Abstract
Heat shock protein 90 (HSP90) is a molecular chaperone that supports stability of client proteins. We found that HSP90 was cleaved to 55 kDa protein after treatment with histone deacetylase (HDAC) inhibitors including suberoylanilide hydroxamic acid (SAHA) in several leukemia cell lines. We further analyzed molecular changes induced by SAHA in K562 cells. The SAHA-induced cleavage of HSP90 was blocked by a pan-caspase inhibitor, z-VAD-fmk, implying that the process is dependent on caspase activity. However, the experiments using antagonistic and agonistic Fas antibodies revealed that the cleavage of HSP90 was not dependent on Fas signaling. SAHA induced generation of reactive oxygen species (ROS), and the cleavage of HSP90 was blocked by a ROS scavenger N-acetylcystein (NAC). We also confirmed that hydrogen peroxide (H2O2) induced cleavage of HSP90 in a similar manner. Caspase 2, 3, 4, 6, 8, and 10 were activated by treatment with SAHA, and the activities were reduced by the pretreatment of NAC. Treatment of the cells with caspase 10 inhihitor, but not other inhibitors of caspases activated by SAHA, prevented cleavage of HSP90 by SAHA. SAHA-induced ROS generation and HSP90 cleavage were dependent on newly synthesized unknown proteins. Taken together, our results suggest that the cleavage of HSP90 by SAHA is mediated by ROS generation and caspase 10 activation. HSP90 cleavage may provide an additional mechanism involved in anti-cancer effects of HDAC inhibitors.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-014-0533-4) contains supplementary material, which is available to authorized users.
Keywords: Suberoylanilide hydroxamic acid, HSP90, Cleavage, ROS, K562
Introduction
Histone deacetylase (HDAC) inhibitors consist of several structural classes, including the following: short-chain fatty acids, hydroxamic acids, cyclic tetrapeptides containing a 2-amino-8-oxo-9,10-epoxy-decanoyl (AOE) moiety, cyclic peptides not containing the AOE moiety, and benzoamides (Marks et al. 2000). Acetylation/deacetylation of histones is an important process in the regulation of gene expression (Kornberg 1999). HDAC inhibitors induce histone acetylation and thereby induce expression of several genes including those involved in cell cycle arrest and apoptosis (Ruefli et al. 2001; Richon et al. 2000). Notably, HDAC inhibitors showed synergistic or additive effects in blocking proliferation or inducing apoptosis when used in combination with different anti-cancer agents, including radiation therapy, chemotherapy, differentiation agents, epigenetic therapy, and new targeted agents (Dokmanovic et al. 2007). Therefore, HDAC inhibitors gained attention as an anti-cancer agent (Bolden et al. 2006), and at least 12 different HDAC inhibitors are undergoing clinical trials as monotherapy or in combination with retinoids, taxol, gemcitabine, radiation, etc (Dokmanovic et al. 2007; Kelly et al. 2005; O’Connor et al. 2006).
Reactive oxygen species (ROS), an apoptosis inducer, is generated in cells by several pathways. Sources of ROS generation are the mitochondrial electron transport chain, NADPH oxidase family, and metabolic pathways (Hole et al. 2011). Generation of ROS in mitochondria induces apoptosis, which is mediated by regulation of cytochrome c release (Cai and Jones 1998). When cells are exposed to a high dose of ROS, they are triggered to apoptosis. On the other hand, ROS promotes cell growth, survival, and regulation of cellular signaling depending on the concentration (Dypbukt et al. 1994; Kamata and Hirata 1999; Trachootham et al. 2008).
Heat shock proteins are found in most living organisms, and their expression increases when cells are exposed to stress (Welch 1993). Heat shock protein 90 (HSP90), a member of the heat shock protein family, is a molecular chaperone that supports stability of client proteins, such as mutated p53, Bcr-Abl, Raf-1, Akt, HER2/Neu (ErbB2), HIF-1α, etc (Neckers and Workman 2012).
HSP90 forms a flexible dimer, and this structure is important to maintain the ATPase cycle of HSP90 for the chaperone function (Rohl et al. 2013). HSP90 monomer consists of three domains, N-domain, M-domain, and C-domain, and the N-domain has an ATP-binding pocket (Prodromou et al. 1997). ATP binding to the N-domain promotes dimerization of the N-domain, and the hydrolysis of ATP to ADP promotes N-domain dissociation (Richter and Buchner 2001; Prodromou et al. 2000). Co-chaperones, such as Hop, p23, cdc37, PP5, and Xap2, contribute to interaction of the chaperone machinery with HSP90. Co-chaperones interact with HSP90 and control ATPase for HSP90 activation and recruit client proteins to HSP90 (Zuehlke and Johnson 2010; Rohl et al. 2013). As many HSP90 client proteins are necessary for cancer cell survival and proliferation, most cancer cells express higher levels of HSP90 compared with normal cells (Ferrarini et al. 1992; Neckers et al. 1999; Miyata et al. 2013). Furthermore, HSP90 is reported to contribute to malignant transition (Boltze et al. 2003). Therefore, many researchers have recently been studying HSP90 as a target of anti-cancer drugs (Neckers et al. 1999; Modi et al. 2011; Dickson et al. 2013; Miyata et al. 2013).
While the cleavage of HSP90 by stresses such as ultraviolet B irradiation (Chen et al. 2009), ascorbate/menadione-mediated oxidative stress (Beck et al. 2009), and andrographolide-mediated ROS (Liu et al. 2014) was previously reported, effects of HDAC inhibitor on the HSP90 cleavage were never investigated before. In this study, we found for the first time that HSP90 was cleaved after treatment with HDAC inhibitors including suberoylanilide hydroxamic acid (SAHA) in several leukemia cell lines. We also revealed that cleavage of HSP90 by SAHA was mediated by ROS and caspase 10 in K562 cells.
Materials and methods
Cell culture
K562 (chronic myelogenous leukemia) cells were maintained in Iscove’s Modified Dulbecco’s Medium (IMDM, Hyclone, Logan, UT, USA) with 10 % fetal bovine serum (FBS, Hyclone), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C under a humidified atmosphere of 5 % CO2. THP-1 (human acute monocytic leukemia) cells were maintained in Roswell Park Memorial Institute medium (RPMI-1640, Hyclone) with 10 % FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM β-mercaptoethanol. KG1a (human acute myelogenous leukemia) cells were maintained in IMDM with 20 % FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. CT-26 (mouse colorectal carcinoma) cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Hyclone) with 10 % FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. U-937 (human histiocytic lymphoma), RPMI-8226 (human myeloma), HT-29 (human colorectal adenocarcinoma), Huh-7, and SNU-739 (human hepatocellular carcinoma) cells were maintained in RPMI-1640 with 10 % FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were treated with HDAC inhibitors at the indicated concentration for 24 h or for the indicated time period. The cells were irradiated with 6 mJ of UVC when necessary. Inhibitors were pretreated 1 h prior to HDAC treatment, if necessary.
Chemicals and antibodies
N-acetylcystein (NAC), dimethyl sulfoxide (DMSO), SAHA, sodium butyrate (NaB), valproic acid (VPA), trichostatin A (TSA), hydrogen peroxide (H2O2), and z-VAD-fmk were purchased from Sigma-Aldrich (St. Louis, MO, USA). Caspase-family inhibitor set III and z-AEVD-fmk were from BioVision (Milpitas, CA, USA). The monoclonal antibodies anti-HSP90α/β (sc-13119), anti-Bcl-2 (sc-7382), and anti-GAPDH (sc-32233) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The polyclonal antibody anti-Akt (#9272) and anti-HSP90β (#7411) were purchased from Cell Signaling Technology (Beverly, MA, USA). The Fas antagonistic antibody (ZB4) and agonistic antibody (CH11) for Fas were obtained from Millipore (Billerica, MA, USA).
Western blot analysis
Harvested cells were lysed in lysis buffer (pH 8.0, 20 mM Tris-HCl, 137 mM NaCl, 10 % glycerol, 10 mM EDTA, 0.5 % sodium deoxycholate, 0.1 % sodium dodecyl sulfate (SDS), 1 % NP-40, protease inhibitor cocktail, and phosphatase inhibitor). Samples were resolved by SDS-polyacrylamide gel electrophoresis, and electro-transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). The membranes were blocked with 5 % dry milk in phosphate buffered saline-Tween-20 (PBS-T; 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and 0.05 % Tween-20) and probed with an appropriate primary antibody. Immunoreactive proteins were detected by horseradish peroxidase-conjugated anti-rabbit and anti-mouse (Jackson Immunoreseach, West Grove, PA, USA) secondary antibodies and an ECL solution (iNtRon, Seongnam, Korea).
Measurement of intracellular ROS
For measurement of intracellular ROS levels, an oxidation-sensitive fluorescent probe, 2,7-dichlorofluorescein diacetate (DCF-DA) (Molecular Probes, Eugene, OR, USA), was used as described previously (Kim et al. 2009) with minor modification. K562 cells were untreated or treated with 20 mM of NAC for 1 h, followed by treatment with 5 μM of SAHA for 6 or 24 h. After treatment, the cells were incubated with 10 μM of DCF-DA for 20 min at 37 °C. The fluorescence images were obtained under fluorescence microscopy (Nikon, Tokyo, Japan).
Measurement of caspase activity
The cell-permeable Caspase Colorimetric Substrate Set II Plus (BioVision) was used for measurement of caspase activities in intact cells according to the manufactory’s protocol. K562 cells were untreated or treated with 20 mM of NAC for 1 h, followed by incubation with 5 μM of SAHA for 24 h. The cells were then incubated with 200 μM of indicated caspase family inhibitors for 90 min at 37 °C. The absorbance values were obtained from a microplate reader at a wavelength of 400 nm. Absorbance levels were normalized using background values obtained from the cells untreated with the caspase family inhibitors.
Flow cytometry
K562 cells were untreated or treated with 5 or 10 μM of SAHA for 24 h. After blocking with 0.1 % BSA in PBS for 20 min, K562 cells were incubated with anti-Fas antibody (ZB4) for 1 h on ice, followed by incubation with FITC-conjugated anti-mouse secondary antibody (BD, San Jose, CA, USA) for 1 h on ice. The cells were analyzed by flow cytometry using a FACSCalibur (BD). Data analysis was done with the program FCS Express (De Novo Software, Los Angeles, CA, USA).
Results
HDAC inhibitors induce HSP90 cleavage in leukemia cell lines
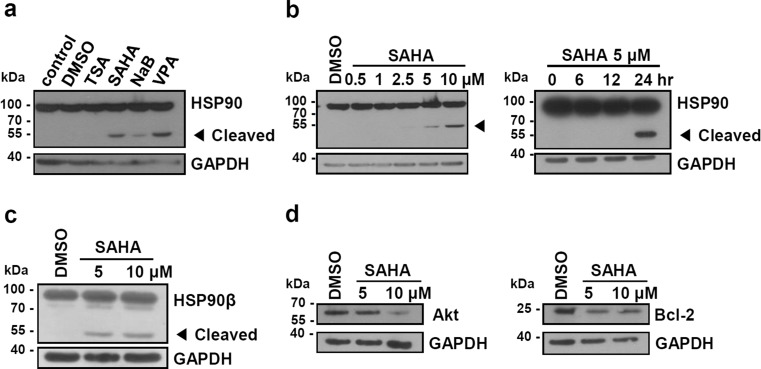
To determine the effect of HDAC inhibitors on HSP90, we treated human leukemia cell line (K562) with indicated doses of HDAC inhibitors (TSA, SAHA, NaB, and VPA). After exposure of cells to HDAC inhibitors for 24 h, we performed Western blot analysis with an anti-HSP90 antibody. K562 cells showed a cleaved form of HSP90 with a molecular weight of approximately 55 kDa after treatment with SAHA, NaB, VPA but not with TSA (Fig. 1a). As the antibody used here recognizes the C-terminal of HSP90, it is likely that the cleavage occurred in the middle domain of HSP90. Cleavage of HSP90 induced by SAHA was increased in a dose- and time-dependent manner (Fig. 1b). NaB and VPA also induced dose- and time-dependent cleavage of HSP90 (data not shown). As SAHA was most effective at a low concentration, we mainly used SAHA for the remaining experiments. Using HSP90β specific antibody, we confirmed that HSP90β was clearly fragmented by the treatment with SAHA (Fig. 1c). Because HSP90 is a molecular chaperone, we examined whether treatment with SAHA affects the stability of HSP90 client proteins such as Bcl-2 and Akt. As shown in Fig 1d, protein levels of Bcl-2 and Akt were decreased in SAHA-treated K562 cells. These data show that cleavage of HSP90 induced by HDAC inhibitor correlates with the reduced activity of HSP90 as a molecular chaperone, which is in agreement with previous reports (Liu et al. 2014).
Fig. 1.
HDAC inhibitors induce cleavage of HSP90. a K562 cells were treated with HDAC inhibitors (250 nM TSA, 5 μM SAHA, 4 mM NaB, 4 mM VPA) for 24 h. b K562 cells were treated with the indicated doses of SAHA for 24 h and with 5 μM SAHA during the indicated periods. c, d K562 cells were treated with the indicated dose of SAHA for 24 h. The cell lysates were subjected to Western blot analysis using anti-HSP90, anti-HSP90β, anti-Bcl-2, anti-Akt, and anti-GAPDH antibodies. Amounts of GAPDH protein are shown as a loading control. Cleaved HSP90 fragment is indicated by an arrowhead
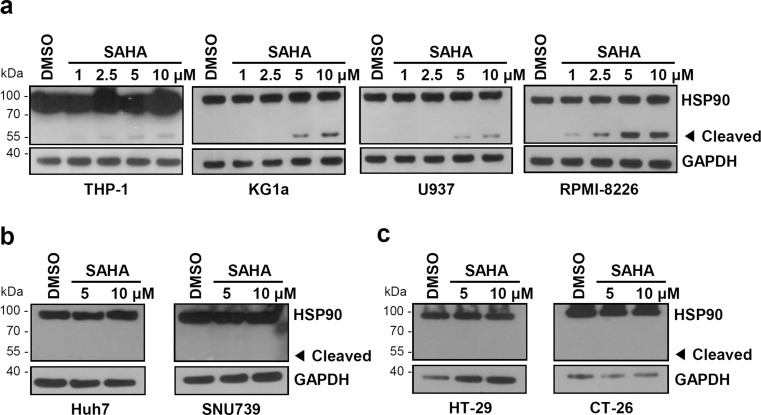
To determine whether this phenomenon is general in several cells, we performed the same experiments using SAHA and various cell lines. THP-1, KG1a, U-937, and RPMI-8226 are leukemia cell lines. Huh-7 and SNU-739 are human hepatocellular carcinoma cell lines. HT-29 and CT-29 are human and mouse colorectal adenocarcinoma cell lines, respectively. In SAHA-treated leukemia cell lines (THP-1, KG1a, U-937, and RPMI-8226), we detected cleavage of HSP90 in a SAHA dose-dependent manner (Fig. 2a). However, there was no change of HSP90 in other cells (Huh-7, SNU-739, HT-29, and CT-26) (Figs. 2b, c). These data suggest that cleavage of HSP90 in response to the HDAC inhibitor may be different depending on the cell types and that the HSP90 cleavage might occur preferentially or sensitively in leukemia cells.
Fig. 2.
Effect of SAHA on the cleavage of HSP90 in several cell lines. a Human leukemia cell lines, b human hepatocellular carcinoma cell lines, c human (HT-29) and mouse (CT-26) colon cancer cell lines. Cells were treated with SAHA at the indicated concentrations for 24 h and analyzed by Western blot analysis
SAHA-induced cleavage of HSP90 is caspase-dependent and Fas-independent
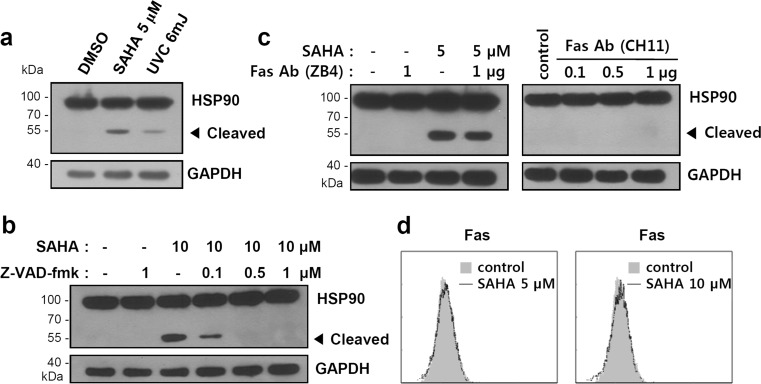
Previously, it was reported that ultraviolet (UV) irradiation creates a fragment of HSP90 with a molecular weight of approximately 55 kDa through caspase-mediated cleavage (Chen et al. 2009). We treated K562 cells with either UV or SAHA and then compared the size of the HSP90 cleavage product. As shown in Fig. 3a, both HDAC inhibitors and UV generate a HSP90 fragment of similar size. Pretreatment of K562 cells with the pan-caspase inhibitor z-VAD-fmk inhibited cleavage of HSP90 in a dose-dependent manner, revealing that the cleavage of HSP90 by SAHA is also caspase-dependent (Fig. 3b).
Fig. 3.
Cleavage of HSP90 by SAHA is mediated by caspase but not by the Fas/Fas ligand pathway. a K562 cells were treated with SAHA (5 μM) for 24 h or irradiated with UV (6 mJ), followed by incubation for 6 h. b K562 cells were untreated or treated with indicated doses of z-VAD-fmk for 1 h, followed by treatment with SAHA (10 μM) for 24 h. c K562 cells were treated with SAHA (5 μM) for 24 h in the presence or absence of prior treatment with the indicated doses of Fas neutralizing antibody (ZB4) for 2 h (left) or in the presence of the indicated dose of Fas activating antibody (CH11) for 24 h (right). The cell lysates were subjected to Western blot analysis. d K562 cells were treated with 5 or 10 μM of SAHA for 24 h, and Fas protein expression was analyzed by immunostaining with anti-Fas antibody (ZB4) and flow cytometry
Cleavage of HSP90 is also known to be mediated by Fas signaling (Chen et al. 2009). We then investigated whether the inhibition of Fas signaling using Fas antagonistic antibody (ZB4) can reduce HSP90 cleavage induced by SAHA. However, there was no prominent effect. Agonistic antibody (CH11) for Fas also did not induce HSP90 cleavage (Fig. 3c). Evaluation of Fas expression in the presence or absence of HDAC treatment revealed no significant changes (Fig. 3d). These results suggest that treatment with HDAC inhibitor induces activation of caspase, leading to cleavage of HSP90 independently of Fas signaling.
ROS generation by SAHA promotes HSP90 cleavage
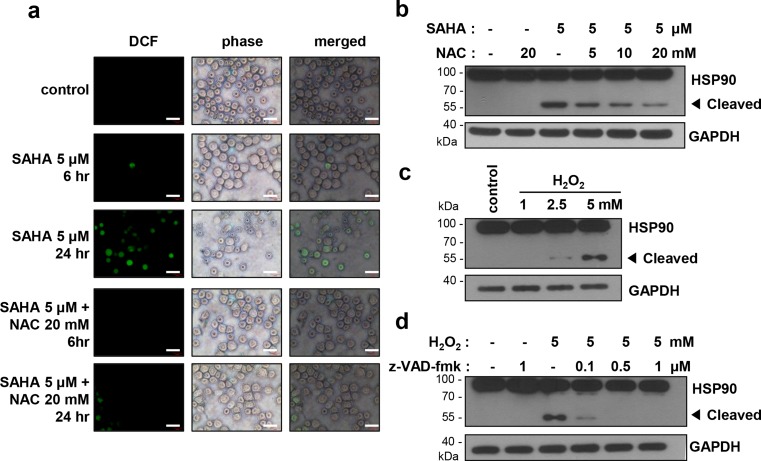
Activation of the caspase cascade is triggered by many factors, such as granzyme B, death receptors, apoptosome, or stresses. Oxidative stress is a caspase activator. SAHA was reported to induce apoptosis through activation of ROS-mediated caspase (Ruefli et al. 2001; Kim and Chung 2007), and oxidative stress was reported to induce HSP90 cleavage leading to degradation of client proteins and cell apoptosis (Beck et al. 2009; Liu et al. 2014). Considering our finding that SAHA induces caspase-dependent HSP90 cleavage (Fig. 3b), it is possible that SAHA-induced ROS may contribute to HSP90 cleavage. Therefore, we first confirmed ROS generation after treatment with SAHA. K562 cells were treated with 5 μM SAHA for 6 and 24 h, and the intracellular ROS level was estimated using a ROS fluorescent probe DCF-DA. As shown in Fig. 4a, the ROS level was increased by treatment with SAHA for 24 h, and ROS generation was blocked by pretreatment with a ROS scavenger, NAC.
Fig. 4.
ROS generated by SAHA induces caspase-mediated cleavage of HSP90. a K562 cells were untreated or treated with 20 mM of NAC for 1 h, followed by treatment with SAHA (5 μM) for 6 and 24 h. The cells were incubated with 10 μM fluorescent probe DCF-DA for 20 min and visualized using fluorescence microscopy. Scale bar: 40 μM. b K562 cells were untreated or treated with indicated doses of NAC for 1 h, followed by treatment with SAHA (5 μM) for 24 h. c K562 cells were treated with the indicated dose of H2O2 for 24 h. d K562 cells were untreated or treated with indicated doses of z-VAD-fmk for 1 h, followed by treatment with H2O2 (5 mM) for 24 h. The cell lysates were subjected to Western blot analysis
To further determine whether ROS, generated by SAHA, induces cleavage of HSP90, we treated K562 cells with 5 μM SAHA after pretreatment with NAC and checked cleavage of HSP90. As shown in Fig. 4b, HSP90 cleavage was reduced in the presence of NAC in a dose-dependent manner. Furthermore, when the cells were treated with a typical ROS generating agent hydrogen peroxide (H2O2), cleavage of HSP90 resulting in the same molecular weight fragment was detected in a concentration-dependent manner (Fig. 4c). The cleavage was reduced in the presence of z-VAD-fmk (Fig. 4d), confirming that the process is caspase-dependent. These data suggest that SAHA-induced generation of ROS activates caspase leading to the cleavage of HSP90.
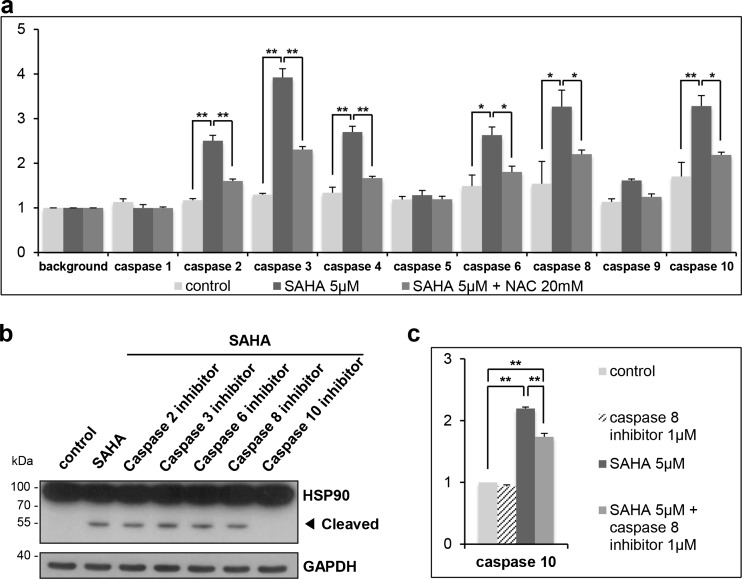
HSP90 cleavage is occurred by caspase 10 activation by SAHA
As we confirmed that HSP90 cleavage is mediated by caspases (Fig. 3b), we next aimed to determine which members of caspase are involved in the cleavage of HSP90. First, we measured the activities of each caspase family members in SAHA-treated K562 cells using cell-permeable substrates (Fig. 5a). The activities of caspase 2, 3, 4, 6, 8, and 10 were increased by SAHA, and the activities were reduced by the pretreatment of NAC. Next, we checked HSP90 cleavage by SAHA in the presence of caspase family-specific inhibitors. As shown in Fig. 5b, pretreatment with caspase 10 inhibitor completely abolished SAHA-mediated HSP90 cleavage. As caspase 8 is known to be an upstream regulator of caspase 10, we expected that caspase 8 inhibitor also prevents HSP90 cleavage. However, our data clearly showed that the pretreatment with caspase 8 inhibitor did not inhibit SAHA-mediated HSP90 cleavage. As shown in Fig. 5c, caspase 10 activity increased by SAHA was only partially reduced by caspase 8 inhibitor. It suggests that caspase 10 is activated by SAHA in both caspase 8 dependent and independent manners as reported previously (Wang et al. 2001). Taken together, these data suggest that SAHA-induced HSP90 cleavage is occurred by caspase 10.
Fig. 5.
SAHA-induced cleavage of HSP90 is mediated by caspase 10. a K562 cells were untreated or treated with 20 mM of NAC for 1 h, followed by treatment with SAHA (5 μM) for 24 h. The cell lysates were incubated with indicated colorimetric caspase substrates, and color development was measured at a wavelength of 400 nm. The caspase activity values were normalized with respective background values. b K562 cells were untreated or treated 1 μM of caspase family inhibitors for 1 h, followed by treatment with SAHA (5 μM) for 24 h. The cell lysates were subjected to Western blot analysis. c K562 cells were untreated or treated with 1 μM of caspase 8 inhibitor for 1 h and then treated with 5 μM of SAHA for 24 h. The cell lysates were incubated with caspase 10 colorimetric substrate to measure the activity of caspase 10. The caspase activity values were normalized with control value. *P < 0.05, **P < 0.01. Caspase 2 inhibitor, z-VDVAD-fmk; caspase 3 inhibitor, z-DEVD-fmk; caspase 6 inhibitor, z-VEID-fmk; caspase 8 inhibitor, z-IETD-fmk; caspase 10 inhibitor, z-AEVD-fmk
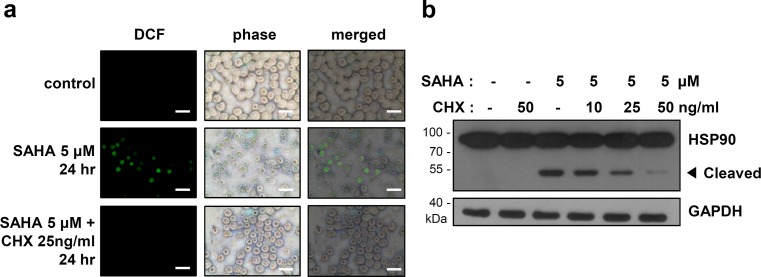
SAHA-induced ROS generation of cleavage of HSP90 needs newly synthesized proteins
Because HDAC inhibitors regulate gene expression through increment of histone acetylation levels (Kornberg 1999), we postulated that new gene expression and protein synthesis can mediate ROS generation and the cleavage of HSP90. K562 cells were pretreated with or without a translation inhibitor cycloheximide (CHX) for 1 h and then treated with SAHA for 24 h. SAHA-induced ROS generation was reduced by the pretreatment with CHX (Fig. 6a). Furthermore, cleavage of HSP90 was also reduced in a dose-dependent manner without a prominent effect on the amount of full-length HSP90 (Fig. 6b). These results suggest that newly synthesized unknown proteins are involved in the SAHA-induced ROS generation and cleavage of HSP90.
Fig. 6.
SAHA-induced cleavage of HSP90 needs newly synthesized proteins. a–b K562 cells were untreated or treated with the indicated doses of cycloheximide (CHX) for 1 h, followed by treatment with SAHA (5 μM). a The cells were incubated with 10 μM fluorescent probe DCF-DA for 20 min and visualized using fluorescence microscopy. Scale bar: 40 μM. b The cell lysates were subjected to Western blot analysis
Discussion
HSP90 is a molecular chaperone that supports proper folding and stability of its client proteins. Most cancer cells express high levels of HSP90 because many of the client proteins of HSP90 are necessary for survival and proliferation of cancer cells (Ferrarini et al. 1992; Neckers et al. 1999; Miyata et al. 2013). Therefore, stimuli that induce cleavage of HSP90 may block the molecular function of HSP90 and result in degradation of the client proteins and increase of apoptosis in cancer cells. Here, we found that HDAC inhibitors induce cleavage of HSP90 in leukemic cells and suggest that cleavage of HSP90 occurs through ROS and caspase 10 using a HDAC inhibitor, SAHA in K562 cells.
UV irradiation (Chen et al. 2009) and HDAC inhibitors (this study) generate a degradation product of HSP90 with a molecular weight of approximately 55 kDa through caspase-mediated cleavage of HSP90 in K562 cells (Fig. 3). HSP90 have two isoforms in cytosol, HSP90α and HSP90β. Previously, it was shown that HSP90β, but not HSP90α, was cleaved to approximately 55 kDa by UVB (Chen et al. 2009). In this study, we confirmed that HDAC inhibitor also induced cleavage of HSP90β. Whether HSP90α is cleaved or not by HDAC inhibitors is to be defined. Another type of HSP90 cleavage was reported to occur in response to oxidative stress induced by ascorbate/menadione (Asc/Men) in K562 cells. In this case, both HSP90 isoform-derived fragments with a molecular weight of approximately 70 kDa were generated by different mechanisms involving chemical degradation by ROS (Beck et al. 2009; Beck et al. 2012). Recently, it was reported that a diterpenoid compound andrographolide induced cleavage of HSP90α to produce a fragment of 50 kDa (Liu et al. 2014). Therefore, it is likely that oxidative stress induces HSP90 cleavage with different isoform specificities and cleavage products depending on the specific types of oxidative stress. Furthermore, HDAC inhibitors induced cleavage of HSP90 in all of the tested leukemia cell lines, but not in HCC (Huh-7 and SNU-739) and colon cancer (CT-26 and HT-29) cell lines we tested (Fig. 2). Although we need more information in other cell types, our results supports that HSP90 cleavage may be dependent on specific cell types.
Caspase activation occurs through various pathways, such as mitochondria, death receptor, ER, and a granzyme B-mediated pathway (Wang et al. 2005). HDAC inhibitors are known to activate caspase by mitochondria or death receptor-mediated pathways (Cheong et al. 2003; Shao et al. 2004; Gillenwater et al. 2007; Kwon et al. 2002). It was also reported that UVB irradiation induces activation of caspase 8 and 10 through the Fas/Fas ligand axis, leading to cleavage of HSP90 and cell apoptosis (Chen et al. 2009). As the HDAC inhibitor apicidin was reported to induce expression of the Fas/Fas ligand in leukemia cells (Kwon et al. 2002), we examined expression of Fas in SAHA-treated K562 cells. However, Fas expression was not affected by SAHA. Furthermore, Fas activating or neutralizing antibodies did not affect cleavage of HSP90 (Fig. 3). We therefore conclude that the Fas/Fas ligand axis is not involved in HSP90 cleavage induced by SAHA.
There have been reports showing that a HDAC inhibitor induces ROS generation and caspase activation (Ruefli et al. 2001; Kim and Chung 2007). Generation of ROS induced by a HDAC inhibitor leads to activation of caspase and triggers apoptosis in various types of cancer cells through an extrinsic or intrinsic pathway (Ruefli et al. 2001; Kim and Chung 2007). On the other hand, it was also reported that oxidative stress induces HSP90 cleavage (Beck et al. 2009). Therefore, we predicted that a HDAC inhibitor would affect HSP90 cleavage and our results supported this. Cleavage of HSP90 was induced by HDAC inhibitors except TSA (Fig. 1). Reportedly, TSA does not induce ROS generation, although it is a potent HDAC inhibitor (Kang et al. 2004). The absence of cleavage of HSP90 by TSA therefore reinforces our conclusion. To further confirm the axis of HDAC inhibitor/ROS/HSP90 cleavage, we investigated the effect of SAHA in K562 cells in detail and confirmed that HSP90 cleavage induced by SAHA was ROS-dependent and caspase 10-dependent. The results showing that SAHA did not induce generation of ROS (data not shown) or cleavage of HSP90 (Fig. 2) in HCC and colon cancer cell lines may support our notion.
SAHA-induced HSP90 cleavage needs newly synthesized unknown protein(s) (Fig. 6). Vitamin D upregulated protein-1 (VDUP-1) is one of the candidates. SAHA is previously reported to upregulate the expression of the VDUP-1 gene (Butler et al. 2002), and VDUP-1 is a negative regulator of thioredoxin (Trx), which acts as an antioxidant (Junn et al. 2000). Therefore, VDUP-1 may contribute to SAHA-induced ROS generation, which is to be determined in the future.
Acetylation or deacetylation of K294(HSP90α)/K287(HSP90β) in M-domain regulates HSP90 function (Bali et al. 2005; Scroggins et al. 2007; Nishioka et al. 2008). K294α/K287β of HSP90 is deacetylated by histone deacetylase 6 (HDAC6), and thereby, HSP90 can act as a molecular chaperone. HDAC1 also contributes to deacetylation and stabilization of HSP90 (Nishioka et al. 2008). HDAC inhibitors therefore can also contribute to apoptosis through acetylation and inactivation of HSP90 function. Acetylation of K294α/K287β in M-domain decreases the affinity of HSP90 to interact with client proteins and co-chaperones. Therefore, client proteins of HSP90 undergo degradation, and cell apoptosis is triggered (Scroggins et al. 2007; Bali et al. 2005). In our data, SAHA induced cleavage of HSP90 producing 55-kDa fragment which can be detected with an antibody recognizing C-terminal epitope. This result suggest that the cleavage site is in the middle of M-domain which removes the N-terminal ATP-binding motif of HSP90 as previously reported in the cases of UV-induced or andrographolide-induced HSP90 cleavage (Chen et al. 2009; Liu et al. 2014). It is also true that the amount of cleaved HSP90 is definitely small compared to that of intact HSP90. Therefore, it is likely that the cleavage of HSP90 results in defect of molecular chaperone activity, contributing to reduced protein levels of client proteins, at least in part (Fig. 1d). In conclusion, we suggest that cleavage of HSP90 can be an additional mechanism involved in HDAC inhibitor-induced apoptosis in leukemic cells.
Electronic supplementary material
(DOCX 4736 kb)
Acknowledgements
This research was supported by grants from the National Research Foundation (2012R1A2A2A01009887, 2011-0011742) funded by the Ministry of Education, Science and Technology in the Republic of Korea.
References
- Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280(29):26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- Beck R, Verrax J, Gonze T, Zappone M, Pedrosa RC, Taper H, Feron O, Calderon PB. Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem Pharmacol. 2009;77(3):375–383. doi: 10.1016/j.bcp.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Beck R, Dejeans N, Glorieux C, Creton M, Delaive E, Dieu M, Raes M, Leveque P, Gallez B, Depuydt M, Collet JF, Calderon PB, Verrax J. Hsp90 is cleaved by reactive oxygen species at a highly conserved N-terminal amino acid motif. PLoS One. 2012;7(7):e40795. doi: 10.1371/journal.pone.0040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Boltze C, Lehnert H, Schneider-Stock R, Peters B, Hoang-Vu C, Roessner A. HSP90 is a key for telomerase activation and malignant transition in pheochromocytoma. Endocrine. 2003;22(3):193–201. doi: 10.1385/ENDO:22:3:193. [DOI] [PubMed] [Google Scholar]
- Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99(18):11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273(19):11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- Chen H, Xia Y, Fang D, Hawke D, Lu Z. Caspase-10-mediated heat shock protein 90 beta cleavage promotes UVB irradiation-induced cell apoptosis. Mol Cell Biol. 2009;29(13):3657–3664. doi: 10.1128/MCB.01640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JW, Chong SY, Kim JY, Eom JI, Jeung HK, Maeng HY, Lee ST, Min YH. Induction of apoptosis by apicidin, a histone deacetylase inhibitor, via the activation of mitochondria-dependent caspase cascades in human Bcr-Abl-positive leukemia cells. Clin Cancer Res. 2003;9(13):5018–5027. [PubMed] [Google Scholar]
- Dickson MA, Okuno SH, Keohan ML, Maki RG, D’Adamo DR, Akhurst TJ, Antonescu CR, Schwartz GK. Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann Oncol. 2013;24(1):252–257. doi: 10.1093/annonc/mds275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5(10):981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- Dypbukt JM, Ankarcrona M, Burkitt M, Sjoholm A, Strom K, Orrenius S, Nicotera P. Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells. The role of intracellular polyamines. J Biol Chem. 1994;269(48):30553–30560. [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51(4):613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- Gillenwater AM, Zhong M, Lotan R. Histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis through both mitochondrial and Fas (Cd95) signaling in head and neck squamous carcinoma cells. Mol Cancer Ther. 2007;6(11):2967–2975. doi: 10.1158/1535-7163.MCT-04-0344. [DOI] [PubMed] [Google Scholar]
- Hole PS, Darley RL, Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood. 2011;117(22):5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164(12):6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11(1):1–14. doi: 10.1016/S0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Kang J, Chen J, Zhang D, Da W, Ou Y. Synergistic killing of human leukemia cells by antioxidants and trichostatin A. Cancer Chemother Pharmacol. 2004;54(6):537–545. doi: 10.1007/s00280-004-0845-7. [DOI] [PubMed] [Google Scholar]
- Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23(17):3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BM, Chung HW. Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem Biophys Res Commun. 2007;363(3):745–750. doi: 10.1016/j.bbrc.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kim D, Lee Y, Choi SY, Park J, Lee SY, Park JW, Kwon HJ. Effects of nanoparticulate saponin-platinum conjugates on 2,4-dinitrofluorobenzene-induced macrophage inflammatory protein-2 gene expression via reactive oxygen species production in RAW 264.7 cells. BMB Rep. 2009;42(5):304–309. doi: 10.5483/BMBRep.2009.42.5.304. [DOI] [PubMed] [Google Scholar]
- Kornberg RD. Eukaryotic transcriptional control. Trends Cell Biol. 1999;9(12):M46–49. doi: 10.1016/S0962-8924(99)01679-7. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Ahn SH, Kim YK, Bae GU, Yoon JW, Hong S, Lee HY, Lee YW, Lee HW, Han JW. Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J Biol Chem. 2002;277(3):2073–2080. doi: 10.1074/jbc.M106699200. [DOI] [PubMed] [Google Scholar]
- Liu SH, Lin CH, Liang FP, Chen PF, Kuo CD, Alam MM, Maiti B, Hung SK, Chi CW, Sun CM, Fu SL. Andrographolide downregulates the v-Src and Bcr-Abl oncoproteins and induces Hsp90 cleavage in the ROS-dependent suppression of cancer malignancy. Biochem Pharmacol. 2014;87(2):229–242. doi: 10.1016/j.bcp.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92(15):1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19(3):347–365. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D’Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W, Patil S, Norton L, Hannah AL, Hudis C. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17(15):5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- Neckers L, Workman P. Hsp90 molecular chaperon inhibitors: are we there yet? Clin Cancer Res. 2012;18(1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L, Mimnaugh E, Schulte TW. Hsp90 as an anti-cancer target. Drug Resist Updat. 1999;2(3):165–172. doi: 10.1054/drup.1999.0082. [DOI] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Takeuchi S, Koeffler HP, Yokoyama A. MS-275, a novel histone deacetylase inhibitor with selectivity against HDAC1, induces degradation of FLT3 via inhibition of chaperone function of heat shock protein 90 in AML cells. Leuk Res. 2008;32(9):1382–1392. doi: 10.1016/j.leukres.2008.02.018. [DOI] [PubMed] [Google Scholar]
- O’Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, MacGregor-Cortelli B, Curly T, Moskowitz C, Portlock C, Horwitz S, Zelenetz AD, Frankel S, Richon V, Marks P, Kelly WK. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006;24(1):166–173. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90(1):65–75. doi: 10.1016/S0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Panaretou B, Chohan S, Siligardi G, O’Brien R, Ladbury JE, Roe SM, Piper PW, Pearl LH. The ATPase cycle of Hsp90 drives a molecular 'clamp' via transient dimerization of the N-terminal domains. EMBO J. 2000;19(16):4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97(18):10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188(3):281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- Röhl A, Rohrberg J, Buchner J. The chaperone Hsp90: changing partners for demanding clients. Trends Biochem Sci. 2013;38(5):253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci U S A. 2001;98(19):10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25(1):151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2004;101(52):18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci U S A. 2001;98(24):13884–13888. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol Int. 2005;29(7):489–496. doi: 10.1016/j.cellbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Welch WJ. How cells respond to stress. Sci Am. 1993;268(5):56–64. doi: 10.1038/scientificamerican0593-56. [DOI] [PubMed] [Google Scholar]
- Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93(3):211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 4736 kb)