Abstract
The landmark HIV Prevention Trials Network (HPTN) 052 trial in HIV-discordant couples demonstrated unequivocally that treatment with antiretroviral therapy (ART) substantially lowers the probability of HIV transmission to the HIV-uninfected partner. However, it has been vigorously debated whether substantial population-level reductions in the rate of new HIV infections could be achieved in “real-world” sub-Saharan African settings where stable, cohabiting couples are often not the norm and where considerable operational challenges exist to the successful and sustainable delivery of treatment and care to large numbers of patients. We used data from one of Africa’s largest population-based prospective cohort studies (in rural KwaZulu-Natal, South Africa) to follow up a total of 16,667 individuals who were HIV-uninfected at baseline, observing individual HIV seroconversions over the period 2004 to 2011. Holding other key HIV risk factors constant, individual HIV acquisition risk declined significantly with increasing ART coverage in the surrounding local community. For example, an HIV-uninfected individual living in a community with high ART coverage (30 to 40% of all HIV-infected individuals on ART) was 38% less likely to acquire HIV than someone living in a community where ART coverage was low (<10% of all HIV-infected individuals on ART).
One of the most successful public health interventions ever undertaken has been the provision of combination antiretroviral therapy (ART) to more than 6.2 million people in sub-Saharan Africa (1). The ART scale-up has resulted in substantial population-level reductions in HIV-related mortality in many populations (2, 3) and overall is estimated to have saved a total of more than nine million life-years (1). The results of the landmark HIV Prevention Trials Network (HPTN) 052 trial in HIV-discordant couples demonstrated unequivocally that reducing the infected partner’s viral load through ART substantially lowers the probability of HIV transmission to the uninfected partner (4). This finding has further fueled hope that widespread use of ART could not only substantially increase life expectancy but also reduce the rate of new HIV infections at a population level and reverse the epidemic (5). Indeed, predictive mathematical models have suggested that under certain conditions high coverage of ART could lead to a substantial decrease in the rate of new HIV infections (6, 7).
The HPTN 052 trial was run under the controlled conditions of a well-conducted clinical study, and hopes of a substantial reduction in the rate of new HIV infections in the hyperendemic communities of sub-Saharan Africa have been tempered with legitimate concerns relating to uptake of HIV testing and treatment, retention, adherence, resistance development, risk compensation in sexual behavior, high rates of migration, and the capacity of health systems to deliver ART. Further, it is unclear to what extent the results of the trial can be extrapolated to communities where stable, cohabiting couples are not the norm (8).
The existing global evidence of the population effect of HIV treatment as prevention has been based on “ecological” associations (correlations between group-level variables and group-level outcomes) between increasing ART coverage and HIV outcomes, such as the number of new HIV diagnoses in a given administrative region of (9). Such designs provide a weak basis for causal inference and may be subject to “ecological fallacy” (10), that is, the inferential fallacy that occurs when a statistical association observed between variables on an aggregate level does not reflect the association that exists at an individual level. In this study, we use data from a population cohort of nearly 17,000 individuals who were HIV-uninfected at baseline and follow them up over several years (2004 to 2011), observing individual HIV seroconversions. We regress the individual-level outcome—time-to-HIV seroconversion—on ART coverage in the local community surrounding each HIV-uninfected individual to estimate the effect of increasing coverage on their risk of HIV acquisition. Although our exposure variable of interest— community-level ART coverage—is necessarily ecological in nature, our outcome and other variables are measured at the individual level, avoiding ecological fallacies in effect attribution. An alternative, rigorous approach to measure the impact of ART coverage on HIV acquisition risk is a cluster-randomized controlled trial. One such trial is already under way at the Africa Centre for Health and Population Studies, and several other such trials will be starting shortly (11). However, the results of these trials will not be available for at least another 4 years.
The study we report here took place in Hlabisa subdistrict, one of the five subdistricts in the rural district of Umkhanyakude in northern KwaZulu-Natal, South Africa (fig. S1). ART has been rapidly scaled up in the district through the Hlabisa HIV Treatment and Care Programme, and by mid-2012 treatment had been initiated in more than 20,000 patients (12). The study area is characterized by high adult HIV prevalence (24% in adults aged 15 years and older in 2011) and high levels of poverty and unemployment (in 2010, 67% of adults over the age of 18 in the study area were unemployed). ART was delivered in 17 community-based clinics by nurses and ART counselors using the standard South African drug regimens, which conform to World Health Organization (WHO) ART guidelines (13). Initially, adults with WHO clinical stage 4 disease or CD4+ cell counts of <200 cells/µl were eligible for ART. The treatment eligibility threshold was increased to CD4+ <350 cells/µl for pregnant women and tuberculosis patients in April 2010, and this new threshold was subsequently extended to all adults in August 2011. During the period of observation, no population groups were eligible for treatment initiation at CD4+ ≥350 cells/µl.
Within the Hlabisa subdistrict lies the Africa Centre demographic surveillance area, with ~60,000 resident individuals under demographic surveillance at any given time (14). Between 2004 and 2006, all women age 15 to 49 years and men age 15 to 54 years resident in the surveillance area were eligible for HIV testing. In 2007, eligibility for the HIV survey was extended to cover all residents age ≥15 years. This HIV population cohort is open, and any individual who migrates into the area immediately becomes eligible for participation in the HIV survey. Within a 5-year period, about 80% of all individuals consented to HIV testing. However, in a single year of testing, the HIV consent rate was ~50%; the rate increased in the most recent 3-year period. To determine the proportion of the total HIV-infected population receiving ART, data from the Hlabisa HIV Treatment and Care Programme’s database [maintained by Africa Centre personnel and described elsewhere (12)] were matched to the Africa Centre’s demographic data using strict matching criteria: A person was linked either by his/her unique South African identification number or by his/her name, if both first and last name matched.
The 16,667 repeat testers who were HIV-uninfected at first test in the Africa Centre’s population-based cohort were individually geolocated to their homesteads, using the comprehensive Africa Centre geographic information system. HIV prevalence and ART coverage for each individual’s own surrounding community were then determined for every year of observation. ART coverage and HIV prevalence around each individual were measured by means of a moving two-dimensional Gaussian kernel of 3 km search radius (fig. S3) for each year of observation (2004 to 2011). The size of the kernel was determined from the results of previous work (15). The kernel moves systematically across the map and measures spatial variation in HIV prevalence [derived from the ~10,000 participants who consent to HIV testing each year (14)] across the surveillance area. It then uses the resulting prevalence and geographic distribution of the total eligible population to calculate the number of HIV-infected individuals in the surrounding local community for each cell on the grid. In the same manner, each patient on ART was geolocated to their homestead of residence, and the number of individuals in the surrounding local community on ART was computed for each cell on the grid. These data are then used to derive ART coverage (which we define as the proportion of all HIV-infected individuals receiving ART) at every location. Given the scattered distribution of the population (homesteads are not concentrated into villages or compounds as in many parts of Africa), the method is well suited to this setting because it does not impose any static geographical boundaries on the data but uses the precise location of each individual (fig. S2) to derive a sensitive community-level measure that is both responsive to local variations and robust to the effects of random noise. The resulting ART coverage measure is a true population estimate because its denominator includes all HIV-infected individuals in this community, independently of whether they have ever accessed an ART program or not.
The group of 16,667 repeat HIV testers in this study are 75% of all individuals (≥15 years of age) ever observed to be HIV-uninfected through the Africa Centre’s population-based HIV surveillance (2004 to 2011). The median duration between tests in these individuals was 1.8 years. We used survival analysis to examine the effect of ART coverage in the surrounding local community on an HIV-uninfected individual’s risk of HIV acquisition while controlling for a range of well-established demographic, behavioral, economic, and environmental determinants of acquisition of new HIV infection (16, 17). These control variables rule out confounding by the individual and community characteristics they capture. In the analysis an individual from this group of repeat HIV testers was considered to be at risk of acquiring HIV after the date of their first HIV-seronegative test.
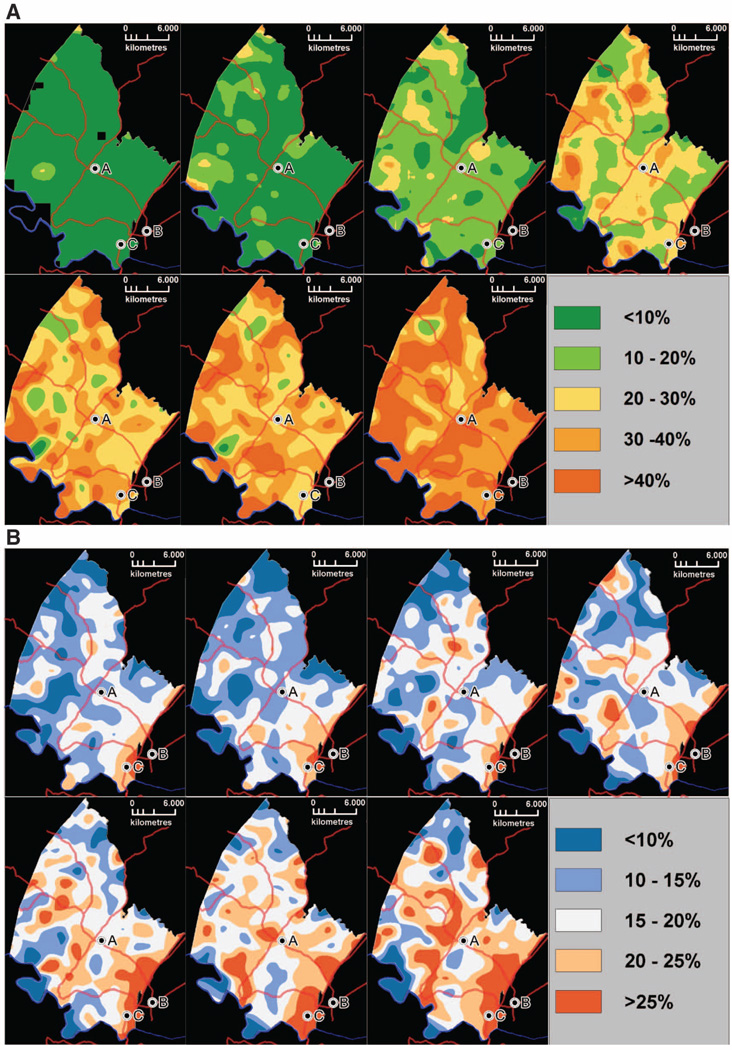
Treatment for the first patients in the surveillance area began in September 2004, and by July 2011, an estimated 37% of all HIV-infected adults in the area had been successfully started on ART. Overall, the speed with which ART has been scaled up in this population has been rapid but heterogeneous in time and space (Fig. 1A). In 2007, 6.4% of the population lived in communities where coverage of all HIV-infected individuals exceeded 30%, and by the middle of 2011 this figure had increased to 90.8%. The geography of ART coverage did not follow a clear spatial pattern over the period of observation (2004 to 2011). HIV prevalence was consistently highest in the peri-urban communities next to the National Road (Fig. 1B), where >30% of the adult population were infected with HIV. The lowest HIV prevalence occurred among communities in the more remote rural areas (<15%). There was little correlation between community-level HIV prevalence and ART coverage (correlation coefficient = 0.17).
Fig. 1.
Time series of maps showing the evolution of the proportion of the HIV-infected adults (≥15 years of age) receiving ART (A) and HIV prevalence (B) across the demographic surveillance area (2005 to 2008, left to right, top row; 2009 to 2011, left to right, bottom row). A, Africa Centre; B, Mtubatuba Town; C, KwaMsane Township. Main roads are also superimposed for ease of reference. Each pixel on the map corresponds to the proportion of the total HIV-infected population receiving ART (A) and total population infected with HIV (B) in the surrounding local community as measured using a standard Gaussian kernel of radius 3 km. Over the study period, estimated HIV prevalence in the adult population (≥15 years of age) increased from 18 to 24%, which is likely a consequence of the increasing life span of HIV-infected people due to ART. The proportion of the total HIV-infected population receiving ART was estimated at 37% in mid-2011.
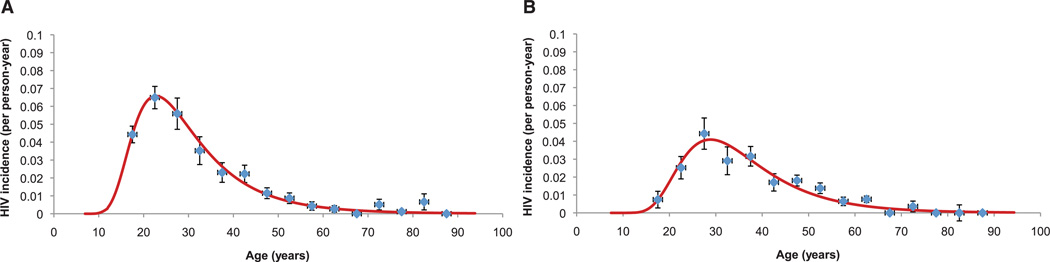
We directly observed 1413 HIV seroconversions in the 53,605 person-years at risk observed over the period 2004 to 2011 in the 16,667 individuals who were HIV-uninfected at baseline. See table S1 for the characteristics of the HIV-uninfected individuals. The crude HIV incidence over the observation period was 2.63 new infections per 100 person-years [95% confidence interval (CI) 2.50 to 2.77]. Incidence peaked at 6.6 per 100 person-years in women at age 24, and 5 years later in men at 4.1 per 100 person-years of observation (Fig. 2).
Fig. 2.
Female (A) and male (B) age variations in HIV incidence (95% CI) by 5-year age-group for entire sample of repeat testers (N = 16,667; 53,605 person years of observation). Superimposed on the graphs are log-normal functions (obtained by maximum likelihood) fitted to the incidence point estimates.
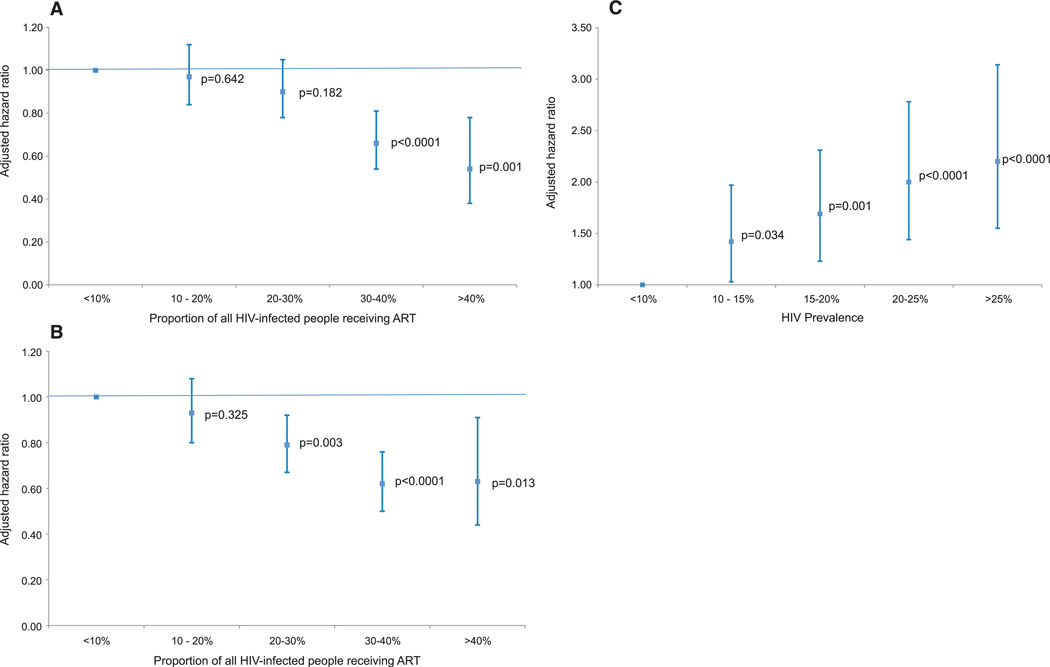
After controlling for differences in the age and sex distribution, the risk of an HIV-uninfected individual acquiring HIV (that is, the acquisition hazard) was substantially lower in areas of high ART coverage (Fig. 3A). Specifically, the risk of infection to an individual living in an area where the ART coverage was 30 to 40% was 34% (P < 0.0001) less than to an individual living in an area with ART coverage of <10%.To increase the strength of causal inference based on the observed association between ART coverage and individuals’ risk of newly acquiring HIV, we also controlled for a wide range of well-known demographic, behavioral, economic, and environmental determinants of an individual’s risk of HIV acquisition (community-level HIV prevalence, marital status, household-level wealth, and the number of sexual partners in the past 12 months). After ruling out confounding by these factors, there was a steep and highly significant decline in an individual’s adjusted HIV acquisition hazard with increasing ART coverage (Fig. 3B). Holding other factors equal, an HIV-uninfected person who lived in a community with ART coverage of 30 to 40% was on average 38% (P < 0.0001) less likely to acquire HIV infection than an HIV-uninfected person living in a community with ART coverage of <10% (Fig. 3B). When we include ART coverage (%) in the model as a continuous variable we find that, overall, a 1% increase in ART coverage is associated with a 1.4% decline [adjusted hazard ratio (aHR) = 0.986] in the risk of acquisition of new HIV infection (Table 1).
Fig. 3.
Results of the multivariable analysis showing an HIV-uninfected individual’s HIV aHR (95% CI) and associated P values for different categories of ART coverage, that is, the proportion of the total HIV infected population receiving ART (A and B), and HIV prevalence (C) in the surrounding local community (derived using a standard Gaussian kernel of radius 3 km, as shown in Fig. 1, A and B). (A) displays the aHRs adjusted for systematic differences in age and sex, and (B) and (C) display the aHRs adjusted for all other variables in the final model (see Table 1 and table S2 multivariable analysis).
Table 1.
Output from the final multivariable survival model showing the effect of coverage of ART in the surrounding community on an HIV-uninfected individual’s hazard of acquiring HIV infection (N = 16,667).
| Variable | Univariable analysis | Multivariable analysis† | ||
|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI) | P | |
| Community-level* ART coverage‡ (versus <10%) | ||||
| 10–20% | 0.85 (0.74–0.98) | 0.030 | 0.93 (0.8–1.08) | 0.325 |
| 20–30% | 0.72 (0.62–0.83) | <0.0001 | 0.79 (0.67–0.92) | 0.003 |
| 30–40% | 0.51 (0.42–0.63) | <0.0001 | 0.62 (0.50–0.76) | <0.0001 |
| >40% | 0.40 (0.28–0.58) | <0.0001 | 0.63 (0.44–0.91) | 0.013 |
| Community-level* HIV prevalence‡ (versus <10%) | ||||
| 10–15% | 1.40 (1.02–1.93) | 0.039 | 1.42 (1.03–1.97) | 0.034 |
| 15–20% | 1.63 (1.20–2.22) | 0.002 | 1.69 (1.23–2.31) | 0.001 |
| 20–25% | 1.86 (1.37–2.54) | <0.0001 | 2.00 (1.44–2.78) | <0.0001 |
| >25% | 1.99 (1.45–2.74) | <0.0001 | 2.20 (1.55–3.14) | <0.0001 |
| Age-sex strata‡ (versus male 15–19 years old) | ||||
| Male 20–24 | 3.29 (2.34–4.64) | <0.0001 | 3.03 (2.15–4.27) | <0.0001 |
| Male 25–29 | 5.77 (3.98–8.36) | <0.0001 | 5.22 (3.6–7.58) | <0.0001 |
| Male 30–34 | 3.82 (2.35–6.2) | <0.0001 | 3.67 (2.23–6.04) | <0.0001 |
| Male 35–39 | 4.15 (2.58–6.65) | <0.0001 | 4.32 (2.67–6.99) | <0.0001 |
| Male 40–44 | 2.23 (1.23–4.05) | 0.008 | 2.47 (1.36–4.5) | 0.003 |
| Male ≥ 45 | 1.25 (0.82–1.89) | 0.294 | 1.71 (1.1–2.64) | 0.017 |
| Female 15–19 | 5.97 (4.40–8.09) | <0.0001 | 6.36 (4.67–8.64) | <0.0001 |
| Female 20–24 | 8.45 (6.24–11.44) | <0.0001 | 8.92 (6.56–2.12) | <0.0001 |
| Female 25–29 | 7.16 (5.13–9.99) | <0.0001 | 7.74 (5.52–0.85) | <0.0001 |
| Female 30–34 | 4.62 (3.16–6.74) | <0.0001 | 5.38 (3.66–7.91) | <0.0001 |
| Female 35–39 | 2.96 (1.99–4.39) | <0.0001 | 3.71 (2.46–5.58) | <0.0001 |
| Female 40–44 | 2.91 (2.00–4.24) | <0.0001 | 3.61 (2.44–5.36) | <0.0001 |
| Female ≥ 45 | 0.87 (0.60–1.24) | 0.429 | 1.14 (0.78–1.67) | 0.494 |
| Area of residence‡ (versus rural) | ||||
| Peri-urban | 1.37 (1.23–1.53) | <0.0001 | 1.13 (0.98–1.3) | 0.105 |
| Urban | 1.00 (0.70–1.43) | 0.985 | 0.93 (0.64–1.35) | 0.703 |
| Marital status (versus single) | ||||
| Married monogamous | 0.33 (0.27–0.41) | <0.0001 | 0.67 (0.53–0.84) | 0.001 |
| Married polygamous | 0.43 (0.25–0.75) | 0.003 | 1.14 (0.65–2.01) | 0.64 |
| Number of partners in the last 12 months (versus zero) | ||||
| One | 3.22 (2.18–4.76) | <0.0001 | 1.77 (1.17–2.67) | 0.008 |
| More than one | 3.72 (2.47–5.61) | <0.0001 | 2.49 (1.61–3.86) | <0.0001 |
| Household wealth quintile (versus poorest) | ||||
| 2nd poorest | 0.99 (0.84–1.16) | 0.881 | 0.96 (0.82–1.14) | 0.658 |
| 3rd poorest | 1.03 (0.88–1.21) | 0.721 | 0.97 (0.82–1.14) | 0.676 |
| 4th poorest | 1.02 (0.87–1.20) | 0.813 | 0.92 (0.78–1.08) | 0.296 |
| Wealthiest | 0.94 (0.78–1.12) | 0.468 | 0.82 (0.68–1.00) | 0.044 |
| Weibull shape parameter | – | – | 2.94 (2.80–3.13) | 0.003 |
Derived using a standard Gaussian kernel (radius 3 km) around each HIV-uninfected individual in the population cohort.
Corresponding values for a model in which community-level ART coverage and HIV prevalence are used as continuous variables (%); ART coverage aHR = 0.986 (95% CI 0.981 to 0.991), P < 0.0001; HIV prevalence aHR = 1.038 (95% CI 1.026 to 1.050), P < 0.0001. We sequentially added polynomial terms for the community-level HIV prevalence and ART coverage to this model, in order to allow greater flexibility in the functional form of the relationship between these factors and HIV acquisition. However, none of these polynomial terms were significant and we thus did not include them in the analyses shown here.
Indicates variables that vary with time.
As expected, a key independent predictor of the risk of acquiring new HIV infection was the level of existing HIV infection in the local community surrounding an HIV-uninfected individual (Fig. 3C). Controlling for other factors, an HIV uninfected individual was 2.2 times as likely to acquire HIV in a community where HIV prevalence was >25% relative to the base category of <10% (P < 0.0001). In line with previous research conducted in this population, age, sex, number of sexual partners in the past 12 months, and marital status were other independent predictors of HIV acquisition (Table 1). The ART coverage hazard ratios were robust to the addition of different control variables and changed little across four nested models (table S2). Living in a peri-urban community was also associated with a greater risk of acquiring infection, but this effect was rendered insignificant by the addition of community-level HIV prevalence (table S2).
In the multivariable base-case model, we control for the known important determinants of HIV acquisition in this population (16, 17). Moreover, the lack of spatial clustering observed in the model residuals (see supplementary materials) in this analysis suggests that unmeasured community-level factors are not influencing our results. Nevertheless, we also wanted to further exclude the potential influence of other HIV prevention services whose scale-up over space and time could have correlated with that of ART. To this end, we analyzed the time trends in six variables that capture the behavioral (18) or biological outcomes of other HIV prevention interventions: (i) the proportion of people who had ever had sex, (ii) the average number of sexual partners in the past year, (iii) the point prevalence of concurrent sexual partnerships, (iv) the mean age difference between partners, (v) proportion using condoms at last sexual activity, and (vi) proportion of circumcised men. We found that neither circumcision rates (which were 5.8% in 2004 and 5.0% in 2011) nor sexual partnership indicators (18) changed significantly over the study period. However, condom use at the last sexual activity with a regular partner increased significantly, from 26.2% in 2005 to 54.3% in 2011. We therefore analyzed the distribution of condom use across the surveillance area using the Gaussian kernel methodology (fig. S4) and ran a subanalysis in which we added condom use as a time- and space-varying community-level control variable to the multivariate base-case model. Controlling for condom use did not affect the strong relationship between the ART coverage and the risk of HIV acquisition (table S3 and fig. S5). This null finding is likely a consequence of the fact that condoms are only effective in preventing HIV infection if they are used correctly and consistently over a long period of time (19).
The adjusted hazard of acquiring HIV (fig. S7) shows a declining trend between 2004 and 2011. To investigate whether changes in the community over time, other than the behavioral and biological risk factors that we directly measured, might explain the ART coverage result, we added time-period dummy variables to our regression equation (table S4). After inclusion of these dummy variables, the ART coverage effect size estimates remain essentially unchanged.
The ART coverage result proved to be robust to a wide range of changes in the multiple sensitivity analyses we performed, including different survival model specifications (table S8), different age-eligibility criteria (table S10), different methods for imputing the date of HIV seroconversion (tables S5 and S6), and a range of different size (radius = 2.5 to 3.8 km) and shape kernels used in constructing the community-level variables (table S7). In the base-case analysis, values that were missing for two of the covariates, marital status and number of sexual partners in the past year, were imputed using multiple imputation (see supplementary materials). As a sensitivity analysis, we repeated our regressions with the sample of complete cases only, that is, the sample of individuals without any missing values. The ART coverage effect size estimates in this analysis remained very similar to those in the base-case analysis (table S9). As described above, the ART coverage results also persisted after we controlled for the influence of other prevention activities that had changed over the study period (table S3), as well as after adjusting for the trends in calendar time (table S4). Consistent with the “treatment-as-prevention” hypothesis, the decline in the risk of acquisition of HIV infection with increasing ART coverage in the older age groups (>35 years of age), where ART coverage was highest, was more pronounced than for the population as a whole (fig. S6). We also formally investigated whether selection effects could affect the results, by imputing HIV status for every individual who was eligible for testing in a particular year (see supplementary materials).We then used the same Gaussian kernel technique as for the base-case analysis to derive a prevalence map and resulting ART coverage estimates. Next, we reran the main model using these alternative community-level estimates. Using the imputed estimates to calculate community-level HIV prevalence and ART coverage had little bearing on the results (table S12). Further, to ascertain whether our findings would also continue to hold if we used an alternative (but less sensitive) approach, we ran an isigodi fixed-effects analysis, with HIV prevalence and ART coverage as time-varying variables. An isigodi is a traditional Zulu area (plural: izigodi); there are a total of 23 izigodi in this surveillance area. This analysis also confirmed a strong ART coverage effect (table S11).
Our findings build on the results from the recent HPTN 052 study that showed that if an HIV-uninfected person adheres to an effective antiretroviral therapy regimen, the risk of transmitting the virus to their uninfected sexual partner can be reduced by 96% (3). The trial was conducted under highly controlled conditions among stable, serodiscordant couples who had mutually disclosed HIV status to each other. High levels of ART adherence were achieved, and only 5% virologic failures in the intervention group were observed over the 42-month study period. By contrast, in the Hlabisa Treatment and Care Programme, 23% of patients initiated on ART had unsuppressed viral loads after having been on treatment for more than 12 months (20). The results presented are in line with recent modeling predictions in which an established stochastic mathematical model was calibrated specifically to the demographics, sexual behavior, and rate of ART scale-up observed in this population (21). The model projects a steady decline in the rate of new infections starting in 2010. Specifically, the model predicts an incidence rate ratio of 0.74 in 2011 (ART coverage at CD4+ <350 cells/µl = 63%) decreasing to 0.57 in 2020 (ART coverage at CD4+ <350 cells/µl = 84%) relative to the baseline year of 2004. Similarly, a systematic comparison of 12 mathematical HIV epidemic models demonstrated consistency in the prediction that high levels of ART coverage will reduce the rate of new infections (6).
In contrast to previous work that focused on ecological associations between uptake of ART and aggregate outcomes associated with the rate of new HIV infections in a given geographical unit (22–29), we directly measured time to HIV seroconversion in each individual and were thus able to avoid ecological fallacies and to control for a wide range of potential individual-level confounders in the analysis. By including both community-level ART coverage and HIV prevalence in the model as space- and time-varying variables, we have accounted for (and exploited for effect size estimation) the rapid scale-up in ART and associated changes in HIV prevalence in this population between 2004 and 2011 and the fact that each HIV-uninfected individual in the population will have differing exposures over time and space. It is a further strength of our data and approach that our effect size estimates capture the combined effect of multiple pathways through which ART coverage might reduce the risk of new HIV infection that are in addition to the biological effect of reducing mean viral load and consequently HIV transmission probability. For instance, HIV counseling and testing uptake might conceivably have increased because the availability of ART adds an important motivation to the other reasons for knowing one’s HIV status. The increasing exposure to HIV counseling, in turn, could have led to changes in sexual behavior in both HIV-uninfected and HIV-infected individuals. Other examples of ART-induced behavior changes include risk compensation (30) (because the availability of ART mitigates the consequences of HIV infection) and decreased sexual risk-taking (because of increased optimism about the future as ART increases life expectancy) (3). Such behavioral effects of ART may contribute to the total effect of ART coverage on HIV acquisition and would have been captured by our approach. Finally, because ART is delivered through a public-sector program in a typical rural sub-Saharan African setting, we were able to test the effectiveness of ART in reducing the risk of acquiring infection in the context of a successful, but imperfect, real-world ART program (3).
A potential threat to the validity of our finding is the possibility that individuals within low ART coverage communities differ systematically from their counterparts in high ART-coverage communities in factors that affect HIV acquisition. Thus, we exploited the detailed longitudinal, individual-level information available in the Africa Centre cohort to control for the well-known determinants of HIV acquisition in this population, as well as for the potential effects of other synchronous HIV prevention interventions. However, as in any large population-based prospective cohort study, such as the Framingham Heart Study, we cannot completely rule out biases due to unobserved confounding factors. Furthermore, it is possible that in other populations, the relationship between ART coverage and HIV acquisition could differ—for instance, due to different sexual behaviors. Future studies, in particular cluster-randomized controlled trials, are necessary to confirm and test the validity and generalizability of our findings.
Another issue to consider is the potential effect of “contamination” arising from differences in the geography of sexual partner choice. In this regard, it is important to note that our ART coverage results were robust to a range of different size and shape “communities” (kernels) used in the analysis (see supplementary materials). In addition, we have previously shown that sexual partner choice in this population has a strong local geographical dimension (17). Indeed, over the duration of this study, 67% of individuals in the cohort report having at least one sexual relationship with someone in the same small Zulu community or isigodi. (Izigodi have a median area of 16.9 km2, just 37% of the area searched around each HIV uninfected participant to derive the community-level variables using the largest Gaussian kernel in our analyses.) This observation is further supported by the striking relationship between local HIV prevalence and risk of new infection (Fig. 3C). Moreover, such unsystematic contamination effects, whereby individuals choose some sex partners outside their community, will likely lead to attenuation of the relationship between the exposure and the outcome, biasing the results toward the null hypothesis (31). It is thus unlikely that we have overestimated the effect of ART coverage on HIV acquisition due to contamination.
Our results suggest that population-level reductions in the transmission of HIV can be achieved in nurse-led, devolved, public-sector ART programs in rural sub-Saharan African settings where complete coverage of therapy under existing treatment guidelines has not yet been attained. Although key questions remain about durability of protection, long-term adherence, and transmission of ART-resistant strains to partners (32), our findings indicate that commitment to further increasing ART coverage in sub-Saharan Africa can contribute substantially to meeting the Political Declaration of the United Nations General Assembly (33) of halving sexual transmission of HIV by 2015.
Supplementary Material
Acknowledgments
Supported by grant 1R01-HD058482-01 from the National Institute of Child Health and Human Development. Funding for the Africa Centre’s Demographic Surveillance Information System and Population-Based HIV Survey was received from the Wellcome Trust, UK (grant 082384/Z/07/Z).
Footnotes
Supplementary Materials
www.sciencemag.org/cgi/content/full/339/6122/966/DC1
Materials and Methods
Figs. S1 to S7
Tables S1 to S12
References
References and Notes
- 1.UNAIDS. Together we will end AIDS. Geneva: UNAIDS; 2012. [Google Scholar]
- 2.Herbst AJ, et al. Bull. World Health Organ. 2009;87:754. doi: 10.2471/BLT.08.058982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bor J, Herbst AJ, Newell M-L, Bärnighausen T. Science. 2013;339:961. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, et al. HPTN 052 Study Team. N. Engl. J. Med. 2011;365:493. [Google Scholar]
- 5.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Lancet. 2009;373:48. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 6.Eaton JW, et al. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granich R, et al. PLoS ONE. 2012;7:e30216. doi: 10.1371/journal.pone.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelton JD. Science. 2011;334:1645. doi: 10.1126/science.1212353. [DOI] [PubMed] [Google Scholar]
- 9.Smith K, Powers KA, Kashuba ADM, Cohen MS. Current Opinion in HIV and AIDS. 2011;6:315. doi: 10.1097/COH.0b013e32834788e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Susser M. Am. J. Epidemiol. 1991;133:635. doi: 10.1093/oxfordjournals.aje.a115939. [DOI] [PubMed] [Google Scholar]
- 11.Granich R, et al. ART in Prevention of HIV and TB Research Writing Group. Curr. HIV Res. 2011;9:446. doi: 10.2174/157016211798038597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houlihan CF, et al. Int. J. Epidemiol. 2011;40:318. doi: 10.1093/ije/dyp402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.South African National Department of Health. National Antiretroviral Treatment Guidelines. South Africa: National Department of Health, Pretoria; 2010. [Google Scholar]
- 14.Tanser F, et al. Int. J. Epidemiol. 2008;37:956. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanser F, Bärnighausen T, Cooke GS, Newell ML. Int. J. Epidemiol. 2009;38:1008. doi: 10.1093/ije/dyp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bärnighausen T, et al. AIDS. 2008;22:139. doi: 10.1097/QAD.0b013e3282f2ef43. [DOI] [PubMed] [Google Scholar]
- 17.Tanser F, et al. Lancet. 2011;378:247. doi: 10.1016/S0140-6736(11)60779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath N, Eaton JW, Tanser F, Bärnighausen T, Newell M-L. Sexual behaviour trends by gender in a rural South African population-based cohort during the era of scaled-up access to VCT and ART, 2005–2010. Paper presented at the XIX International AIDS Conference; 22 to 27 July 2012; Washington, DC. [Google Scholar]
- 19.Weller S, Davis K. Cochrane Database Syst. Rev. 2002;1:CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]
- 20.Mutevedzi PC, et al. Bull. World Health Organ. 2010;88:593. doi: 10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hontelez JA, et al. PLoS ONE. 2011;6:e21919. doi: 10.1371/journal.pone.0021919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castel A, et al. Use of community viral load as population-based biomarker of HIV, Washington, DC, 2004–2008. Paper presented at the 18th Annual Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- 23.Das M, et al. PLoS ONE. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montaner JS, et al. Lancet. 2010;376:532. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood E, et al. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang CT, et al. Division of AIDS and STD, Center for Disease Control, Department of Health, Executive Yuan. J. Infect. Dis. 2004;190:879. [Google Scholar]
- 27.Katz MH, et al. Am. J. Public Health. 2002;92:388. doi: 10.2105/ajph.92.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Law MG, et al. Australian HIV Observational Database (AHOD) J. Int. AIDS Soc. 2011;14:10. doi: 10.1186/1758-2652-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porco TC, et al. AIDS. 2004;18:81. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatesh KK, Flanigan TP, Mayer KH. AIDS. 2011;25:1939. doi: 10.1097/QAD.0b013e32834b4ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P, White RF. Stat. Med. 2003;22:3089. doi: 10.1002/sim.1541. [DOI] [PubMed] [Google Scholar]
- 32.Anglemyer A, Rutherford GW, Baggaley RC, Egger M, Siegfried N. Cochrane Database Syst. Rev. 2011;2011:CD009153. doi: 10.1002/14651858.CD009153.pub2. [DOI] [PubMed] [Google Scholar]
- 33.United Nations General Assembly. Political declaration on HIV/AIDS: Intensifying our efforts to eliminate HIV/ AIDS. New York: United Nations General Assembly; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.