PTX may be used to treat a variety of disorders including vasoocclusive diseases 10, infectious diseases 33, immune deficiencies 34 and hypercoagulation 35.
Platelet activating factor (PAF), which leads to vasoconstriction with vasoactive properties and tissue hypoperfusion, causes a gastrointestinal mucosal ulceration. Many researchers have shown that PAF might cause mucosal damage within the gastrointestinal tract 27.
Adams et al. 33 investigated the effect of pentoxifylline in an ischemic reperfusion model. In this study, the efficacy of pentoxifylline was demonstrated based on the resulting inhibition of platelet-activating factor.
Studies have shown that PDE inhibitors and NO-dependent cGMP protein kinases play an important role in platelet inhibition. Vardenafil and sildenafil are expected to correct potential platelet inhibition and microcirculation 28.
In a postoperative skin flap viability study conducted by Sarifakioğlu 28 and Hart et al. 29 were demonstrated significant improvement due to platelet inhibition and sildenafil-related vasodilatation. Moreover, the efficacy of vardenafil and other PDE-5 inhibitors has been demonstrated in the treatment of pulmonary arterial hypertension 30 as well as in studies of lung contusion 31, testicular torsion and radical prostatectomy 32. In the present study, we considered these properties of vardenafil and other PDE–5 inhibitors as a starting point, compared their effects with the proven effects of PTX and investigated the efficacy of vardenafil administration in an animal model of IC.
In routine clinical studies, many patients diagnosed with IC are admitted to hospitals; however, the use of vardenafil in these patients has been limited.
In the present study, we successfully employed the experimental model of ischemic colitis as described by Griffen and Hagihara 11.
In comparing our sham group to the control group, we found statistically significant differences in the mean MDA values 15 of macroscopic damage scores (MVD) (used by Irkorucu et al. 9 and described by Wallace et al. 13) as well as in the Gomella ischemic area 12 and Chiu classification 14. These differences illustrated the suitability of our model system.
In our ischemic colitis model, rats were administered vardenafil at 5 mg or 10 mg and the macroscopic damage scores, Gomella ischemic areas 12 and mean MDA values 15 were used as biochemical parameters of therapeutic outcome. Additionally, histopathological measurements were evaluated using the Chiu classification system 14. The resulting data revealed significant differences between the treatment and control groups. These results indicate that vardenafil administration is an effective strategy overall and the two different doses of vardenafil evaluated in this study were therapeutically equivalent.
Pentoxifylline (PTX) is a drug with proven efficacy in clinical practice 33-35. In our ischemic colitis rat model, we administered PTX and evaluated several biochemical parameters including macroscopic damage scores 13, Gomella ischemic areas 12 and mean MDA values 15 to quantify therapeutic efficacy. We also employed the Chiu classification 14 for histopathological evaluation. Statistically significant differences were observed between the PTX, treatment group and the control group, demonstrating that PTX may be an effective clinical option.
The limitations of this study include the fact that we did not analyze acute phase reactants or cytokines (IL-1, 6, TNF-α) and we did not study any alternative PDE-5 inhibitors.
The relative efficacies of vardenafil versus PTX administration were evaluated in an ischemic colitis rat model. Morphological and biochemical data obtained from the treatment groups were statistically comparable and significant differences were noted when compared to the control group. These results demonstrate that both vardenafil and pentoxifylline are effective therapeutic options for this IC model and there is no significant difference between the effects of these two pharmacological agents.
Our study results demonstrated that vardenafil and pentoxifylline are effective therapeutic options in a rat model of IC. These results are likely due to the positive effect that these drugs exert on the hemodynamics associated with vascular smooth muscle and platelet functions. Vardenafil and PTX might therefore be useful treatment options in models of mesenteric ischemia and vasospastic disease. Our hope is that the results of the current study, combined with those of additional studies, will lead to future clinical utilization of vardenafil and other PDE-5 inhibitors in the treatment of ischemic colitis.
ACKNOWLEDGMENTS
Financial or other support was not received from any person or organization.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am. 2001;30:445–73. doi: 10.1016/s0889-8553(05)70190-4. [DOI] [PubMed] [Google Scholar]
- 2.Elder K, Lashner BA, Al Solaiman F. Clinical approach to colonic ischemia. Cleve Clin J Med. 2009;76(7):401–9. doi: 10.3949/ccjm.76a.08089. [DOI] [PubMed] [Google Scholar]
- 3.Boley SJ, Brandt LJ, Sammartano RJ. History of mesenteric ischemia. The evolution of a diagnosis and management. Surg Clin North Am. 1997;77(2):275–88. doi: 10.1016/s0039-6109(05)70548-x. [DOI] [PubMed] [Google Scholar]
- 4.Longo WE, Ward D, Vernava AM, 3rd, Kaminski DL. Outcome of patients with total colonic ischemia. Dis Colon Rectum. 1997;40(12):1448–54. doi: 10.1007/BF02070711. [DOI] [PubMed] [Google Scholar]
- 5. Brandt LJ, Smithline AE. Ischemic lesions of the bowel Feldman M, Scharschmidt B F, Sleisenger M H, edsGastrointestinal and Liver Disease. 6th edn 1998WB Saunders: Philadelphia; 2009–24. [Google Scholar]
- 6.Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am. 1998;27(4):827–60, vi. doi: 10.1016/s0889-8553(05)70034-0. [DOI] [PubMed] [Google Scholar]
- 7.Diaz Nieto R, Varcada M, Ogunbiyi OA, Winslet MC. Systematic review on the treatment of ischaemic colitis. Colorectal Dis. 2011;13(7):744–7. doi: 10.1111/j.1463-1318.2010.02272.x. [DOI] [PubMed] [Google Scholar]
- 8.Reissfelder C, Sweiti H, Antolovic D, Rahbari NN, Hofer S, Büchler MW, et al. Ischemic colitis: who will survive. Surgery. 2011;149(4):585–92. doi: 10.1016/j.surg.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Irkorucu O, Taşcilar O, Cakmak GK, Karakaya K, Emre AU, Ucan BH, et al. The effect of sildenafil on an animal model for ischemic colitis Dig Dis Sci. 2008;53(6):1618–23. doi: 10.1007/s10620-007-0033-9. [DOI] [PubMed] [Google Scholar]
- 10.Stoop MJ, Dirksen R, Hendriks T. Advanced age alone does not suppress anastomotic healing in intestine. Surgery. 1996;119(1):15–9. doi: 10.1016/s0039-6060(96)80207-8. [DOI] [PubMed] [Google Scholar]
- 11.Griffen TS, Hagihara PF. Ischemic colitis in rats. Dis Colon Rectum. 1982;25(7):638–40. doi: 10.1007/BF02629530. [DOI] [PubMed] [Google Scholar]
- 12.Gomella LG, Flanigan GC, Hagihara PF, Lucas BA, McRoberts JW. The influence of uremia and immunosuppression on an animal model for ischemic colitis. Dis Colon Rectum. 1986;29(11):724–7. doi: 10.1007/BF02555319. [DOI] [PubMed] [Google Scholar]
- 13.Wallace JL, Keenan CM. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. 1990;258(4 Pt 1):G527–34. doi: 10.1152/ajpgi.1990.258.4.G527. Am Am J Physiol. [DOI] [PubMed] [Google Scholar]
- 14.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101(4):478–83. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama M, Mihara M. Determination of malonaldehyde precurser in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 16.Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology. 1999;211(2):381–8. doi: 10.1148/radiology.211.2.r99ma28381. [DOI] [PubMed] [Google Scholar]
- 17.Medina C, Vilaseca J, Videla S, Farba R, Armengol-Miro JR, Malagelada JR. Outcome of patients with ischemic colitis: review of fifty-three cases. Dis Colon Rectum. 2004;47(2):180–4. doi: 10.1007/s10350-003-0033-6. [DOI] [PubMed] [Google Scholar]
- 18.Sreenarasimhaiah J. Diagnosis and management of ischemic colitis. Curr Gastroenterol Rep. 2005;7(5):421–6. doi: 10.1007/s11894-005-0013-1. [DOI] [PubMed] [Google Scholar]
- 19.Korotinski S, Katz A, Malnick SD. Chronic ischaemic bowel disease in the aged-go with the flow. Age Ageing. 2005;34(1):10–6. doi: 10.1093/ageing/afh226. [DOI] [PubMed] [Google Scholar]
- 20.Longo WE, Ballantyne GH, Gusberg RJ. Ischemic colitis: Patterns and prognosis. Dis Colon Rectum. 1992;35(8):726–30. doi: 10.1007/BF02050319. [DOI] [PubMed] [Google Scholar]
- 21.Mishima Y, Horie Y. Experimental studies of ischemic enterocolitis. World J Surg. 1980;4:601–8. doi: 10.1007/BF02401641. [DOI] [PubMed] [Google Scholar]
- 22.Fan YF, Zhang R, Jiang X, Wu DC, Liu D, Yuan P, et al. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc Res. 2013;99(3):395–403. doi: 10.1093/cvr/cvt109. [DOI] [PubMed] [Google Scholar]
- 23.Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol. 2007;102(10):2247–52. doi: 10.1111/j.1572-0241.2007.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotta Y, Ohno R, Kataoka T, Mikumo M, Takahata Y, Ohno M, et al. Effects of chronic vardenafil treatment persist after end of treatment in rats with acute arteriogenic erectile dysfunction. J Sex Med. 2012;9(7):1782–8. doi: 10.1111/j.1743-6109.2012.02742.x. [DOI] [PubMed] [Google Scholar]
- 25.Parra-Membrives P, Ruiz-Luque V. Effect of Pentoxifylline on the Healing of ischemic colorectal anastomoses. Dis Colon Rectum. 2006;50(3):369–75. doi: 10.1007/s10350-006-0803-z. [DOI] [PubMed] [Google Scholar]
- 26.Tireli GA, Salman T. The effect of pentoxifylline on intestinal anastomotic healing after ischemia. Pediatr Surg Int. 2003;19(1-2):88–90. doi: 10.1007/s00383-002-0741-3. [DOI] [PubMed] [Google Scholar]
- 27.El-Awady MS, Said E. Vardenafil ameliorates immunologic- and non-immunologic-induced allergic reactions. Can J Physiol Pharmacol. 2014;92(3):175–80. doi: 10.1139/cjpp-2013-0316. [DOI] [PubMed] [Google Scholar]
- 28.Sarifakioglu N, Gokrem S, Ates L, Akbuga UB, Aslan G. The influence of sildenafil on random skin flap survival in rats: an experimental study. Br J Plast Surg. 2004;57(8):769–72. doi: 10.1016/j.bjps.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Hart K, Baur D, Hodam J, Lesoon-Wood L, Parham M, Keith K, et al. Short- and long-term effects of sildenafil on skin flap survival in rats. Laryngoscope. 2006;116(4):522–8. doi: 10.1097/01.mlg.0000200792.67802.3b. [DOI] [PubMed] [Google Scholar]
- 30.Chen WS, Li XQ, Cao W, Xiao X, Dong L, Zhang JZ. Vardenafil ameliorates calcium mobilization in pulmonary artery smooth muscle cells from hypoxic pulmonary hypertensive mice. Arch Med Res. 2012;43(4):265–73. doi: 10.1016/j.arcmed.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Salamanca JI, La Fuente JM, Cardoso J, Fernández A, Cuevas P, Wright HM, et al. Nebivolol potentiates the efficacy of PDE5 inhibitors to relax corpus cavernosum and penile arteries from diabetic patients by enhancing the NO/cGMP pathway. J Sex Med. 2014;11(5):1182–92. doi: 10.1111/jsm.12477. [DOI] [PubMed] [Google Scholar]
- 32.Dogrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother. 2005;6(1):75–84. doi: 10.1517/14656566.6.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Rasslan R, Utiyama EM, Marques GM, Ferreira TC, Costa VA, Victo NC, et al. Inflammatory activity modulation by hypertonic saline and pentoxifylline in a rat model of strangulated closed loop small bowel obstruction. Int J Surg. 2014;12(6):594–600. doi: 10.1016/j.ijsu.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Ersoy YE, Ayan F, Himmetoglu S. Trace element levels in ischemia-reperfusion injury after left colonic anastomosis in rats and effects of papaverine and pentoxiphylline on vascular endothelial growth factor in anastomosis healing. Acta Gastroenterol Belg. 2011;74(1):22–7. [PubMed] [Google Scholar]
- 35.Sunil VR, Vayas KN, Cervelli JA, Malaviya R, Hall L, Massa CB, et al. Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation. Exp Mol Pathol. 2014;97(1):89–98. doi: 10.1016/j.yexmp.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyriazis I, Kagadis GC, Kallidonis P, Georgiopoulos I, Marazioti A, Geronasiou A, et al. PDE5 inhibition against acute renal ischemia reperfusion injury in rats: does vardenafil offer protection. World J Urol. 2013;31(3):597–602. doi: 10.1007/s00345-012-0980-4. [DOI] [PubMed] [Google Scholar]
- 37.Minareci E, Sadan G. An evaluation of vardenafil as a calcium channel blocker in pulmonary artery in rats. Indian J Pharmacol. 2014;46(2):185–90. doi: 10.4103/0253-7613.129315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang L, Radovits T, Szabo G, Mozes MM, Rosivall L, Kokeny G. Selective phosphodiesterase-5 (PDE-5) inhibitor vardenafil ameliorates renal damage in type 1 diabetic rats by restoring cyclic 3′,5′ guanosine monophosphate (cGMP) level in podocytes. Nephrol Dial Transplant. 2013;28(7):1751–61. doi: 10.1093/ndt/gfs391. [DOI] [PubMed] [Google Scholar]
- 39.Veres G, Hegedu˝s P, Barnucz E, Zöller R, Radovits T, Korkmaz S, et al. Addition of vardenafil into storage solution protects the endothelium in a hypoxia-reoxygenation model. Eur J Vasc Endovasc Surg. 2013;46(2):242–8. doi: 10.1016/j.ejvs.2013.05.006. [DOI] [PubMed] [Google Scholar]