Abstract
Background:
Coagulase-negative staphylococci (CoNS), especially Staphylococcus epidermidis, are considered as normal flora of human epithelia and also important opportunistic pathogens for nosocomial infections. S. epidermidis can also act as a reservoir for mecA, responsible for high-level resistance to methicillin and transferring it to S. aureus.
Objectives:
The aim of this study was to determine the prevalence of S. epidermidis as well as antibiotic susceptibility pattern and mecA prevalence in S. epidermidis isolated from intensive care unit (ICU) patients.
Materials and Methods:
A cross-sectional study was conducted from September 2010 to September 2011 and 184 coagulase-negative staphylococci were collected from different clinical samples in three hospitals. S. epidermidis was identified by conventional bacteriological tests. Antibiotic susceptibility testing was performed using disk diffusion method. Frequency of mecA was detected by specific PCR.
Results:
Frequency of S. epidermidis was 34.8%, the most susceptibility was seen to linezolid and vancomycin, and the least susceptibility was seen to tetracycline.Majority of the S. epidermidis isolates carried mecA (92.2%). The most common resistant pattern was trimethoprim-sulfamethoxazole, tetracycline, erythromycin, and methicillin resistance, found in 23.4% of the isolates, followed by resistance to methicillin as the second-most common resistant pattern, observed in 20.3% of the isolates.
Conclusions:
Frequency of S. epidermidis was significantly lower, compared to other studies. Presence rate of mecA and susceptibility to linezolid and vancomycin did not show significant differences with other investigations, while resistant to trimethoprim-sulfamethoxazole was significantly lower compared to other investigations, and resistance to tetracycline was significantly higher in comparison to other investigations. Presence of methicillin-resistant S. epidermidis in ICU patients, especially in individuals with compromised immune systems, may cause infection and would be more complicated in the case of antibiotic resistance.
Keywords: Staphylococcus epidermidis, mecA, Coagulase, Methicillin-Resistant
1. Background
Coagulase-negative staphylococci (CoNS), especially Staphylococcus epidermidis, are considered as normal epithelial flora of every human in different parts of the body such as nares, head and axilla, with an essential role in maintaining the normal flora of healthy skin (1). S. epidermidis is also recognized as an important opportunistic pathogen in nosocomial infections, particularly in indwelling medical device users and contaminant agent of blood cultures. Nosocomial infection with S. epidermidis should be more complicated when bacteria are resistant to beta-lactam antibiotics such as methicillin. Methicillin-resistant CoNS show a higher resistance rate to antibiotics than S. aureus. These bacteria are common causes of nosocomial infections worldwide in immunocompromised patients or patients who carry indwelling devices (2).
Reduced affinity for beta-lactam antibiotics in methicillin-resistant staphylococci mediates by a penicillin-binding protein (PBP2a) encoded by mecA gene located in staphylococcal chromosome cassette mec (SCCmec). Furthermore, it is believed that S. epidermidis acts as a reservoir for mecA and transfers it to S. aureus by horizontal gene transfer. Recent data indicate that not only mecA, but also other mobile genetic elements can be transferred from S. epidermidis to S. aureus (3).
2. Objectives
The aim of this study was to determine the prevalence of S. epidermidis as well as antibiotic susceptibility pattern and mecA prevalence in S. epidermidis isolated from intensive care unit (ICU) patients.
3. Materials and Methods
3.1. Bacterial Isolates
A cross-sectional study was conducted from September 2010 to September 2011. A collection of 184 coagulase-negative staphylococci isolated from blood (n = 135), urine (n = 17), tracheal (n = 19), wound (n = 6), cerebrospinal fluid (CSF) (n = 2), pleural fluid (n = 2), synovial fluid (n = 2), and peritoneal fluid (ascitic fluid) (n = 1), from three different teaching hospitals of Tehran, Iran, was included in this study. S. epidermidis was identified by conventional bacteriological tests. The sample was enriched in tryptic soy broth, and grown on mannitol salt agar, then catalase, tube coagulase and urease tests, and carbohydrate fermentation were performed. S. epidermidis is catalase positive, urease positive, unable to ferment D-mannitol and D-trehalose, and able to ferment D-mannose and D-maltose (4, 5).
3.2. Antibiotic Susceptibility Testing
Antibiotic susceptibility testing was performed for S. epidermidis isolates using disk diffusion method on Mueller-Hinton agar plates (Merck, Germany) and commercial antibiotic disks including: vancomycin (30 μg), linezolid (30 μg), rifampin (5 μg), ciprofloxacin (5 μg), trimethoprim-sulfamethoxazole (1.25/23.75 μg), tetracycline (30 μg), and erythromycin (15 μg), (Mast, UK), according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (6).
3.3. DNA Extraction
DNA of each S. epidermidis isolate was extracted from 1mL of overnight bacterial culture. Extraction was performed by adding TE [10 mM Tris, 1 mM EDTA (pH = 8)] buffer and lysostaphin, 95°C for 5 minutes, then 0°C for 5 minutes, and centrifuged in 10000 rpm for 5 minutes. The supernatant was used as the template DNA in PCR reactions. DNA was measured using a BioPhotometer (Eppendorf, Germany) to determine the concentration and purity.
3.4. Detection of mecA
PCR was performed as described by Zhang et al. (7) with the following specific primers: forward: 5’-GTGAAGATATACCAAGTGATT-3’ reverse: 5’-ATGCGCTATAGATTGAAAGGAT-3’. Amplification was performed in a MJ mini. Gradiant thermal cycler PTC- 1148. U.S.A ; initial denaturation step at 94°C for 5 minutes, followed by 30 cycles of 45 seconds at 94°C,45 seconds at 55°C,and 90 seconds at 72°C, ending with a final extension step at 72°C for 10 minutes, followed by a hold at 4°C. The amplified products were visualized by UV light after electrophoresis on 1% agarose gel. The amplicon size was 147 bp (Figure 1). In the PCR reaction, S. epidermidis ATCC 12228 and S. aureus ATCC 33591 were used as negative and positive controls, respectively (7).
Figure 1. PCR for mecA Gene.
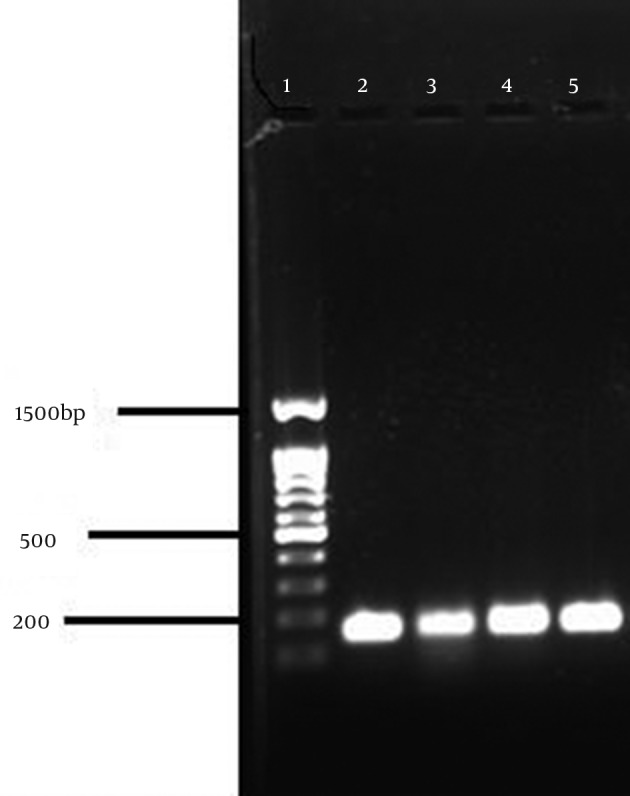
1, Marker, 100 bp; 2, S. aureus ATCC 33591 (147 bp); 3-5, Positive clinical strains.
3.5. Statistical Analysis
Confidence interval test was used to assess the statistical significance with confidence level of 95% (α = 0.05).
4. Results
In this study, frequency of S. epidermidis was 34.8% (95% CI: 46.5-23.1). Of the 64 S. epidermidis isolates, 52 (81.3%) were from blood, 6 (9.4%) tracheal, 4 (6.2%) urine, and 2 (3.1%) wound samples. mecA was detected in 92.2% (95%CI: 98.8-85.6) of S. epidermidis isolates. The results of antibiotic susceptibility testing are shown in Table 1. All of 64 S. epidermidis isolates were susceptible to vancomycin and linezolid. Resistance to rifampin and ciprofloxacin were 9.4% (95%CI: 16.5-2.3) and 23.4% (95%CI: 33.8-13), respectively. Resistance to trimethoprim-sulfamethoxazole was observed in 51.6% (95% CI: 63.8-39.4) of S. epidermidis isolates, while 46.9% (95% CI: 59.1-34.7) were susceptible to trimethoprim-sulfamethoxazole. Susceptibility to tetracycline was observed in 42.1% (95% CI: 54.2-30) of S. epidermidis isolates, while resistance was observed in 57.8% (95%CI: 69.9-45.7) and 59.4% (95%CI: 71.4-47.4) to tetracycline and erythromycin, respectively. Resistance pattern of 64 S. epidermidis isolates is shown in Table 2.
Table 1. Antimicrobial Susceptibility Rate of S. epidermidis Isolates by Disk Diffusion Method.
| Blood | Urine | Trachea | Wound | Total, No. (%) | |
|---|---|---|---|---|---|
| Vancomycin | 52 | 4 | 6 | 2 | 64 (100) |
| Linezolid | 52 | 4 | 6 | 2 | 64 (100) |
| Rifampin | 48 | 4 | 4 | 2 | 58 (90.6) |
| Ciprofloxacin | 41 | 2 | 3 | 2 | 48 (75) |
| Trimethoprim-sulfamethoxazole | 26 | 3 | 0 | 1 | 30 (46.9) |
| Tetracycline | 21 | 3 | 1 | 2 | 27 (42.2) |
| Erythromycin | 21 | 2 | 1 | 1 | 25 (39.1) |
Table 2. Resistance Pattern of 64 S. epidermidis Isolates a.
| Resistance Pattern | Number |
|---|---|
| Resistance to 6 antibiotics | |
| RP, CIP, SXT, Te, E, mecA | 3 |
| Resistance to 5 antibiotics | |
| CIP, SXT, Te, E, mecA | 2 |
| RP, CIP, Te, E, mecA | 2 |
| Resistance to 4 antibiotics | |
| SXT, Te, E, mecA | 15 |
| CIP, Te, E, mecA | 2 |
| CIP, SXT, E, mecA | 1 |
| CIP, SXT, Te, mecA | 1 |
| RP, CIP, E, mecA | 1 |
| Resistance to 3 antibiotics | |
| Te, E, mecA | 3 |
| SXT, E, mecA | 3 |
| CIP, E, mecA | 1 |
| SXT, Te, mecA | 2 |
| Resistance to 2 antibiotics | |
| Te, E | 3 |
| SXT, E | 2 |
| Te, mecA | 3 |
| SXT, mecA | 4 |
| CIP, mecA | 1 |
| Resistance to 1 antibiotic | |
| mecA | 13 |
| Te | 1 |
| Non | 1 |
| Total | 64 |
aAbbreviations: CIP, ciprofloxacin; E, Erythromycin; mecA, presence of mecA; RP, rifampin; SXT, trimethoprim-sulfamethoxazole; Te, tetracycline,
Three Of the 64 isolates (4.7%) were resistance to six antibiotics, 4 (6.3%) to five antibiotics, 20 (31.3%) to four antibiotics, 9 (14%) to three antibiotics, 13 (20.3%) to two antibiotics, 14 (21.9%) to one antibiotic, and 1 (1.7%) was susceptible to all the antibiotics. The most common resistance pattern was trimethoprim-sulfamethoxazole, tetracycline, erythromycin, and methicillin resistance, found in 15 isolates (23.4%), followed by resistance to methicillin only, the second-most common resistance pattern, observed in13 (20.3%) of the isolates.
5. Discussion
In the recent years, S. epidermidis has been isolated as an infective agent in nosocomial infections. Some reasons for such infections increasing are increased number of immune-deficient patients, use of indwelling medical devices, and use of antibiotics and disinfectants (8). Methicillin resistance was observed in 75-90% of isolated S. epidermidis from nosocomial infections, which was higher than the resistance rate for S. aureus isolates (40-60%). Noticing the presence of S. epidermidis as a human commensal flora, it is assumed to be carrier and reservoir for different genes such as antimicrobial resistance gene (2).
Frequency of S. epidermidis isolates in this study was significantly lower than reports of Okee et al. (9), Eftekhar and Raei (10), Bouchami et al. (11), Barbier et al. (12), Ruppe et al. (13), and Mombach Pinheiro Machado AB et al. (14). In the present study, all the S. epidermidis isolates were susceptible to vancomycin, which was in accordance with the result of Mendes et al. (15), Eftekhar and Raei (10), Abd EL Hafez et al. (16), Hellmark et al. (17), and Zhanelet al. (18), and significantly higher than report of Delgado et al. (19). In our study, all the S. epidermidis isolates were susceptible to linezolid, which was in accordance with the results of Hellmark et al. (17) and Zhanel et al. (18), but significantly higher than Delgado et al. (19) report.
In the present study, resistance to rifampin was significantly higher than Abd EL Hafez et al. (16) report, but significantly lower than reports of Bouchami et al. (11) and Hellmark et al. (17). In addition, resistance to ciprofloxacin was in accordance with Haque et al. (20) report, but significantly lowers than Mendes et al. (15), Hellmark et al. (17), and Bouchami et al. (11) reports, and it also was significantly higher than Abd EL Hafez et al. (16) reports. Susceptibility to trimethoprim-sulfamethoxazole was in accordance with Mendes et al. (15) report, but significantly higher than reports of Hellmark et al. (17), Bouchami et al. (11), Abd EL Hafez et al. (16), and Zhanel et al. (18). Furthermore, resistant to trimethoprim-sulfamethoxazole was significantly lower than report of Delgado et al. (19). In this study, resistance to erythromycin did not show significant difference with results of Mendes et al. (15), Bouchami et al. (11), and Hellmark et al. (17), but was significantly higher than the report of Haque et al. (20) and significantly lower than Abd EL Hafez et al. (16) report. In this study, susceptibility to tetracycline was significantly lower than the report of Mendes et al. (15) and Delgado et al. (19); also, resistance to tetracycline was significantly higher than the report of Bouchami et al. (11).
In this study, 92.2% of S. epidermidis isolates carried the mecA gene. Rohde et al. (21) reported that 87.5% of S. epidermidis isolates harbored mecA. Pourmand et al. (8) also reported that 95.8% of S. epidermidis isolates harbored mecA. Eftekhar and Raei (10) reported 90.9% methicillin resistance. Our data did not show significant difference with these studies, but was significantly higher than Līduma et al. (22) and Okee et al. (9) results, which reported only 10% mecA distribution, and Mendes et al. (15) study, which reported 73.2% methicillin resistant. In our study, 56.3% of S. epidermidis isolates were resistant to three antibiotics or more. Considering the presence of S. epidermidis in ICU patients, multidrug-resistant bacteria can cause infection and would be more complicated in treatment.
In conclusion, frequency of S. epidermidis was significantly lower compared to other studies. Presence rate of mecA and susceptibility to linezolid and vancomycin did not show significant differences with other investigations, while susceptibility to trimethoprim-sulfamethoxazole was significantly higher, and susceptibility to tetracycline was significantly lower than other investigations. Presence of mecA-positive S. epidermidis isolates in ICU patients, especially in ones with compromised immune systems, may cause infection and would be more complicated in the case of antibiotic resistance.
Acknowledgments
The authors would like to express their gratitude to the staff of the bacteriology department of the Tarbiat Modares University for their cooperation.
Footnotes
Authors' Contributions:Here we confirm that all authors participated in the research design and contributed to sections of the research.
Financial Disclosure:The authors declare that there is no conflict of interests to publish this article.
Funding Support:This study was performed with a grant from Tarbiat Modares University, Faculty of Medical Sciences, Tehran, Iran.
References
- 1.Otto M. Molecular basis of Staphylococcus epidermidis infections. Semin Immunopathol. 2012;34(2):201–14. doi: 10.1007/s00281-011-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otto M. Staphylococcus epidermidis--the 'accidental' pathogen. Nat Rev Microbiol. 2009;7(8):555–67. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung GY, Otto M. Understanding the significance of Staphylococcus epidermidis bacteremia in babies and children. Curr Opin Infect Dis. 2010;23(3):208–16. doi: 10.1097/QCO.0b013e328337fecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes BA, Sahm DF, Weissfeld AS. Bailey and Scott's Diagnostic Microbiology. Mosby; 2007. pp. 254–3. [Google Scholar]
- 5.Sharon J. P., Staphylococcus. Topley wilson's Microbiology and Microbial Infections. Hodder Arnold; 2005. pp. 771–816. [Google Scholar]
- 6.Wayne PA. CLSI document M100-S21 Performance Standards for Antimicrobial Susceptibility Testing Twenty-First Informational Supplement. Clinical and Laboratory Standards Institute. 2011 [Google Scholar]
- 7.Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5026–33. doi: 10.1128/JCM.43.10.5026-5033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pourmand MR, Abdossamadi Z, Salari MH, Hosseini M. Slime layer formation and the prevalence of mecA and aap genes in Staphylococcus epidermidis isolates. J Infect Dev Ctries. 2011;5(1):34–40. doi: 10.3855/jidc.984. [DOI] [PubMed] [Google Scholar]
- 9.Okee MS, Joloba ML, Okello M, Najjuka FC, Katabazi FA, Bwanga F, et al. Prevalence of virulence determinants in Staphylococcus epidermidis from ICU patients in Kampala, Uganda. J Infect Dev Ctries. 2012;6(3):242–50. doi: 10.3855/jidc.2007. [DOI] [PubMed] [Google Scholar]
- 10.Eftekhar F, Raei F. Correlation of Minimum Inhibitory Concentration Breakpoints and Methicillin Resistance Gene Carriage in Clinical Isolates of Staphylococcus epidermidis. Iran J Med Sci. 2011;36(3):213–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchami O, Achour W, Mekni MA, Rolo J, Ben Hassen A. Antibiotic resistance and molecular characterization of clinical isolates of methicillin-resistant coagulase-negative staphylococci isolated from bacteremic patients in oncohematology. Folia Microbiol (Praha). 2011;56(2):122–30. doi: 10.1007/s12223-011-0017-1. [DOI] [PubMed] [Google Scholar]
- 12.Barbier F, Ruppe E, Hernandez D, Lebeaux D, Francois P, Felix B, et al. Methicillin-resistant coagulase-negative staphylococci in the community: high homology of SCCmec IVa between Staphylococcus epidermidis and major clones of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2010;202(2):270–81. doi: 10.1086/653483. [DOI] [PubMed] [Google Scholar]
- 13.Ruppe E, Barbier F, Mesli Y, Maiga A, Cojocaru R, Benkhalfat M, et al. Diversity of staphylococcal cassette chromosome mec structures in methicillin-resistant Staphylococcus epidermidis and Staphylococcus haemolyticus strains among outpatients from four countries. Antimicrob Agents Chemother. 2009;53(2):442–9. doi: 10.1128/AAC.00724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mombach Pinheiro Machado AB, Reiter KC, Paiva RM, Barth AL. Distribution of staphylococcal cassette chromosome mec (SCCmec) types I, II, III and IV in coagulase-negative staphylococci from patients attending a tertiary hospital in southern Brazil. J Med Microbiol. 2007;56(Pt 10):1328–33. doi: 10.1099/jmm.0.47294-0. [DOI] [PubMed] [Google Scholar]
- 15.Mendes RE, Deshpande LM, Costello AJ, Farrell DJ. Molecular epidemiology of Staphylococcus epidermidis clinical isolates from U.S. hospitals. Antimicrob Agents Chemother. 2012;56(9):4656–61. doi: 10.1128/AAC.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abd El Hafez M, Khalaf NG, El Ahmady M, Abd El Aziz A, Hashim Ael G. An outbreak of methicillin resistant Staphylococcus epidermidis among neonates in a hospital in Saudi Arabia. J Infect Dev Ctries. 2011;5(10):692–9. doi: 10.3855/jidc.1293. [DOI] [PubMed] [Google Scholar]
- 17.Hellmark B, Unemo M, Nilsdotter-Augustinsson A, Soderquist B. Antibiotic susceptibility among Staphylococcus epidermidis isolated from prosthetic joint infections with special focus on rifampicin and variability of the rpoB gene. Clin Microbiol Infect. 2009;15(3):238–44. doi: 10.1111/j.1469-0691.2008.02663.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhanel GG, DeCorby M, Laing N, Weshnoweski B, Vashisht R, Tailor F, et al. Antimicrobial-resistant pathogens in intensive care units in Canada: results of the Canadian National Intensive Care Unit (CAN-ICU) study, 2005-2006. Antimicrob Agents Chemother. 2008;52(4):1430–7. doi: 10.1128/AAC.01538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgado S, Arroyo R, Jimenez E, Marin ML, del Campo R, Fernandez L, et al. Staphylococcus epidermidis strains isolated from breast milk of women suffering infectious mastitis: potential virulence traits and resistance to antibiotics. BMC Microbiol. 2009;9:82. doi: 10.1186/1471-2180-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque N, Hossain MA, Bilkis L, Musa AK, Mahamud C, Bari MS, et al. Antibiotic susceptibility pattern of Staphylococcus epidermidis. Mymensingh Med J. 2009;18(2):142–7. [PubMed] [Google Scholar]
- 21.Rohde H, Kalitzky M, Kroger N, Scherpe S, Horstkotte MA, Knobloch JK, et al. Detection of virulence-associated genes not useful for discriminating between invasive and commensal Staphylococcus epidermidis strains from a bone marrow transplant unit. J Clin Microbiol. 2004;42(12):5614–9. doi: 10.1128/JCM.42.12.5614-5619.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liduma I, Tracevska T, Bers U, Zilevica A. Phenotypic and genetic analysis of biofilm formation by Staphylococcus epidermidis. Medicina (Kaunas). 2012;48(6):305–9. [PubMed] [Google Scholar]


