Abstract
Women with bacterial vaginosis (BV) display reduced vaginal acidity, which make them susceptible to associated infections such as HIV. In the current study, poly(ethylene glycol) (PEG) nanocarrier-based degradable hydrogels were developed for the controlled release of lactic acid in the vagina of BV-infected women. PEG-lactic acid (PEG-LA) nanocarriers were prepared by covalently attaching lactic acid to 8-arm PEG-SH via cleavable thioester bonds. PEG-LA nanocarriers with 4 copies of lactic acid per molecule provided controlled release of lactic acid with a maximum release of 23% and 47% bound lactic acid in phosphate buffered saline (PBS, pH 7.4) and acetate buffer (AB, pH 4.3), respectively. The PEG nanocarrier-based hydrogels were formed by cross-linking the PEG-LA nanocarriers with 4-arm PEG-NHS via degradable thioester bonds. The nanocarrier-based hydrogels formed within 20 min under ambient conditions and exhibited an elastic modulus that was 100-fold higher than the viscous modulus. The nanocarrier-based degradable hydrogels provided controlled release of lactic acid for several hours; however, a maximum release of only 10%–14% bound lactic acid was observed possibly due to steric hindrance of the polymer chains in the cross-linked hydrogel. In contrast, hydrogels with passively entrapped lactic acid showed burst release with complete release within 30 min. Lactic acid showed antimicrobial activity against the primary BV pathogen Gardnerella vaginalis with a minimum inhibitory concentration (MIC) of 3.6 mg/ml. In addition, the hydrogels with passively entrapped lactic acid showed retained antimicrobial activity with complete inhibition G. vaginalis growth within 48 h. The results of the current study collectively demonstrate the potential of PEG nanocarrier-based hydrogels for vaginal administration of lactic acid for preventing and treating BV.
1. INTRODUCTION
Around 34 million people are living with HIV worldwide and over half are women [1]. Given the high rate of new infections (2.2–2.8 million in 2011), the development and implementation of female-controlled preventive methods is critical in order to successfully curb HIV. Toward this end, the past decade has seen an increased effort in the development of vaginal microbicides-topically applied, self-administered products that protect against HIV transmission [2, 3]. Most microbicides that have been evaluated in clinical trials, however, failed to prevent infection despite showing promising results in vitro [4–6]. It was only recently that a 1% tenofovir gel showed marginal success in prevention (CAPRISA 004 trial results, July 2010) with a 39% reduction in HIV transmission compared to the placebo [7].
In women, a primary risk factor for HIV infection via the cervicovaginal route is bacterial vaginosis (BV) [8–13]. The normal vaginal flora is composed predominantly of lactobacilli, which continuously produce lactic acid that maintains the acidic pH (4.0–4.5) of the vagina [14, 15]. However, normal vaginal flora is compromised in BV, a common and recurrent infection characterized by overgrowth of anaerobic pathogens such as Gardnerella vaginalis, Prevotella, Peptostreptococcus, Mobiluncus and Bacteroides spp. [16, 17]. Women with BV have reduced vaginal lactobacilli and lactic acid and show high vaginal pH (up to 7.0) [18]. The decreased vaginal acidity in women with BV greatly increases their susceptibility to HIV infection [9–11]. A study investigating the association of BV with HIV-1 infection among female sex-workers in Thailand found that among the 43% of participants that were HIV-1 positive, 33% had BV [9]. A similar study in Uganda showed that over half the participants had moderate to severe BV and the rate of HIV-1 infection was considerably higher in women with BV (26.7%) than in women with normal vaginal flora (14.2%) [10]. Thus, it is generally believed that maintaining vaginal acidity and preventing BV infection are critical to HIV prophylaxis.
Previous microbicide gels designed for maintaining vaginal acidity such as BufferGel and Acidform are semi-solid gels consisting primarily of buffering agents (Carbopol 974P for BufferGel and acidifying agents for Acidform) formulated with other excipients [19, 20]. BufferGel was found to be safe for use but did not provide protection against HIV and was discontinued from further development as a microbicide [21, 22]. Acidform was shown to be effective against HSV-2 in a mouse model but is yet to be evaluated for efficacy due to safety concerns [23, 24]. Clinical trials of semi-solid gel microbicides suggest that one of the reasons for their poor in vivo performance might be the inadequate retention of the gel itself resulting in insufficient concentrations of active agents in the vagina. Semi-solid gels have been reported to leak from the vagina within a few hours of application [25–27]. In fact, BufferGel and Acidform are currently being evaluated for contraceptive use and BufferGel has been shown to be effective only when used in combination with a diaphragm [28, 29]. Hence, there is a need for alternative delivery systems that show improved vaginal retention. Novel vaginal drug delivery systems such as intravaginal rings (IVRs), temperature and pH sensitive gels and nanoparticles are being investigated for use as microbicides [30–32]. Among these, IVRs are currently being developed for the vaginal delivery of antiretroviral drugs. However, with IVRs there are concerns of toxicity and systemic absorption due to initial high concentrations of drug in the vagina [33, 34].
In the current study, poly(ethylene glycol) (PEG) nanocarrier-based degradable hydrogels were designed and developed for the controlled release of lactic acid in the vagina for preventing and treating BV, thus reducing the risk of HIV. Lactic acid was chosen as the active moiety because it is the intrinsic acidifying agent in the vagina and has broad-spectrum antimicrobial activity against a variety of bacterial and viral pathogens including the BV-associated pathogen G. vaginalis, HSV-2 and HIV [35–41]. PEG-lactic acid (PEG-LA) nanocarriers were formed by covalently attaching lactic acid to 8-arm PEG-SH via cleavable thioester bonds, and then cross-linked with 4-arm PEG-NHS to form nanocarrier-based hydrogels. The properties of the nanocarrier-based hydrogels such as gelation time, rheology, swelling and degradation were evaluated under various conditions with the end goal of developing these hydrogels for vaginal administration. The release kinetics of lactic acid from the nanocarrier-based hydrogels were compared to hydrogels with passively entrapped lactic acid prepared by mixing lactic acid with the 8-arm PEG-SH and 4-arm PEG-NHS polymer solutions. The antimicrobial activity of lactic acid and hydrogels with passively entrapped lactic acid were evaluated against the G. vaginalis. In addition, the cytoxicity of the 8-arm PEG-SH and 4-arm PEG-NHS polymers was determined using a vaginal epithelial cell line.
2. MATERIALS AND METHODS
2.1 Materials
The polymers 8-arm PEG-SH (20 kDa) and 4-arm PEG-NHS (20 kDa) were obtained from NOF Corporation (White Plains, NY). Lactic acid, L-Cysteine, N, N′-Dicyclohexylcarbodiimide and 2-(Dimethylamino)pyridine were obtained from Sigma-Aldrich (St. Louis, MO). Ellman’s reagent [5,5′-Dithio-bis-(2-nitrobenzoic acid)] and Slide-A-Lyzer® mini-dialysis units (MWCO: 3,500 Da) were obtained from Thermo Fisher Scientific (Waltham, MA). All solvents used were obtained from VWR International (Radnor, PA) or Sigma-Aldrich (St. Louis, MO). Gel permeation chromatography (GPC) was done on a Waters Breeze GPC system (Milford, MA) with dual-absorbance UV and refractive index detectors, using an Ultrahydrogel 1000 Column (12 μm, 7.8 × 300 mm). Rheological data were obtained using Kinexus Ultra rotational rheometer (Malvern Instruments Inc., Westborough, MA). Lactate assay kit, used to determine lactic acid concentrations was obtained from BioVision, Inc. (Mountain View, CA).
2.2 Synthesis and characterization of PEG-LA nanocarriers
Lactic acid (100 mg) was dissolved in Dichloromethane (DCM). N-Hydroxysuccinimide (1.2 eq.), and an excess of N, N′-Dicyclohexylcarbodiimide (DCC) were added under anhydrous conditions and the reaction mixture was stirred at RT for 8 h. The precipitate was filtered using a sintered funnel and the solvent removed using a rotary evaporator. The N-hydroxysuccinimidyl ester of lactic acid was then reacted with 8-arm PEG-SH, to obtain PEG-LA nanocarriers. The 8-arm PEG-SH was dissolved in DCM. The N-hydroxysuccinimidyl ester of lactic acid (8 eq.) was added along with an excess of 4-dimethylaminopyridine (DMAP). The reaction mixture was flushed with argon and stirred at RT for 4 h. Nanocarriers were obtained by precipitation from cold ether followed by drying. The amount of lactic acid bound to the PEG-LA nanocarriers was estimated indirectly using Ellman’s assay and Gel permeation chromatography (Supplementary Information).
2.3 Release of lactic acid from PEG-LA nanocarriers
The PEG-LA nanocarriers (1 mg/100 μl) were dissolved in sodium phosphate buffer (PB, 20 mM, pH 7.4). The solution was transferred to Slide-A-Lyzer® mini-dialysis units and dialyzed against 3.6 ml phophate buffered saline (PBS, 10 mM, pH 7.4) or acetate buffer (AB, 20 mM, pH 4.3) at 37 °C, with continuous stirring (60 rpm). Aliquots (1 ml) were withdrawn from the release medium at pre-determined time intervals and the medium was replenished with an equal volume in order to maintain sink conditions throughout the study. The aliquots were concentrated and the amount of lactic acid determined using a lactate assay kit, as per the manufacturer’s protocol (O.D. 570 nm). The above experiments (and subsequent experiments described below) were performed in triplicate and the results reported as mean±SEM, unless otherwise mentioned.
2.4 Preparation of nanocarrier-based hydrogels
The nanocarrier-based hydrogels were prepared using degradable thioester cross-links as follows: the PEG-LA nanocarriers (4%, 6% and 8%; w/v) were mixed with 2 equiv. of 4-arm PEG-NHS in PB and allowed to stand at room temperature until the hydrogels formed. The PEG-LA nanocarriers formed aggregates upon addition of PB and were sonicated prior to mixing with 4-arm PEG-NHS. The time of formation of the hydrogels was determined using the “inverted tube method” and was noted as the time when the solution ceased to flow, upon inversion of the tube.
2.5 Rheological characterization of nanocarrier-based hydrogels
Rheological measurements were performed at 37 °C using a rheometer with parallel plate geometry (plate diameter: 20 mm, gap: 300 μm). Nanocarrier-based hydrogels (4% and 6% w/v) were allowed to form between the parallel plates at RT, before ramping the temperature up to 37 °C. The elastic/storage modulus (G′) and viscous/loss modulus (G″) of the hydrogels were measured as a function of strain and frequency using dynamic oscillatory tests. First, a strain sweep test was performed at a constant frequency of 1 Hz, in order to determine the linear viscoelastic regime. Next, a frequency sweep test (0.1–10 rad/sec) was carried out at a constant strain of 1%.
2.6 Swelling and Degradation of nanocarrier-based hydrogels
The swelling and degradation of the nanocarrier-based hydrogels was investigated in both physiological and acidic conditions. Hydrogels (100 μl samples) were placed in a flat-bottomed vial and the initial weight recorded (Wi). The samples were then immersed in 1 ml of PBS or AB, and incubated at 37 °C on an orbital shaker (at 60 rpm). The buffer was removed at pre-determined time intervals and weight of the vials with the swollen hydrogels was recorded (Wt). The buffer was replaced after each measurement and the swelling ratio at each time point was calculated as and plotted against time. Swelling equilibrium was reached when the weight of the hydrogels remained constant for two or more consecutive time points.
The time taken for the hydrogels to degrade completely was determined by monitoring the swelling ratio of the hydrogels beyond the swelling equilibrium. A reduction in swelling ratio was observed at time points beyond the swelling equilibrium as the hydrogels began to degrade into the surrounding medium. The degradation time was defined as the time taken for the hydrogels to completely dissolve into the surrounding medium.
2.7 Release of lactic acid from nanocarrier-based hydrogels
The nanocarrier-based hydrogels (4 and 6% w/v) were prepared as described before and the release of lactic acid from hydrogels investigated in physiological and acidic conditions. A 100 μl sample of the hydrogels was placed in a flat-bottomed vial and immersed in 2 ml of PBS or AB. The vials were placed on an orbital shaker (at 60 rpm) and incubated at 37 °C. Aliquots (1 ml) were withdrawn from the release medium at pre-determined time intervals and the medium was replenished with an equal volume in order to maintain sink conditions throughout the study. The amount of lactic acid released was determined using a lactate assay kit as per the manufacturer’s protocol (O.D. 570 nm).
2.8 Hydrogels with passively entrapped lactic acid
Hydrogels with passively entrapped lactic acid were prepared as follows: The 8-arm PEG-SH (4% and 6%, w/v) was mixed with 2 equiv. of 4-arm PEG-NHS in PB at RT. Lactic acid (0.2 mg) was added to the polymer solutions during mixing. The release of lactic acid was determined as follows: Hydrogels (100 μl samples) were immersed in 2 ml PBS (after an initial wash in PBS) and incubated at 37 °C on an orbital shaker (at 60 rpm). The PBS was withdrawn and replaced at pre-determined time intervals and the amount of lactic acid released determined using a lactate assay kit (O.D. 570 nm).
2.9 Death kinetics of G. vaginalis in the presence of lactic acid
G. vaginalis ATCC 14018 was the reference BV-associated strain used in these studies. The cells were stored at −80°C in Brain Heart Infusion (BHI) medium (Difco, Sparks, MD) supplemented with 3% horse serum (JRH Biosciences, KS) and 15% glycerol. For in vitro studies, strains from frozen stocks were cultured on human blood bilayer-Tween (HBT) agar (Remel, Lenexa, KS) and grown at 37°C in 5% CO2 and 2.5% H2 for 48 h using EZ Anaerobe Container System GasPaks (Becton, Dickinson and Co, Sparks, MD).
G. vaginalis (107 CFU/ml) was added to T-25 flasks pre-incubated with BHI medium supplemented with 3% horse serum in the anaerobic chamber, overnight at 37 °C. The initial cell counts in each flask was determined using the drop plate method, by plating 30 μl of cell suspension in duplicates on HBT bilayer agar. Lactic acid was added to the medium in the flasks at final concentrations of 0.9 mg/ml, 4.5 mg/ml and 9.1 mg/ml. Flasks with G. vaginalis in medium without lactic acid was used as the positive control for growth. The flasks were incubated in the anaerobic chamber at 37 °C and cell counts were performed from the flasks at various time points using the drop plate method.
2.10 Growth kinetics of G. vaginalis
The growth kinetics of G. vaginalis in the presence of lactic acid, and on hydrogels with passively entrapped lactic acid was determined as follows: 4% w/v hydrogels with passively entrapped lactic acid (0.2 mg and 1 mg per 50 μl of hydrogel), and lactic acid solutions (50 μl/well in PB, final concentrations of 0.9–4.4 mg/ml per well) were prepared in a 96-well plate. G. vaginalis (107 CFU/ml, 200 μl/well) was added to the wells and sterile mineral oil (50 μl/well) was added on top to each well to facilitate anaerobic growth of G. vaginalis. Hydrogels with no entrapped lactic acid and medium alone were used as controls. The growth kinetics was determined by measuring turbidity every hour over 48 h (O.D. 595 nm). The minimum inhibitory concentration (MIC) of lactic acid was determined as the lowest concentration of lactic acid that inhibited G. vaginalis growth.
2.10 Cytotoxicity evaluation on vaginal epithelial cells
The 8-arm PEG-SH and 4-arm PEG-NHS polymers and lactic acid were evaluated for cytotoxicity on the Vk2/E6E7 human vaginal epithelial cell line. Vk2 cells were seeded on a 96-well plate at a density of 20,000 cells/well in Keratinocyte Serum Free (KSF) growth medium. After overnight incubation, the medium was withdrawn and replaced with medium containing 8-arm PEG-SH and 4-arm PEG-NHS polymers and lactic acid at various concentrations. Nonoxynol-9 cream (N-9, 4% w/v) dissolved in medium was used as the positive control for toxicity and untreated cells (medium alone) were used as the negative control. The cells were grown for an additional 24 h and cell viability was determined relative to the untreated cells using alamarBlue® assay (AbD Serotec, Raleigh, NC). Briefly, the medium containing the various compounds was removed and cells were washed 1x with Dulbecco’s Phosphate Buffered Saline (DPBS). The wells treated with 8-arm PEG-SH solution at concentrations above 40 mg/ml showed gelation of the medium above the cell layer and were treated with dithiotreitol (100mM, dissolved in DPBS) for 5–10 min to facilitate removal of the medium. The cells were then incubated with medium containing alamarBlue® (10% v/v; 100 μl/well) for 4 h at 37 °C. The reduction of alamarBlue® was measured using fluorescence (Ex: 560nm, Em: 590 nm) and the viability calculated as a percentage of the untreated cells. The percentage viability was plotted against polymer concentration, and the CC50 of the polymers was determined by fitting the data with a sigmoidal-dose response curve:
where Bottom = 0 Since a 50% reduction in cell viability was not observed with lactic acid, the CC50 of lactic acid could not be calculated from the observed data.
3. RESULTS
3.1 Synthesis and characterization of PEG-LA nanocarriers
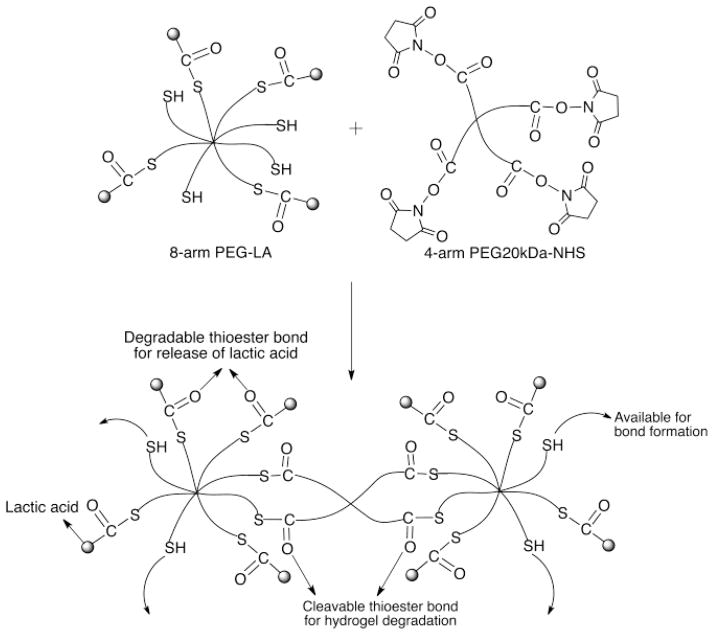
The PEG-LA nanocarriers were synthesized by attaching multiple copies of lactic acid to 8-arm PEG-SH polymer via thioester bonds. Lactic acid was first activated to form the N-hydroxysuccinimidyl ester of lactic acid, and then reacted with 8-arm PEG-SH, to obtain PEG-LA nanocarriers (Scheme 1). The lactic acid loading efficiency was estimated using Ellman’s assay and was found to be 20 μg/mg of nanocarrier or 2% by polymer weight corresponding to four copies of lactic acid per molecule (Supplementary information). The hydrodynamic radii of a series of PEG polymers was measured previously in our laboratory and the size was found to correlate to the molecular weight and architecture of the polymer [42]. The hydrodynamic radius of the unmodified 8-arm PEG-SH polymer used in the current study was found to be 7.432±0.538 nm. Since the conjugation of 4 copies of lactic acid (MW: 90.08 Da) to the 8-arm PEG-SH polymer will not significantly increase the molecular weight of the polymer, the nanocarriers will be in the same size range as the unmodified polymer. The nanocarriers were characterized using GPC and showed a retention time that was similar to the unmodified polymer (RT ~8.8 min, Supplementary information). In addition, the nanocarrier peak at 210 nm, the absorbance wavelength of lactic acid was 4-fold higher than the unmodified polymer indicating attachment of lactic acid.
Scheme 1.
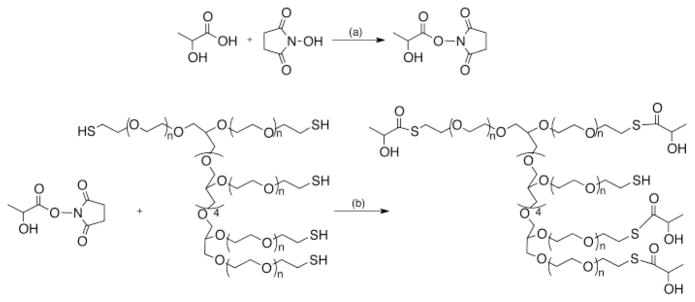
Preparation of PEG-lactic acid (PEG-LA) nanocarriers. (a) DMF, DCC, room temperature, stir for 8 h (b) DMF, DMAP, room temperature, stir for 4 h.
The release of lactic acid from the PEG-LA nanocarriers was determined at 37°C in PBS (pH 7.4) and AB (pH 4.3). Since the thioester bonds are hydrolytically labile under both acidic and basic conditions, a sustained release of lactic acid from the nanocarriers was expected under these conditions. The cumulative release of lactic acid from the nanocarriers was plotted against time and the release profile was fit using a one-phase exponential equation:
| (1) |
In eq. (1) is the fractional release of the drug and k is the rate constant. A maximum release of 23% of bound lactic acid in PBS and 47% in AB was observed (Figure 1A). Since the hydrolysis of esters in buffer is pseudo first-order, the kinetics of nanocarrier hydrolysis were determined by plotting the log of the percentage remaining PEG-LA nanocarriers against time (Figure 1B) [43, 44]. The data were fit using linear regression and the rate constant (k) and half-life (t1/2) of nanocarrier hydrolysis was estimated from the slope. Nanocarrier hydrolysis proceeded at a faster rate in AB (t1/2= 29.5 h) than in PBS (t1/2= 63.1 h). Thus, the increased release of lactic acid from the nanocarriers in AB was due to acid catalyzed ester hydrolysis.
Figure 1.
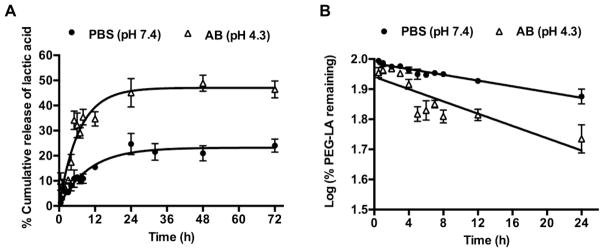
Release of lactic acid from PEG-LA nanocarriers, n=3, mean±SEM (A) Cumulative release of lactic acid in PBS and AB (pH 4.3). The release was first order with a maximum release of 23% in PBS and 47% in AB. (B) Kinetics of PEG-LA nanocarrier hydrolysis in PBS and AB. The hydrolysis of the PEG-LA nanocarriers was pseudo-first order with a t1/2 = 63.1 h in PBS and 29.5 h in AB.
3.2 Preparation and characterization of nanocarrier-based hydrogels
The nanocarrier-based hydrogels were prepared by cross-linking the PEG-LA nanocarriers with 4-arm PEG-NHS via thioester bonds (Scheme 2). Using this schematic, it was expected that lactic acid will be released from the hydrogels due to thioester hydrolysis and that the hydrogels will undergo degradation also via hydrolysis of the thioester crosslinks. The nanocarrier-based hydrogels (4–8%, w/v) were formed within 20 min under ambient conditions and an increase in nanocarrier concentration from 4% to 8% w/v resulted in faster hydrogel formation (Table 1). The 8% w/v nanocarrier-based hydrogels were not evaluated further since the PEG-LA nanocarriers at this concentration were extremely viscous and mixing the nanocarriers uniformly with the PEG-NHS polymer solutions was difficult.
Scheme 2.
Hydrogel formation using PEG-LA nanocarrier and 4-arm PEG-NHS. The release of lactic acid and degradation of the hydrogel is via hydrolysis of the thioester bonds.
Table 1.
Time of formation of PEG-LA nanocarrier-based hydrogels; mean±SD, n=3
| 8-arm PEG-LA (mg/0.05 ml) | 4-arm PEG-NHS (mg/0.05 ml) | Time of hydrogel (0.1 ml) formation (min) |
|---|---|---|
| 4 | 8 | 16.6 ± 0.2 |
| 6 | 12 | 16.5 ± 0.2 |
| 8 | 16 | 10.4 ± 0.4 |
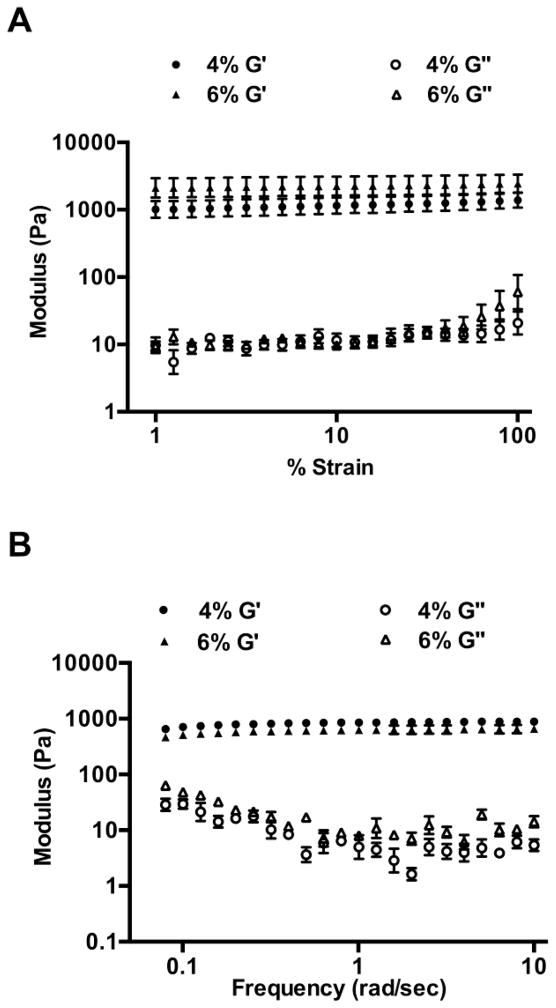
The viscoelastic properties of the nanocarrier-based hydrogels were evaluated by conducting dynamic oscillatory studies. A strain sweep test was conducted in order to establish the linear viscoelastic regime. This was followed by a frequency sweep test. The nanocarrier-based hydrogels displayed an elastic modulus (G′) that was 100-fold higher than the viscous modulus (G″) over the entire range indicating elastic solid-like behavior (Figure 2). Moreover, G′ increased only slightly with frequency and reached a plateau indicating that the hydrogels can resist structural changes under strain [45]. The viscoelastic properties of the nanocarrier-based hydrogels were compared with a hydroxyethylcellulose placebo gel (HEC gel) obtained from the NIH AIDS Reagent Reference program (HPTN 035 Study Gel). In contrast to the hydrogels, the HEC gel showed G″>G′ over the entire frequency range tested (data not shown).
Figure 2.
Dynamic oscillatory measurements on 4% and 6% w/v nanocarrier-based hydrogels, n=3; mean± SEM. (A) Strain sweep test for determining the linear viscoelastic regime (LVE). The elastic modulus (G′) was greater than the viscous modulus (G″) of the hydrogels over the entire range tested. (B) Frequency sweep test over a range of 0.1–10 rad/sec, at a constant strain of 1%. The hydrogels showed G′>G″ even at the higher applied frequencies.
The swelling and degradation of the nanocarrier-based hydrogels were determined in PBS and AB by measuring hydrogel weights at various time points following immersion in the appropriate buffer. The degree of swelling was expressed as a percentage of the initial hydrogel weight and plotted over time. A continuous increase in hydrogel weight was observed within the first 4–6 h in both conditions due to uptake of the surrounding fluid and swelling equilibrium was reached within 12 h (Figure 3A). The maximum swelling ratio for the 4% w/v hydrogels was 491.6 ±22.6% and 379.3 ±32.8% in PBS and AB, respectively, and for the 6% w/v hydrogels was 529.2 ±70% and 398.4±15% in PBS and AB, respectively (n=3, mean±SD). An increase in nanocarrier concentration from 4% to 6% w/v resulted in a larger degree of swelling in both conditions due to the increased hydrophilicity of the hydrogels. A decrease in hydrogel weights was observed after 24 h indicating that the hydrogels had started to degrade due to hydrolysis of the thioester crosslinks (Figure 3B). Degradation was considered complete when the thioester crosslinks had fully hydrolyzed and the hydrogels had dissolved into the surrounding medium. The hydrogels in PBS were found to degrade within 6 days (Figure 3B). However, a slower rate of degradation was observed in AB and the hydrogels degraded completely within 42 days.
Figure 3.
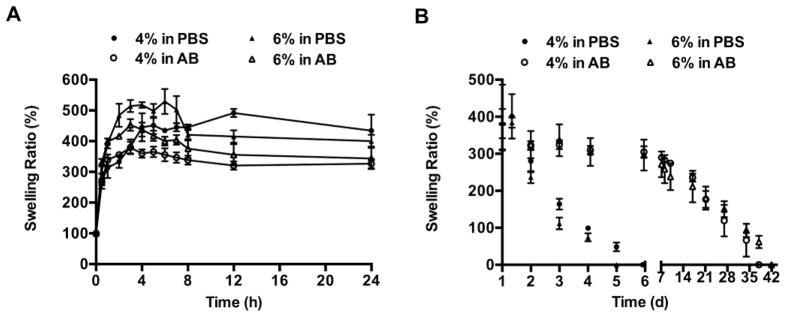
Swelling and degradation profiles of nanocarrier based hydrogels in PBS (pH 7.4) and AB (pH 4.3) at 37 °C, n=3; mean±SEM. (A) Swelling ratio of 4% and 6% w/v nanocarrier-based hydrogels in PBS and AB, until equilibrium was reached. (B) Degradation profiles of 4% and 6% w/v hydrogels determined by measuring swelling ratios from equilibrium until the hydrogels completely dissolved into the surrounding medium. The hydrogels degraded completely in 6 days in PBS and 42 days in AB.
The release of lactic acid from the nanocarrier-based hydrogels was determined in PBS and AB at 37 °C. The nanocarrier-based hydrogels showed controlled release of lactic acid for several hours. Release from 4% w/v nanocarrier-based hydrogels in PBS was first order with a maximum of 10% of bound lactic acid released in 72 h (Figure 4A). Release in AB was two-phase, with an initial fast release of 9% of bound lactic acid within 48 h, followed by a slow release phase. A maximum release of 14% of bound lactic acid was observed in AB (Figure 4A).
Figure 4.
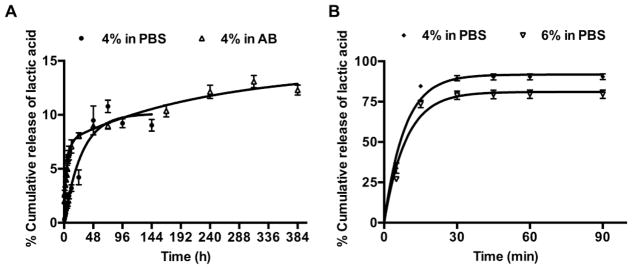
Release of lactic acid from (A) nanocarrier-based hydrogels, and (B) hydrogels with passively entrapped lactic acid at 37 °C, n=3; mean±SEM. (A) The nanocarrier-based hydrogels showed a controlled release of lactic acid with maximum release of 10% in PBS and 14% in AB. (B) The hydrogels with passively entrapped lactic acid showed a burst release of lactic acid in PBS with 90% and 80% lactic acid released from 4% w/v and 6% w/v hydrogels, respectively within 30 min.
3.3 Hydrogels with passively entrapped lactic acid
Hydrogels with passively entrapped lactic acid were prepared by mixing lactic acid with 8-arm PEG-SH (4% and 6%, w/v) and 2 equiv. of 4-arm PEG-NHS in PB at RT. The time of formation for the 4% w/v and 6% w/v hydrogels was 9.8 ± 0.2 min and 6.6 ± 0.2 min, respectively. The hydrogels with passively entrapped lactic acid thus formed faster than the nanocarrier-based hydrogels due to the availability of more unmodified–SH groups for crosslinking. The release of lactic acid from the above hydrogels was measured in PBS at 37 °C. The hydrogels showed a burst release of lactic acid, with 90% and 80% lactic acid released from 4% w/v and 6% w/v hydrogels, respectively within 30 min. (Figure 4B).
3.4 Inhibitory effect of lactic acid and hydrogels with passively entrapped lactic acid on G. vaginalis
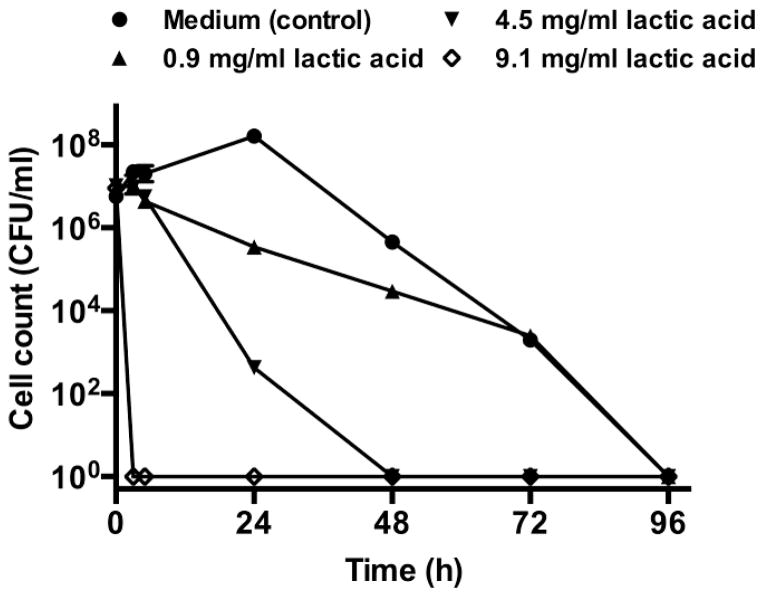
Previously, Atassi et al. demonstrated that the growth of G. vaginalis was inhibited within 4 h when incubated with lactic acid at concentrations of 100 mM [46]. More recently, O’ Hanlon et al. showed that lactic acid at concentrations of 55–111 mM (corresponding to 5–10 mg/ml) in acidic (pH 4.5) medium killed G. vaginalis within 2 h [37].The inhibitory concentration of lactic acid against G. vaginalis was determined in the current study by monitoring death kinetics of G. vaginalis in the presence of varying concentrations of lactic acid. A 7 log10 reduction in G. vaginalis cell counts was observed at a lactic acid concentration of 9.1 mg/ml within 3 h (Figure 5).
Figure 5.
Death kinetics of G. vaginalis in the presence of lactic acid. G. vaginalis was incubated in flasks with medium alone (positive control for growth) or medium with various concentrations of lactic acid (0.9–9.1 mg/ml). The flasks were incubated in the anaerobic chamber at 37 °C and cell counts were performed from the flasks at various time points, using the drop plate method, n=2, mean±SD. A 7 log10 reduction in viable cell count was observed within 3 h at a lactic acid concentration o 9.1 mg/ml.
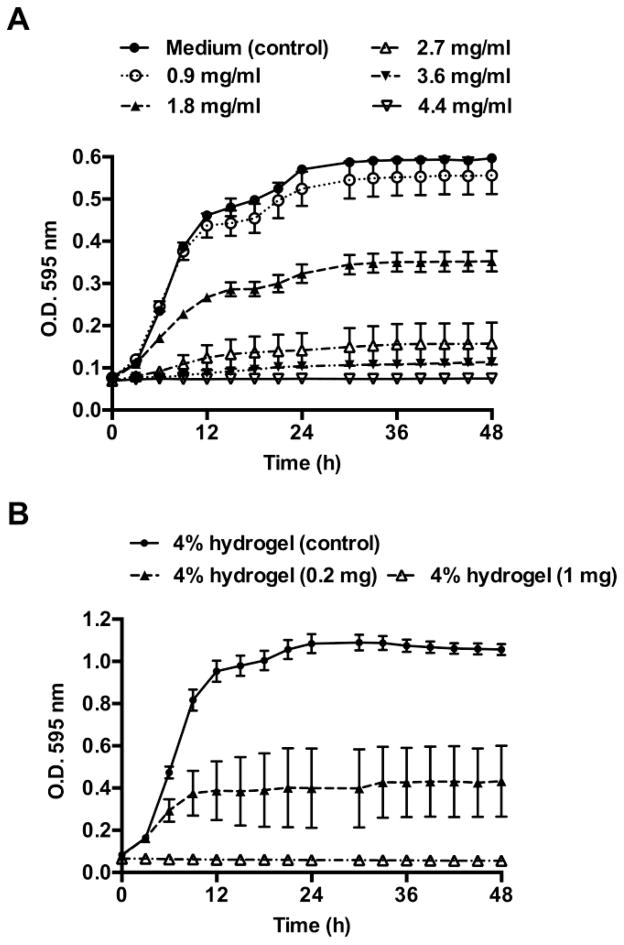
The growth kinetics of G. vaginalis was monitored in medium with various concentrations of lactic acid (0.9–4.4 mg/ml), and on hydrogels with passively entrapped lactic acid (0.2 mg and 1 mg lactic acid per 50 μl hydrogel). The nanocarrier-based hydrogels were not evaluated since a maximum of only 14% bound lactic acid was released from the nanocarrier-based hydrogels. The growth kinetics of G. vaginalis was followed turbidometrically over 48 h (O.D. 595 nm). Complete inhibition of G. vaginalis was observed with 3.6 mg/ml of lactic acid compared to the medium control (Figure 6A). Similarly, hydrogels containing 1 mg lactic acid completely inhibited G. vaginalis growth (Figure 6B). The hydrogels without entrapped lactic acid had no effect on G. vaginalis growth indicating that the antimicrobial activity was due to the lactic acid released from the hydrogels.
Figure 6.
Growth kinetics of G. vaginalis in the presence of lactic acid (0.9–4.4 mg/ml), and on hydrogels with passively entrapped lactic acid (0.2 and 1 mg per 50 μl of hydrogel), n=3, mean ±SEM. The growth kinetics was determined by measuring absorbance every hour, for 48 h (O.D. 595 nm). For the sake of clarity, the readings obtained every 3 h are shown in the above figure. The MIC of lactic acid was found to be 3.6 mg/ml.
3.5 Cytotoxicity evaluation in vaginal epithelial cells
The cytotoxicity of the 8-arm PEG-SH and 4-arm PEG-NHS polymers used to prepare the hydrogels was evaluated using the Vk2/E6E7 vaginal epithelial cell line. Vk2 cells were incubated for 24 h with 8-arm PEG-SH and 4-arm PEG-NHS polymers at concentrations of 2.5–80 mg/ml. The cytotoxicity of lactic acid was evaluated by incubating the cells with lactic acid at concentrations of 10–100 μg/ml. N-9 was used as the positive control for toxicity due to its demonstrated in vitro and in vivo cervicovaginal toxicity [47, 48]. Medium alone was used as the negative control for toxicity. After the incubation period, cell viability assessed using the alamarBlue® proliferation assay. The cells incubated with N-9 (positive control for toxicity) showed a 98% reduction in viability compared to the untreated cells (Figure 7A). The 8-arm PEG-SH and 4-arm PEG-NHS polymers showed a 50% reduction in cell viability at concentrations of 50.25 mg/ml and 57.45 mg/ml, respectively (Figure 7A). No significant reduction in cell viability was observed with lactic acid (Figure 7B).
Figure 7.
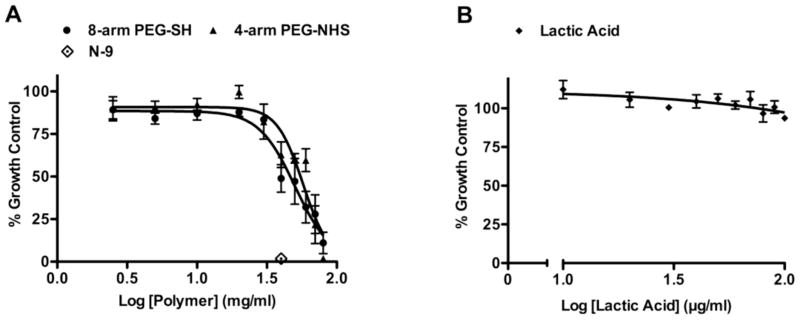
Cytotoxicity of the 8-arm PEG-SH and 4-arm PEG-NHS polymers on Vk2 cells, determined using the alamarBlue proliferation assay. (A) Cells were incubated with 8-arm PEG-SH and 4-arm PEG-NHS at concentrations from 2.5–80 mg/ml, for 24 h. N-9 was used as the positive control for toxicity and medium was used as the negative control. The data are plotted as a percentage of the medium control, n=6, mean±SEM. The CC50 values for the 8-arm PEG-SH and 4-arm PEG-NHS polymers were found to be 50.24 mg/ml and 57.45 mg/ml respectively. (B) Cells were incubated with lactic acid at concentrations of 10–100 μg/ml for 24 h. The data are plotted as a percentage of the medium control, n=6, mean±SEM.
4. DISCUSSION
The present study evaluates the feasibility of developing PEG nanocarrier-based hydrogels for the controlled release of lactic acid for restoring the vaginal microenvironment. Exploratory treatments for restoring the healthy vaginal environment and preventing BV infection include oral/vaginal administration of probiotics and vaginal acidification [49–52]. However, current intra vaginal gel formulations show poor retention in the vagina and are hence ineffective due to insufficient concentrations of the active agent in the vagina [25–27]. Therefore, the present study aims at developing PEG-based hydrogels: (1) for the controlled release of lactic acid to maintain vaginal acidity for a prolonged period of time, and (2) with viscoelastic properties to prevent leakage and improve vaginal retention.
PEG-LA nanocarriers were prepared by attaching lactic acid to 8-arm PEG-SH via degradable thioester bonds in order to obtain controlled release of lactic acid. The PEG-LA nanocarriers showed sustained release of lactic acid for several hours in PBS and AB. However, a maximum release of 23% of bound lactic acid in PBS and 47% in AB was observed from the nanocarriers. The incomplete release of lactic acid from the PEG-LA nanocarriers is likely due to steric hindrance of the branched polymer chains of the 8-arm PEG-SH polymer. Previous studies indicate that drug release from polymer-drug conjugates is influenced by a number of factors such as molecular weight of the polymer, architecture (linear vs. branched) and the type of releasable bond [53, 54]. Kurtoglu et al. demonstrated that the release of ester-linked ibuprofen from PAMAM-dendimer and mPEG conjugates was dependent on pH and polymer architecture [53]. The spherical architecture of the dendrimer conjugates inhibited hydrolysis of the ester linkages, hindering ibuprofen release in plasma. Similarly, when paclitaxel was conjugated to poly-L-glutamic acid via ester bond, drug release did not occur due to the steric hindrance in the conjugate [54].
The PEG-LA nanocarriers were cross-linked with 4-arm PEG-NHS via thioester bonds to form nanocarrier-based hydrogels (4–8%, w/v). The nanocarrier-based hydrogels formed rapidly (gelation time< 20 min) under ambient conditions and an increase in nanocarrier concentration from 4% to 8% w/v resulted in faster hydrogel formation. The nanocarrier-based hydrogels showed sustained release of lactic acid for several days; however, a maximum release of 10% bound lactic acid in PBS and 14% in AB was achieved. A reason for the low release of lactic acid from the nanocarrier-based hydrogels is likely the additional steric hindrance of the polymer chains of the cross-linked gel network, limiting access to the bound lactic acid. One way of reducing steric effects and increasing lactic acid release would be to introduce a spacer molecule between lactic acid and the thioester bond [53]. Alternatively, higher lactic acid release concentrations can be achieved by increasing the lactic acid payload of the nanocarriers by conjugating polylactic acid to the 8-arm PEG-SH polymer.
Hydrogels with passively entrapped lactic acid were prepared by mixing lactic acid with the 8-arm PEG-SH and 4-arm PEG-NHS polymer solutions under ambient conditions. The hydrogels showed a burst release of lactic acid with 90% entrapped lactic acid released within 30 min in contrast to the nanocarrier-based hydrogels, which showed sustained release. Thus the low molecular weight and high solubility of lactic acid resulted in rapid migration through the hydrogel network when passively entrapped within the polymer matrix.
Current semi-solid vaginal gels are pre-formed gels that leak rapidly from the vagina resulting in poor retention. The nanocarrier-based hydrogels developed in the current study are designed to be administered as a solution into the vagina and undergo gelation in situ. Hence, the viscoelastic properties of the nanocarrier-based hydrogels are particularly important for vaginal application since gel leakage and retention in the vagina are largely dependent on these properties [55, 56]. Previous studies have shown that semi-solid gels formed by physical interactions of polymer chains (i.e., chain entanglements) such as hydroxyethylcellulose (HEC) and sodium carboxymethylcellulose (NaCMC) gels demonstrate viscous liquid-like behavior at higher oscillatory frequencies [56–58]. Semi-solid gels, therefore, have low structural integrity under shear. Unlike semi-solid gels, covalently cross-linked PEG-based hydrogels developed previously in our laboratory showed elastic behavior at higher frequencies indicating mechanical resistance to shear [45, 59, 60]. Similarly, the nanocarrier-based hydrogels in the current study showed G′>G″ indicating that they are predominantly elastic over the frequency range tested. In contrast, the HEC gel behaved as a viscous liquid with G″>G′ over the entire frequency range (data not shown). The nanocarrier-based hydrogels are thus expected to provide improved vaginal retention when compared to semi-solid gels due to their superior elastic properties.
The nanocarrier-based hydrogels in the current study are formed via reversible thioester bonds that degrade by hydrolysis or enzymatic cleavage [61]. The in vitro swelling and degradation profile of the nanocarrier-based hydrogels was monitored in PBS and AB. Due their hydrophilic nature, the nanocarrier-based hydrogels swelled by absorbing surrounding fluid (reaching equilibrium in 12–24 h, depending on the polymer concentration) after which they started degrading.
The vaginal retention of the hydrogels and the HEC gel was studied in a pre-clinical mouse model using MRI imaging (manuscript submitted/in preparation). The hydrogels were retained in the vagina up to 48 h post-dose unlike the HEC gel, which completely cleared from the vagina within 8 h (data not shown). Due to their elastic properties, the hydrogels were observed to remain structurally intact in the vagina for several hours after undergoing gelation in situ, and prior to degradation and clearance from the vagina. Hence, the optimal in vivo dose and delivery device for forming a hydrogel film on the mucosa that is sufficient for protection but does not to impair vaginal lubrication will need to be determined.
The inhibitory effect of the hydrogels with passively entrapped lactic acid on the predominant BV pathogen G. vaginalis was determined in vitro. Since the nanocarrier-based hydrogels showed insufficient release of lactic acid, the efficacy of hydrogels with passively entrapped lactic acid was studied. Hydrogels with 1 mg entrapped lactic acid completely inhibited G. vaginalis growth demonstrating that lactic acid released from the gel had activity against G. vaginalis.
The cytotoxicity of the 8-arm PEG-SH and 4-arm PEG-NHS polymers, and lactic acid was evaluated using the Vk2/E6E7 vaginal epithelial cell line. Since the cross-linked hydrogels absorbed the surrounding medium when applied to cell monolayers, the individual components of the hydrogel were evaluated using a cell viability assay. Lactic acid did not show any toxicity at all the concentrations that were tested. The 8-arm PEG-SH and 4-arm PEG-NHS polymers showed a CC50 of 50.25 mg/ml and 57.45 mg/ml, respectively. The cytotoxicity of the individual polymers at high polymer concentrations is most likely due to the -SH and –NHS functionalization of PEG since PEG has no reported cytotoxicity. However, the free– SH and –NHS functional groups are expected to be present at lower concentrations in the hydrogel than the individual polymers due to the formation of thioester cross-links. Further evaluation of the hydrogels in organotypic or explant models will determine potential in vivo toxicity to epithelial tissue [62, 63].
5. CONCLUSIONS
PEG nanocarrier-based hydrogels were developed for the controlled release of lactic acid. The nanocarrier-based hydrogels formed rapidly (< 20 min), showed elastic properties and were degradable in both physiological and acidic conditions. Release of lactic acid from the nanocarrier-based hydrogels was sustained for several days; however, a maximum release of only 14% bound lactic acid was observed. In contrast, hydrogels with passively entrapped lactic acid showed a burst release with 90% lactic acid released in 30 min. Lactic acid showed antimicrobial activity against the primary BV pathogen G. vaginalis with a MIC of 3.6 mg/ml. In addition, the hydrogels with passively entrapped lactic acid showed retained antimicrobial activity with complete inhibition of G. vaginalis growth within 48 h. The results of the current study collectively demonstrate the potential of PEG nanocarrier-based hydrogels for vaginal administration of lactic acid for preventing and treating BV and suggest that passive entrapment combined with the longer acting lactic acid nanocarrier release may provide at least a once a week non drug treatment option.
Supplementary Material
Acknowledgments
This work was funded by a grant from the National Institutes of Health HIT-IT program (R01AI084137).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. 2012 UNAIDS Report on the Global AIDS Epidemic. 2012. [Google Scholar]
- 2.Nuttall J. Microbicides in the prevention of HIV infection: current status and future directions. Drugs. 2010;70:1231–1243. doi: 10.2165/10898650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8:685–697. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramjee G, Kamali A, McCormack S. The last decade of microbicide clinical trials in Africa: from hypothesis to facts. AIDS. 2010;24(Suppl 4):S40–49. doi: 10.1097/01.aids.0000390706.81383.f3. [DOI] [PubMed] [Google Scholar]
- 5.Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 7.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen CR, Duerr A, Pruithithada N, Rugpao S, Hillier S, Garcia P, Nelson K. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS. 1995;9:1093–1097. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 11.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 13.Moodley P, Connolly C, Sturm AW. Interrelationships among human immunodeficiency virus type 1 infection, bacterial vaginosis, trichomoniasis, and the presence of yeasts. J Infect Dis. 2002;185:69–73. doi: 10.1086/338027. [DOI] [PubMed] [Google Scholar]
- 14.Linhares IM, Summers PR, Larsen B, Giraldo PC, Witkin SS. Contemporary perspectives on vaginal pH and lactobacilli. Am J Obstet Gynecol. 2011;204:120 e121–125. doi: 10.1016/j.ajog.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Lang WR. Vaginal acidity and pH; a review. Obstet Gynecol Surv. 1955;10:546–560. doi: 10.1097/00006254-195508000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Turovskiy Y, Sutyak Noll K, Chikindas ML. The aetiology of bacterial vaginosis. J Appl Microbiol. 2011;110:1105–1128. doi: 10.1111/j.1365-2672.2011.04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobel JD. Bacterial vaginosis. Annu Rev Med. 2000;51:349–356. doi: 10.1146/annurev.med.51.1.349. [DOI] [PubMed] [Google Scholar]
- 18.Hillier SL. Diagnostic microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:455–459. doi: 10.1016/0002-9378(93)90340-o. [DOI] [PubMed] [Google Scholar]
- 19.Garg S, Anderson RA, Chany CJ, 2nd, Waller DP, Diao XH, Vermani K, Zaneveld LJ. Properties of a new acid-buffering bioadhesive vaginal formulation (ACIDFORM) Contraception. 2001;64:67–75. doi: 10.1016/s0010-7824(01)00217-7. [DOI] [PubMed] [Google Scholar]
- 20.Mumper RJ, Bell MA, Worthen DR, Cone RA, Lewis GR, Paull JR, Moench TR. Formulating a sulfonated antiviral dendrimer in a vaginal microbicidal gel having dual mechanisms of action. Drug Dev Ind Pharm. 2009;35:515–524. doi: 10.1080/03639040802488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdool Karim SS, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, Kapina M, Maslankowski L, Coletti A, Profy A, Moench TR, Piwowar-Manning E, Masse B, Hillier SL, Soto-Torres L. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25:957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer KH, Peipert J, Fleming T, Fullem A, Moench T, Cu-Uvin S, Bentley M, Chesney M, Rosenberg Z. Safety and tolerability of BufferGel, a novel vaginal microbicide, in women in the United States. Clin Infect Dis. 2001;32:476–482. doi: 10.1086/318496. [DOI] [PubMed] [Google Scholar]
- 23.Tuyama AC, Cheshenko N, Carlucci MJ, Li JH, Goldberg CL, Waller DP, Anderson RA, Profy AT, Klotman ME, Keller MJ, Herold BC. ACIDFORM inactivates herpes simplex virus and prevents genital herpes in a mouse model: optimal candidate for microbicide combinations. J Infect Dis. 2006;194:795–803. doi: 10.1086/506948. [DOI] [PubMed] [Google Scholar]
- 24.Keller MJ, Carpenter CA, Lo Y, Einstein MH, Liu C, Fredricks DN, Herold BC. Phase I randomized safety study of twice daily dosing of acidform vaginal gel: candidate antimicrobial contraceptive. PLoS One. 2012;7:e46901. doi: 10.1371/journal.pone.0046901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnhart KT, Pretorius ES, Timbers K, Shera D, Shabbout M, Malamud D. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception. 2004;70:498–505. doi: 10.1016/j.contraception.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Brown J, Hooper G, Kenyon CJ, Haines S, Burt J, Humphries JM, Newman SP, Davis SS, Sparrow RA, Wilding IR. Spreading and retention of vaginal formulations in post-menopausal women as assessed by gamma scintigraphy. Pharm Res. 1997;14:1073–1078. doi: 10.1023/a:1012113714552. [DOI] [PubMed] [Google Scholar]
- 27.Mehta S, Verstraelen H, Peremans K, Villeirs G, Vermeire S, De Vos F, Mehuys E, Remon JP, Vervaet C. Vaginal distribution and retention of a multiparticulate drug delivery system, assessed by gamma scintigraphy and magnetic resonance imaging. Int J Pharm. 2012;426:44–53. doi: 10.1016/j.ijpharm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Barnhart KT, Rosenberg MJ, MacKay HT, Blithe DL, Higgins J, Walsh T, Wan L, Thomas M, Creinin MD, Westhoff C, Schlaff W, Archer DF, Ayers C, Kaunitz A, Das S, Moench TR. Contraceptive efficacy of a novel spermicidal microbicide used with a diaphragm: a randomized controlled trial. Obstet Gynecol. 2007;110:577–586. doi: 10.1097/01.AOG.0000278078.45640.13. [DOI] [PubMed] [Google Scholar]
- 29.von Mollendorf CE, Van Damme L, Moyes JA, Rees VH, Callahan MM, Mauck CK, Puren AJ, Tweedy K, Taylor D. Results of a safety and feasibility study of the diaphragm used with ACIDFORM Gel or K-Y Jelly. Contraception. 2010;81:232–239. doi: 10.1016/j.contraception.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Gupta KM, Barnes SR, Tangaro RA, Roberts MC, Owen DH, Katz DF, Kiser PF. Temperature and pH sensitive hydrogels: an approach towards smart semen-triggered vaginal microbicidal vehicles. J Pharm Sci. 2007;96:670–681. doi: 10.1002/jps.20752. [DOI] [PubMed] [Google Scholar]
- 31.Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, McCullagh SD. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006;325:82–89. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26:502–511. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. Advances in microbicide vaginal rings. Antiviral Res. 2010;88(Suppl 1):S30–39. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005;56:954–956. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 35.Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67:5170–5175. doi: 10.1128/iai.67.10.5170-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16:1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 37.O’Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juarez Tomas MS, Ocana VS, Wiese B, Nader-Macias ME. Growth and lactic acid production by vaginal Lactobacillus acidophilus CRL 1259, and inhibition of uropathogenic Escherichia coli. J Med Microbiol. 2003;52:1117–1124. doi: 10.1099/jmm.0.05155-0. [DOI] [PubMed] [Google Scholar]
- 39.Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol. 2002;187:561–568. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 40.Conti C, Malacrino C, Mastromarino P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol. 2009;60(Suppl 6):19–26. [PubMed] [Google Scholar]
- 41.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh Y, Gao D, Gu Z, Li S, Rivera KA, Stein S, Love S, Sinko PJ. Influence of molecular size on the retention of polymeric nanocarrier diagnostic agents in breast ducts. Pharm Res. 2012;29:2377–2388. doi: 10.1007/s11095-012-0763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldwin AD, Robinson KG, Militar JL, Derby CD, Kiick KL, Akins RE., Jr In situ crosslinkable heparin-containing poly(ethylene glycol) hydrogels for sustained anticoagulant release. J Biomed Mater Res A. 2012;100:2106–2118. doi: 10.1002/jbm.a.34050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Najlah M, Freeman S, Attwood D, D’Emanuele A. Synthesis, characterization and stability of dendrimer prodrugs. Int J Pharm. 2006;308:175–182. doi: 10.1016/j.ijpharm.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 45.Anumolu SS, DeSantis AS, Menjoge AR, Hahn RA, Beloni JA, Gordon MK, Sinko PJ. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials. 2010;31:964–974. doi: 10.1016/j.biomaterials.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atassi F, Servin AL. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol Lett. 2010;304:29–38. doi: 10.1111/j.1574-6968.2009.01887.x. [DOI] [PubMed] [Google Scholar]
- 47.Krebs FC, Miller SR, Catalone BJ, Fichorova R, Anderson D, Malamud D, Howett MK, Wigdahl B. Comparative in vitro sensitivities of human immune cell lines, vaginal and cervical epithelial cell lines, and primary cells to candidate microbicides nonoxynol 9, C31G, and sodium dodecyl sulfate. Antimicrob Agents Chemother. 2002;46:2292–2298. doi: 10.1128/AAC.46.7.2292-2298.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stafford MK, Ward H, Flanagan A, Rosenstein IJ, Taylor-Robinson D, Smith JR, Weber J, Kitchen VS. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–331. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 49.Falagas ME, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect. 2007;13:657–664. doi: 10.1111/j.1469-0691.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 50.Andersch B, Lindell D, Dahlen I, Brandberg A. Bacterial vaginosis and the effect of intermittent prophylactic treatment with an acid lactate gel. Gynecol Obstet Invest. 1990;30:114–119. doi: 10.1159/000293230. [DOI] [PubMed] [Google Scholar]
- 51.Delia A, Morgante G, Rago G, Musacchio MC, Petraglia F, De Leo V. Effectiveness of oral administration of Lactobacillus paracasei subsp. paracasei F19 in association with vaginal suppositories of Lactobacillus acidofilus in the treatment of vaginosis and in the prevention of recurrent vaginitis. Minerva Ginecol. 2006;58:227–231. [PubMed] [Google Scholar]
- 52.Tasdemir M, Tasdemir I, Tasdemir S, Tavukcuoglu S. Alternative treatment for bacterial vaginosis in pregnant patients; restoration of vaginal acidity and flora. Arch AIDS Res. 1996;10:239–241. [PubMed] [Google Scholar]
- 53.Kurtoglu YE, Mishra MK, Kannan S, Kannan RM. Drug release characteristics of PAMAM dendrimer-drug conjugates with different linkers. Int J Pharm. 2010;384:189–194. doi: 10.1016/j.ijpharm.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 54.Maeda H, Greish K, Fang J. The enhanced permeability and retention effect and polymeric drugs: a paradigm shift for cancer chemotherapy in the 21st century. Adv Polym Sci. 2006;193:103–121. [Google Scholar]
- 55.das Neves JSB, Cunha T, Teizeira B, Rocha P, Dias G. Vaginal microbicides: the importance of effective distribution, retention and coating of the mucosa. AIDS. 2008;22:2. doi: 10.1097/QAD.0b013e3282f73870. [DOI] [PubMed] [Google Scholar]
- 56.das Neves J, da Silva MV, Goncalves MP, Amaral MH, Bahia MF. Rheological properties of vaginal hydrophilic polymer gels. Curr Drug Deliv. 2009;6:83–92. doi: 10.2174/156720109787048294. [DOI] [PubMed] [Google Scholar]
- 57.Owen DH, Peters JJ, Kieweg SL, Geonnotti AR, Schnaare RL, Katz DF. Biophysical analysis of prototype microbicidal gels. J Pharm Sci. 2007;96:661–669. doi: 10.1002/jps.20736. [DOI] [PubMed] [Google Scholar]
- 58.Mourtas S, Fotopoulou S, Duraj S, Sfika V, Tsakiroglou C, Antimisiaris SG. Liposomal drugs dispersed in hydrogels. Effect of liposome, drug and gel properties on drug release kinetics. Colloids Surf B Biointerfaces. 2007;55:212–221. doi: 10.1016/j.colsurfb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Anumolu SS, Singh Y, Gao D, Stein S, Sinko PJ. Design and evaluation of novel fast forming pilocarpine-loaded ocular hydrogels for sustained pharmacological response. J Control Release. 2009;137:152–159. doi: 10.1016/j.jconrel.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anumolu SS, Menjoge AR, Deshmukh M, Gerecke D, Stein S, Laskin J, Sinko PJ. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials. 2011;32:1204–1217. doi: 10.1016/j.biomaterials.2010.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lendlein A, Langer R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science. 2002;296:1673–1676. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- 62.Ayehunie S, Cannon C, Larosa K, Pudney J, Anderson DJ, Klausner M. Development of an in vitro alternative assay method for vaginal irritation. Toxicology. 2011;279:130–138. doi: 10.1016/j.tox.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cummins JE, Jr, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, Grohskopf LA, Paxton L, Dezzutti CS. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51:1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.