Abstract
Chronic glutamine supplementation reduces exercise-induced intestinal permeability and inhibits the NF-κB pro-inflammatory pathway in human peripheral blood mononuclear cells. These effects were correlated with activation of HSP70. The purpose of this paper is to test if an acute dose of oral glutamine prior to exercise reduces intestinal permeability along with activation of the heat shock response leading to inhibition of pro-inflammatory markers. Physically active subjects (N = 7) completed baseline and exercise intestinal permeability tests, determined by the percent ratio of urinary lactulose (5 g) to rhamnose (2 g). Exercise included two 60-min treadmill runs at 70 % of VO2max at 30 °C after ingestion of glutamine (Gln) or placebo (Pla). Plasma levels of endotoxin and TNF-α, along with peripheral blood mononuclear cell (PBMC) protein expression of HSP70 and IκBα, were measured pre- and post-exercise and 2 and 4 h post-exercise. Permeability increased in the Pla trial compared to that at rest (0.06 ± 0.01 vs. 0.02 ± 0.018) and did not increase in the Gln trial. Plasma endotoxin was lower at the 4-h time point in the Gln vs. 4 h in the Pla (6.715 ± 0.046 pg/ml vs. 7.952 ± 1.11 pg/ml). TNF-α was lower 4 h post-exercise in the Gln vs. Pla (1.64 ± 0.09 pg/ml vs. 1.87 ± 0.12 pg/ml). PBMC expression of IkBα was higher 4 h post-exercise in the Gln vs. 4 h in the Pla (1.29 ± 0.43 vs. 0.8892 ± 0.040). HSP70 was higher pre-exercise and 2 h post-exercise in the Gln vs. Pla (1.35 ± 0.21 vs. 1.000 ± 0.000 and 1.65 ± 0.21 vs. 1.27 ± 0.40). Acute oral glutamine supplementation prevents an exercise-induced rise in intestinal permeability and suppresses NF-κB activation in peripheral blood mononuclear cells.
Keywords: Exercise, HSP70, Intestinal permeability
Introduction
Glutamine is the most abundant amino acid in the body and serves important roles in healthy immune and gastrointestinal function. Recently, we have shown that chronic glutamine supplementation (7 days) may serve two roles under conditions of intense exercise: (1) prevention of exercise-induced gastrointestinal permeability through stabilization of the epithelial tight junction protein complex and (2) suppression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pro-inflammatory pathway in peripheral blood mononuclear cells (PBMCs) (Zuhl et al. 2014). These responses were associated with upregulation of the heat shock protein pathway in both intestinal epithelial cells (Caco-2) and human PBMCs (Dokladny et al. 2013; Zuhl et al. 2014). Glutamine supplementation prior to exercise and treatment in clinical populations has been extensively researched; however, the effect of an acute dosage prior to stress on GI and immune function has never been reported. Glutamine has been supplemented several days or weeks prior to medical treatment such as bone marrow transplant (Ziegler 2002) or chemotherapy (Jensen et al. 1994) to prevent immune suppression (Parry-Billings et al. 1990). In addition, it has been given to burn patients (Parry-Billings et al. 1990) to prevent sepsis and has been tested in patients post joint replacement surgery to counteract muscle atrophy and myopathies (Blomqvist et al. 1995; Burnham et al. 2005). In exercise studies, glutamine has been supplemented during recovery from marathon running (Castell 2003; Castell and Newsholme 1997) and in weeks or months leading up to an endurance event (Hiscock and Mackinnon 1998). The benefits include decreased reports of infection (Castell et al. 1996), inflammatory cytokines (Cruzat et al. 2010), and intestinal permeability (Lambert et al. 2001), along with an increase in anti-inflammatory cytokines (IL-2, IL-1Ra, IL-6) (Castell 2003; Hiscock and Mackinnon 1998). However, Lambert et al. (2001) is the only known study that has examined the acute effects of glutamine on intestinal permeability, but glutamine was tested in combination with a carbohydrate supplement, and not independently. In addition, the Lambert group combined exercise with aspirin ingestion in their model, which may have evoked added stress (Lambert et al. 2001).
In leukocytes, glutamine provides nutritional support and cell maintenance (Newsholme et al. 1985) which improves the immune response to illness and systemic inflammation. During an immunological challenge, glutaminase activity increases, which catalyzes the metabolism of glutamine to glutamate and ammonia (Ardawi and Newsholme 1983). Glutamine as a metabolic substrate and Krebs cycle intermediary is required for T cell proliferation and proper function (Carr et al. 2010). Removing glutamine from leukocyte culture reduced the ability of cells to produce cytokines in response to bacterial stimulus (LPS) (Yaqoob and Calder 1998). Conversely, the addition of glutamine prior to exercise has been shown to reduce pro-inflammatory cytokines. The mechanism of cytokine inhibition may be through activation of the heat shock response leading to inactivation of the NF-κB inflammatory pathway (Dokladny et al. 2010; Paimela et al. 2011). In this model, upregulation of heat shock factor-1 (HSF-1) and heat shock protein 70 or HSP70 (HSPA1A/B) (Kampinga et al. 2009) prevented phosphorylation of nuclear factor of kappa-light-polypeptide gene enhancer in B cells inhibitor, alpha (IκBα) leading to cytosolic housing of NF-κB (Dokladny et al. 2010; Ohno et al. 2011). We have previously demonstrated that 7 days of glutamine supplementation upregulated HSP70 and increased de-phosphorylated IκBα in PBMCs, along with preventing intestinal permeability in response to exercise. However, the effects of an acute glutamine dosage on HSP70 regulation in immune cells and intestinal permeability are not known. It is important to note that total endogenous levels of HSP70 protein were measured in these previous studies with no isoform specificity.
Therefore, in this study, we assessed whether or not an acute oral glutamine dosage prior to vigorous exercise inhibits exercise-induced intestinal permeability. In addition, we investigated if acute glutamine supplementation upregulates PBMC expression of HSP70 and upstream regulators (IκBα) of the NF-κB pathway in response to exercise.
Materials and methods
Human protocol
Subjects
The present study was approved by the Human Research Review Committee of the University of New Mexico, Albuquerque, USA. Seven endurance-trained adult men (n = 2) and women (n = 5) aged 18–45 years volunteered for the present study. All subjects completed a health questionnaire, and procedures, discomforts, and risks were discussed before written informed consent was obtained. Subjects were excluded if taking medications (NSAIDs, antidepressants, or diuretics) or nutritional supplements. All testing was performed in the Exercise Laboratory at the University of New Mexico at 1,585 m of altitude. Data collection occurred during the winter months (January–March), and all participants were residents of Albuquerque, NM.
Experimental design
Using a double-blinded research design, each subject participated in baseline testing and both a glutamine (Gln) and placebo (Pla) trial. The two trials were separated by a 4-week washout period. During the first visit to the lab, baseline measurements (maximal oxygen consumption, body composition, and intestinal permeability) were determined for each subject. Afterwards, subjects were provided a supplement bag containing one dose of glutamine or placebo. After an overnight fast, subjects reported at 7 a.m. on the day of each exercise trial. Height and nude weight were measured, and a rectal thermistor (YSI Precision Series 4400; Yellow Springs Instruments Co., Yellow Springs, OH, USA) was inserted for core temperature measurement. After 20 min of seated rest, a 20-ml blood sample was taken to measure baseline plasma levels of endotoxin, tumor necrosis factor alpha (TNF-α), and glutamine, along with PBMC levels of HSP70 and IκBα. Each exercise trial consisted of a 60-min treadmill run at 70 % of VO2max in an environmental chamber (custom-made structure) set at 30 °C (Siemens Temperature Sensor, Siemens Corporation, Washington, D.C., USA) and a humidity range of 12–20 % (ambient humidity). Oxygen consumption was sampled every 5 min throughout the trial using a metabolic cart to control for exercise intensity. Trials were terminated early if subjects reached a core temperature of 40 °C. Twenty minutes into the exercise trial, a 50-ml sugar probe solution (5 g lactulose, 2 g rhamnose) was consumed for measurement of intestinal permeability. Subjects consumed water ad libitum during and after each trial; however, drinking patterns were not recorded. Seated, posture-controlled venous blood samples were taken immediately post- and 2 and 4 h post-exercise for plasma measurements. In addition, urine was collected for 5 h post-exercise for measurement of intestinal permeability by quantifying the levels of lactulose and rhamnose. One month later, subjects returned and performed the second exercise trial using the identical protocol.
Maximal oxygen consumption (VO2max)
All tests were performed on a belt-driven treadmill (model C966; Precor, Woodinville, WA, USA). After a brief warm-up, subjects were connected to a metabolic cart (True One; Parvomedics, Sandy, UT, USA) for the continuous measurement of oxygen consumption and carbon dioxide production. During each test, either speed or grade was increased every minute until subjects reached volitional fatigue. The goal of the test was for subjects to reach maximal effort within 8–10 min (Yoon et al. 2007). Maximal oxygen consumption was determined based on the highest value achieved using an 11-breath average.
Body composition
A three-site skinfold measurement was taken to estimate body density (Lange; Beta Technology Inc, Cambridge, MD, USA) and used to calculate percent body fat from the appropriate Jackson equation for men and women (Jackson et al. 1980, 1999). Each site was measured three times with the mean of the two closest values used.
Glutamine and placebo supplementation
Subjects ingested 0.9 g/kg of fat-free mass of glutamine (GLN) mixed with 2 g of sugar-free lemon drink or the lemon drink alone (PLA). The supplements were taken 2 h prior to the beginning of the exercise trials.
Intestinal barrier permeability
The assessment of intestinal permeability was quantified based on the absorption and urinary excretion of two sugar probes. Lactulose is a large disaccharide probe that is a marker of small intestine paracellular permeability. Rhamnose is a smaller monosaccharide that crosses the epithelia via the transcellular pathway and is a marker of intestinal absorption. Lactulose levels increase while rhamnose stays constant or increases slightly, which provides evidence of stable absorption and increased paracellular movement. The sugars are calculated based on the amount recovered in the urine (g) multiplied by the urine volume recorded during the 5-h collection period. This value is then divided by the quantity ingested (5 g lactulose, 2 g rhamnose) to determine a percent recovered for each sugar. The excretion percentages of the lactulose and rhamnose are used to then calculate a ratio (lactulose/rhamnose), where an increase in the ratio is a marker for an increase in intestinal permeability.
Subjects consumed a 50-ml solution containing 5 g lactulose (L7877 Sigma-Aldrich, St. Louis, MO, USA) and 2 g rhamnose (R3875 Sigma-Aldrich, St. Louis, MO, USA). During the GLN and PLA trials, the sugar drink was consumed 20 min into each exercise bout to ensure transit of the sugar probe solution when an increase in intestinal permeability would likely occur. Samples were separated into 30-ml aliquots and stored at −20 °C for subsequent analysis of the lactulose to rhamnose excretion ratio.
Urinary lactulose
Lactulose was quantified using a standard enzymatic method developed by Behrens et al. (1984). A 200-μl sample of urine was added to 100 μl triethanolamine (TEA) buffer (5.6 g TEA and 740 mg MgSO4 in 50 ml dH2O; pH adjusted to 7.5 with 1 M NaOH and total volume brought to 100 ml stock solution); 6.2 μl of beta-galactosidase was added to the urine and TEA buffer and incubated at room temperature for 2 h. After the incubation period, 2.73 ml of pre-prepared cocktail (1 ml TEA, 2 g ATP, 2 g NADP, 0.009 ml HK/G6PDH, and 1.721 ml dH2O) was added per sample. Absorbance was measured at 340 nm (A1) against a blank water sample using a spectrophotometer (Beckman Coulter DU530, Brea, CA). Seven microliters of phospho-glucoisomerase was then added to each sample, and absorbance was measured again at 340 nm (A2) against a blank water sample every 3 min until the reaction was stable. A blank sample containing water along with the cocktail and enzymes was subtracted from the summed absorbance. Concentration was calculated using Beer’s law for NADH.
Urinary rhamnose
Rhamnose was quantified using a colorimetric enzyme immunoassay kit (K-RHAM, Megazyme, Wicklow, Ireland). The assay is sensitive to 1.3 mg/l. Samples were not diluted, and all manufacturer directions were followed.
Blood sampling and analysis
A seated posture-controlled (20 min seated) venous blood was collected pre-, post-, 2 h post-, and 4 h post-exercise from an antecubital vein. Blood samples were drawn into sterile syringes and immediately transferred into Vacutainer tubes containing EDTA (BD Biosciences, Franklin Lakes, NJ). Blood was added to Histopaque (Sigma-Aldrich 1077, St. Louis, MO) in a 1:1 ratio (15 ml/15 ml) and centrifuged (968g, 30 min, 20 °C). Plasma was pipetted into 1.5-ml microtubes and frozen at −80 °C for further analysis of endotoxin, glutamine, and TNF-α. The buffy coat containing the mononuclear cells was collected, transferred to a clean conical centrifuge tube, and re-suspended with 10 ml of phosphate-buffered saline (PBS) (Sigma-Aldrich 4417, St. Louis, MO), and the mixture was centrifuged (968g, 10 min, 20 °C). The supernatant was removed, and the mononuclear pellet was stored at −80 °C for subsequent analysis of cellular levels of HSP70 and IκBα.
Endotoxin
Endotoxin was assessed in the plasma using a limulus amebocyte lysate chromogenic endpoint assay (HIT302, Hycult Biotech, Plymouth Meeting, PA) sensitive to 1.4 pg/ml. Samples were diluted 1:3 with endotoxin-free water then heated at 75 °C for 5 min in a warm plate. Manufacturer directions were followed, and a logarithmic standard curve was used to calculate concentrations in picograms per milliliter. Each sample was tested for interference with recovery within 50–200 %.
Tumor necrosis factor alpha
TNF-α was assessed in the plasma using an enzyme-linked immunosorbent assay (ELISA) sensitive to 0.191 pg/ml (HSTA00D, R&D Systems). Samples were diluted 1:3 with sample diluent provided by the manufacturer. Directions were followed, and a standard curve was used to calculate concentrations in picograms per milliliter.
Glutamine
Plasma glutamine was assessed with a quantitative colorimetric enzyme assay kit (EGLN-100, BioAssay Systems, Hayward, CA) sensitive to 0.023 mM glutamine. Samples were diluted 1:2 with distilled water. All materials and chemicals were provided by the manufacturer. Manufacturer directions were followed. Glutamate was measured in each sample and subtracted from the glutamine absorbance of the respective sample.
Western blot gel electrophoresis
Mononuclear cells were homogenized for 25 min with 200 μl of lysis buffer (150 mM NaCl, 20 mM HEPES, 2 mM EDTA, 0.2 % SDS, 0.5 % sodium deoxycholate, 10 % Triton X-100, 100 μM phenylmethylsulfonyl fluoride (PMSF), 100 μM vanadate, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 40 mM paranitrophenyl phosphate, 1 μg/ml aprotinin) and then centrifuged (20,000g, 10 min, 4 °C). The supernatant was collected and protein measurement was performed using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Laemmli (161-0737, Bio-Rad Laboratories, Hercules, CA, USA) gel loading buffer was added to the lysate containing 15–20 μg of protein and boiled for 10 min. Proteins were then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a membrane (162-0094, nitrocellulose membrane; Bio-Rad Laboratories). The membrane was incubated for 1 h in blocking solution (5 % dry milk in Tris-buffered saline Tween 20 buffer) followed by incubation with HSP70 (4872S, Cell Signaling Technology, Danvers, MA, USA) and IκBα (I0505, Sigma, St. Louis, MO, USA) antibody in a blocking solution. Each membrane was cut, and the upper half was treated with antibody for β-actin (61-0120, Invitrogen) while the lower half was treated with IκBα. After incubation, the membrane was washed with TBS-Tween then treated with horseradish peroxidase-conjugated secondary antibody (7074S, 7076S, Cell Signaling Technology, Danvers, MA, USA). The membrane was developed using Santa Cruz Western Blotting Luminol Reagents (SC-2048, Santa Cruz Biotechnology, Santa Cruz, CA, USA) on the Kodak BioMax MS film (F-BX57, Fisher Scientific, Pittsburgh, PA, USA). Adobe Photoshop (San Jose, CA) was used to quantify protein expression and standardized to β-actin to control for protein loading. Protein levels also were expressed relative to the pre-exercise time point.
Statistical analysis
All results are expressed as means ± SD and checked for homogeneity of variance and normality. A one-way ANOVA was used to measure intestinal permeability (ratio of lactulose to rhamnose) with condition (baseline, glutamine, placebo) used as the independent variable. A two-factor repeated measure ANOVA was used to analyze plasma glutamine with condition (glutamine, placebo) and various time points (pre, post, 2, and 4 h) as the independent variables. If there was a main effect significance, Tukey’s test, which maintains alpha levels and is moderately conservative, was used for post hoc comparisons (Kuennen et al. 2011). Paired t tests were used to compare all other data.
Results
Subject characteristics
Seven subjects (two males and five females) completed baseline, Pla, and Gln trials. Subject characteristics are presented in Table 1. The intensity (70 % VO2max) during each trial was determined by oxygen consumption sampling every 5 min. Treadmill speed and grade were adjusted throughout the trials to maintain the appropriate oxygen consumption for each subject. There was no difference between the Pla and Gln trials for exercise intensity reported as relative percentage of VO2max (69.77 % ± 3.31 vs. 69.84 % ± 6.32, p > 0.05, respectively). End exercise core temperature was not different between Pla and Gln trials (39.51 °C ± 0.36 vs. 39.31 °C ± 0.20, p > 0.05, respectively).
Table 1.
Subject physiological characteristics
| Sex | Male (2), female (5) |
|---|---|
| Age | 26 ± 4.4 |
| Height (cm) | 169 ± 8.5 |
| Weight (kg) | 60.26 ± 6.705 |
| Body fat (%) | 16.01 ± 5.750 |
| VO2max (m kg min) | 51.61 ± 6.71 |
Data are mean ± SD, n = 7
Plasma glutamine levels
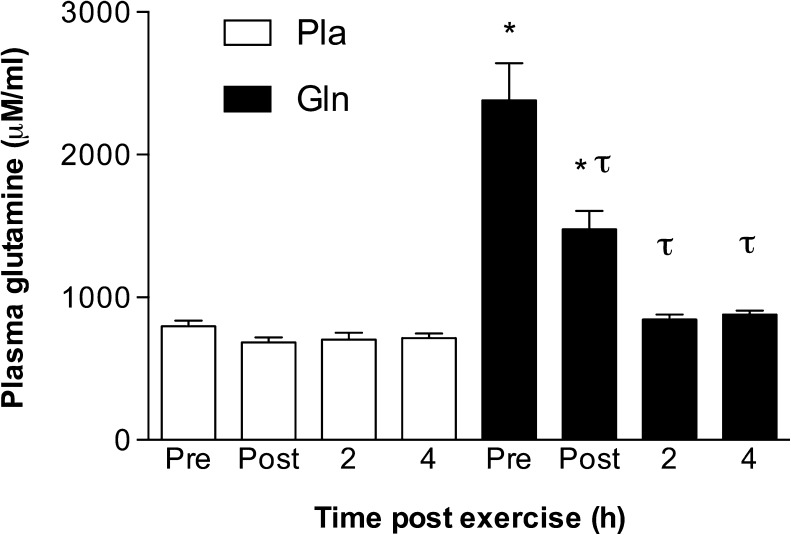
Acute oral glutamine supplementation increased resting plasma glutamine levels. The main effect (time × supplement) was statistically significant (F (3, 18) = 24.30, p < 0.0001). Post hoc testing showed pre- and post-exercise plasma glutamine levels higher in the Gln trial compared to those at the same time points in Pla (2,382 ± 684.10 μM vs. 796.40 ± 107.90 μM and 1,475 ± 345.90 μM vs. 683.30 ± 92.92, p < 0.05, respectively). In addition, plasma glutamine levels declined significantly in the Gln trial at all post exercise time points (post, 2 h, and 4 h) when compared to pre exercise levels (1,475 ± 345.90 μM, 842.90 ± 96.85 μM, and 878.40 ± 71.84 μM vs. 2,382 ± 684.10 μM, p < 0.05, respectively) (Fig. 1).
Fig. 1.
The effect of acute oral glutamine supplementation on plasma glutamine levels. *p < 0.05, significantly different from the same time point in the placebo trial. τ p < 0.05, significantly different from the pre-exercise level in the same trial. Data are mean ± SD, n = 7
Intestinal permeability and plasma endotoxin
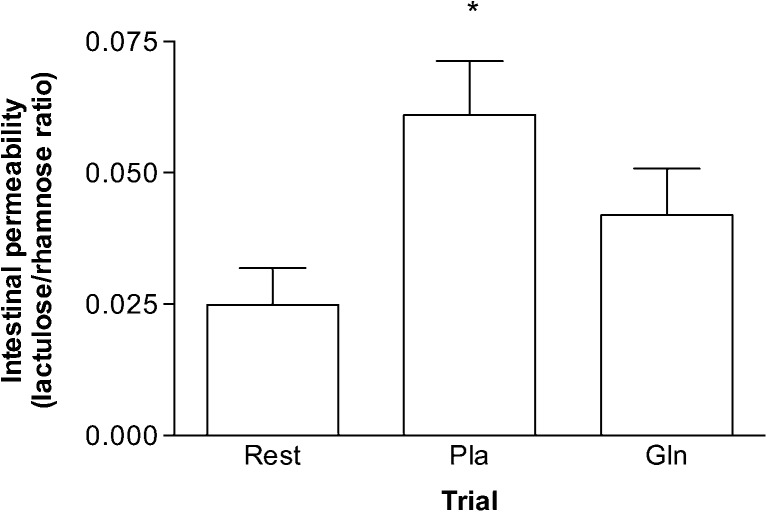
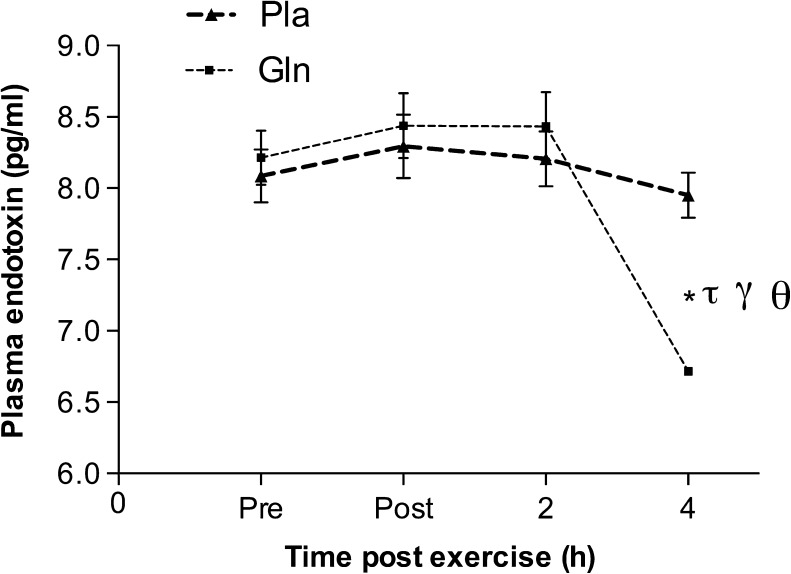
Exercise caused an increase in the urinary ratio of lactulose and rhamnose with the main effect statistically significant (F (1.54, 9.24) = 7.43, p < 0.05). Permeability was higher in the Pla trial when compared to that at rest (0.06 ± 0.01 vs. 0.02 ± 0.01, p < 0.05), and there was no statistical significance between resting levels and after the Gln trial (0.02 ± 0.01 vs. 0.04 ± 0.02, p > 0.05). Plasma endotoxin was lower at the 4-h time point in the Gln trial when compared to that at the same time point in the Pla trial (6.71 ± 0.04 pg/ml vs. 7.95 ± 1.11 pg/ml, p < 0.05). In addition, endotoxin was lower at the 4-h time point in the Gln trial when compared to that at the pre-, post-, and 2-h post-exercise time points in the same trial (6.71 ± 0.04 pg/ml vs. 8.21 ± 1.33 pg/ml, 8.44 ± 1.33 pg/ml, 8.433 ± 1.67 pg/ml, p < 0.05, respectively) (Figs. 2 and 3).
Fig. 2.
The effect of acute glutamine supplementation on exercise-induced intestinal permeability. Permeability was measured as the ratio of urine levels of lactulose to rhamnose. *p < 0.05, significantly different from rest. Data are mean ± SD, n = 7
Fig. 3.
The effect of acute glutamine supplementation on plasma endotoxin levels. *p < 0.05, significantly different from the same time point in the placebo trial. τ p < 0.05, significantly different from the pre-exercise level in the same trial. γ p < 0.05, significantly different from the post-exercise time point in the same trial. Θ p < 0.05, significantly different from the 2-h time point in the same trial. Data are mean ± SD, n = 7
Plasma TNF-α
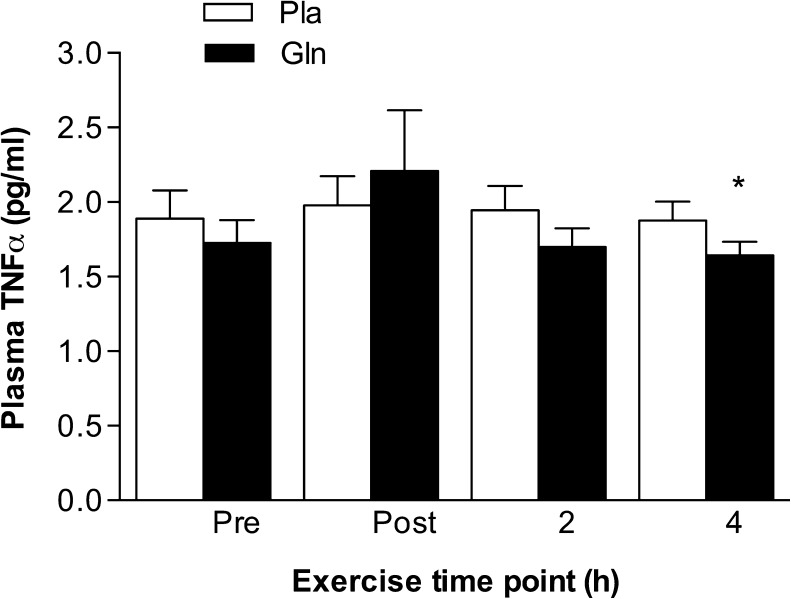
Plasma levels of TNF-α were significantly lower at the 4-h time point in the Gln trial when compared to those at the 4-h time point in the Pla trial (1.64 ± 0.22 pg/ml vs. 1.87 ± 0.30 pg/ml, p < 0.05) (Fig. 4).
Fig. 4.
The effect of acute glutamine supplementation on plasma TNF-α levels. Plasma TNF-α was lower at the 4-h time point in the Gln trial compared to that at the same time point in the Pla trial. *p < 0.05, significantly different from the same time point in the Pla trial. Data are mean ± SD, n = 7
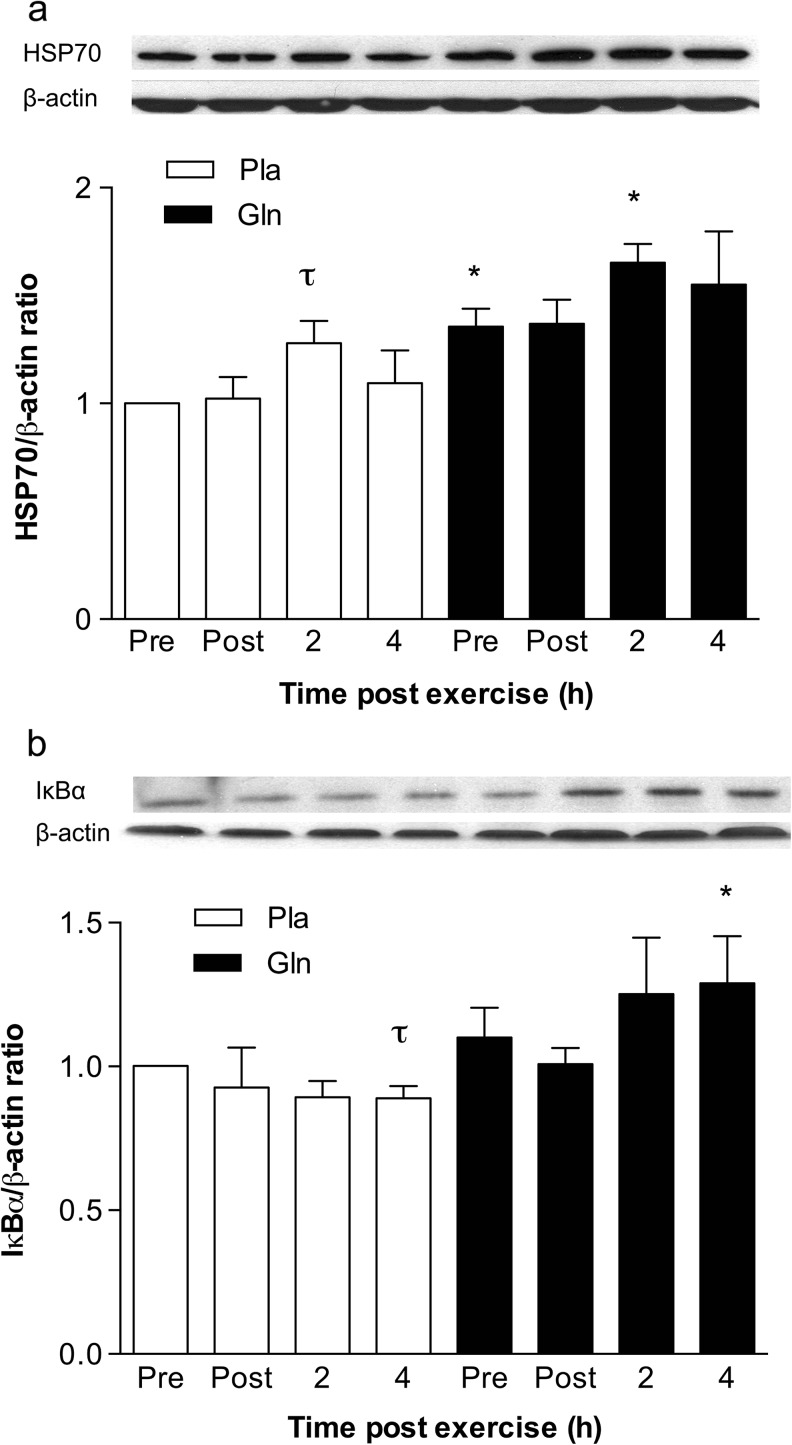
Peripheral blood mononuclear cells HSP70 and IκBα expression
HSP70 expression increased at the 2-h time point in the Pla compared to that at pre-exercise (1.27 ± 0.25 vs. 1.00 ± 0.00, p < 0.05). In the Gln trial, glutamine increased resting levels of total HSP70 (not isoform specific) when compared to that at pre-exercise in the Pla trial (1.36 ± 0.21 vs. 1.00 ± 0.00, p < 0.05). In addition, HSP70 was higher at the 2-h time point in the Gln trial compared to that at the same time point in the Pla trial (1.65 ± 0.21 vs. 1.27 ± 0.40, p < 0.05). IκBα expression was significantly lower in the Pla trial at the 4-h time point when compared to that at pre-exercise (0.88 ± 0.04 vs. 1.00 ± 0.00, p < 0.05). However, levels increased at the 4-h time point in the Gln trial when compared to those of the 4-h time point in the Pla trial (1.29 ± 0.43 vs. 0.88 ± 0.04, p < 0.05) (Fig. 5).
Fig. 5.
The effect of acute glutamine supplementation on HSP70 (a) and IκBα (b) levels in PBMCs. Densitometric values of protein content were obtained using Photoshop software and normalized to β-actin and set to 1. *p < 0.05, significantly different from same time point in the placebo trial. τ p < 0.05, significantly different from the pre-exercise level in the same trial. Data are mean ± SD, n = 7
Discussion
Intestinal permeability has been shown consistently to increase in response to high-intensity exercise (Lambert et al. 2001; Zuhl et al. 2014), causing endotoxin leakage, activation of the NF-κB pathway, and transcription of pro-inflammatory plasma proteins (i.e., TNF-α, IL-1β, IL-6) (Selkirk et al. 2008). We demonstrate in this study that an acute dose of oral glutamine 2 h before intense exercise ameliorates stress-induced intestinal permeability along with lowering of plasma endotoxins. Plasma TNF-α levels are statistically lower at the 4-h post-exercise time point, but this change may not be biologically significant (1.64 ± 0.22 pg/ml vs. 1.87 ± 0.30 pg/ml). In addition, we show that glutamine increases human PBMC expression of HSP70 and IκBα, demonstrating heat shock chaperone-associated inhibition of the NF-κB pathway. These results indicate a dual role of glutamine as an anti-inflammatory agent: through stabilization of the intestinal wall preventing toxin leakage and by enhancing the heat shock response in peripheral leukocytes. Both mechanisms may be mediated by glutamine activation of the heat shock response.
Glutamine has been established as a protective agent for the intestinal tract, promoting tight junction stability along with prevention of endotoxin leakage upon stress exposure (chemical or physical) (Jensen et al. 1994; Peng et al. 2004; Singleton and Wischmeyer 2006). In humans, chronic glutamine supplementation (30 days) has been shown to improve intestinal integrity demonstrated by decreased urine lactulose content in neonates (Sevastiadou et al. 2011). Fourteen days of enteral glutamine supplementation lowered intestinal permeability and endotoxin levels among severe burn patients (Peng et al. 2004). Further, we have recently demonstrated that 7 days of oral glutamine supplementation inhibited exercise-induced intestinal permeability during exercise in the heat (Zuhl et al. 2014). To our knowledge, this is the first study to report the effects of an acute dosage of oral glutamine prior to vigorous exercise in the heat on intestinal permeability. Lambert et al. (2001) examined the effect of an acute carbohydrate + glutamine combined beverage on aspirin-induced intestinal permeability during intense treadmill running. The supplement reduced lactulose excretion but not the lactulose to rhamnose ratio. This indicates that the amount of overall intestinal absorption (rhamnose) changed along with paracellular transport (lactulose), making the interpretation of intestinal permeability unclear. The results in the current study conflict with Lambert’s data for several reasons; our subjects ingested an isolated glutamine beverage compared to a mixed beverage (carbohydrate + glutamine), and ingestion occurred 2 h before exercise in the present study compared to the night before. Another explanation of the difference in results is that Lambert’s group tested the combined effect of aspirin and exercise on permeability, which may have incited a greater stress on the intestinal wall when compared to exercise alone (Lambert et al. 2001). It is also important to acknowledge that the current study took place at moderate altitude (1,585 m), which may have enhanced gut ischemia leading to greater oxidative stress. This may have contributed to the increase in intestinal permeability in the control trial (Dinmore et al. 1994). However, our subjects were residents of Albuquerque, NM, and it is likely that they were acclimated to the altitude.
The protective effects of glutamine on the intestinal epithelium may be through stimulation of the heat shock response. Wischmeyer’s group has demonstrated through numerous animal model studies that glutamine activates HSF-1 leading to HSP70 upregulation (Morrison et al. 2006; Singleton and Wischmeyer 2007; Wischmeyer 2006). Rats fed oral glutamine for 5 days and exposed to hyperthermia demonstrated enhanced HSP70 expression in intestinal tissue, along with reduced plasma endotoxin (Singleton and Wischmeyer 2006). In addition, HSP70 knockout mice did not benefit from glutamine supplementation and had greater tissue injury after lung puncture and cecal ligation (Singleton and Wischmeyer 2007). Glutamine-mediated regulation and activation of HSF-1 in the intestinal epithelium is multifactorial. Xue et al. (2012) demonstrated that glutamine has dual mechanisms of regulation: through increased HSF-1 trimerization and increased transactivation activity and through inhibition of a CCAAT enhancer-binding protein (C/EBP), which suppressed HSF-1. The action through trimerization is thought to increase HSF-1 activation, while the C/EBP pathway regulates HSF-1 expression (Xue et al. 2012). A third possible mechanism is through glutamine activation of O-linked β-N-acetylglucosamine (O-GlcNAc), a monosaccharide that regulates protein function through glycosylation, and has an analogous effect as phosphorylation (Wells et al. 2002). O-GlcNAc may modify HSF-1 expression and HSP70 levels by reducing phosphorylation at serine 303 (Ser303) by glycogen synthase kinase-3 beta (GSK-3β), an HSF-1 inhibitor (Kazemi et al. 2010). Ser303 phosphorylation, combined with the chaperone complex (HSP90, HSP40, HSP70), regulates the inactive cytosolic HSF-1 monomer (Shi et al. 1998). Chemical inhibition of O-GlcNAc prevented glutamine-mediated activation of HSF-1, reducing HSP70 levels in LPS-treated cardiomyocytes (Gong and Jing 2011).
HSF-1 has been shown to play a role in intestinal epithelial cell tight junction protein regulation under conditions of heat stress. Dokladny et al. (2008) demonstrated that HSF-1 mediates occludin expression and junctional localization in Caco-2 cells exposed to heat stress. Further, HSF-1 knockdown using siRNA negated occludin levels and prevented tight junction stabilization (Dokladny et al. 2008). Caco-2 cells supplemented with 4 and 6 mM of glutamine for 7 days and exposed to heat stress demonstrate greater HSF-1 and occludin expression when compared to cells without glutamine. This indicates that glutamine stabilizes the tight junction possibly through HSF-1 activation resulting in improved barrier function, which may lead to protection against paracellular transport of toxins. In summary, glutamine activates HSF-1 through a variety of molecular mechanisms (discussed above), leading to tight junction protein stabilization and lower intestinal permeability to luminal toxins under conditions of physical stress.
Glutamine’s role in immune function is complex and unclear. Critically ill patients commonly exhibit glutamine deficiency, which is brought about by increased requirements for immune cells combined with insufficient glutamine production (Bongers et al. 2007; Jackson et al. 1999). Conejero et al. (2002) demonstrated that enterally fed critically ill patients had lower rates of pneumonia and enteric bacteria when compared to a non-glutamine group. Glutamine has been shown to alter leukocyte pro-inflammatory cytokine production under conditions of catabolic stress (Conejero et al. 2002). Nascimento et al. (2005) demonstrated that blocking endogenous glutamine production through inhibition of glutamine synthetase enhanced IL-1β levels in rats exposed to paw tissue damage. Further, glutamine supplemented at a dose greater than 4 mM decreased LPS-induced TNF-α production in an in vitro human PBMC model (Wischmeyer et al. 2003). In the same study, pre-heating cells prior to the LPS insult upregulated HSP70 expression and also decreased TNF-α release. The results from our current study show that acute oral glutamine supplementation combined with exercise leads to increased HSP70 induction in human PBMCs and lower plasma TNF-α levels.
The heat shock response may inhibit pro-inflammatory cytokine production through regulation of the NF-κB pathway. It has been demonstrated that HSP70 physically binds to the rel65 unit of the NF-κB complex, preventing nuclear translocation and cytokine transcriptional activity. We have previously shown that overexpression of HSP70 stabilizes IκBα, inhibiting NF-κB translocation in LPS-stimulated liver cells (Dokladny et al. 2010). In the current study, we show increased expression of HSP70 and IκBα in the acute oral glutamine group, indicating NF-κB suppression in PBMCs. The HSP70 response was significantly higher at the 2-h time point where IκBα levels were higher at the 4-h mark. This indicates a time course response, allowing HSP70 to bind to the NF-κB subunit leading to an increase in IκBα levels later into exercise recovery. In addition, TNF-α levels were significantly lower at the 4-h time point.
This study demonstrates that an acute dose of oral glutamine prior to strenuous exercise inhibits exercise-induced intestinal permeability along with reducing pro-inflammatory cytokine production. Glutamine’s action may be through activation of the heat shock response, which serves dual roles: stabilization of the intestinal epithelial cell tight junctions preventing toxin leakage and suppression of the NF-κB inflammatory pathway in peripheral leukocytes.
Acknowledgments
Acknowledgments
This work was supported in part by the University of New Mexico’s Research Allocation Committee and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000041.
References
- Ardawi MS, Newsholme EA. Glutamine metabolism in lymphocytes of the rat. Biochem J. 1983;212:835–842. doi: 10.1042/bj2120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens RH, Docherty H, Elia M, Neale G. A simple enzymatic method for the assay of urinary lactulose. Clin Chim Acta. 1984;137:361–367. doi: 10.1016/0009-8981(84)90125-6. [DOI] [PubMed] [Google Scholar]
- Blomqvist BI, Hammarqvist F, von der Decken A, Wernerman J. Glutamine and alpha-ketoglutarate prevent the decrease in muscle free glutamine concentration and influence protein synthesis after total hip replacement. Metabolism. 1995;44:1215–1222. doi: 10.1016/0026-0495(95)90019-5. [DOI] [PubMed] [Google Scholar]
- Bongers T, Griffiths RD, McArdle A. Exogenous glutamine: the clinical evidence. Crit Care Med. 2007;35:S545–552. doi: 10.1097/01.CCM.0000279193.23737.06. [DOI] [PubMed] [Google Scholar]
- Burnham EL, Moss M, Ziegler TR. Myopathies in critical illness: characterization and nutritional aspects. J Nutr. 2005;135:1818s–1823s. doi: 10.1093/jn/135.7.1818S. [DOI] [PubMed] [Google Scholar]
- Carr EL, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell L. Glutamine supplementation in vitro and in vivo, in exercise and in immunodepression. Sports Med. 2003;33:323–345. doi: 10.2165/00007256-200333050-00001. [DOI] [PubMed] [Google Scholar]
- Castell LM, Newsholme EA. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition. 1997;13:738–742. doi: 10.1016/S0899-9007(97)83036-5. [DOI] [PubMed] [Google Scholar]
- Castell LM, Poortmans JR, Newsholme EA. Does glutamine have a role in reducing infections in athletes? Eur J Appl Physiol Occup Physiol. 1996;73:488–490. doi: 10.1007/BF00334429. [DOI] [PubMed] [Google Scholar]
- Conejero R, et al. Effect of a glutamine-enriched enteral diet on intestinal permeability and infectious morbidity at 28 days in critically ill patients with systemic inflammatory response syndrome: a randomized, single-blind, prospective, multicenter study. Nutrition. 2002;18:716–721. doi: 10.1016/S0899-9007(02)00847-X. [DOI] [PubMed] [Google Scholar]
- Cruzat VF, Rogero MM, Tirapegui J. Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem Funct. 2010;28:24–30. doi: 10.1002/cbf.1611. [DOI] [PubMed] [Google Scholar]
- Dinmore AJ, Edwards JS, Menzies IS, Travis SP. Intestinal carbohydrate absorption and permeability at high altitude (5,730 m) J Appl Physiol. 1994;76(1985):1903–1907. doi: 10.1152/jappl.1994.76.5.1903. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Ye D, Kennedy JC, Moseley PL, Ma TY. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am J Pathol. 2008;172:659–670. doi: 10.2353/ajpath.2008.070522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Lobb R, Wharton W, Ma TY, Moseley PL. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-kappaB. Cell Stress Chaperones. 2010;15:153–163. doi: 10.1007/s12192-009-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Zuhl MN, Mandell M, Bhattacharya D, Schneider S, Deretic V, Moseley PL. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J Biol Chem. 2013;288:14959–14972. doi: 10.1074/jbc.M113.462408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Jing L. Glutamine induces heat shock protein 70 expression via O-GlcNAc modification and subsequent increased expression and transcriptional activity of heat shock factor-1. Minerva Anestesiol. 2011;77:488–495. [PubMed] [Google Scholar]
- Hiscock N, Mackinnon LT. A comparison of plasma glutamine concentration in athletes from different sports. Med Sci Sports Exerc. 1998;30:1693–1696. doi: 10.1097/00005768-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–181. [PubMed] [Google Scholar]
- Jackson NC, Carroll PV, Russell-Jones DL, Sonksen PH, Treacher DF, Umpleby AM. The metabolic consequences of critical illness: acute effects on glutamine and protein metabolism. Am J Physiol. 1999;276:E163–170. doi: 10.1152/ajpendo.1999.276.1.E163. [DOI] [PubMed] [Google Scholar]
- Jensen JC, et al. Prevention of chronic radiation enteropathy by dietary glutamine. Ann Surg Oncol. 1994;1:157–163. doi: 10.1007/BF02303560. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi Z, Chang H, Haserodt S, McKen C, Zachara NE. O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem. 2010;285:39096–39107. doi: 10.1074/jbc.M110.131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuennen M, Gillum T, Dokladny K, Bedrick E, Schneider S, Moseley P. Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R524–533. doi: 10.1152/ajpregu.00039.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert GP, Broussard LJ, Mason BL, Mauermann WJ, Gisolfi CV. Gastrointestinal permeability during exercise: effects of aspirin and energy-containing beverages. J Appl Physiol. 2001;90(1985):2075–2080. doi: 10.1152/jappl.2001.90.6.2075. [DOI] [PubMed] [Google Scholar]
- Morrison AL, Dinges M, Singleton KD, Odoms K, Wong HR, Wischmeyer PE. Glutamine’s protection against cellular injury is dependent on heat shock factor-1. Am J Physiol Cell Physiol. 2006;290:C1625–1632. doi: 10.1152/ajpcell.00635.2005. [DOI] [PubMed] [Google Scholar]
- Nascimento SB, et al. Glutamine depletion potentiates leucocyte-dependent inflammatory events induced by carrageenan or Clostridium difficile toxin A in rats. Immunology. 2005;116:328–336. doi: 10.1111/j.1365-2567.2005.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985;70:473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Yamada S, Sugiura T, Ohira Y, Yoshioka T, Goto K. Possible role of NF-kB signals in heat stress-associated increase in protein content of cultured C2C12 cells. Cells Tissues Organs. 2011;194:363–370. doi: 10.1159/000323324. [DOI] [PubMed] [Google Scholar]
- Paimela T, Hyttinen JM, Viiri J, Ryhanen T, Karjalainen RO, Salminen A, Kaarniranta K. Celastrol regulates innate immunity response via NF-kappaB and Hsp70 in human retinal pigment epithelial cells. Pharmacol Res. 2011;64:501–508. doi: 10.1016/j.phrs.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Parry-Billings M, Evans J, Calder PC, Newsholme EA. Does glutamine contribute to immunosuppression after major burns? Lancet. 1990;336:523–525. doi: 10.1016/0140-6736(90)92083-T. [DOI] [PubMed] [Google Scholar]
- Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns. 2004;30:135–139. doi: 10.1016/j.burns.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Selkirk GA, McLellan TM, Wright HE, Rhind SG. Mild endotoxemia, NF-kappaB translocation, and cytokine increase during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol. 2008;295:R611–623. doi: 10.1152/ajpregu.00917.2007. [DOI] [PubMed] [Google Scholar]
- Sevastiadou S, Malamitsi-Puchner A, Costalos C, Skouroliakou M, Briana DD, Antsaklis A, Roma-Giannikou E. The impact of oral glutamine supplementation on the intestinal permeability and incidence of necrotizing enterocolitis/septicemia in premature neonates. J Matern Fetal Neonatal Med. 2011;24:1294–1300. doi: 10.3109/14767058.2011.564240. [DOI] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KD, Wischmeyer PE. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock. 2006;25:295–299. doi: 10.1097/01.shk.0000196548.10634.02. [DOI] [PubMed] [Google Scholar]
- Singleton KD, Wischmeyer PE. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1839–1845. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW. Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteomics. 2002;1:791–804. doi: 10.1074/mcp.M200048-MCP200. [DOI] [PubMed] [Google Scholar]
- Wischmeyer PE. Glutamine: the first clinically relevant pharmacological regulator of heat shock protein expression? Curr Opin Clin Nutr Metab Care. 2006;9:201–206. doi: 10.1097/01.mco.0000222100.44256.6b. [DOI] [PubMed] [Google Scholar]
- Wischmeyer PE, Riehm J, Singleton KD, Ren H, Musch MW, Kahana M, Chang EB. Glutamine attenuates tumor necrosis factor-alpha release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition. 2003;19:1–6. doi: 10.1016/S0899-9007(02)00839-0. [DOI] [PubMed] [Google Scholar]
- Xue H, Slavov D, Wischmeyer PE. Glutamine-mediated dual regulation of heat shock transcription factor-1 activation and expression. J Biol Chem. 2012;287:40400–40413. doi: 10.1074/jbc.M112.410712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob P, Calder PC. Cytokine production by human peripheral blood mononuclear cells: differential sensitivity to glutamine availability. Cytokine. 1998;10:790–794. doi: 10.1006/cyto.1998.0358. [DOI] [PubMed] [Google Scholar]
- Yoon BK, Kravitz L, Robergs R. VO2max, protocol duration, and the VO2 plateau. Med Sci Sports Exerc. 2007;39:1186–1192. doi: 10.1249/mss.0b13e318054e304. [DOI] [PubMed] [Google Scholar]
- Ziegler TR. Glutamine supplementation in bone marrow transplantation. Br J Nutr. 2002;87(Suppl 1):S9–15. doi: 10.1079/BJN2001452. [DOI] [PubMed] [Google Scholar]
- Zuhl MN, Lanphere KR, Kravitz L, Mermier CM, Schneider S, Dokladny K, Moseley PL. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol. 2014;116:183–191. doi: 10.1152/japplphysiol.00646.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]