Abstract
Mice provide a highly valuable resource for investigating learning and memory processes; however, many of the established tasks for evaluating learning and memory were developed for rats. Behaviors of mice in these tasks appear to be driven by different motivational factors, and as a result, they often do not perform reliably on tasks involving rewards traditionally used for rats. Because of difficulties in measuring learning and memory in mice as well as the need to have a task that can reliably measure these behavioral processes, we have developed a mouse version of the Stone T-maze utilizing what appears to be the primary motivation of mice, escape to a safe location. Specifically, we have constructed a task that requires the mouse to wade through water to reach a dark and dry goal box. To escape this aversive environment, the Stone T-maze requires learning the correct sequence of 13 left and right turns to reach the goal box. Through a series of experiments examining a variety of protocols, it was found that mice will reliably perform this task. This task can be used to assess learning and memory without the potential performance confounds that can affect performance of mice in other tasks. We believe this task offers a valuable new tool for evaluating learning and memory in mice not previously available to researchers.
Keywords: Mice, learning, memory, maze, water-motivated
1. Introduction
The advent of genetic techniques in mice to study a wide variety of molecular, physiological and behavioral processes has led to a major growth in their use in biomedical research. The ability to manipulate individual genes within their genome has opened new avenues evaluating the role of specific genes in functional outcomes, e.g., behavior. However, one of the difficulties in evaluating genetic alterations on functional outcomes results from the fact that most of the behavioral tasks were developed and refined for use of rats as subjects. While many of the tasks available to evaluate sensory and motor function have transferred relatively easily from rats to mice, tasks associated with higher neural processing such as learning and memory have proven more difficult to apply to mice.
The Morris water maze is probably the most commonly used task for evaluating spatial learning and memory in mice. Originally developed for use in rats, the advent of genetic and molecular mouse models led to a marked rise in the use of this task to assess learning and memory in mice (Crawley, 2000). Rats are excellent performers in this task, but mice have proven to be much more problematic, and in general often appear to be less responsive to the contingencies required to successfully acquire competency in this task. Although it is unclear whether these decrements in performance are related to swimming or spatial learning capabilities (Ikeda et al., 2005; Whishaw and Tomie, 1997), many laboratories have successfully adapted and applied this paradigm. However, while mice generally make an effort to swim in the Morris maze, they are often reported to float passively (Rogers et al., 1999), have difficulty remaining afloat (Hartman et al., 2001), and, due to their smaller size, exhibit hypothermia (Iivonen et al., 2003). In addition, this task requires a significant motor component, a potential confound for studies involving mice that have motor or muscle dysfunction resulting from age or genetic manipulation. Finally there is the heavy dependence on visual abilities for performance in Morris maze. Relying on extramaze visual cues, rodents must use vision to acquire their spatial location in the maze in order to navigate efficiently to the goal platform. Despite relatively poor visual acuity in rats and mice generally, this requirement does not seem to impede learning in young animals; however, impaired visual abilities can present an issue when using the Morris maze to examine learning and memory in aged animals.
A variety of tasks designed for evaluating learning and memory in mice have been investigated in many different laboratories (Holmes et al., 2002; Koopmans et al., 2003; Kuc et al., 2006; Lange-Asschenfeldt et al., 2007; Patil et al., 2009) with varying degrees of success. In addition, our laboratory has evaluated a number of other tasks, without success, in an attempt to find a task capable of reliably measuring learning of memory in mice. One of our observations from the various tasks we investigated was that escape from the task appeared to be the primary motivational drive for mice. Instead of working to retrieve a food or water reward, or remaining on the platform in the water maze to learn its spatial location, the mice invested considerable time and energy in trying to escape from the apparatus. Therefore, we decided to modify the rat version of the Stone T-maze (STM), a task that has proven reliable and valid for evaluating learning and memory in rats (Spangler et al., 1986; Ingram, 1988) for use in mice.
In the STM the animal is required to learn the correct sequence of left and right turns to successfully navigate the maze from a start box to a goal box. Figure 1 presents the configuration of the maze. Reinforcement to learn the task has been imposed by food rewards following food deprivation (Berman et al., 1988), water rewards following water deprivation (Goodrick, 1968), and avoidance and/or escape from footshock (Spangler et al., 1986; Ingram, 1988). In the current version, the negative reinforcement was applied by having the mice wade, not swim, through water to reach a goal box that was both dark and dry, allowing escape out of the water. We designed a series of experiments to assess the validity of the maze to evaluate learning and memory in mice using a number of different training protocols. However, it is important to note while the mouse STM provides a valuable tool for measuring learning and memory, it cannot be considered a direct replacement for the Morris maze as the mouse STM is most likely not heavily dependent on spatial learning (Ingram, 1985; 1988). However, the initial data collected with the mouse STM indicates it may be more appropriate for assessing learning and memory in mice due to its motivational properties.
Figure 1.
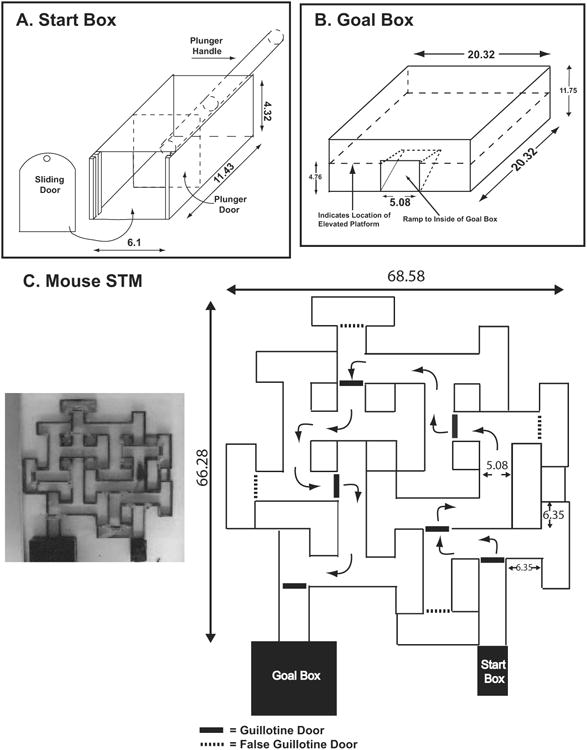
Schematics of the start box (A), goal box (B) and maze (C) are presented. All of the numbers designating dimensions indicate length in centimeters.
2. Experimental Procedures
2.1 Animals
For Experiments 1-4, the mice were 3-6 month old male C57BL/6J (Exp 1, N=7; Exp 2, N=7, Exp 3, N=6, Exp 4, N=7) obtained from the Jackson Laboratory (Bar Harbor, ME) For Experiment 5 the mice were 3-4 month old virgin male A/J (Cohort 1, N=8; Cohort 2, N=8) obtained from the Jackson Laboratory (Bar Harbor, ME). The mice were housed in the vivarium at the Pennington Biomedical Research Center under controlled environmental conditions (22± °C, 70±10% humidity) with a 12-h light/dark cycle. The mice were group housed (4/cage) and had ad libitum access to both standard chow (Lab Diets, 5001) and water. All procedures were approved by the Institutional Animal Care and Use Committee of the Pennington Biomedical Research Center and followed the NIH guidelines for the Care and Use of Laboratory Animals.
2.2 Apparatus
The straight run and maze were constructed from clear or black acrylic. Both the straight run and maze were placed into a steel pan that was filled with water to a level where half of the walls were covered by water (approximately 2.22 cm deep), and the water was maintained at 20-24 degrees Celsius. The walls of both the straight run and maze were constructed using black opaque acrylic 3.81 cm high with feet 0.64 cm in height placed at various locations under the bottom of walls to allow for circulation of water under the walls. The ceiling of the straight run and maze were covered with clear acrylic to prevent mice from rearing out of the water. These dimensions created a situation where the mice were capable of remaining in contact with the floor while maintaining their head above water but could not rear up. The straight run consisted of an alley 68.58 cm long and the alley through which the mice traversed was 5.08 cm wide. At the beginning of each trial, the mouse was placed into a start box constructed completely out of opaque acrylic with a removable top and sliding door that was raised by hand at the start of a trial. Inside the back wall of the start box, there was a sliding panel that could be moved using a rod extending out the rear wall to push the mice from the start box if needed. The start box was 11.43 in length, with the height and width matching the dimensions of the alleys in the straight run and maze. An overhead view of the maze is shown in Figure 1A, and a schematic of the maze with dimensions is shown in Figure 1B.
Overall the maze measured 68.58 cm × 66.68 cm. Throughout the entire maze, the alleys through which the mice traveled were 5.08 cm wide. In general, the arms forming the stem of a T-junction were 6.35 cm long, and the two dead-end arms of the T were 5.72-6.35 cm long. The dimensions of the T's were relatively consistent, but minor variations occurred when necessary to fit within the overall design and construction of the apparatus. During construction, one of the choice-points present in the rat STM was omitted from the mouse version, so the two versions are not identical. Five sliding guillotine doors were placed at various locations throughout the maze (see Figure 1C). Once a mouse successfully navigated a section of the maze, the door was lowered to prevent the mouse from potentially backtracking too far after an error resulting in increased potential for a failed trial. These doors were located within a supporting framework attached to the ceiling of the maze, and were open and closed manually by the experimenter using the fishing line with a weight at the end. To prevent the mice from utilizing the guillotine doors as navigation cues, false guillotine doors were placed at specific points in the maze over the top of alleys leading to dead-end T-junctions.
The enclosed goal box was constructed from opaque black acrylic and measured 20.32 cm wide × 20.32 cm in length and was 11.76 cm high. In one wall was an open door the same height and with dimensions as the alleys of the maze and straight run. At the door a ramp led up to an elevated floor within the box that was 4.78 cm above the floor of the pan holding the water. This allowed the mice to escape above the water level when reaching the goal box. The ceiling of the goal box was removable to allow access to the mice.
2.3 Procedure
On day 1 the mice underwent straight-run training. The straight-run training was implemented to establish the contingency that moving forward would allow them to escape from the water into the goal box. Successful completion of this training phase required that the mice reach the goal box in 15 sec or less on 13/15 trials, with a maximum of 30 trials administered. Any mice that were unable to reach this criterion were excluded from further behavioral testing. For all experiments, maze training commenced the following day.
To provide a comprehensive assessment of the ability of this new maze paradigm to measure learning and memory, a variety of different training protocols were evaluated. The primary measures of learning were the latency to reach the goal box and the number of errors committed. The errors were scored online by the experimenter, but all of the straight run training and maze trials were recorded using a video tracking system for later review (Viewpoint Lifesciences, Montreal, Quebec, Canada). An error was defined as complete entry of the mouse's head into an incorrect path. During acquisition, if a mouse failed to reach the goal box within 6 min, the trial was terminated and was scored as a failure. Any mouse achieving three failures was removed from further analysis.
2.3.1 Experiment 1: Traditional Protocol
To provide a direct comparison to the shock-motivated rat version of the STM, mice were given a total of 15 consecutive acquisition trials during a single session. This is one of the most commonly used protocols in the rat version (Devan et al., 2004; Pistell et al., 2009). Each mouse completed all 15 trials, with a 2-min intertrial interval (ITI), before the next mouse was given its first trial. Retention was evaluated 7 days following maze acquisition during which no further exposure to the maze occurred. Retention testing consisted of 5 trials in the maze with the same session protocol as during acquisition. In addition, long-term retention was tested in this group of mice after 35 days from the previous retention test. Including the long-term retention testing (5 trials), the mice were given 20 additional trials in the maze prior to testing them for the potential use of the goal box as a cue. After receiving the additional 20 trials (10 trials/day over 2 days), the goal box was removed, and the mice were given 5 trials. Following the 5 trials with the goal box removed, the mice were given an additional 5 trials with the goal box placed on the opposite side of the maze from where it was located during acquisition. This procedure was implemented to assess whether the mice were using the goal box as a visual cue to help guide them through the maze.
2.3.2 Experiment 2: Daily Spaced Training Protocol
This protocol was evaluated to assess the ability of the mice to acquire task performance when training was distributed over several days. This approach may provide a means to assess retention over the course of training. Mice were administered daily sessions of 5 acquisition trials/day over 4 consecutive days for a total of 20 acquisition trials. Each mouse received all trials in succession with approximately a 2 min ITI, before the next mouse was administered acquisition. Retention was evaluated 1 week following the last day of acquisition. As another check to verify what type of strategy the mice were utilizing to learn the maze, the mice were given 10 additional trials in the maze 37 days after the retention test to firmly establish task performance. Following additional training, 5 trials were administered with the entire maze rotated 180° to assess whether or not the mice were using external visual cues, or the possible contribution of hippocampal place cells.
2.3.3 Experiment 3: Weekly Spaced Training Protocol
To further extend the evaluation of distributed training, mice were administered acquisition training in weekly intervals. A single session consisted of 5 trials, and was repeated at weekly intervals until the mice completed a total of 7 sessions/35 trials. Each mouse received 5 trials in succession with an approximately 2 min ITI.
4.3.4 Experiment 4: Traditional Protocol with Extended ITI
Due to the potential for prolonged exposure to the water when testing mice that may be impaired, or simply take a longer duration to complete the maze due to motor deficits, a protocol similar to that traditionally used for water maze studies was evaluated. For this experiment 8 mice received all 15 maze acquisition trials in a single day. However, the mice were run in a squad where every mouse received Trial 1 prior to the first mouse receiving Trial 2. This resulted in an ITI of approximately 5-12 minutes in duration. During the ITI mice were placed in a holding cage with a towel. Retention was assessed one week following acquisition.
2.3.5 Experiment 5: Evaluation of the A/J strain
Because a wide variety of mouse strains are utilized for research, it was important to establish the ability of the mouse STM to assess learning in a different strain than the C57BL/6J. The A/J strain was chosen because of previous reports indicating its inferior performance on tasks such as the water maze (Upchurch and Wehner, 1988; Logue et al., 1997; Thifault et al., 2002). If this mouse strain was capable of acquiring task performance in the mouse STM, the potential for success in a wide variety of strains would be deemed more likely. The protocol used for evaluating A/J mice was the same as Experiment 4. However, due to some observations made while running the first cohort of this strain, two cohorts were run with different lighting conditions. For the first cohort indirect overhead lighting via lamps attached to the walls provided higher overall illumination levels in the maze. For the second cohort, the overhead lighting was turned off with the light source coming from an in-ceiling bank of fluorescent lights at the far end of the room.
2.4 Statistics
For all of the experiments, the acquisition data were analyzed using ANOVA with repeated measures and the retention data using within-subjects t-tests. Statistical significance was accepted as p<0.05.
3. Results
3.1. Experiment 1: Traditional Training Protocol and Goal Box Assessment
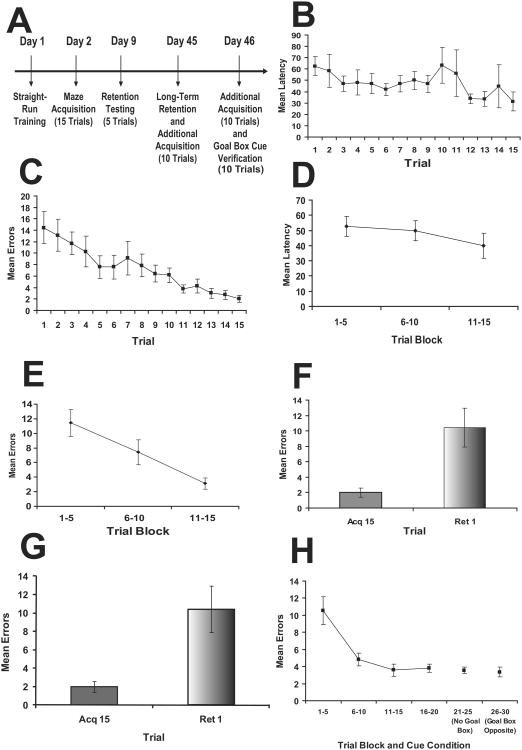
The timeline for training is depicted in Figure 2A. When the data were represented across all trials, the latency to reach the goal box did not indicate a significant learning curve (F(14,84) = 1.04, p = 0.422; Figure 2B). However, examination of errors committed across all trials indicated the mice were able to acquire task performance (F(14,84) = 7.14, p < 0.001; Figure 2C). For the sake of simplified presentation and analysis, data collected from the rat shock-motivated version of the STM was usually collapsed into trial blocks of 3 or 5 trials. When the current data were represented in this fashion (Figures 2D & 2E), the same overall conclusions were reached for both latency (F(2,12) = 2.48, p = 0.13) and errors (F(2,12) = 18.88, p < 0.001).
Figure 2.
Results from Experiment 1 with the traditional protocol. A. Timeline for the experiment. B. Mean latency (+/- sem) across acquisition trials. C. Mean errors (+/- sem) across acquisition trials. D. Mean latency (+/- sem) collapsed into 5 trial blocks. E. Mean errors (+/-) collapsed into 5 trial blocks. F. Mean errors (+/- sem) for 1-week retention through comparison of the last acquisition trial (acq15) to the first retention trial (ret1). G. Mean errors (+/- sem) for 1-month retention through the comparison of the last trial of 1-week retention to the first trial of further acquisition training for cue analysis. H. Mean errors (+/- sem) collapsed into 5 trial blocks for overtraining and evaluation of performance after goal box removal, and goal box moved to alternate location.
In our experience the best index of retention is provided by comparing the last acquisition trial, when maze acquisition is at its peak, to the first trial of retention, due to the fact that the retention trials are essentially further acquisition trials, and only the first retention trial is truly representative of retention. Retention testing after both 1-week (t(6) = 3.16, p = 0.02; Figure 2F) and 1-month (t(6) = 2.97, p = 0.025; Figure 2G) intervals indicated “forgetting”, as measured by the number of errors when retested; however the degree of forgetting did not appear to be substantially worse at 1-month compared to 1-week.
To evaluate whether the mice were using the goal box as a cue to learn the proper route through the maze, we conducted experiments in which the goal box was removed or located to an opposite side of the maze. Neither removal of the goal box (t(6) = 0.43, p = 0.68), nor moving it to the opposite side from where it was located during acquisition (t(6) = 0.718, p = 0.50), had a significant impact on performance as measured by errors (Figure 2H) since there was no increase in the number of errors committed when the location of the goal box was shifted.
3.2. Experiment 2: Distributed Daily Protocol and Maze Rotation
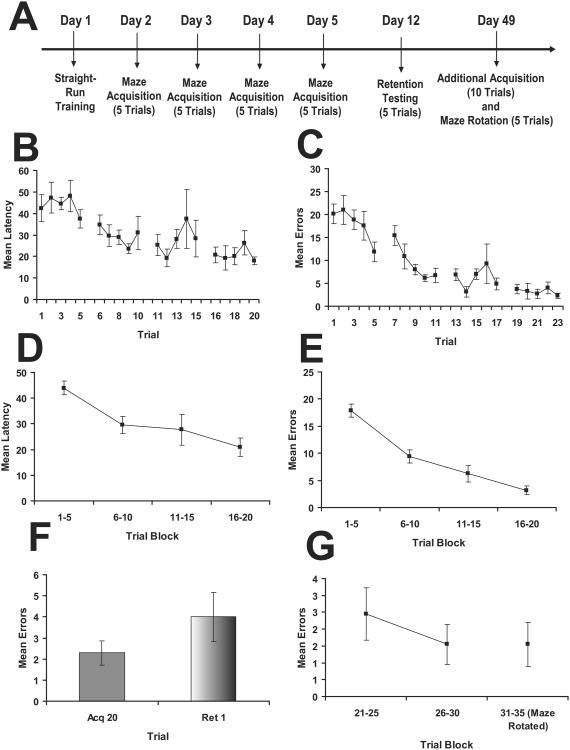
The second experiment evaluated the ability of mice to acquire task performance when acquisition trials were distributed across daily intervals. The timeline is presented in Figure 3A. As observed in Figures 3B & 3C, mice improved in task performance as indicated by decreased latency and errors across both days and trials. While for latency there was only a significant main effect for Day (F(3,18) = 7.64, p = 0.002), for errors there was significantly improved learning across both Day (F(3,18) = 28.38, p < 0.001) and Trial (F(4,24) = 3.82, p = 0.015, as well as a significant Day × Trial interaction (F(12,72) = 2.21, p = 0.020). When the data were collapsed into 5 trial blocks, mice exhibited learning as indicated by decreased latency (F(3,18) = 7.64, p = 0.002; Figure 3D) and errors (F(3,28) = 193.88, p < 0.001; Figure 3E). With this protocol the mice did not exhibit a significant decrement in error performance after a 1-week interval (t(6) = 0.81, p = 0.447; Figure 3F). Rotation of the maze 180° after additional training had no impact on performance (t(6) = 0.00, p = 1.0; Figure 3G), indicating the mice were most likely not using external cues in their strategy to learn the maze.
Figure 3.
Results from Experiment 2 evaluating the daily spaced training protocol. A. Timeline for the experiment. B. Mean latency (+/- sem) across acquisition trials. C. Mean errors (+/- sem) across acquisition trials. D. Mean latency (+/- sem) collapsed into 5 trial blocks. E. Mean errors (+/-) collapsed into 5 trial blocks. F. Mean errors (+/- sem) for 1-week retention through comparison of the last acquisition trial (acq15) to the first retention trial (ret1). G. Mean errors (+/- sem) collapsed into 5 trial block for overtraining and evaluation of performance when maze was rotated 180°.
3.3. Experiment 3: Distributed Weekly Protocol
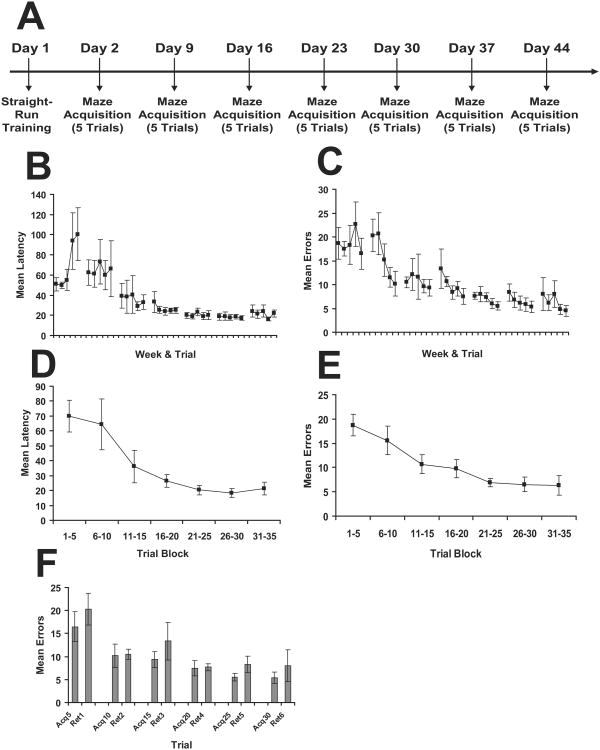
To further examine the impact of distributed training on the ability of the mice to acquire this task, this experiment assessed performance when 5 trials/day were administered at weekly intervals. The timeline of testing is shown in Figure 4A. As can be seen in Figure 4B, latency indicated significantly improved performance across days (F(6,24) = 5.12, p = 0.002), but there was no significant effect for Trials (F(4,16) = 1.84, p = 0.017) nor a significant interaction (F(24,98) = 1.46, p = 0.10). While there was no significant interaction for errors (F(24,120) = 1.20, p = 0.255), the mice did exhibit improved performance across both days F(6,30) = 12.13, p < 0.001) and trials (F(4,20) = 6.42, p = 0.002) as shown in Figure 4C. When the data for latency and errors were collapsed into 5 trial blocks (Figures 4D & 4E), there also was evidence of learning (Latency Trial Block: F(6,30) = 8.02, p < 0.001; Errors Trial Block: F(6,30) = 12.13, p < 0.001). Because the mice received acquisition training at 1-week intervals, evaluation of 1-week retention was already integrated within this protocol. Comparison of the last trial from the previous week with the first trial of the next session indicated the mice did not exhibit a significant decrement in performance across any of the weekly intervals under this protocol (Figure 4F).
Figure 4.
Results from Experiment 3 evaluating the weekly spaced training protocol. A. Timeline for the experiment. B. Mean latency (+/- sem) across acquisition trials. C. Mean errors (+/- sem) across acquisition trials. D. Mean latency (+/- sem) collapsed into 5 trial blocks. E. Mean errors (+/-) collapsed into 5 trial blocks. F. Mean errors (+/- sem) for 1-week retention by comparison of the last trial of the previous week, with the first trial of the subsequent week.
3.4. Experiment 4: Evaluation of a Distributed ITI protocol
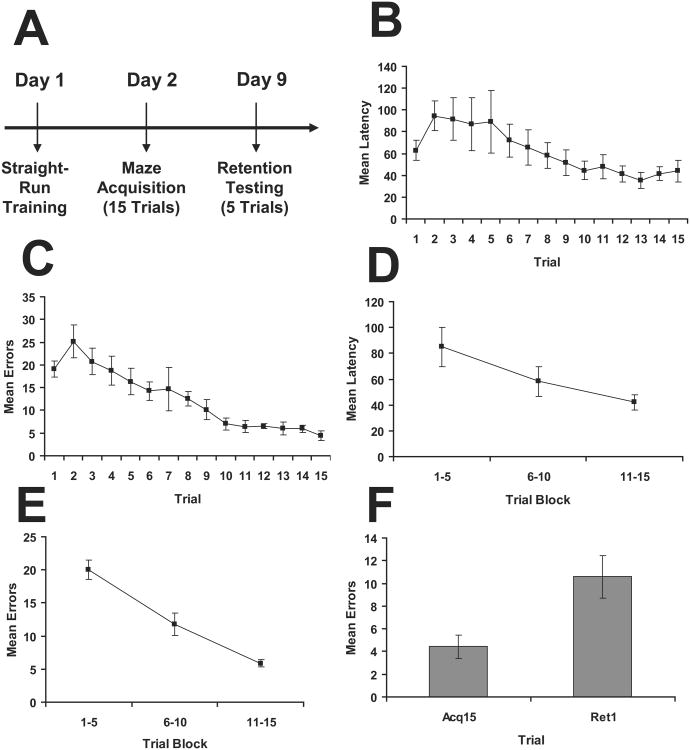
This protocol evaluated the ability of the mice to acquire the task when 15 acquisition trials were conducted in a single day, but the mice were given a longer ITI between trials (approximately 10-25 min). The timeline of testing is shown in Figure 5A. Analysis of the data across trials indicated learning as measured by both latency (Figure 5B; F(14,84) = 3.36, p < 0.001) and errors (Figure 5C; F(14,84) = 7.70, p < 0.001). When the data were collapsed into 5 trial blocks, there was also evidence for learning as measured by both latency (Figure 5D; F(2,12) = 8.69, p = 0.005) and errors (Figure 5E; F(2,12) = 26.80, p = < 0.001). Comparison of the last acquisition trial with the first retention trial indicated the mice exhibited a decrement in performance after the 1-week interval as measured by errors (Figure 5F; t(7) = 3.45, p = 0.014), which replicated the pattern of forgetting observed in Figure 2F.
Figure 5.
Results from Experiment 4 evaluating the traditional protocol with extended ITI protocol. A. Timeline for the experiment. B. Mean latency (+/- sem) across acquisition trials. C. Mean errors (+/- sem) across acquisition trials. D. Mean latency (+/- sem) collapsed into 5 trial blocks. E. Mean errors (+/-) collapsed into 5 trial blocks. F. Mean errors (+/- sem) for 1-week retention through comparison of the last acquisition trial (acq15) to the first retention trial (ret1).
3.5. Experiment 5: Evaluation of the A/J Strain
To evaluate the capabilities of the mouse STM to assess learning and memory in a mouse strain generally considered to be a poor learner, the A/J strain was tested. The protocol used for running the A/J mice was the same as for Experiment 4, and the timeline is shown in Figure 6A. Two groups of A/J mice were run with different lighting conditions, the first with higher lighting levels in the area of the maze, and the second with very diffuse lighting. When the data were analyzed by trials, there was no significant difference between the two groups for latency (Group: F(1,11) = 0.05, p = 0.83; Figure 6B), but both groups exhibited improved performance (Trial: F(14,154) = 14.80, p < 0.001). However, while there was no significant main effect of Group for errors (F(1,10) = 1.76, p = 0.214), there was a significant main effect for Trials (F(14,140) = 12.94, p < 0.001) as well as a significant Group × Trial interaction (F(14,140) = 2.37, p = 0.006), indicating that while both groups of mice were capable of acquiring the task, the cohort run with the dimmed lights exhibited better acquisition (Figure 6C). The same pattern of results for both latency (Figure 6D) and errors (Figure 6E) was evident when the data were collapsed into 5 trial blocks. As shown in Figure 6F, evaluation of 1-week retention indicated cohort 1 (lights on) exhibited a retention deficit (t(7) = 3.19, p = 0.015), while the number of errors committed by cohort 2 on the last acquisition trial compared to the first retention trial did not quite reach statistical significance (t(7) = 2.26, p = 0.058). Overall, these results indicate the mouse STM is capable of measuring learning and memory even in strains traditionally considered to be difficult to train.
Figure 6.
Results from Experiment 5 evaluating performance of two groups of the A/J strain. The first group was run with higher levels of illumination in the maze environment and the second group with very diffuse indirect lighting. A. Timeline for the experiment. B. Mean latency (+/- sem) across acquisition trials. C. Mean errors (+/- sem) across acquisition trials. D. Mean latency (+/- sem) collapsed into 5 trial blocks. E. Mean errors (+/-) collapsed into 5 trial blocks. F. Mean errors (+/- sem) for 1-week retention through comparison of the last acquisition trial (acq15) to the first retention trial (ret1).
4. Discussion
The present series of experiments evaluating the mouse STM indicates it is a reliable paradigm for measuring learning and memory in both C57BL/6J and A/J mice. Across a variety of training protocols, the mice were motivated through the negative reinforcement of water escape to improve task performance across training trials. While the latency to reach the goal box is included as a measurement in the mouse STM, the number of errors committed emerged as the most reliable and meaningful measurement of learning and memory that is not confounded by motoric factors.
The key element in developing the mouse STM has been to manipulate the primary motivation of escape to achieve consistent performance. From our experience in evaluating learning and memory in other tasks, including the water maze, the common difficulty encountered is not due to an inability of the mice to learn. Instead, rather than work for the intended reinforcement, e.g., for a food or water reward, mice will often spend most of their time trying to escape from the apparatus. Although the water maze does utilize escape as the reinforcement, when the mice reach the platform, many elect to remain in the water, and rather than attending to visual cues while on the platform, they often spend that time seeking a way to escape from the maze such as re-entering the water to swim or leaping from the platform to the side of the pool which indicates their discomfort with the goal situation.
Another advantage of the mouse STM centers on the fact that the mice remain in contact with the floor, alleviating the heavy motor and muscle function demands inherent in the water maze. This is problematic for studies where comparisons are being made between mice of different ages, or between wildtype and genetically modified mice in which alterations in motor function may confound the primary measure of learning— latency. Because the primary measure of learning and memory in the mouse STM is number of errors, their cognitive function is evaluated independently of motor function, e.g., a mouse under the influence of a drug with soporific side effects can complete the maze with the same number of errors as a control mouse, even though it may take twice as long to reach the goal box. In the water maze this confound of altered motor function could be mistakenly be interpreted as impaired learning.
Also, consistent with past descriptions of rat performance in the STM (Ingram, 1985; 1988), vision is not heavily taxed during performance in this paradigm. Rats can navigate this maze when run under dark conditions (Ingram, 1985; 1988). This design feature is highly important when evaluating maze performance in aged animals with a high potential for visual impairments. In the current study, we found that manipulating extra-maze cues, such as moving the goal box or rotating the maze 180 degrees did not affect task performance. Thus, another advantage of this maze is the reduced demand on learning performance guided by visual cues.
Aspects of the motivational properties of the mouse STM have been utilized previously in other paradigms. Combining the aspects of the water maze and Barnes maze, Deacon and Rawlins (Deacon and Rawlins, 2002) found mice readily learned a task when required to wade through water to escape out of a circular pool through the correct doorway, out of 12, located in the walls of the maze. Upon reaching the correct door, the mice were allowed to run through an alley and were then immediately transferred back to their home cage. Mice with hippocampal lesions exhibited impaired acquisition compared to control mice for both latency and error measures, although the differences were much more pronounced with errors.
Versions of the Hebb-Williams maze have been used that are similar to the mouse STM in utilizing shallow water to motivate the mice to search for escape into the goal box. After the initial development (Hebb and Williams, 1946) and refinement (Rabinovitch and Rosvold, 1951), a scaled down version was developed for mice (Meunier et al., 1986). A mouse swimming version of this maze was found to correlate with performance on other water-motivated tasks, including the Morris water maze (Locurto and Scanlon, 1998). The Hebb-Williams maze has been utilized successfully to evaluate cognitive function across different strains of mice (Galsworthy et al., 2002; Galsworthy et al., 2005), individual differences in mice (Locurto et al., 2003; Locurton et al., 2006), effects of arginine 8-vasopressin (Paban et al., 2003), transgenic mice (Coleman et al., 1999) and the effects of focal lesions of the cingulate cortex (Meunier and Destrade, 1988). However, there are several key elements that differ between the two maze tasks. The Hebb-Williams maze utilizes water at a depth of 3.5 cm while the mouse STM utilizes water at a depth of approximately 2.25 cm. The protocol for the Hebb-Williams maze calls for a water temperature of 15° C, while in the mouse STM it was maintained at 20-24° C. The ceiling of the maze was much higher in the Hebb-Williams maze (10 cm) than in the mouse STM (approximately 4.45 cm). The lower height in the mouse STM prevents the mice from rearing out of the water, and is most likely a critical component of keeping the mice moving forward in search of escape. One critical distinction is that the configurations used for the Hebb-Williams maze generally consist of relatively few choice points 3-5, compared to the 13 in the current configuration of the mouse STM.
In general, the mice were motivated to reach the goal box of the STM and did not spend too much time inactive or in non-goal oriented activities during a trial. A small percentage of mice (approximately 10-15%) exhibited some resistance to maintaining on-task behavior during trials. This resistance often occurred after a mouse spent a long trial in the maze, or after engaging in extreme perseverative behavior in one section. In most of these cases, the mice could be encouraged to proceed again using a series of additional interventions on the part of the experimenter. Whenever a mouse paused and became motionless, e.g., no longer made movements even with the head, the first stimulus used to encourage the mice was quickly opening and closing one of the guillotine doors to make a noise. If this was not sufficient to stimulate the mouse to move, then the experimenter would, by lifting up one side of the maze, raise and lower the maze slightly. These two approaches worked for most of the mice that exhibited pausing behavior, and within a few trials, they were completing trials without pausing. However, there were some mice that continued to pause and required additional stimulation to perform throughout all of their trials. There were some that refused to move under any circumstances (less than 5%), and reached the criterion of three failed trials requiring them to be removed from further analysis. The A/J mice required much more experimenter encouragement than the C57BL/6 mice, but none of them reached the criterion of three failed trails for removal from further training. A similar percentage of rats failed to complete the shock-motivated version of the STM, so these results are consistent, and despite the occasional failure, the percentages of mice that would not complete the maze protocol was markedly smaller than other tasks we have attempted to utilize (50-80% completion failure). Based on these results, we believe the mouse STM to be an extremely valuable tool for evaluating learning and memory in mice.
Future studies need to address several specific issues regarding learning and memory in the mouse STM. Evaluation of additional mouse strains will potentially expand its usefulness for assessing learning and memory across a wide variety of strains, thereby making it relevant for assessment of cognition across various genetic models. Additional experiments need to address the type of strategy employed by the mice to learn the maze and the brain areas involved. Previous work in rats indicated that while hippocampal circuitry is involved (Duffy et al., 2008), an intact striatum appears to be critical for successful acquisition (Pistell et al., 2009). These studies need to be replicated in the mouse STM. In addition, previous work in rats implicated both the cholinergic, glutamatergic, and nitric oxide systems (Spangler et al., 1986; Ingram et al., 1998a; Ingram et al.; 1998b, Meyer et al.; 1998, Pistell et al., 2007), and confirming these results in the mouse STM would further establish the validity of the mouse in comparison to previously published results in rats.
While a number of different acquisition protocols were evaluated, we have generally settled on utilizing the distributed ITI protocol from Experiment 4. Due to the fact that the mice get a longer rest between trials, this makes the protocol more conducive when running potentially impaired mice who may be spending a longer duration in the maze on each trial. However, all of the protocols were adequate for measuring learning and memory, and depending on the question being asked, are clearly feasible for implementation. The different acquisition protocols led to different degrees of retention deficits. As would be expected based on evidence indicating the effectiveness of massed versus distributed training on learning and memory (Fanselow and Tighe, 1988; Rowe and Craske, 1998), retention deficits were observed when acquisition occurred in a single day, (Experiments 1, 4 & 5), but not when training was distributed over several days or weeks (Experiments 2 & 3). Again the protocol used in Experiment 4 produced a retention deficit that would permit assessment of this important parameter.
Further development plans will improve ease of use for this maze. Currently we are working on developing automated scoring of errors via a video tracking system and software. We also plan to include distance traveled in our dependent measures of maze performance. Because of the relatively small size of the mouse STM, it requires much less laboratory space than required for tasks such as the water maze. In addition, its design easily lends itself to adaptation to other motivational stimuli such as foot shock. Rather than being placed in the pan filled with water, it can easily be placed upon a shock grid. Additionally we are exploring other configurations of the maze to provide the opportunity for multiple maze testing.
Overall, the mouse STM appears to be a reliable and valid task for studying learning and memory in mice and provides a new tool for researchers to expand their pursuit of the underlying mechanisms, pathologies and therapeutic interventions relevant to cognitive function.
Acknowledgments
We would like to thank Richard Zichos at the National Institute on Aging (Baltimore, MD) for working with us on the design, and for constructing the original version of the mouse STM.
Abbreviations
- STM
Stone T-maze
- ITI
intertrial interval
Footnotes
Section Editor: Dr. Geoffrey M. Schoenbaum
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berman RF, Goldman H, Altman HJ. Age-related changes in regional cerebral blood flow and behavior in Sprague-Dawley rats. Neurobiol Aging. 1988;9:691–696. doi: 10.1016/s0197-4580(88)80134-9. [DOI] [PubMed] [Google Scholar]
- Coleman GJ, Bernard CC, Bernard O. Bcl-2 transgenic mice with increased number of neurons have a greater learning capacity. Brain Res. 1999;832:188–194. doi: 10.1016/s0006-8993(99)01498-5. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York: Wiley-Liss; 2000. [Google Scholar]
- Deacon RMJ, Rawlins JNP. Learning impairments of hippocampal-lesioned mice in a paddling pool. Behav Neurosci. 2002;116:472–478. doi: 10.1037//0735-7044.116.3.472. [DOI] [PubMed] [Google Scholar]
- Devan BD, Sierra-Mercado D, Jr, Jimenez M, Bowker JL, Duffy KB, Spangler EL, Ingram DK. Phosphodiesterase inhibition by sildenafil citrate attenuates the learning impairment induced by blockade of cholinergic muscarinic receptors in rats. Pharmacol Biochem Behav. 2004;79:691–699. doi: 10.1016/j.pbb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Duffy KB, Spangler EL, Devan BD, Guo Z, Bowker JL, Janas AM, Hagepanos A, Minor RK, DeCabo R, Mouton PR, Shukitt-Hale B, Joseph JA, Ingram DK. A blueberry-enriched diet provides cellular protection against oxidative stress and reduces a kainate-induced learning impairment in rats. Neurobiol Aging. 2008;29:1680–1689. doi: 10.1016/j.neurobiolaging.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Tighe TJ. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of explicit conditional stimuli. J Exp Psychol Anim Behav Process. 1988;14:187–199. [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Liu L, Monleón S, Gregoryan G, Fernandes C, Schalkwyk LC, Plomin R. Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav Genet. 2005;35:675–692. doi: 10.1007/s10519-005-3423-9. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Monleón S, Plomin R. Evidence for general cognitive ability (g) in heterogeneous stock mice and an analysis of potential confounds. Genes Brain Behav. 2002;1:88–95. doi: 10.1034/j.1601-183x.2002.10204.x. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Learning, retention, and extinction of a complex maze habit for mature-young and senescent Wistar albino rats. J Gerontol. 1968;23:298–304. doi: 10.1093/geronj/23.3.298. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer's-like neuropathology. Exp Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- Hebb DO, Williams K. A method of rating animal intelligence. J Gen Psychol. 1946;34:59–65. doi: 10.1080/00221309.1946.10544520. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Iivonen H, Nurminen L, Harri M, Tanila H, Puolivali J. Hypothermia in mice tested in Morris water maze. Behav Brain Res. 2003;141:207–213. doi: 10.1016/s0166-4328(02)00369-8. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Shoji M, Kawarai T, Kawarabayashi T, Matsubara E, Murakami T, Sasaki A, Tomidokoro Y, Ikarashi Y, Kuribara H, Ishiguro K, Hasegawa M, Yen SH, Chishti MA, Harigaya Y, Abe K, Okamoto K, St George-Hyslop P, Westaway D. Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am J Pathol. 2005;166:521–531. doi: 10.1016/S0002-9440(10)62274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK. Analysis of age-related impairments in learning and memory in rodent models. Ann N Y Acad Sci. 1985;444:312–331. doi: 10.1111/j.1749-6632.1985.tb37599.x. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Complex maze learning in rodents as a model of age-related memory impairment. Neurobiol Aging. 1988;9:475–485. doi: 10.1016/s0197-4580(88)80101-5. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Spangler EL, Kametani H, Meyer RC, London ED. Intracerebroventricular injection of N omega-nitro-L-arginine in rats impairs learning in a 14-unit T-maze. Eur J Pharmacol. 1998a;341:11–16. doi: 10.1016/s0014-2999(97)01427-1. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Spangler EL, Meyer RC, London ED. Learning in a 14-unit T-maze is impaired in rats following systemic treatment with N omega-nitro-L-arginine. Eur J Pharmacol. 1998b;341:1–9. doi: 10.1016/s0014-2999(97)01426-x. [DOI] [PubMed] [Google Scholar]
- Koopmans G, Blokland A, van Nieuwenhuijzen P, Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol Behav. 2003;79:683–693. doi: 10.1016/s0031-9384(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Kuc KA, Gregersen BM, Gannon KS, Dodart JC. Holeboard discrimination learning in mice. Genes Brain Behav. 2006;5:355–363. doi: 10.1111/j.1601-183X.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, Lohmann P, Riepe MW. Spatial performance in a complex maze is associated with persistent long-term potentiation enhancement in mouse hippocampal slices at early training stages. Neuroscience. 2007;147:318–324. doi: 10.1016/j.neuroscience.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Locurto C, Fortin E, Sullivan R. The structure of individual differences in heterogeneous stock mice across problem types and motivational systems. Genes Brain Behav. 2003;2:40–55. doi: 10.1034/j.1601-183x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- Locurto C, Scanlon C. Individual differences and a spatial learning factor in two strains of mice (Mus musculus) J Comp Psychol. 1998;112:344–352. [Google Scholar]
- Locurton C, Benoit A, Crowley C, Miele A. The structure of individual differences in batteries of rapid acquisition tasks in mice. J Comp Psychol. 2006;120:378–388. doi: 10.1037/0735-7036.120.4.378. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Meunier M, Destrade C. Electrolytic but not ibotenic acid lesions of the posterior cingulate cortex produce transitory facilitation of learning in mice. Behav Brain Res. 1988;27:161–172. doi: 10.1016/0166-4328(88)90041-1. [DOI] [PubMed] [Google Scholar]
- Meunier M, Saint-Marc M, Destrade C. The Hebb-Williams test to assess recovery of learning after limbic lesions in mice. Physiol Behav. 1986;37:909–913. [PubMed] [Google Scholar]
- Meyer RC, Spangler EL, Patel N, London ED, Ingram DK. Impaired learning in rats in a 14-unit T-maze by 7-nitroindazole, a neuronal nitric oxide synthase inhibitor, is attenuated by the nitric oxide donor, molsidomine. Eur J Pharmacol. 1998;341:17–22. doi: 10.1016/s0014-2999(97)01428-3. [DOI] [PubMed] [Google Scholar]
- Paban V, Soumireu-Mourat B, Alescio-Lautier B. Behavioral effects of arginine8-vasopressin in the Hebb-Williams maze. Behav Brain Res. 2003;141:1–9. doi: 10.1016/s0166-4328(02)00316-9. [DOI] [PubMed] [Google Scholar]
- Patil SS, Sunyer B, Hager H, Lubec G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res. 2009;198:58–68. doi: 10.1016/j.bbr.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Daffin LW, Jr, Nelson CM, Duffy KB, Bowker JL, Spangler EL, Ingram DK, Devan BD. Combined administration of subthreshold doses of the nitric oxide inhibitor, nitro-L-arginine, and muscarinic receptor antagonist, scopolamine, impairs complex maze learning in rats. Behav Pharmacol. 2007;18:801–805. doi: 10.1097/FBP.0b013e3282f18d2f. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Nelson CM, Miller MG, Spangler EL, Ingram DK, Devan BD. Striatal lesions interfere with acquisition of a complex maze task in rats. Behav Brain Res. 2009;197:138–143. doi: 10.1016/j.bbr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch MS, Rosvold HE. A closed-field intelligence test for rats. Can J Psychol. 1951;5:122–128. doi: 10.1037/h0083542. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Jones DNC, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Craske MG. Effects of an expanding-spaced vs massed exposure schedule on fear reduction and return of fear. Behav Res Ther. 1998;36:701–717. doi: 10.1016/s0005-7967(97)10016-x. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Rigby P, Ingram DK. Scopolamine impairs learning performance of rats in a 14-unit T-maze. Pharmacol Biochem Behav. 1986;25:673–679. doi: 10.1016/0091-3057(86)90158-9. [DOI] [PubMed] [Google Scholar]
- Thifault S, Lalonde R, Sanon N, Hamet P. Comparisons between C57BL/6J and A/J mice in motor activity and coordination, hole-poking, and spatial learning. Brain Res Bull. 2002;58:213–218. doi: 10.1016/s0361-9230(02)00782-7. [DOI] [PubMed] [Google Scholar]
- Upchurch M, Wehner JM. Differences between inbred strains of mice in Morris water maze performance. Behav Genet. 1988;18:55–68. doi: 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Of Mice and Mazes: Similarities Between Mice and Rats on Dry Land But Not Water Mazes. Physiol Behav. 1997;60:1191–1197. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]