Abstract
Cockayne syndrome (CS) is a debilitating and complex disorder that result from inherited mutations in the CS complementation genes A and B, CSA and CSB. The links between the molecular functions of the CS genes and the complex pathophysiology of CS are as of yet poorly understood and are the subject of intense debate. While mouse models reflect the complexity of CS, studies on simpler genet models might shed new light on the consequences of CS mutations. Here we describe a functional homolog of the human CSA gene in Caenorhabditis elegans. Similar to its human counterpart, mutations in the nematode csa-1 gene lead to developmental growth defects as a consequence of DNA lesions.
Keywords: Nucleotide excision repair, C. elegans, Cockayne syndrome
1. Introduction
Congenital defects in DNA repair pathways lead to highly complex disease syndromes that are characterized by various degrees of developmental failure, segmental premature ageing, and tissue-specific cancer susceptibility. These DNA repair deficiencies have suggested a causal role of DNA damage in human pathology[1]. However, the link between specific unrepaired lesions and mechanistic roles of DNA repair molecules to the disease manifestations is incompletely understood. Nucleotide excision repair (NER) deficiencies provide a particular intriguing example of the complexity of human disorders caused by mutations in DNA repair genes. NER removes helix-distorting lesions such as UV-induced 6-4 photoproducts (6-4PPs) and cyclobutane pyrimidine dimers (CPDs) [2]. These lesions are recognized by two distinct detection systems: Global-genome (GG)-NER scans the entire genome, while transcription-coupled (TC)-NER recognizes lesions at sites of RNA polymerase (RNAP) II stalling. Congenital defects that primarily inactivate GG-NER lead to Xeroderma pigmentosum (XP) and an increased risk of skin cancer. On the other hand, mutations in CSA and CSB, which inactivate TC-NER, cause Cockayne syndrome (CS) [3]. Classical CS cases exhibit postnatal growth retardation, followed by cachexia, neuronal degeneration, loss of retinal cells, and premature death between the ages of 12 and 16. Mutations in CSA or CSB can also cause mild UV hypersensitivity syndrome (UVSS), as well as the more severe type II CS and Cerebro-oculo-facio-skeletal syndrome (COFS) [4]. The causal relationship between mutations in CSA and CSB and these disorders remains obscure, especially in light of the variations in pathology and severity. S. cerevisiae budding yeast strains with certain mutations in Rad28 and Rad26 (homologs of CSA and CSB) display only mild increases in UV sensitivity [5]. In contrast, mutations in the S. pombe CSA homolog ckn1+ do not confer UV-sensitivity in an otherwise repair-proficient background [6]. Mice that are defective in Csa or Csb develop mild symptoms that mirror CS and show elevated UV-induced skin cancer susceptibility [7,8]. Only upon additional inactivation of the GG-NER factor Xpc or the general NER factor Xpa do the mice develop severe growth retardation and degenerative phenotypes reminiscent of the severe CS pathology in humans [9]. While the murine studies have been important for understanding physiological alterations caused by NER defects, the disease complexity in mammals makes it very challenging to gain a basic mechanistic understanding of the outcomes of transcription-blocking DNA lesions and TC-NER deficiencies in multicellular organisms. Therefore, it is critical to establish simple metazoan systems to study TC-NER functions and the consequences of transcription-blocking DNA lesions. The utility of such model systems depends on the identification of functional homologs of TC-NER genes. While CSB homologs are clearly conserved throughout evolution, a nematode homolog for CSA has remained elusive. The CSA protein is comprised of WD40 domains spanning nearly the entire protein sequence [10]. WD40 domains occur in a large variety of proteins and make the unequivocal assignment of homologous genes in the absence of other functional domains difficult [11]. We have identified the open reading frame D2013.3, which we propose to name csa-1, as the closest homolog of CSA in the nematode worm C. elegans. A functional homology has been confirmed by demonstrating that mutations in csa-1 sensitize animals to UV irradiation during developmental growth. Moreover, we show that csa-1 forms an epistasis group with csb-1, and, similar to Csa mutations in yeast and mice, loss of functional csa-1 enhances the UV sensitivity of GG-NER defective nematode strains. Our results form the basis of further analysis of the roles of the CSA protein in the context of a simple metazoan model system.
2. Materials and methods
2.1. Growth conditions
C. elegans strains were cultured according to standard conditions at 20°C on nematode growth medium (NGM) plates with E. coli strain OP50 [12]. Strains used were: N2 (Bristol; wild type), csb-1 (ok2335), xpa-1 (ok698), xpc-1 (tm3886), csa-1 (tm4539), csa-1 (tm5232), csa-1 (tm4916). ). csa-1 mutant strains were obtained from National Bioresource Project; other strains were provided from the Caenorhabditis Genetics Center. All strains were backcrossed a minimum of four times.
2.2. Somatic UV-sensitivity assay
Eggs were obtained from gravid adult worms by hypochlorite treatment and were allowed to hatch over night in M9 buffer. The following day, synchronized L1 larvae were transferred to NGM agar plates with OP50 and 4 hours later, UV-irradiated (310nm) with the indicated doses using a UV6 lamp (Philips) in a Waldmann UV236B device. A minimum of 40 worms was used for each UV-dose, and each treatment was conducted in triplicate. Post-UV, worms were allowed to develop for 65 hours at 20°C. Afterwards, the percentage of different larval stages in each treatment was counted and quantified to assess the UV-sensitivity.
2.3. Germline UV-sensitivity assay
Staged young adults were treated with indicated UV-doses and allowed to recover for 16 hours. Following recovery, timed egg laying was conducted by transferring three worms per UV-dose to new NGM plates with OP50 for 3 hours. Each treatment was conducted in triplicate. The total number of eggs laid was counted and, 48 hours later, the number of viable eggs was determined. To assess UV-sensitivity, the percentage of eggs that were laid after UV-treatment was compared to 0J/m2 and the percentage of viable eggs was determined after hatching.
2.4. Illudin-M sensitivity assay
Synchronized L1 larvae were obtained by hypochlorite treatment and treated with the indicated concentrations of illudin-M in liquid S-basal medium with OP50 for 24 hours at 20°C on a shaking platform. Worms were then transferred to NGM plates with OP50 and allowed to grow for another 40 hours. A minimum of 40 worms was used for each illudin-M dose and each treatment was conducted in triplicate. Illudin-M sensitivity was measured by determining the percentage of different larval stages in each treatment. Illudin-M was a kind gift from Rainer Schobert.
3. Results and discussion
3.1. Identification of the C. elegans CSA homolog
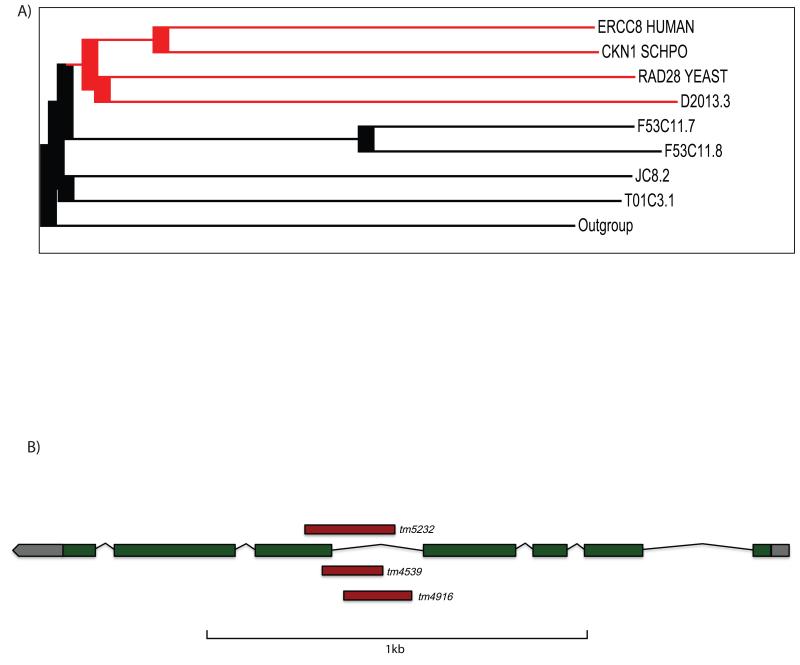
While the CSB protein has a clearly identifiable homolog in C. elegans, no homolog for CSA has been uncovered thus far. CSA is largely comprised of WD40-repeat domains that are found in a large number of sequence-related proteins. For the bioinformatic identification of a C. elegans CSA homolog, we constructed a generalized sequence profile from a multiple sequence alignment of established CSA sequences. This profile was used to screen a database of C. elegans protein sequences (current version of Wormbase [13]). The procedure identified 55 different C. elegans gene products with significant similarity to the profile, most containing no biological relationship to CSA except for the presence of WD40-repeats. A dendrogram using the neighbor-joining method [14] was calculated from a multiple alignment [15] of the C. elegans proteins and representative CSA sequences (Figure 1A, Supplementary Figure 1). As shown in Figure 1A, the uncharacterized C. elegans ORF D2013.3 clusters with the CSA proteins and was thus selected for further experimental characterization. D2013.3 is 1968 nucleotides long and is organized into seven exons. The coding sequence is 1083 nucleotides long and encodes a 360 amino acid protein. We obtained three deletion alleles for D2013.3 from National BioResource Project [16]. The tm4539 and tm5232 alleles contain 150 bp and 212 bp deletions, which delete parts of intron 4 and exon 5 (respectively). Both alleles are expected to encode null mutations as they introduce frameshifts that should be polar on the downstream parts of the open reading frames. The tm4916 allele encodes a 171 nucleotide deletion in intron 4 (Figure 1B).
Figure 1. Bioinfomatical identification of D2101.3 as a CSA homolog.
(A) A dendrogram showing the alignment of C. elegans proteins and known CSA sequences, calculated using the neighbour-joining method. The uncharacterized C. elegans ORF D2013.3 clusters with the CSA sequences. (B) Representation of the genomic architecture of D2013.3. Green boxes represent exons, black lines represent introns, and untranslated regions are in gray. The region deleted in the corresponding alleles is represented in red.
3.2. D2013.3 mutants are UV-hypersensitive
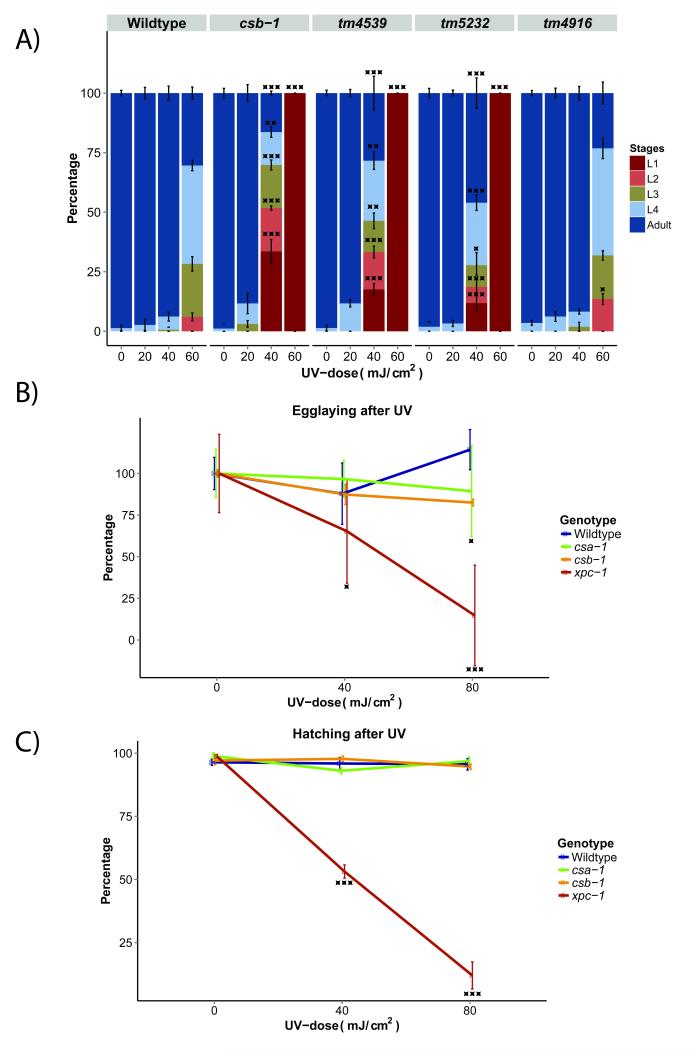
The C. elegans life cycle consists of four larval stages: L1, L2, L3, and L4, which precede adulthood. In C. elegans, the two branches of NER lead to UV hypersensitivity at distinct developmental stages [17,18]. Maintenance of the germline of adult animals and the embryos requires predominantly GG-NER, while late embryos and L1 larvae mostly rely on TC-NER. Most developmental cell divisions occur during embryogenesis [19] and the adult germline contains populations of mitotic and meiotic cells (in contrast, the adult soma is post-mitotic); thus the requirement of GG-NER in these contexts corresponds with their high proliferative activity. Consequently, the germ cells of GG-NER deficient xpc-1 mutant strains are highly UV sensitive. TC-NER, in contrast, is required during late embryonic and early larval development when cells are expected to be highly transcriptionally active. So far, the only TC-NER specific mutation in C. elegans is a deletion in the CSB homolog, csb-1 [17].To investigate whether D2013.3 is involved in TC-NER, we subjected L1 larvae carrying one of the three deletion alleles to different UV doses and followed their development. 65 hours post-UV, the untreated worms reached adulthood, whereas worms carrying the tm4539 and tm5232 alleles displayed UV-dependent development arrest after a dose of 60 mJ/cm2, similar to a csb-1 mutant strain (Figure 2A). In contrast to the other two alleles, the tm4916 allele displayed no UV-hypersensitivity and developed similar to wild-type animals. This observation could be explained by the fact that the deletion in tm4916 only affects intronic sequence, while the exons remain intact. The UV-hypersensitivity of D2013.3 supports our bioinformatic identification and we now refer to D2013.3 as csa-1. The csa-1(tm4539) mutant allele was used for further experiments.
Figure 2. D2013.3 is required for TC-NER but dispensable for GG-NER.
(A) The percentage of larval stages 65 hours after UV-treatment administered at the L1 larval stage. The tm4539 and tm5232 alleles display UV-sensitivity similar to the TC-NER-defective csb-1 mutant strain. A minimum of 40 worms were used for each UV-dose, and each treatment was conducted in triplicate. Error bars represent SD. *=p<0.05, **=p<0.01, ***=p<0.001, two-tailed t-test, compared to wildtype. (B) Egg-laying activity of the indicated mutants 16 hours after UV-treatment at young adult stage. Timed egg-laying was conducted with three worms per UV-dose for 3 hours. Each treatment was conducted in triplicate. (C) Percentage of hatched eggs after UV-treatment. Egg-laying and hatching of csa-1 mutant worms are unaffected, unlike GG-NER deficient xpc-1 mutant worms. B,C-Error bars represent SD. Statistical significance was calculated using two-tailed t-test, comparing treatments to 0mJ/cm2.
3.3. csa-1 is dispensable for GG-NER
To validate the specificity for csa-1 in TC-NER, we next examined the UV sensitivity of germ cells, which mainly rely on GG-NER. UV-treatment of GG-NER-deficient xpc-1 mutant animals leads to a reduction in fertility [17]. To examine the role of csa-1 in GG-NER, young adult worms were UV-treated, allowed to recover for 16 hours and their egg-laying activity was assessed over the course of three hours. The xpc-1 mutant animals displayed a dose-dependent reduction in the number of eggs laid and the percentage of eggs hatched. In contrast, UV irradiation had no significant effect on the germline activity of csa-1 mutant worms, as there was no significant reduction in the number of eggs laid after UV or the percentage of eggs hatched (Figure 2B). Hence, csa-1 is dispensable for GG-NER.
3.4. csa-1 mutants are sensitive to Illudin-M
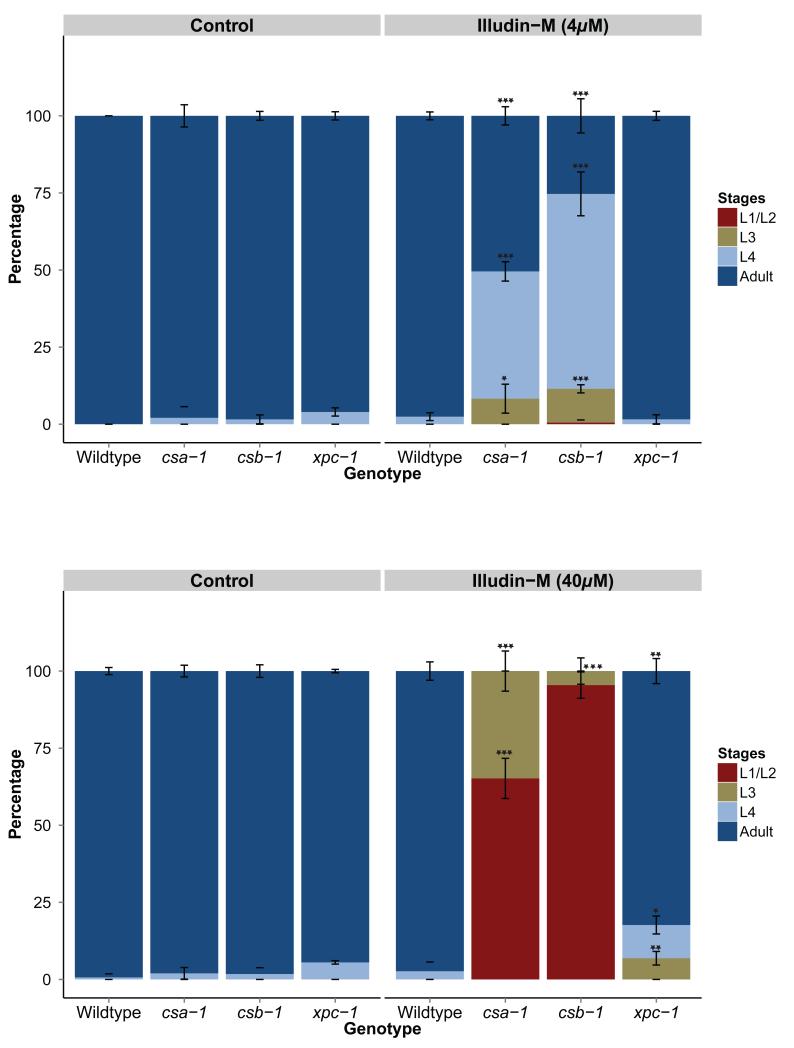
To further elucidate the role of csa-1 in NER, we tested the sensitivity of csa-1 mutant animals to illudins: sesquiterpenoid compounds isolated from the mushroom Omphalotus illudens with tumor-therapeutic properties [20]. Though the nature of the DNA lesions induced by illudins is not clearly understood, previous studies have demonstrated that illudin-induced lesions interfere with transcription and require TC-NER for their removal [21]. We hypothesized that the illudin treatment would induce a developmental arrest in TC-NER deficient worms similar to UV-induced DNA lesions. 40μM illudin-M induced a developmental delay in csa-1 and csb-1 mutant worms and a higher dose (40μM) caused developmental arrest (Figure 3). As expected, the development of the GG-NER deficient xpc-1 mutant strain was only very mildly affected. These results further corroborate a TC-NER-specific function of C. elegans csa-1.
Figure 3. csa-1 mutants are illudin-M sensitive.
Percentage of larval stages 65 hours after 4μM or 40μM illudin-M treatment. Illudin-M induces a developmental delay at 4μM and a developmental arrest at 40μM in csa-1 and csb-1 mutants. A minimum of 40 worms was used for each illudin-M dose and each treatment was conducted in triplicate. Error bars represent SD. *=p<0.05, **=p<0.01, ***=p<0.001, two-tailed t-test, compared to wildtype.
3.5. csa-1 is transcription-coupled NER specific
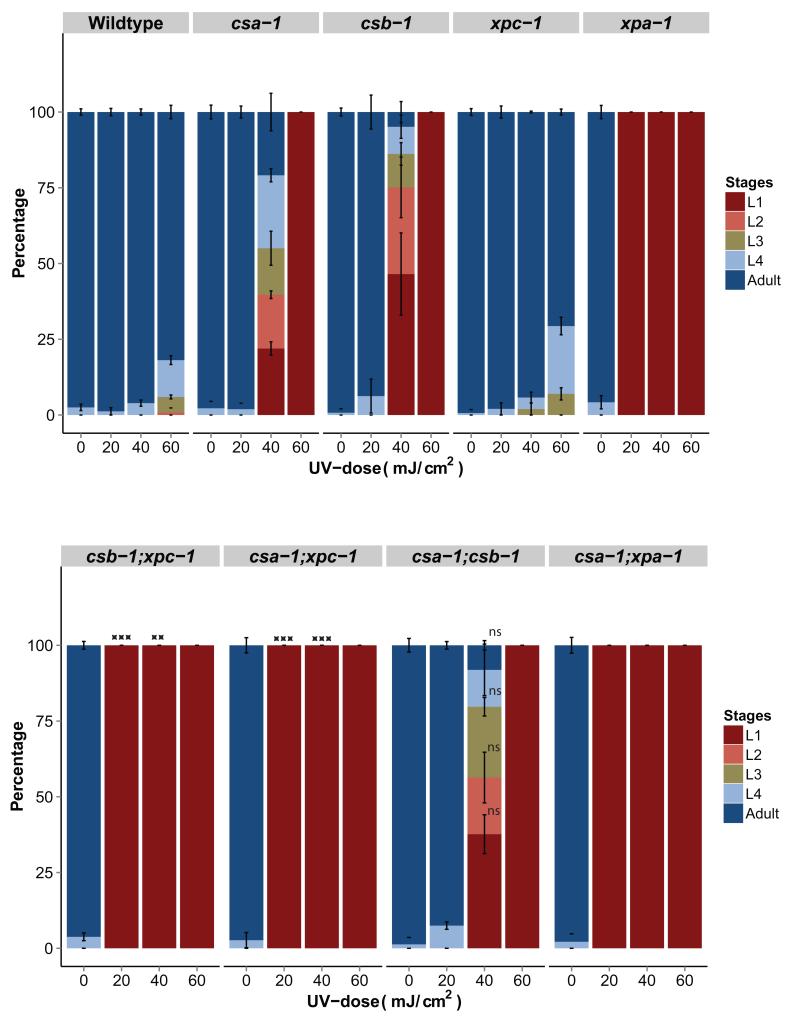
In order to integrate csa-1 into the genetics of NER, we next tested the genetic interactions of csa-1 with TC-NER-deficient csb-1, GG-NER deficient xpc-1 and total NER deficient xpa-1 mutant strains (Figure 4). As expected, csa-1;csb-1 double mutants displayed similar UV-sensitivity as the corresponding single mutants, indicating that csa-1 and csb-1 are epistatic. Likewise, UV-sensitivity of csa-1;xpa-1 was similar to xpa-1, suggesting that csa-1 acts in the same pathway as csb-1 and upstream of xpa-1. UV-sensitivity of csa-1;xpc-1 double mutants was enhanced compared to both single mutants, reminiscent of the UV sensitivity of csb-1;xpc-1 double mutants. This relationship suggests that in csa-1;xpc-1 double mutants, both the NER sub-pathways are compromised, as they are in csb-1;xpc-1 double mutants. Hence, csa-1 is TC-NER-specific and, along with csb-1, acts in parallel to xpc-1 to repair UV-induced DNA lesions.
Figure 4. csa-1 is epistatic to csb-1 and TC-NER specific.
Percentage of larval stages 65 hours after UV-treatment during the L1 larval stage of the indicated genotypes. csa-1;csb-1 double mutants display similar UV-sensitivity as csa-1 and csb-1 single mutants. csa1;xpc-1 double mutants display enhanced UV-sensitivity compared to csa-1 and xpc-1 single mutants. A minimum of 40 worms was used for each UV-dose, and each treatment was conducted in triplicate. Error bars represent SD. ns = not significant, two-tailed t-test, compared to csb-1.
3.6. Discussion
The remarkable complexity of pathologies caused by CSA mutations necessitates the establishment of experimentally tractable multicellular systems for studying NER mutations. Here, we report the identification of a functional homolog of the Cockayne Syndrome gene, CSA, in the metazoan model C. elegans. We demonstrated that csa-1 clusters most closely with budding yeast Rad28. In yeast TC-NER is not required to resistance to UV irradiation [5]; however, TC-NER mutations further aggravate the UV-hypersensitivity of GG-NER mutants. Similarly, TC-NER mutations in mice only give rise to mild CS-like phenotypes, while mutations in Xpc strongly enhance the consequences of Csa and Csb loss [9]. In contrast, most human patients carrying mutations in CSA or CSB develop classical type I CS with severe growth defects and mental retardation and do not survive beyond the second decade of life [4]. Similar to yeast, proliferating cells in C. elegans mostly rely on GG-NER to repair UV-induced lesions. In contrast to unicellular yeast, however, post-mitotic tissues in C. elegans become primarily reliant on TC-NER to withstand UV irradiation. Hence, developing larvae that have completed most of their cell divisions during embryogenesis require csa-1 and csb-1 when challenged with UV. Nonetheless, xpc-1 mutations strongly enhance UV sensitivity of TC-NER mutants during development, arguing for a backup function of GG-NER, similar to the genetic observations in mice. The C. elegans system provides a clear developmental distinction between the utilization of the two NER branches. The availability of csa-1 mutant strains will help to further define the function of TC-NER components during developmental growth and genome integrity in differentiated tissues.
4. Conclusions
NER genes are highly conserved from nematodes to mammals. Intriguingly, the distinct functions of the two NER branches: TC-NER during development and GG-NER in proliferating cell types, is phenotypically consistent in C. elegans. This conservation reflects the distinct pathologies of developmental impairments in CS patients and the genome instability that leads to UV-induced skin cancer development in XP patients. The identification of a functional homolog of csa-1 in C. elegans further establishes the nematode model for investigating the function of TC-NER during development and ageing.
Supplementary Material
Highlights.
C. elegans possesses an orthologue for the Cockayne syndrome complementation group A (CSA) gene
C. elegans csa-1 mutants are sensitive the UV-induced DNA damage during development
Genetically csa-1 is epistatic to csb-1 and mutant csa-1 enhances UV-sensitivity of global-genome NER defective xpc-1 mutants
Acknowledgements
We thank Rainer Schobert for providing illudin-M. We thank Giorgi Berishvili and Berhanu Chekol for their contributions earlier in the project and Ashley B. Williams for critically reading the manuscript. Worm strains were provided by Caenorhabditis Genetics Center (funded by the NIH National Center for Research Resources) and National Bioresource Project. VB received an IGSDHD fellowship. BS acknowledges funding from the Deutsche Forschungsgemeinschaft (CECAD, SFB 670, SFB 829, and KFO 286), the European Research Council (ERC Starting grant 260383), Marie Curie (FP7 ITN CodeAge 316354, aDDRess 316390, MARRIAGE 316964), the German-Israeli Foundation (110468.11/2010), the Deutsche Krebshilfe (109453), and the BMBF (SyBaCol).
Abbreviations
- bp
Base pairs
- 6-4 PP
6-4 photoproduct
- cm
Centimetre
- CPD
Cyclobutane pyrimidine dimer
- CS
Cockayne syndrome
- COFS
Cerebro-oculo-facio-skeletal-syndrome
- GG-NER
Global-genome nucleotide excision repair
- mJ
Milli-Joule
- NER
Nucleotide excision repair
- NGM
Nematode growth medium
- nt
Nucleotide
- ORF
Open reading frame
- RNAP II
RNA polymerase II
- TC-NER
Transcription-coupled nucleotide excision repair
- UTR
Un-translated region
- UV
Ultra-violet
- UVSS
UV-hypersensitivity syndrome
- XP
Xeroderma pigmentosum
Footnotes
Conflict of interest statement: The authors declare no conflict of interests.
References
- [1].Schumacher B, Garinis GA, Hoeijmakers JHJ. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. doi:10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- [2].Cleaver JE, Lam ET, Revet I. Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nat Rev Genet. 2009;10:756–768. doi: 10.1038/nrg2663. doi:10.1038/nrg2663. [DOI] [PubMed] [Google Scholar]
- [3].Hanawalt PC, Spivak G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008;9:958–970. doi: 10.1038/nrm2549. doi:10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- [4].Laugel V, Dalloz C, Durand M, Sauvanaud F, Kristensen U, Vincent MC, et al. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum Mutat. 2010;31:113–126. doi: 10.1002/humu.21154. doi:10.1002/humu.21154. [DOI] [PubMed] [Google Scholar]
- [5].Bhatia PK, Verhage RA, Brouwer J, Friedberg EC. Molecular cloning and characterization of Saccharomyces cerevisiae RAD28, the yeast homolog of the human Cockayne syndrome A (CSA) gene. J. Bacteriol. 1996;178:5977–5988. doi: 10.1128/jb.178.20.5977-5988.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fukumoto Y, Dohmae N, Hanaoka F. Schizosaccharomyces pombe Ddb1 recruits substrate-specific adaptor proteins through a novel protein motif, the DDB-box. Molecular and Cellular Biology. 2008;28:6746–6756. doi: 10.1128/MCB.00757-08. doi:10.1128/MCB.00757-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van der Horst GT, van Steeg H, Berg RJ, van Gool AJ, de Wit J, Weeda G, et al. Defective transcription-coupled repair in Cockayne syndrome B mice is associated with skin cancer predisposition. Cell. 1997;89:425–435. doi: 10.1016/s0092-8674(00)80223-8. [DOI] [PubMed] [Google Scholar]
- [8].van der Horst GTJ, Meira L, Gorgels TGMF, de Wit J, Velasco-Miguel S, Richardson JA, et al. UVB radiation-induced cancer predisposition in Cockayne syndrome group A (Csa) mutant mice. DNA Repair. 2002;1:143–157. doi: 10.1016/s1568-7864(01)00010-6. [DOI] [PubMed] [Google Scholar]
- [9].van der Pluijm I, Garinis GA, Brandt RM, Gorgels TG, Wijnhoven SW, Diderich KE, et al. Impaired genome maintenance suppresses the growth hormone--insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 2007;5:e2. doi: 10.1371/journal.pbio.0050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saijo M. The role of Cockayne syndrome group A (CSA) protein in transcription-coupled nucleotide excision repair. Mech. Ageing Dev. 2013;134:196–201. doi: 10.1016/j.mad.2013.03.008. doi:10.1016/j.mad.2013.03.008. [DOI] [PubMed] [Google Scholar]
- [11].Stirnimann CU, Petsalaki E, Russell RB, Muller CW. WD40 proteins propel cellular networks. Trends Biochem. Sci. 2010;35:565–574. doi: 10.1016/j.tibs.2010.04.003. doi:10.1016/j.tibs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- [12].Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen N, Harris TW, Antoshechkin I, Bastiani C, Bieri T, Blasiar D, et al. WormBase: a comprehensive data resource for Caenorhabditis biology and genomics. Nucleic Acids Res. 2005;33:D383–D389. doi: 10.1093/nar/gki066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- [15].Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. doi:10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mitani S. Nematode, an experimental animal in the national BioResource project. Exp. Anim. 2009;58:351–356. doi: 10.1538/expanim.58.351. [DOI] [PubMed] [Google Scholar]
- [17].Lans H, Marteijn JA, Schumacher B, Hoeijmakers JHJ, Jansen G, Vermeulen W. Involvement of global genome repair, transcription coupled repair, and chromatin remodeling in UV DNA damage response changes during development. PLoS Genet. 2010;6:e1000941. doi: 10.1371/journal.pgen.1000941. doi:10.1371/journal.pgen.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stergiou L, Doukoumetzidis K, Sendoel A, Hengartner MO. The nucleotide excision repair pathway is required for UV-C-induced apoptosis in Caenorhabditis elegans. Cell Death Differ. 2007;14:1129–1138. doi: 10.1038/sj.cdd.4402115. [DOI] [PubMed] [Google Scholar]
- [19].Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Developmental Biology. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- [20].Kelner MJ, McMorris TC, Beck WT, Zamora JM, Taetle R. Preclinical evaluation of illudins as anticancer agents. Cancer Res. 1987;47:3186–3189. [PubMed] [Google Scholar]
- [21].Jaspers NG, Raams A, Kelner MJ, Ng JM, Yamashita YM, Takeda S, et al. Anti-tumour compounds illudin S and Irofulven induce DNA lesions ignored by global repair and exclusively processed by transcription- and replication-coupled repair pathways. DNA Repair. 2002;1:1027–1038. doi: 10.1016/s1568-7864(02)00166-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.