Abstract
Intensive muscular activity can trigger oxidative stress, and free radicals may hence be generated by working skeletal muscle. The role of the enzyme xanthine oxidase as a generating source of free radicals is well documented and therefore is involved in the skeletal muscle damage as well as in the potential transient cardiovascular damage induced by high-intensity physical exercise. Allopurinol is a purine hypoxanthine-based structural analog and a well-known inhibitor of xanthine oxidase. The administration of the xanthine oxidase inhibitor allopurinol may hence be regarded as promising, safe, and an economic strategy to decrease transient skeletal muscle damage (as well as heart damage, when occurring) in top-level athletes when administered before a competition or a particularly high-intensity training session. Although continuous administration of allopurinol in high-level athletes is not recommended due to its possible role in hampering training-induced adaptations, the drug might be useful in non-athletes. Exertional rhabdomyolysis is the most common form of rhabdomyolysis and affects individuals participating in a type of intense exercise to which they are not accustomed. This condition can cause exercise-related myoglobinuria, thus increasing the risk of acute renal failure and is also associated with sickle cell trait. In this manuscript, we have reviewed the recent evidence about the effects of allopurinol on exercise-induced muscle damage. More research is needed to determine whether allopurinol may be useful for preventing not only exertional rhabdomyolysis and acute renal damage but also skeletal muscle wasting in critical illness as well as in immobilized, bedridden, sarcopenic or cachectic patients.
Keywords: Xanthine oxidase, Free radicals, Muscle injury, Rhabdomyolysis, Sarcopenia, Cachexy
Introduction
Over half a century ago, it was discovered that free radicals (FRs) may be effectively produced by the skeletal muscle (Commoner et al. 1954). Several lines of evidence then confirmed that intensive muscular activity can trigger oxidative stress (OS), mainly mirrored by increased glutathione oxidation and oxidation of proteins, lipids, and DNA (Gomez-Cabrera et al. 2003, 2005). In 1992, it was also demonstrated that physical exercise practiced until the point of exhaustion may be a cause of OS. In line with this evidence, a linear correlation was found between the reduced and oxidized glutathione quotient and the lactate–pyruvate quotient (Sastre et al. 1992), a finding that was corroborated in subsequent studies (Heunks et al. 1999; Viña et al. 1996).
There are several sources of FRs in skeletal muscle. The role of the enzyme xanthine oxidase (XO) as a generating source of FRs is well documented. XO and xanthine dehydrogenase (XDH) are isoenzymes of xanthine oxide-reductase (XOR). The former enzyme is prevalently found in smooth muscle cells of vessel walls, as well as in endothelial cells of skeletal muscles. Conversion of XDH into XO is catalyzed by vascular proteases. Hypoxanthine is formed in working muscles during intensive physical exercise or at the end stages of long-lasting physical exercise. XO easily crosses the cell membrane, and XOR catalyzes the enzymatic step that catalyzes the conversion of hypoxanthine to xanthine and of xanthine to the final end-product, uric acid (Lippi et al. 2008a). Although XDH preferentially transfers the electrons resulting from NAD oxidation, XO uses molecular oxygen, which implies superoxide radical production (Harris et al. 1999), which, in turn, may cause exertional muscle damage.
Therefore, in accordance with the principle of hormesis, exercise leads to an acute OS that up-regulates endogenous antioxidant defenses (Radak et al. 2008). To put it simply, the hormetic zone, also known as the “Goldilocks” zone, is a biological set point which is neither too comfortable, but even not too harsh (Nunn et al. 2009). It may hence be reflected by an intermediate degree of stress (i.e., moderate physical exercise) which would help the organism to enhance its anti-stressor mechanisms and improve the ability to resist to OS but contextually limit the deleterious effects of excessive stress (i.e., strenuous exercise) on cardiac, renal, and muscle integrity (Garatachea et al. 2014; Lippi et al. 2012a; Sanchis-Gomar et al. 2014b), although such effects do not seem to impair longevity (Garatachea et al. 2014). This appealing concept has gained widespread popularity during the past decade, so it seems conceivable that lifestyle interventions, foods, nutritional supplements, or other compounds that help maintain the organism in hormesis would generate relevant benefits on health and fitness.
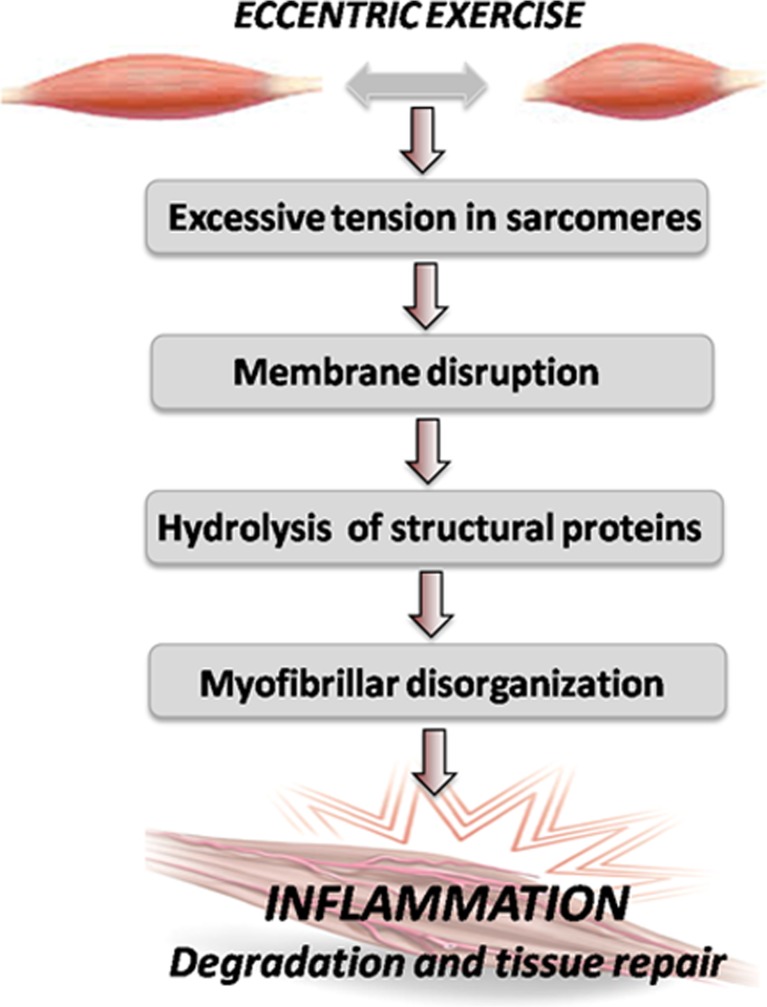
Exercise, particularly the eccentric type, can provoke muscle damage (Armstrong et al. 1983; Kyparos et al. 2001) through excess tension in the sarcomere, which is hence the leading source of muscular lesion from membrane disruption, then triggering structural protein hydrolysis and causing the habitually observed myofibril deformation (Lieber and Friden 1999) and permanent muscle injury (Lippi et al. 2010). The subsequent inflammatory phenomenon helps degrade and repair tissue (see Fig. 1). Soccer as a sport presents a high eccentric component while being played. Given the numerous competitions throughout the year, the frequent episodes of muscle damage that players suffer may increase the risk of injuries, especially among professional soccer players. Although physical exercise is recommended to prevent a wide range of chronic conditions, such as cardiovascular diseases, cancer, osteoporosis, or diabetes (Viña et al. 2012), some sports like cycling or long-distance running (i.e., marathon, ultramarathon, and mountain running) have been associated with a transient post-exercise increase in biomarkers of skeletal muscle and cardiac damage, as reflected by the substantial increase of biomarkers of myocardiocyte necrosis, including cardiac troponins (Brancaccio et al. 2010; Eijsvogels et al. 2011; Lippi et al. 2008b, 2011a, b, c, 2012b; Sanchis-Gomar and Lippi 2014). Radiological findings suggestive of fibrosis and myocardial damage have also been reported (Yared and Wood 2009), along with an increased concentration of biomarkers of cardiac stress and fibrosis (Salvagno et al. 2014). As previously mentioned, several cell sources of reactive oxygen species (ROS) production exist in the skeletal muscle, including XO. Exhaustive or acute physical exercise increases ROS generation and therefore OS in skeletal muscle and other organs, which may finally result in cell injury (Davies et al. 1982; Gomez-Cabrera et al. 2005, 2008). OS is hence involved in remodeling and heart failure physiopathology and also in skeletal muscle damage induced by exhaustive exercise (Tsutsui et al. 2011).
Fig. 1.
Physical exercise, especially if having a high eccentric component, can cause muscle damage. Excessive sarcomere tension is the main cause of muscle lesion through membrane disruption, which permits structural protein hydrolysis, leading to myofibril deformation. Thereafter, inflammation occurs to help degrade and subsequently repair necrotic tissue
In this regard, it has been shown that antioxidants such as vitamins C and E may hamper training-induced adaptations such as skeletal muscle mitochondrial biogenesis both in animals and humans, thus decreasing performance (Gomez-Cabrera et al. 2008; Khassaf et al. 2003; Marshall et al. 2002; Paulsen et al. 2014; Ristow et al. 2009; Sharman et al. 1971; Strobel et al. 2010) or even reducing heat-shock protein 70 (HSP 70), increasing apoptosis (Hooper and Hooper 2004) as well as the risk of heart failure and death (Bjelakovic et al. 2007; Lonn et al. 2005; Wray et al. 2009), although these findings were not confirmed in all studies (Gey et al. 1970; Higashida et al. 2011; Keren and Epstein 1980; Maughan 1999; Theodorou et al. 2011; Yfanti et al. 2010).
Allopurinol is a purine hypoxanthine-based structural analog and a well-known inhibitor of XO frequently employed in clinical practice (Moorhouse et al. 1987) and a promising drug to prevent oxidative muscle damage while practicing exhaustive physical exercise (Gomez-Cabrera et al. 2006). In human (Viña et al. 2000b; c) and animal models (Viña et al. 2000a), our research group demonstrated that allopurinol prevents glutathione oxidation, protein oxidation, and lipoperoxidation associated with exertional exhaustion. In professional cyclists participating in the Tour de France, administration of a daily 300-mg oral dose of allopurinol prevented the increases in serum activity of both creatine kinase (CK) and aspartate aminotransferase (AST) (i.e., two biomarkers of muscle damage) at the stage (team time trial) at which all the studied cyclists had undertaken maximum-intensity exercise for more than 1 h (Gomez-Cabrera et al. 2003). Similarly, the plasma levels of malondialdehyde (MDA) increased in all study participants once the race had finished. However, this increase was significantly greater in the placebo group compared with the allopurinol group. These results suggest that XO may be involved in muscle damage associated with performing physical exercise to the point of exhaustion. These findings were confirmed in a later study conducted in marathon runners. In this case, the plasma levels of MDA significantly increased after a running test until exhaustion, with allopurinol administration preventing this increase (Gomez-Cabrera et al. 2006). However, it was also reported that allopurinol administration may attenuate exercise activation of the mitochondrial biogenesis pathway in skeletal muscle (Gomez-Cabrera et al. 2005; Kang et al. 2009). At variance with these data, Wadley et al. recently showed that allopurinol does not inhibit exercise-training increases in skeletal muscle mitochondrial biogenesis (Wadley et al. 2013).
The inhibition of HSP expression is another non-XO effect of allopurinol (George and Struthers 2009). Nishizawa and collaborators also reported that allopurinol significantly reduced the accumulation of messenger RNA (mRNA) for HSP70 or HSP90 after repetitive ischemia/reperfusion (Nishizawa et al. 1999), whereas Ohlmann et al. showed that pretreatment of rat hepatocyte cultures with allopurinol before exposure to anoxia and reoxygenation led to a marked decrease of heme oxygenase 1 (HO-1) and HSP70 mRNA expression during reoxygenation (Ohlmann et al. 2003). In addition, Mao et al. recently reported that allopurinol administration in combination or not with N-acetylcysteine affects HO-1 expression, normalizing cardiac levels of HO-1 in diabetic rats, and thus resulting in a significant attenuation of post-ischemic myocardial infarction (Mao et al. 2013).
It has also been demonstrated that acute exercise and resistance (weight lifting) training can trigger changes in serum and urine concentration of several laboratory parameters, so their evaluation would enable identification and monitoring of the damage at a specific tissue level (the liver, kidney, skeletal muscle, or myocardium). This aspect has led more sport physicians and researchers to use biomarkers of conventional tissue injury in recent decades (Banfi et al. 2012). Furthermore, the aim of new initiatives in sport research is to boost the research for innovative and promising biomarkers that will improve follow-up of training and sport performance, diagnosis of sport-related injury, overtraining prediction, and even identification of the best time to return to top-level competition after the recovery period from injury.
The hypoxanthine/xanthine oxidase system
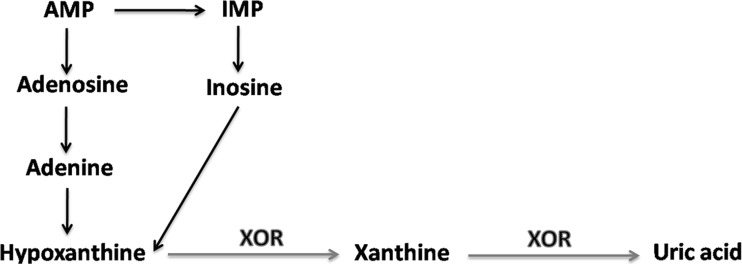
XOR, an enzyme originally described in 1902 as being an aldehyde oxidase (Schardinger 1902), is widely distributed among living beings of distinct complexity. In various species, it catalyzes hydroxylation of a wide range of substrates like purines, pyrimidines, pterines, and aldehydes. XDH is able to employ both NAD+ like oxygen and acceptors of electrons, but especially the former. XO is capable of using only oxygen as an acceptor of electrons. It is the enzyme responsible for purine degradation, as Fig. 2 shows.
Fig. 2.
Diagram of purine degradation. AMP adenosine monophosphate, IMP inositol monophosphate, XOR xanthine oxide-reductase
XO
Despite several research groups identified that the mitochondrion in skeletal muscle is the main source of ROS generation during exercise, a conceptual problem lies in this observation. The superoxide radical produced by contracting muscles can be detected in the extracellular area (Reid et al. 1992) as well as in the vascular compartment (Lee and Okabe 1995). It is unlikely that the superoxide anion generated in the mitochondrion can be measured outside the cell. This would mean that reactive and electrically charged species would escape to antioxidant systems in the mitochondrial matrix and diffuse through the internal and external mitochondrial membrane, the cytosol and the sarcolemma. Therefore, it is unlikely that they would be involved in chemical reaction(s). Diffusion through the capillary endothelium in the vascular compartment seems even less likely (Reid 2001). XO represents an alternative source of ROS with experimental support. In skeletal muscle, XO is localized mainly in the vascular endothelium (Linder et al. 1999). The administration of enzyme inhibitors attenuates the release of superoxide radicals in the vascular area in contracting muscles (Stofan et al. 2000), and this strategy has been proven effective to partially inhibit fatigue in vivo (Barclay and Hansel 1991). Unlike what occurs in the mitochondrion, which generates FRs in a basal state, the ROS arising from XO play an important role in the inflammatory response to physical exercise bouts that have a high eccentric component or impose either high-intensity or long-lasting efforts (Hellsten et al. 1997), as well as in the damage caused by ischemia–reperfusion processes (Kadambi and Skalak 2000).
XO was initially identified as a potential source of FRs in the cytosol of muscle cells (Laughlin et al. 1991). Nevertheless, later studies using monoclonal antibodies for XDH/XO revealed the presence of immunoreactivity in smooth muscle cells of the vessel wall and in endothelial cells at the same time (Hellsten-Westing 1993), but its presence within the muscle fibers was virtually excluded. Hypoxanthine is formed in muscles during intensive physical exercise, and consequently, its concentration also shows marked increases in blood (Sahlin et al. 1991), with the amount of circulating hypoxanthine increasing in parallel with exercise intensity (Hellsten-Westing et al. 1991). Hypoxanthine formation can be associated with IMP accumulation in muscle which, in turn, is directly related to exercise intensity and duration (Sahlin et al. 1989). Recent studies suggested that nucleotides are degraded when ATP resynthesis is impaired due to low muscular glycogen levels (Broberg and Sahlin 1989). As regards uric acid, previous studies that reported no release of this metabolic compound from working muscles might have been biased by the use of poorly sensitive detection methods (Hellsten 1994; Sahlin 1991). In rats, for example, uric acid accumulation has been found after electrical stimulation (Arabadjis et al. 1993).
A linear correlation exists between the plasma peak of hypoxanthine and that of uric acid following exhaustive physical exercise (Hellsten-Westing et al. 1994). This observation indicates that a plasma concentration of hypoxanthine is important in the XDH/XO pathway flow because a high level of this molecule would entail increased superoxide radical production, should conversion into XO occurs. In 1981, Granger et al. demonstrated that treating feline intestine with superoxide dismutase prior to an ischemic process attenuated the damage during subsequent reperfusion, thus suggesting that the superoxide radical is indeed responsible for tissue injury (Granger et al. 1981). The authors also proposed that ischemia triggers the conversion of XDH into XO, as well as the degradation of adenine nucleotides into hypoxanthine. Thus, with the reintroduction of molecular oxygen during reperfusion, a considerable amount of superoxide radical may be generated in the XO reaction.
Allopurinol
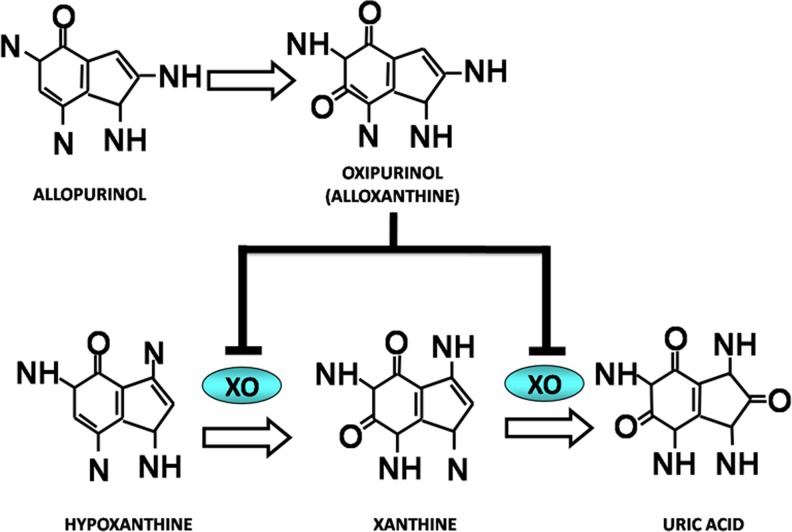
Allopurinol (1H-pyrazol (3,4-d)pyrimidin-4-one) is a natural purine hypoxanthine-based structural analog with a molecular weight of 136.1 Da that acts on the catabolism of purines without affecting their biosynthesis. Basically, it lowers uric acid production by inhibiting the biochemical reactions that lead to its generation. As mentioned, this drug acts as an inhibitor of XOR, the enzyme responsible for converting hypoxanthine into xanthine and xanthine into uric acid, with the latter compound being the end-product of purine catabolism in humans. Allopurinol effectively inhibits XO both in vivo and in vitro conditions (Elion et al. 1966), by forming a reversible complex with molybdenum and by interfering with the purines that interact with the enzyme, so that their oxidation cannot take place (Massey et al. 1970). Allopurinol is absorbed by the intestinal tract and is metabolized to alloxanthine (oxypurinol), which is also an inhibitor of XO. Allopurinol and oxypurinol are cleared by the kidney, and as such, impairment of kidney function has a profound effect on the dosage (see Fig. 3). As a result of XO inhibition, levels of xanthine and hypoxanthine of 0.3 to 0.4 mg/dl (clearly above the normal levels of ∼0.15 mg/dl) have been detected in patients treated with allopurinol (Turnheim et al. 1999). The highest value detected of these oxypurines after taking very high doses of allopurinol (i.e., 0.9 mg/dl) was much higher than saturation (>7 mg/dl).
Fig. 3.
Xanthine oxidase (XO) inhibition by allopurinol. Allopurinol is a natural purine hypoxanthine-based structural analog, and it acts as an XO inhibitor. The two compounds’ structures are similar. Thus, allopurinol can act on the catabolism of purines without modifying their biosynthesis. Allopurinol, as a previous step to oxypurinol, acts by inhibiting XO, the enzyme responsible for converting hypoxanthine into xanthine and xanthine into the end-product of purine catabolism, uric acid
Effect of allopurinol administration on skeletal muscle and cardiovascular damage induced by highly intensive physical exercise in trained subjects
Effects on classic biomarkers
The prevention and effective treatment of soccer injuries is a foremost challenge for sport physicians and coaches (Giza and Micheli 2005). A good approach to prevent muscle lesions in soccer can be based on counteracting muscle damage caused by repeated contractions during training and competition without biasing players’ performance. In this regard, the implication of XO as a source of FRs in skeletal muscle as well as the role of allopurinol as an antioxidant during exercise is well documented (Borras et al. 2006; Gomez-Cabrera et al. 2003, 2005; Ji et al. 2007). Although XO, the enzyme that generates the FRs involved in damage induced by ischemia–reperfusion (McCord 1985), causes muscle injury associated with exhaustive physical exercise (Gomez-Cabrera et al. 2003, 2006; Viña et al. 2000a), it has been demonstrated that allopurinol may be effective to prevent the skeletal muscle damage induced by highly intensive physical exercise in top-level soccer players (Sanchis-Gomar et al. 2013a, b, 2014a).
After professional soccer players had played a match, serum markers of skeletal muscle damage (CK activity, lactate dehydrogenase (LDH), AST, or myoglobin) significantly increased, a phenomenon which, in turn, could be efficiently prevented by allopurinol administration. Despite the fact that serum levels of these biomarkers vary with age, gender, race, muscle mass, and physical activity (Brancaccio et al. 2007), no differences in these variables were found between the allopurinol and placebo groups before the match. Allopurinol administration also prevented exercise-induced lipid peroxidation (Sanchis-Gomar et al. 2014a). These findings are in agreement with the abovementioned study, showing the benefits of allopurinol administration in Tour de France participants (Gomez-Cabrera et al. 2003). Moreover, allopurinol also prolongs exercise time to exhaustion in patients with stable angina pectoris (Noman et al. 2010). However, no changes in the gamma glutamyltransferase (GGT) activity, a hepatic damage marker, was found after a professional soccer match either in the placebo or in the allopurinol groups (Sanchis-Gomar et al. 2014a).
Although there is some controversy (Ruiz et al. 2013), intensive and long-lasting endurance training might favor cardiac remodeling and increase the risk of arrhythmias, especially atrial fibrillation (Aizer et al. 2009; Benito et al. 2011). Intensive physical exercise may also generate transitory cardiac ischemia, myocardium stress, and left diastolic ventricular dysfunction and often induces a transient rise in biomarkers of cardiac injury (Kim et al. 2012; Lippi and Maffulli 2009; Neilan et al. 2006). In our study, we found a significant increase in a serum marker of heart damage (i.e., cardiospecific troponin T), which could be prevented by allopurinol administration (Sanchis-Gomar et al. 2014a).
An increase in cardiac biomarkers in relation to exercise has been broadly described, but no definite mechanistic explanation has been offered (Banfi et al. 2012). It seems plausible that such an increase reflects the occurrence of reversible lesions in cardiomyocytes, which may then become less reversible with repeated, long-term exposure to intense exercise bouts (Banfi et al. 2012; Lippi et al. 2011a). The beneficial effects of allopurinol for different cardiovascular pathologies have been demonstrated. Thus, in chronic heart failure, long-term treatment with allopurinol improves left ventricular hemodynamics and avoids left ventricular remodeling. These long-term effects are, at least partly, caused by a transitory reduction in FRs at the myocardium level (Mellin et al. 2005). Allopurinol is a useful, safe anti-ischemic drug for patients with chronic stable angina (Noman et al. 2010). A causal association between hyperuricemia and cardiovascular risk has been found (Papezikova et al. 2012). In effect, high levels of uric acid are associated with higher risk of cardiovascular events, coronary heart disease, and cerebrovascular accidents (Lippi et al. 2008a). It has also been demonstrated that uric acid has a predictive value of mortality related to chronic heart failure (Anker et al. 2003). Therefore, allopurinol may help prevent the increase of markers of skeletal muscle and cardiac injury associated with practicing highly intensive physical exercise. It has been also demonstrated that inhibiting XO activity by allopurinol administration prevents muscular atrophy through inhibition of the p38 MAPK-MAFbx pathway and may have therefore clinical benefits such as preventing muscular atrophy in critical, bedridden, sarcopenic, or cachectic patients (Derbre et al. 2012; Sanchis-Gomar et al. 2013c).
Effects on emerging biomarkers
A large number of conventional and innovative cardiovascular biomarkers are currently regarded as promising indicators of the level of injury generated by exercise (Brancaccio et al. 2010). Biomarkers are essential parameters that assess the impact of different exercise intensities and patterns in sport and exercise medicine, cardiology, and clinical biochemistry. In this context, the identification of new biomarkers with suitable sensitivity is essential. Thus, new compounds, such as copeptin (CoP), midregional part of proadrenomedullin (MR-proADM), growth differentiation factor 15 (GDF15), vascular endothelial growth factor receptor-1 (sVEGFR-1/sFLT-1), and placental growth factor (PlGF), have recently emerged as candidates to be circulating biomarkers of exercise-induced damage, and the effects of allopurinol administration on their levels has been assessed (Sanchis-Gomar et al. 2013a).
Two recent studies have assessed plasma CoP levels in relation to ultramarathon runners’ hydration state. Hew-Butler et al. found a significant increase in CoP levels during and at the end of long-distance races. These authors also reported the existence of a significant association between CoP levels and the percentage of change in plasma volume (Hew-Butler et al. 2011). Similarly, Burge et al. observed that the plasma concentration of CoP increased by almost 12-fold after running a 100-km ultramarathon, and a correlation between changes in CoP and in serum sodium levels was also reported (Burge et al. 2011). Recently, it has been demonstrated that CoP has a relatively short plasma half-life in plasma, i.e., 23 to 47 min (L’Abate et al. 2013). Although we found that CoP levels increased after physical exercise both with and without pre-exercise administration of allopurinol, we were unable to provide an explanation for this phenomenon (Sanchis-Gomar et al. 2013a).
To the best of our knowledge, no studies have investigated the post-exercise variation of plasma MR-proADM. Normally, increased ADM levels are associated with injury at the endothelial level (Hinson et al. 2000). Under certain conditions, an increase in ADM concentration suggests that this compound may exert hormone-like effects, i.e., by lowering vascular resistance and blood pressure (Hinson et al. 2000). We recently observed a significant increase in serum MR-proADM levels in a placebo group after playing a soccer match (Sanchis-Gomar et al. 2014a). Allopurinol administration was also effective in preventing exercise-induced increases in serum MR-proADM levels (Sanchis-Gomar et al. 2013a). Irrespective of the underlying causes of increased MR-proADM, the finding that XO activity affects the levels of this marker is interesting and may have some implications and clinical applications, e.g., to be used in the follow-up of patients with hyperuricemia. We recently found that MR-proADM levels significantly increased after acute high-intensity exercise (Sanchis-Gomar et al. 2013a). High levels of GDF15 are associated with hypertrophic cardiopathy (Montoro-Garcia et al. 2012). Yet regular, moderate exercise practice (i.e., 1 h, three times a week for more than 6 months) does not seem to affect circulating levels of GDF15 in patients with stable coronary heart disease (Munk et al. 2011). GDF15 increases not only in patients with heart failure and a normal ejection fraction but also in patients with systolic heart failure (Stahrenberg et al. 2010). It is also independently associated with low levels of exercise capacity and poor quality of life. The diagnostic efficacy of GDF15 has been reported to be as high as that of NT-proBNP, and the combination of these two biomarkers can improve the diagnosis accuracy of measuring natriuretic peptides alone in heart failure patients (Stahrenberg et al. 2010). Tchou et al. reported significant increases in the serum concentrations of GDF15 after an ultramarathon (Tchou et al. 2009). We observed a significant increase in serum GDF15 levels after a soccer match in both the placebo and allopurinol groups (Sanchis-Gomar et al. 2013a). Since allopurinol administration did not affect the concentrations of GDF15, GDF15 metabolism may be independent of allopurinol administration and therefore of XO activity (Sanchis-Gomar et al. 2013a).
Bailey et al. found that exercise increases the circulating levels of sVEGFR-1/sFLT-1 in healthy volunteers (Bailey et al. 2006). Because sVEGFR-1/sFLT-1 acts as an inhibitor of endogenous VEGF, it may be effective to lower the plasma levels of free VEGF. Another study described a positive, significant association between the percentage increase in plasma sVEGFR-1/sFLT-1 levels and maximum oxygen consumption while exercising (Bailey et al. 2006). However, Kivelä et al. did not observe any significant change in the expression of VEGFR-1 in skeletal muscle in either healthy or diabetic mice after exercise (Kivela et al. 2008). In our study, the concentration of sVEGFR-1/sFLT-1 or PlGF did not significantly increase in either group (placebo or allopurinol) after playing a soccer match. This finding suggested that these biomarkers are practically insensitive to physical exercise, at least under our experimental conditions of intensity and duration. We also found that allopurinol administration did not alter the serum levels of sVEGFR-1/sFLT-1 or PlGF, thus suggesting that the metabolism of these two biomarkers is scarcely influenced by XO activity (Sanchis-Gomar et al. 2013a).
Soluble urokinase plasminogen activator receptor (suPAR) acts as a risk “master alarm” in several disease conditions including diabetes, cancer, and kidney, cardiovascular, infectious, inflammatory, or autoimmune diseases, with high levels indicating poor prognosis and low levels reflecting favorable outcome and success of treatment (Eugen-Olsen et al. 2010; Huai et al. 2006; Kofoed et al. 2008; Sidenius et al. 2000). We recently found that neither physical exercise nor allopurinol administration influences serum suPAR levels (Sanchis-Gomar et al. 2013b). This finding may have some meaningful clinical implications. In fact, allopurinol is increasingly used in patients with different tissue and vascular lesions, such as acute coronary syndrome, chronic heart failure, inflammatory diseases, septic shock (Pacher et al. 2006), burns, injuries (Sahib et al. 2010), and several forms of localized infection (Gobbi et al. 2007), and the relative insensitiveness of this biomarker to allopurinol administration may make it a more reliable marker for disease monitoring than any other biomarkers of inflammation, the concentration of which is substantially affected by allopurinol.
Conclusions and future perspectives
XO is involved in the skeletal muscle damage as well as in the potential transient cardiovascular damage that might be induced by high-intensity physical exercise, as reflected by the assessment of “classic” biomarkers like CK activity, LDH, AST, myoglobin, or cardiac troponins and also of more novel biomarkers such as MR-proADM and GDF15. The administration of the XO inhibitor allopurinol may hence be regarded as a promising, safe, and economic strategy to decrease transient skeletal muscle damage (as well as heart damage, if occurring) in top-level athletes when administered before a competition or a particularly high-intensity training session (see Table 1). It is also noteworthy, however, that continuous administration of allopurinol in high-level athletes is not recommended at this point in time due to its possible role in hampering training-induced adaptations according to the previously discussed hormesis theory.
Table 1.
Summary of changes induced by exercise and the administration of allopurinol on muscular, hepatic, and cardiovascular biochemical variables
| Biomarker | After exercise | After exercise + allopurinol administration |
|---|---|---|
| CK | ↑ | ↓ |
| CK-MB | ↑ | ↓ |
| LDH | ↑ | ↓ |
| AST | ↑ | ↓ |
| ALT | ↑ | ↑ |
| GGT | = | = |
| Myo | ↑ | ↓ |
| Hs-TnT | ↑ | ↓ |
| MDA | ↑ | ↓ |
| CoP | ↓ | ↑ |
| MR-proADM | ↑ | ↑ |
| GDF15 | ↑ | ↓ |
| sVEGFR-1/sFLT-1 | = | = |
| PlGF | = | = |
| suPAR | = | = |
“↑” increase, “↓” decrease, “=” no change (of note, undifferentiated fibers inside the damaged, regenerating skeletal muscle can express liver (e.g., AST and ALT) or cardiac isoenzymes (e.g., CK-MB)—this potential confounder might lead to the false assumption that intense/eccentric exercise consistently causes cardiac or liver damage). ALT alanine aminotransferase, AST aspartate aminotransferase, CK creatine kinase, CK-MB creatine kinase, myocardic isoenzyme, CoP copeptin, GDF15 growth differentiation factor 15, GGT gamma glutamyltransferase, Hs-TnT highly sensitive troponin T, LDH lactate dehydrogenase, MDA malondialdehyde, MR-proADM midregional part of proadrenomedullin, Myo myoglobin, PlGF placental growth factor, suPAR soluble urokinase plasminogen activator receptor, sVEGFR-1/sFLT-1 vascular endothelial growth factor receptor-1
On the other hand, drugs such as statins, common and effective treatments for hypercholesterolemia, can also cause muscle damage as reflected by “hyper-CK-emia”, myalgias, cramps, exercise intolerance, muscle weakness, and even rhabdomyolysis (Meador and Huey 2010; Mor et al. 2011). Exertional rhabdomyolysis is the most common form of rhabdomyolysis and affects individuals who participate in novel and intense exercise to which they are unaccustomed (Cleary et al. 2011). Moreover, exertional rhabdomyolysis can cause myoglobinuria, which increases the risk of acute renal failure (Elsayed and Reilly 2010; Patel et al. 2009), and is also associated with sickle cell trait (Tsaras et al. 2009). Therefore, although more research is needed, allopurinol could prove useful in preventing exertional myoglobinuria and subsequent renal damage, along with skeletal muscle wasting in critical illness or in immobilized, bedridden, sarcopenic, or cachectic patients (Sanchis-Gomar et al. 2013c). In addition, allopurinol is used increasingly in patients with various cardiovascular conditions, in whom CRP and IL-6 are frequently assessed as follow-up markers. Thus, evidence that allopurinol does not significantly modify serum suPAR levels would be relevant for its use as a reliable marker in patients who receive this drug.
Acknowledgments
Conflict of interest
The authors declare that no conflict of interest exists.
Abbreviations
- ADM
Adrenomedullin
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ATP
Adenosine triphosphate
- CK
Creatine kinase
- CK-MB
Creatine kinase, myocardic isoenzyme
- CoP
Copeptin
- CRP
C-reactive protein
- FRs
Free radicals
- GDF15
Growth differentiation factor 15
- GGT
Gamma glutamyltransferase
- HSP
Heat-shock protein
- HO
Heme oxygenase
- Hs-TnT
Highly sensitive troponin T
- IL-6
Interleukin-6
- IMP
Inositol monophosphate
- LDH
Lactate dehydrogenase
- MDA
Malondialdehyde
- MR-proADM
Midregional part of proadrenomedullin
- Myo
Myoglobin
- NAD
Nicotinamide adenine dinucleotide
- OS
Oxidative stress
- PCT
Procalcitonin
- PlGF
Placental growth factor
- ROS
Reactive oxygen species
- suPAR
Soluble urokinase plasminogen activator receptor
- sVEGFR-1/sFLT-1
Vascular endothelial growth factor receptor-1
- VEGF
Vascular endothelial growth factor
- XDH
Xanthine dehydrogenase
- XO
Xanthine oxidase
- XOR
Xanthine oxide-reductase
References
- Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103:1572–1577. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- Arabadjis PG, Tullson PC, Terjung RL. Purine nucleoside formation in rat skeletal muscle fiber types. Am J Physiol. 1993;264:C1246–1251. doi: 10.1152/ajpcell.1993.264.5.C1246. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Shparago M, Gu JW. Exercise increases soluble vascular endothelial growth factor receptor-1 (sFlt-1) in circulation of healthy volunteers. Med Sci Monit. 2006;12:CR45–50. [PubMed] [Google Scholar]
- Banfi G, Colombini A, Lombardi G, Lubkowska A. Metabolic markers in sports medicine. Adv Clin Chem. 2012;56:1–54. doi: 10.1016/b978-0-12-394317-0.00015-7. [DOI] [PubMed] [Google Scholar]
- Barclay JK, Hansel M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can J Physiol Pharmacol. 1991;69:279–284. doi: 10.1139/y91-043. [DOI] [PubMed] [Google Scholar]
- Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S, Mont L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Borras C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, Viña J. Genistein, a soy isoflavone, up-regulates expression of antioxidant genes: involvement of estrogen receptors, ERK1/2, and NFkappaB. FASEB J. 2006;20:2136–2138. doi: 10.1096/fj.05-5522fje. [DOI] [PubMed] [Google Scholar]
- Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull. 2007;81–82:209–230. doi: 10.1093/bmb/ldm014. [DOI] [PubMed] [Google Scholar]
- Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757–767. doi: 10.1515/CCLM.2010.179. [DOI] [PubMed] [Google Scholar]
- Broberg S, Sahlin K. Adenine nucleotide degradation in human skeletal muscle during prolonged exercise. J Appl Physiol (1985) 1989;67:116–122. doi: 10.1152/jappl.1989.67.1.116. [DOI] [PubMed] [Google Scholar]
- Burge J, Knechtle B, Knechtle P, Gnadinger M, Rust CA, Rosemann T. Maintained serum sodium in male ultra-marathoners–the role of fluid intake, vasopressin, and aldosterone in fluid and electrolyte regulation. Horm Metab Res. 2011;43:646–652. doi: 10.1055/s-0031-1284352. [DOI] [PubMed] [Google Scholar]
- Cleary MA, Sadowski KA, Lee SY, Miller GL, Nichols AW. Exertional rhabdomyolysis in an adolescent athlete during preseason conditioning: a perfect storm. J Strength Cond Res. 2011;25:3506–3513. doi: 10.1519/JSC.0b013e318216302f. [DOI] [PubMed] [Google Scholar]
- Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Derbre F, Ferrando B, Gomez-Cabrera MC, Sanchis-Gomar F, Martinez-Bello VE, Olaso-Gonzalez G, Diaz A, Gratas-Delamarche A, Cerda M, Viña J. Inhibition of xanthine oxidase by allopurinol prevents skeletal muscle atrophy: role of p38 MAPKinase and E3 ubiquitin ligases. PLoS ONE. 2012;7:e46668. doi: 10.1371/journal.pone.0046668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijsvogels TM, Shave R, van Dijk A, Hopman MT, Thijssen DH. Exercise-induced cardiac troponin release: real-life clinical confusion. Curr Med Chem. 2011;18:3457–3461. doi: 10.2174/092986711796642526. [DOI] [PubMed] [Google Scholar]
- Elion GB, Kovensky A, Hitchings GH. Metabolic studies of allopurinol, an inhibitor of xanthine oxidase. Biochem Pharmacol. 1966;15:863–880. doi: 10.1016/0006-2952(66)90163-8. [DOI] [PubMed] [Google Scholar]
- Elsayed EF, Reilly RF. Rhabdomyolysis: a review, with emphasis on the pediatric population. Pediatr Nephrol. 2010;25:7–18. doi: 10.1007/s00467-009-1223-9. [DOI] [PubMed] [Google Scholar]
- Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, Petersen J, Pielak T, Moller LN, Jeppesen J, Lyngbaek S, Fenger M, Olsen MH, Hildebrandt PR, Borch-Johnsen K, Jorgensen T, Haugaard SB. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- Garatachea N, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Emanuele E, Lucia A (2014) Elite athletes live longer than the general population: a meta-analysis. Mayo Clin Proc. doi: 10.1016/j.mayocp.2014.06.004 [DOI] [PubMed]
- George J, Struthers AD. Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vasc Health Risk Manag. 2009;5:265–272. doi: 10.2147/vhrm.s4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gey GO, Cooper KH, Bottenberg RA. Effect of ascorbic acid on endurance performance and athletic injury. JAMA. 1970;211:105. [PubMed] [Google Scholar]
- Giza E, Micheli LJ. Soccer injuries. Med Sport Sci. 2005;49:140–169. doi: 10.1159/000085395. [DOI] [PubMed] [Google Scholar]
- Gobbi P, Lo Presti MS, Fernández AR, Enders JE, Fretes R, Gea S, Paglini-Oliva PA, Rivarola HW. Allopurinol is effective to modify the evolution of Trypanosoma cruzi infection in mice. Parasitol Res. 2007;101:1459–1462. doi: 10.1007/s00436-007-0644-2. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Pallardo FV, Sastre J, Viña J, Garcia-del-Moral L. Allopurinol and markers of muscle damage among participants in the Tour de France. JAMA. 2003;289:2503–2504. doi: 10.1001/jama.289.19.2503-b. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Martinez A, Santangelo G, Pallardo FV, Sastre J, Viña J. Oxidative stress in marathon runners: interest of antioxidant supplementation. Br J Nutr. 2006;96(Suppl 1):S31–33. doi: 10.1079/bjn20061696. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- Granger DN, Perry MA, Kvietys PR, Taylor AE. Interstitium-to-blood movement of macromolecules in the absorbing small intestine. Am J Physiol. 1981;241:G31–36. doi: 10.1152/ajpgi.1981.241.1.G31. [DOI] [PubMed] [Google Scholar]
- Harris CM, Sanders SA, Massey V. Role of the flavin midpoint potential and NAD binding in determining NAD versus oxygen reactivity of xanthine oxidoreductase. J Biol Chem. 1999;274:4561–4569. doi: 10.1074/jbc.274.8.4561. [DOI] [PubMed] [Google Scholar]
- Hellsten Y. Xanthine dehydrogenase and purine metabolism in man. With special reference to exercise. Acta Physiol Scand Suppl. 1994;621:1–73. [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U, Orthenblad N, Sjodin B, Richter EA. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. J Physiol. 1997;498(Pt 1):239–248. doi: 10.1113/jphysiol.1997.sp021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten-Westing Y. Immunohistochemical localization of xanthine oxidase in human cardiac and skeletal muscle. Histochemistry. 1993;100:215–222. doi: 10.1007/BF00269094. [DOI] [PubMed] [Google Scholar]
- Hellsten-Westing Y, Sollevi A, Sjodin B. Plasma accumulation of hypoxanthine, uric acid and creatine kinase following exhausting runs of differing durations in man. Eur J Appl Physiol Occup Physiol. 1991;62:380–384. doi: 10.1007/BF00634977. [DOI] [PubMed] [Google Scholar]
- Hellsten-Westing Y, Kaijser L, Ekblom B, Sjodin B. Exchange of purines in human liver and skeletal muscle with short-term exhaustive exercise. Am J Physiol. 1994;266:R81–86. doi: 10.1152/ajpregu.1994.266.1.R81. [DOI] [PubMed] [Google Scholar]
- Heunks LM, Viña J, van Herwaarden CL, Folgering HT, Gimeno A, Dekhuijzen PN. Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Physiol. 1999;277:R1697–1704. doi: 10.1152/ajpregu.1999.277.6.R1697. [DOI] [PubMed] [Google Scholar]
- Hew-Butler T, Hoffman MD, Stuempfle KJ, Rogers IR, Morgenthaler NG, Verbalis JG. Changes in copeptin and bioactive vasopressin in runners with and without hyponatremia. Clin J Sport Med. 2011;21:211–217. doi: 10.1097/JSM.0b013e31821a62c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH. Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab. 2011;301:E779–784. doi: 10.1152/ajpendo.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper JJ. Vitamin E and atherosclerosis. Prev Cardiol. 2004;7:144. doi: 10.1111/j.1520-037x.2004.3520.x. [DOI] [PubMed] [Google Scholar]
- Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li Y, Yuan C, Bian C, Chen L, Furie B, Furie BC, Cines DB, Huang M. Structure of human urokinase plasminogen activator in complex with its receptor. Science. 2006;311:656–659. doi: 10.1126/science.1121143. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Viña J. Role of nuclear factor kappaB and mitogen-activated protein kinase signaling in exercise-induced antioxidant enzyme adaptation. Appl Physiol Nutr Metab. 2007;32:930–935. doi: 10.1139/H07-098. [DOI] [PubMed] [Google Scholar]
- Kadambi A, Skalak TC. Role of leukocytes and tissue-derived oxidants in short-term skeletal muscle ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;278:H435–443. doi: 10.1152/ajpheart.2000.278.2.H435. [DOI] [PubMed] [Google Scholar]
- Kang C, O’Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med. 2009;47:1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Keren G, Epstein Y. The effect of high dosage vitamin C intake on aerobic and anaerobic capacity. J Sports Med Phys Fitness. 1980;20:145–148. [PubMed] [Google Scholar]
- Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol. 2003;549:645–652. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Malhotra R, Chiampas G, D’Hemecourt P, Troyanos C, Cianca J, Smith RN, Wang TJ, Roberts WO, Thompson PD, Baggish AL. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366:130–140. doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- Kivela R, Silvennoinen M, Lehti M, Jalava S, Vihko V, Kainulainen H. Exercise-induced expression of angiogenic growth factors in skeletal muscle and in capillaries of healthy and diabetic mice. Cardiovasc Diabetol. 2008;7:13. doi: 10.1186/1475-2840-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofoed K, Eugen-Olsen J, Petersen J, Larsen K, Andersen O. Predicting mortality in patients with systemic inflammatory response syndrome: an evaluation of two prognostic models, two soluble receptors, and a macrophage migration inhibitory factor. Eur J Clin Microbiol Infect Dis. 2008;27:375–383. doi: 10.1007/s10096-007-0447-5. [DOI] [PubMed] [Google Scholar]
- Kyparos A, Matziari C, Albani M, Arsos G, Deligiannis A. A decrease in soleus muscle force generation in rats after downhill running. Can J Appl Physiol. 2001;26:323–335. doi: 10.1139/h01-020. [DOI] [PubMed] [Google Scholar]
- L’Abate P, Wiegert S, Struck J, Wellmann S, Cannizzaro V. Determinants of plasma copeptin: a systematic investigation in a pediatric mechanical ventilation model. Respir Physiol Neurobiol. 2013;185:222–227. doi: 10.1016/j.resp.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Hale CC, Novela L, Gute D, Hamilton N, Ianuzzo CD. Biochemical characterization of exercise-trained porcine myocardium. J Appl Physiol. 1991;71:229–235. doi: 10.1152/jappl.1991.71.1.229. [DOI] [PubMed] [Google Scholar]
- Lee C, Okabe E. Hydroxyl radical-mediated reduction of Ca(2+)-ATPase activity of masseter muscle sarcoplasmic reticulum. Jpn J Pharmacol. 1995;67:21–28. doi: 10.1254/jjp.67.21. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Mechanisms of muscle injury after eccentric contraction. J Sci Med Sport. 1999;2:253–265. doi: 10.1016/s1440-2440(99)80177-7. [DOI] [PubMed] [Google Scholar]
- Linder N, Rapola J, Raivio KO. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Lab Invest. 1999;79:967–974. [PubMed] [Google Scholar]
- Lippi G, Maffulli N. Biological influence of physical exercise on hemostasis. Semin Thromb Hemost. 2009;35:269–276. doi: 10.1055/s-0029-1222605. [DOI] [PubMed] [Google Scholar]
- Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta. 2008;392:1–7. doi: 10.1016/j.cca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Lippi G, Schena F, Montagnana M, Salvagno GL, Guidi GC. Influence of acute physical exercise on emerging muscular biomarkers. Clin Chem Lab Med. 2008;46:1313–1318. doi: 10.1515/CCLM.2008.250. [DOI] [PubMed] [Google Scholar]
- Lippi G, Guidi GC, Salvagno GL, Impellizzeri F, Schena F. Highly sensitive cardiac troponin T is not increased by strenuous eccentric exercise. Am J Cardiol. 2010;105:1043–1044. doi: 10.1016/j.amjcard.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Lippi G, Cervellin G, Banfi G, Plebani M. Cardiac troponins and physical exercise. It’s time to make a point. Biochem Med (Zagreb) 2011;21:55–62. doi: 10.11613/bm.2011.012. [DOI] [PubMed] [Google Scholar]
- Lippi G, Schena F, Montagnana M, Salvagno GL, Banfi G, Guidi GC. Significant variation of traditional markers of liver injury after a half-marathon run. Eur J Intern Med. 2011;22:e36–38. doi: 10.1016/j.ejim.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Lippi G, Schena F, Salvagno GL, Tarperi C, Aloe R, Guidi GC. Comparison of conventional and highly-sensitive troponin I measurement in ultra-marathon runners. J Thromb Thrombolysis. 2011;33:338–342. doi: 10.1007/s11239-011-0651-0. [DOI] [PubMed] [Google Scholar]
- Lippi G, Sanchis-Gomar F, Salvagno GL, Aloe R, Schena F, Guidi GC. Variation of serum and urinary neutrophil gelatinase associated lipocalin (NGAL) after strenuous physical exercise. Clin Chem Lab Med. 2012;50:1585–1589. doi: 10.1515/cclm-2011-0954. [DOI] [PubMed] [Google Scholar]
- Lippi G, Schena F, Dipalo M, Montagnana M, Salvagno GL, Aloe R, Guidi GC (2012) Troponin I measured with a high sensitivity immunoassay is significantly increased after a half marathon run. Scand J Clin Lab Invest 72: 467–470 [DOI] [PubMed]
- Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- Mao X, Wang T, Liu Y, Irwin MG, Ou JS, Liao XL, Gao X, Xu Y, Ng KF, Vanhoutte PM, Xia Z. N-acetylcysteine and allopurinol confer synergy in attenuating myocardial ischemia injury via restoring HIF-1alpha/HO-1 signaling in diabetic rats. PLoS ONE. 2013;8:e68949. doi: 10.1371/journal.pone.0068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RJ, Scott KC, Hill RC, Lewis DD, Sundstrom D, Jones GL, Harper J. Supplemental vitamin C appears to slow racing greyhounds. J Nutr. 2002;132:1616S–1621S. doi: 10.1093/jn/132.6.1616S. [DOI] [PubMed] [Google Scholar]
- Massey V, Komai H, Palmer G, Elion GB. On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo[3,4-d]pyrimidines. J Biol Chem. 1970;245:2837–2844. [PubMed] [Google Scholar]
- Maughan RJ. Nutritional ergogenic aids and exercise performance. Nutr Res Rev. 1999;12:255–280. doi: 10.1079/095442299108728956. [DOI] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Meador BM, Huey KA. Statin-associated myopathy and its exacerbation with exercise. Muscle Nerve. 2010;42:469–479. doi: 10.1002/mus.21817. [DOI] [PubMed] [Google Scholar]
- Mellin V, Isabelle M, Oudot A, Vergely-Vandriesse C, Monteil C, Di Meglio B, Henry JP, Dautreaux B, Rochette L, Thuillez C, Mulder P. Transient reduction in myocardial free oxygen radical levels is involved in the improved cardiac function and structure after long-term allopurinol treatment initiated in established chronic heart failure. Eur Heart J. 2005;26:1544–1550. doi: 10.1093/eurheartj/ehi305. [DOI] [PubMed] [Google Scholar]
- Montoro-Garcia S, Hernandez-Romero D, Jover E, Garcia-Honrubia A, Vilchez JA, Casas T, Martinez P, Climent V, Caballero L, Valdes M, Marin F. Growth differentiation factor-15, a novel biomarker related with disease severity in patients with hypertrophic cardiomyopathy. Eur J Intern Med. 2012;23:169–174. doi: 10.1016/j.ejim.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, Gutteridge JM. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1987;213:23–28. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- Mor A, Wortmann RL, Mitnick HJ, Pillinger MH. Drugs causing muscle disease. Rheum Dis Clin North Am. 2011;37:219–231. doi: 10.1016/j.rdc.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Munk PS, Valborgland T, Butt N, Larsen AI. Response of growth differentiation factor-15 to percutaneous coronary intervention and regular exercise training. Scand Cardiovasc J. 2011;45:27–32. doi: 10.3109/14017431.2010.516368. [DOI] [PubMed] [Google Scholar]
- Neilan TG, Januzzi JL, Lee-Lewandrowski E, Ton-Nu TT, Yoerger DM, Jassal DS, Lewandrowski KB, Siegel AJ, Marshall JE, Douglas PS, Lawlor D, Picard MH, Wood MJ. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation. 2006;114:2325–2333. doi: 10.1161/CIRCULATIONAHA.106.647461. [DOI] [PubMed] [Google Scholar]
- Nishizawa J, Nakai A, Matsuda K, Komeda M, Ban T, Nagata K. Reactive oxygen species play an important role in the activation of heat shock factor 1 in ischemic-reperfused heart. Circulation. 1999;99:934–941. doi: 10.1161/01.cir.99.7.934. [DOI] [PubMed] [Google Scholar]
- Noman A, Ang DS, Ogston S, Lang CC, Struthers AD. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375:2161–2167. doi: 10.1016/S0140-6736(10)60391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn AV, Bell JD, Guy GW. Lifestyle-induced metabolic inflexibility and accelerated ageing syndrome: insulin resistance, friend or foe? Nutr Metab (Lond) 2009;6:16. doi: 10.1186/1743-7075-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann A, Giffhorn-Katz S, Becker I, Katz N, Immenschuh S. Regulation of heme oxygenase-1 gene expression by anoxia and reoxygenation in primary rat hepatocyte cultures. Exp Biol Med (Maywood) 2003;228:584–589. doi: 10.1177/15353702-0322805-51. [DOI] [PubMed] [Google Scholar]
- Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papezikova I, Pekarova M, Kolarova H, Klinke A, Lau D, Baldus S, Lojek A, Kubala L (2012) Uric acid modulates vascular endothelial function through the down regulation of nitric oxide production. Free Radic Res 47:82–8 [DOI] [PubMed]
- Patel DR, Gyamfi R, Torres A. Exertional rhabdomyolysis and acute kidney injury. Phys Sportsmed. 2009;37:71–79. doi: 10.3810/psm.2009.04.1685. [DOI] [PubMed] [Google Scholar]
- Paulsen G, Cumming KT, Holden G, Hallen J, Ronnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Ostgaard HN, Buer C, Midttun M, Freuchen F, Wiig H, Ulseth ET, Garthe I, Blomhoff R, Benestad HB, Raastad T. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J Physiol. 2014;592:1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Reid MB. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc. 2001;33:371–376. doi: 10.1097/00005768-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II Extracellular release of free radicals. J Appl Physiol (1985) 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JR, Joyner M, Lucia A. CrossTalk opposing view: prolonged intense exercise does not lead to cardiac damage. J Physiol. 2013;591:4943–4945. doi: 10.1113/jphysiol.2013.257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahib AS, Al-Jawad FH, Alkaisy AA. Effect of antioxidants on the incidence of wound infection in burn patients. Ann Burns Fire Disasters. 2010;23:199–205. [PMC free article] [PubMed] [Google Scholar]
- Sahlin K. Control of energetic processes in contracting human skeletal muscle. Biochem Soc Trans. 1991;19:353–358. doi: 10.1042/bst0190353. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Broberg S, Ren JM. Formation of inosine monophosphate (IMP) in human skeletal muscle during incremental dynamic exercise. Acta Physiol Scand. 1989;136:193–198. doi: 10.1111/j.1748-1716.1989.tb08652.x. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Ekberg K, Cizinsky S. Changes in plasma hypoxanthine and free radical markers during exercise in man. Acta Physiol Scand. 1991;142:275–281. doi: 10.1111/j.1748-1716.1991.tb09157.x. [DOI] [PubMed] [Google Scholar]
- Salvagno GL, Schena F, Gelati M, Danese E, Cervellin G, Guidi GC, Lippi G. The concentration of high-sensitivity troponin I, galectin-3 and NT-proBNP substantially increase after a 60-km ultramarathon. Clin Chem Lab Med. 2014;52:267–272. doi: 10.1515/cclm-2013-0601. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Lippi G. Physical activity - an important preanalytical variable. Biochem Med (Zagreb) 2014;24:68–79. doi: 10.11613/BM.2014.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Bonaguri C, Aloe R, Pareja-Galeano H, Martinez-Bello V, Gomez-Cabrera MC, Candel J, Viña J, Lippi G. Effects of acute exercise and xanthine oxidase inhibition on novel cardiovascular biomarkers. Transl Res. 2013;162:102–109. doi: 10.1016/j.trsl.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Bonaguri C, Pareja-Galeano H, Gomez-Cabrera MC, Candel J, Viña J, Lippi G. Effects of acute exercise and allopurinol administration on soluble urokinase plasminogen activator receptor (suPAR) Clin Lab. 2013;59:207–210. doi: 10.7754/clin.lab.2012.120728. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Pareja-Galeano H, Cortell-Ballester J, Perez-Quilis C (2013c) Prevention of acute skeletal muscle wasting in critical illness. Minerva Anestesiol 80:748 [PubMed]
- Sanchis-Gomar F, Pareja-Galeano H, Gomez-Cabrera MC, Candel J, Lippi G, Salvagno GL, Mann GE, Viña J (2014) Allopurinol prevents cardiac and skeletal muscle damage in professional soccer players. Scand J Med Sci Sports. doi: 10.1111/sms.12213 [DOI] [PubMed]
- Sanchis-Gomar F, Pareja-Galeano H, Santos-Lozano A, Fiuza-Luces C, Garatachea N, Lucia A (2014b) Strenuous exercise and the heart: are we not seeing the wood for the trees? Int J Cardiol. doi: 10.1016/j.ijcard.2014.07.159 [DOI] [PubMed]
- Sastre J, Asensi M, Gasco E, Pallardo FV, Ferrero JA, Furukawa T, Viña J. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol. 1992;263:R992–995. doi: 10.1152/ajpregu.1992.263.5.R992. [DOI] [PubMed] [Google Scholar]
- Schardinger F. Uber das verhalten der kulmilch gegen methylenblau und seine verwendung zur unterscheidung von ungekochter und gekochter milch. Z Untersuch Nahrungs Genussmittel. 1902;5:1113–1121. [Google Scholar]
- Sharman IM, Down MG, Sen RN. The effects of vitamin E and training on physiological function and athletic performance in adolescent swimmers. Br J Nutr. 1971;26:265–276. doi: 10.1079/bjn19710033. [DOI] [PubMed] [Google Scholar]
- Sidenius N, Sier CF, Ullum H, Pedersen BK, Lepri AC, Blasi F, Eugen-Olsen J. Serum level of soluble urokinase-type plasminogen activator receptor is a strong and independent predictor of survival in human immunodeficiency virus infection. Blood. 2000;96:4091–4095. [PubMed] [Google Scholar]
- Stahrenberg R, Edelmann F, Mende M, Kockskamper A, Dungen HD, Luers C, Binder L, Herrmann-Lingen C, Gelbrich G, Hasenfuss G, Pieske B, Wachter R. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail. 2010;12:1309–1316. doi: 10.1093/eurjhf/hfq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofan DA, Callahan LA, Di MA, Nethery DE, Supinski GS. Modulation of release of reactive oxygen species by the contracting diaphragm. Am J Respir Crit Care Med. 2000;161:891–898. [PubMed] [Google Scholar]
- Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2010;43:1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- Tchou I, Margeli A, Tsironi M, Skenderi K, Barnet M, Kanaka-Gantenbein C, Papassotiriou I, Beris P. Growth-differentiation factor-15, endoglin and N-terminal pro-brain natriuretic peptide induction in athletes participating in an ultramarathon foot race. Biomarkers. 2009;14:418–422. doi: 10.1080/13547500903062976. [DOI] [PubMed] [Google Scholar]
- Theodorou AA, Nikolaidis MG, Paschalis V, Koutsias S, Panayiotou G, Fatouros IG, Koutedakis Y, Jamurtas AZ. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am J Clin Nutr. 2011;93:1373–1383. doi: 10.3945/ajcn.110.009266. [DOI] [PubMed] [Google Scholar]
- Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. 2009;122:507–512. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- Turnheim K, Krivanek P, Oberbauer R. Pharmacokinetics and pharmacodynamics of allopurinol in elderly and young subjects. Br J Clin Pharmacol. 1999;48:501–509. doi: 10.1046/j.1365-2125.1999.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viña J, Severa E, Asensi M, Sastre J, Pallardó F, Ferrero JA, García de la Asunción J, Antón V, Marín J. Exercise causes blood glutathione oxidation in chronic obstructive pulmonary disease: prevention by O2 therapy. J Appl Physiol (1985) 1996;81:2199–2202. [PubMed] [Google Scholar]
- Viña J, Gomez-Cabrera MC, Lloret A, Marquez R, Minana JB, Pallardo FV, Sastre J. Free radicals in exhaustive physical exercise: mechanism of production, and protection by antioxidants. IUBMB Life. 2000a;50:271–277. doi: 10.1080/713803729. [DOI] [PubMed] [Google Scholar]
- Viña J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardo FV, Cuesta A, Ferrero JA, Terada LS, Repine JE. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000b;49:539–544. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- Viña J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley GD, Nicolas MA, Hiam DS, McConell GK. Xanthine oxidase inhibition attenuates skeletal muscle signaling following acute exercise but does not impair mitochondrial adaptations to endurance training. Am J Physiol Endocrinol Metab. 2013;304:E853–862. doi: 10.1152/ajpendo.00568.2012. [DOI] [PubMed] [Google Scholar]
- Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 2009;116:433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- Yared K, Wood MJ. Is marathon running hazardous to your cardiovascular health? The jury is still out. Radiology. 2009;251:3–5. doi: 10.1148/radiol.2512200803. [DOI] [PubMed] [Google Scholar]
- Yfanti C, Akerstrom T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc. 2010;42:1388–1395. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]